Abstract
In dorsal root ganglia (DRG) neurons TRESK channels constitute a major current component of the standing outward current IKSO. A prominent physiological role of TRESK has been attributed to pain sensation. During inflammation mediators of pain e.g. lysophosphatidic acid (LPA) are released and modulate nociception. We demonstrate co-expression of TRESK and LPA receptors in DRG neurons. Heterologous expression of TRESK and LPA receptors in Xenopus oocytes revealed augmentation of basal K+ currents upon LPA application. In DRG neurons nociception can result from TRPV1 activation by capsaicin or LPA. Upon co-expression in Xenopus oocytes LPA simultaneously increased both depolarising TRPV1 and hyperpolarising TRESK currents. Patch-clamp recordings in cultured DRG neurons from TRESK[wt] mice displayed increased IKSO after application of LPA whereas under these conditions IKSO in neurons from TRESK[ko] mice remained unaltered. Under current-clamp conditions LPA application differentially modulated excitability in these genotypes upon depolarising pulses. Spike frequency was attenuated in TRESK[wt] neurons and, in contrast, augmented in TRESK[ko] neurons. Accordingly, excitation of nociceptive neurons by LPA is balanced by co-activation of TRESK channels. Hence excitation of sensory neurons is strongly controlled by the activity of TRESK channels, which therefore are good candidates for the treatment of pain disorders.
The family of tandem-pore potassium (K2P) channels comprises 15 members that constitute background or leak currents and control cellular excitability. They are widely expressed in the nervous system and their activity is regulated by a plethora of extracellular and intracellular physiological messengers1. In addition, K2P channels are regulated by neurotransmitters and G-protein coupled receptors2. Rat brainstem motoneurons, for example, have been shown to be regulated by the neurotransmitter serotonin that inhibits the K2P channel TASK3 probably via the α-subunit of Gαq-coupled receptors4. Other studies have shown that diacylglycerol mediates the activity of TASK channels in transfected cell lines5 as well as in native neurons6.
In dorsal root ganglia (DRGs) and trigeminal peripheral neurons the K2P channels TREK-2 and TRESK (TWIK-related spinal cord potassium) are the major current components of the standing outward current IKSO7,8. A main physiological function of TRESK has been attributed to the modulation of nociception. Down-regulation of TRESK expression by siRNA increased the sensitivity to painful stimuli9. In line with these findings overexpression of TRESK in DRG neurons attenuates nerve injury-induced mechanical allodynia10. A frameshift mutation in the KCNK18 gene coding for TRESK has recently been shown to be involved in the development of a certain form of migraine with aura11. The truncated channel protein leads to a complete loss of TRESK function and, moreover, exerts a dominant negative effect on wildtype channels12. A key feature of TRESK channels is their activation by Gαq-coupled receptors. M1 cholinergic receptors potentiate TRESK currents up to 5-fold through an intracellular pathway including phospholipase C, calcium and calcineurin13.
Important physiological mediators of peripheral nociception are substances released during inflammation after tissue injury. These factors represent a wide array of signalling molecules, such as neurotransmitters (e.g. serotonin, histamine), peptides (e.g bradykinin), lipids, neurotrophins, cytokines and chemokines14. Only recently lysophosphatidic acid was found to play a major role in inflammatory disorders with direct accumulation at sites of inflammation15. Each of these factors sensitise or excite nociceptors by interacting with cell surface receptors expressed in these neurons16.
Lysophosphatidic acid (LPA) is a small, ubiquitous lysophospholipid that is released upon tissue injury17 and acts as an extracellular molecule by binding to and activating at least five known G protein-coupled receptors, LPA1-LPA518. In the nervous system LPA signalling influences cortical development, survival, migration and proliferation of cells as well as neurological disorders such as schizophrenia and neuropathic pain19. However, its function on the molecular level is still poorly understood. During tissue injury LPA is released from activated platelets or microglia, thereby altering the activity of ion channels that regulate the excitability of neurons. In heterologous systems TREK-1, another prominent member of the K2P channel family, was shown to be down-modulated by LPA20. In primary nociceptors TRPV1 (transient receptor potential vanilloid receptor type 1) currents appear to be directly activated by this lipid21. Both channels account for significant current components in DRG neurons5,7,22.
In the present study we use heterologous gene expression to show that LPA strongly activates TRESK channels by its Gαq-coupled receptors. In primary DRG neurons the excitatory effect of LPA was shown to be balanced by co-activation of TRESK channels as revealed from differences in wildtype and functional TRESK knockout mice.
Results
Detection of TRESK channel protein and LPA receptor transcripts in DRG
TRESK antibody
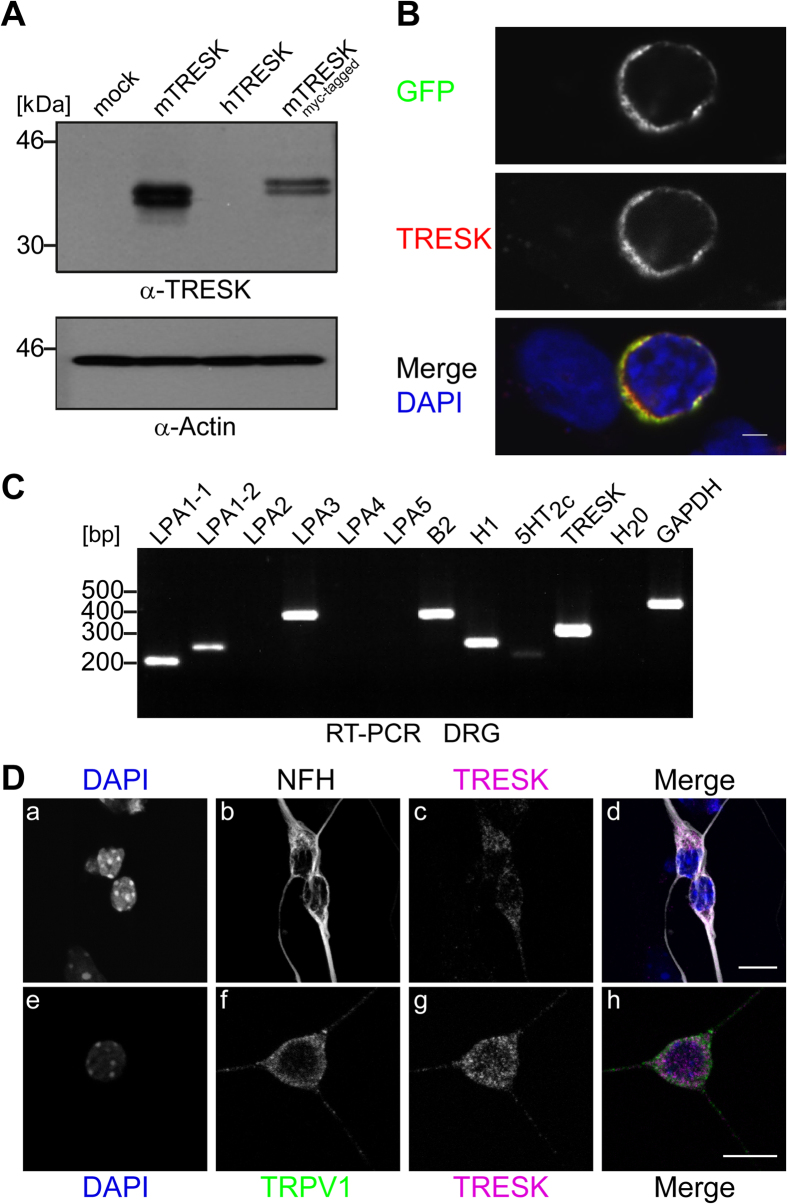
To detect TRESK channel protein in native cells we tested commercially available peptide antibodies (Alomone Labs Ltd., Santa Cruz Biotechnology, Abcam) for TRESK specificity. In our hands none of those was able to detect TRESK-specific signals by comparison of human embryonal kidney HEK-293 cells transfected with the channel or mock-transfected cells (data not shown). Thus we developed a polyclonal rabbit antibody directed against a polypeptide of 68 amino acids (Arg197-Ser264) within the intracellular loop between transmembrane segments M2 and M3 of mouse TRESK (mTRESK) subunit. Western blot analysis of whole cell protein extracts from mTRESK-transfected HEK-293 revealed a double band at the expected molecular weight of approx. 44 kD (compare 23). The same pattern at slightly higher molecular weight was found in extracts of HEK-293 cells transfected with myc-tagged mTRESK cDNA. However, recombinant human TRESK expressed in HEK-293 cells was not detected by the antibody and also no signals were found in control extracts of mock-transfected cells indicating the specificity of the antibody against mouse TRESK channels (Fig. 1A). Similarly, by immunocytochemistry antibody labelling was found only in HEK-293 cells transfected with mTRESK-GFP. Membrane staining of TRESK antibody and GFP display identical patterns as the fluorescent protein was fused to the C-terminus of the channel (Fig. 1B).
Figure 1. Expression profile of LPA receptors and immunodetection of TRESK channel in DRG neurons.
(A) Whole cell extracts of HEK-293 cells transfected with pcDNA3 (mock), mTRESK, hTRESK and myc-mTRESK plasmid DNA (as indicated) were immunoblotted and analysed with polyclonal TRESK antibody (upper panel) or anti-Actin antibody as loading control (lower panel). Extracts of cells transfected with mTRESK or myc-mTRESK displayed double bands with the expected molecular weight indicating high specificity for mouse TRESK of the novel antibody. (B) HEK-293 cells were transfected with mTRESK-eGFP fusion construct and analysed by confocal microscopy. Only transfected cells (GFP in upper panel) were found to have membrane reactivity with TRESK antibodies (middle panel). Lower panel, merge displayed co-reactivity of the antibody with GFP signal. DAPI staining in blue revealed also non-transfected cells. Scale bar 2 μm. (C) Expression of mTRESK, and a selection of Gq-coupled receptors (as indicated) was monitored in adult DRGs by RT-PCR. Primers for GAPDH were used as a positive control and H2O was applied as a negative control in reverse transcription. (D) Immunocytochemistry of cultured DRG neurons from E13.5 mouse embryos probed with antibodies against TRESK (c, g), NFH (b), TRPV1 (f) and DAPI (a, e). Images a-c and e-g are merged in panel d and h, respectively. Merge in d documented that only NFH-stained neurons (grey) displayed TRESK reactivity (magenta); DAPI-stained, non-neuronal cells (a, upper and lower edge) displayed hardly any TRESK signal (c). Neuronal co-expression of TRESK (magenta) and TRPV1 (green) was documented in merge of panel h (out of 29 TRESK-positive cells 18 were also positive for TRPV1). Fused z-stacks of confocal images are shown. Scale bar 10 μm.
TRESK and LPA receptors in DRGs
TRESK channels are involved in nociceptive signalling, which is modulated under inflammatory conditions24,25. Pro-inflammatory mediators e.g. bradykinin or serotonin, as well as LPA are released during inflammation and thus the question arises if the corresponding receptors are co-expressed with TRESK channels in DRG neurons. Using RT-PCR with gene-specific primers, transcripts of H1, B2 and 5HT2c receptors were detected in dorsal root ganglia from adult mouse. In addition a distinct set of LPA receptors, namely LPA1-1, LPA1-2 and LPA3, were found to be co-expressed with TRESK channels in this tissue (Fig. 1C) without resolution of different cell populations.
In preparations of primary cells from dissected embryonic DRGs, sensory neurons, glial cells and fibroblasts were isolated and grown together. To distinguish neurons from other cell populations, these cultures were immunostained with antibodies against neurofilament-H (NFH). Co-labelling with TRESK antibodies revealed specific dot-like signals in almost every NFH-positive cells, documenting a distinct expression of the channel, apparently with a moderate level of protein (Fig. 1D, panel a-d). TRESK immunreactivity was detected not only in neuronal cell bodies but also in the corresponding neurites (Fig. 1D, panel c and g), supporting the idea of its sensory function in the periphery. To verify if TRESK may in principle attenuate depolarisation by excitatory channels during nociception we conducted another double staining with TRPV1 and TRESK antibodies. Confocal imaging detected co-distribution of both channels in the soma as well as in neurites of the same neuron (Fig. 1D, panel e-h).
Augmentation of TRESK currents by LPA receptor activation
Expression profiles of TRESK channels24 indicate their contribution to signal transduction during inflammatory processes. Thus we tested TRESK regulation by Gαq-coupled receptors mediated by several substances released upon inflammation. Initially, receptors of well-known mediators like bradykinin, serotonin or histamine were co-expressed with TRESK channels in Xenopus oocytes.
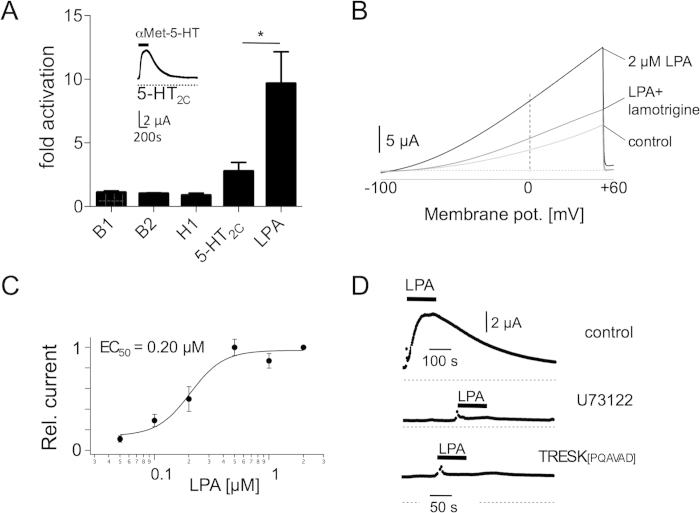
TRESK current amplitude was increased 2.77 ± 0.69 fold (n = 9) compared to basal current amplitude at a depolarising potential of +30 mV after activation of 5-HT2C receptors with 2.5 nM α-Met-5-HT (Fig. 2A inset). Activation of co-injected B1 receptors with bradykinin (100 nM) augmented TRESK currents 1.1 ± 0.12 fold (n = 8) and co-injected B2 receptors 1.02 ± 0.06 fold (n = 6), respectively. Histamine (100 nM) augmented TRESK currents less potently 0.88 ± 0.17 fold (n = 12) in comparison to other mediators (Fig. 2A). For each receptor agonist concentrations were chosen to yield maximal activation of TRESK currents.
Figure 2. Regulation of TRESK channels by Gq-coupled LPA receptors heterologously expressed in Xenopus oocytes.
(A) Co-expression of 5-HT2c, bradykinin B1, bradykinin B2, histamine H1 and LPA2 receptors together with TRESK augmented outward currents upon activation with the respective selective agonist. Inset displays representative trace with co-expressed 5-HT2c receptors. (B) Ramp recordings from −100 to +60 mV elicited outwardly rectifying currents amplified after application of LPA and blocked by lamotrigine (100 μM) (C) Dose-response curve of TRESK activation by LPA recorded at a holding potential of +30 mV with a half-maximal activating concentration of 0.2 μM. (D) LPA-induced augmentation of outward currents (upper panel) was abolished either upon incubation with the phospholipase C blocker U73122 (middle trace) or by a mutant lacking the calcineurin binding motif (lower trace).
Phospholipids such as LPA are also secreted during inflammation and were found to modulate the activity of diverse ion channels involved in pain perception20,21. Co-expression of LPA receptors and TRESK channels augmented basal K+ current 9.67 ± 2.48 fold (n = 8, two-tailed t-test, p = 0.046) upon LPA application (0.5 μM) showing this compound to be the most effective activator of TRESK channels (Fig. 2A).
As two homologues of mammalian LPA receptors are endogenously expressed in Xenopus oocytes26 we tested TRESK activation by endogenous receptors. After sole injection of TRESK cRNA into oocytes, ramp recordings from −100 to +60 mV elicited an outwardly rectifying current that was amplified 6.76 ± 1.42 fold (n = 11) upon application of 0.5 μM LPA. This G-protein coupled receptor activation could be reversed by 100 μM lamotrigine, an inhibitor of TRESK channels27 (Fig. 2B). Increasing LPA concentrations augmented TRESK current amplitude with an EC50 of 0.2 μM (Fig. 2C). To confirm a Gαq-coupled signalling pathway of LPA-induced augmentation of TRESK currents we used the specific blocker of phospholipase C (PLC) U73122. Pre-incubation of oocytes in a solution containing U73122 significantly reduced LPA-mediated TRESK augmentation to 0.4 ± 0.12 fold (n = 6, two-tailed unpaired t-test, p = 0.006; Fig. 2D, middle trace). In a previous study it has been shown that TRESK can be activated about 10-fold by the calcium/calmodulin-dependent protein phosphatase calcineurin upon binding to the consensus motif PQIVID28. To proof whether calcineurin is also necessary for LPA-induced augmentation we mutated this interaction motif by the exchange of two isoleucines with alanine (PQAVAD), as this alteration was shown to inactivate calcineurin binding28. Now application of 2 μM LPA totally abolished TRESK current augmentation (n = 12; Fig 2D, lower trace) indicating that this step of signal transduction is essential to regulate TRESK channels by endogenous LPA receptors.
In further experiments we took advantage of endogenously expressed LPA receptors that are necessary and sufficient to regulate TRESK channels.
Time-locked activation of TRESK and TRPV1 channels
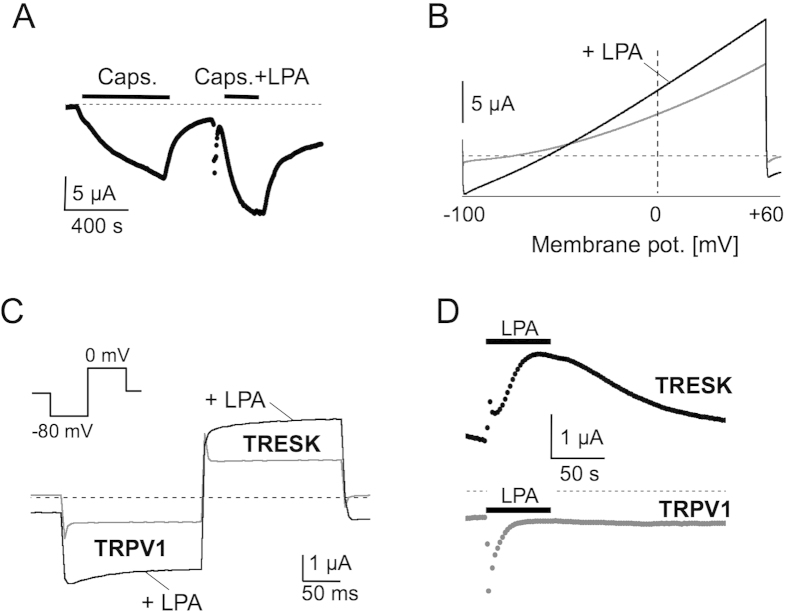
In DRG neurons the capsaicin receptor TRPV1 is one of the major molecular targets to transduce painful stimuli, such as heat, noxious chemicals or acid22. Quite recently potent activation of TRPV1 channels by direct interaction with LPA was discovered21. Therefore we used the depolarising excitatory TRPV1 current to test the role of TRESK as a regulator of excitability. TRPV1 expression in Xenopus oocytes elicited robust inward currents of 6.89 ± 1.64 μA at a holding potential of −80 mV upon application of 2.5 μM capsaicin. Upon co-application of 5 μM LPA, inward currents were augmented to 10.03 ± 2.41 μA without any deactivation kinetics (n = 4, paired t-test, p = 0.028; Fig 3A). The reversal potential of ramp recordings was −2 mV which is close to the calculated Nernst potential of non-selective cation channels (data not shown).
Figure 3. TRPV1 and TRESK are simultaneously activated by LPA.
(A) LPA augmented capsaicin activated currents in TRPV1 injected oocytes. (B) TRPV1 and TRESK co-expressing oocytes displayed an augmented inward and outward current upon application of LPA. (C) Pulse protocol to the respective reversal potentials demonstrated time-locked co-activation of TRESK and TRPV1 with LPA expressed in the same oocyte. (D) LPA-induced augmentation of TRESK and TRPV1 currents were depicted on a larger time scale. Note the fast deactivation of TRPV1 currents upon sole activation with LPA.
As TRESK channels and TRPV1 receptors are both activated by LPA we next investigated LPA induced effects in the same oocyte co-injected with TRPV1 and TRESK cRNA. Upon application of LPA ramp recordings from these cells displayed an activation of inward currents as well as outward currents with a shift of the reversal potential from initially −81.4 ± 2.7 mV to −55.6 ± 4.06 mV (n = 5; Fig. 3B). Washout of the agonist completely reversed the activation of both currents. To measure TRESK und TRPV1 currents separately in the same cell a specific pulse protocol was applied. Alternative stepping to the reversal potential of either TRESK (−80 mV) or TRPV1 channels (0 mV) allows the recording of TRPV1 currents at −80 mV and TRESK currents at 0 mV. At −80 mV application of LPA (5 μM) led to an 83 ± 20% increase of inward current (n = 14, paired t-test, p = 0.002; Fig. 3C and D) and at the same time point, TRESK outward currents were augmented by 69 ± 15% at 0 mV (n = 14, paired t-test, p = 0.004; Fig. 3C, D). Noteworthy the activation of TRPV1 by LPA displayed prominent deactivation within several seconds. Thus LPA induced co-augmentation of TRPV1 and TRESK in recombinant systems indicates a limitation of excitatory effects during inflammation when TRESK channels are involved in cellular signalling.
Regulation of excitability in DRG neurons by TRESK channels
F-11 cell line
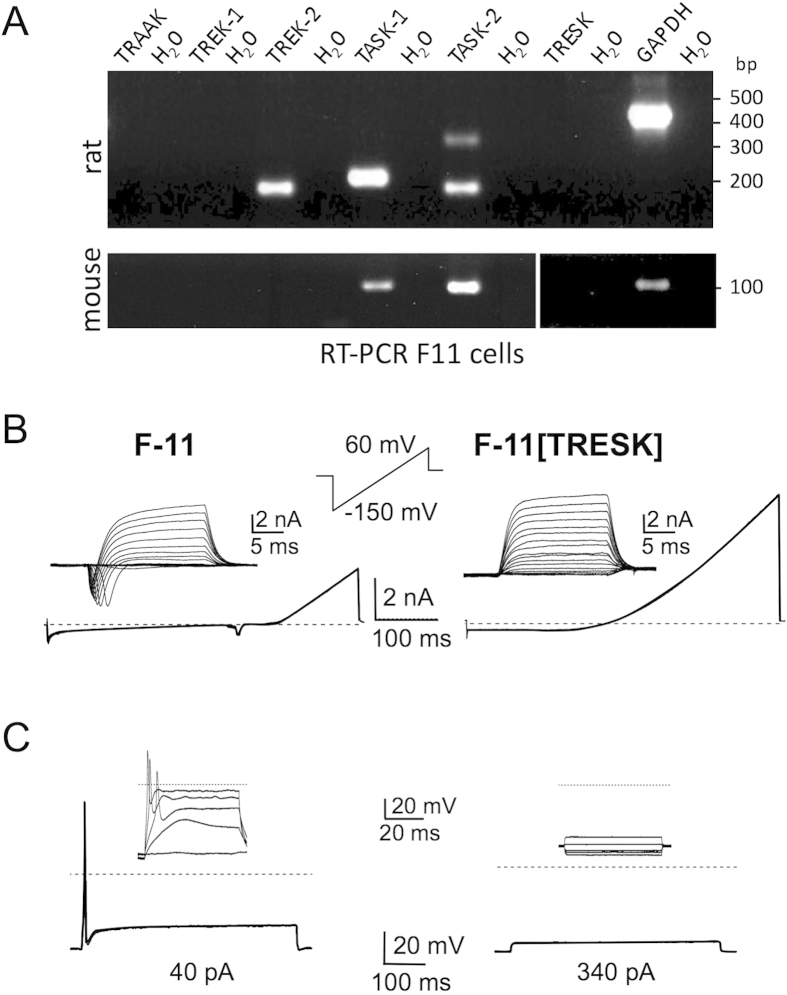
Next we tried to elucidate how TRESK channels are involved in the regulation of cellular excitability. For this purpose we chose neuronal cells derived from rat DRGs that were hybridised with mouse neuroblastoma cells for immortalisation. These F-11 cells endogenously express voltage-gated HERG K+ and Na+ channels29, Ca2+ -activated K+ channels30 as well as temperature-sensitive TRPV2 channels31. However, the expression of TRESK channels in F-11 cells is still unknown. When we conducted a RT-PCR using mouse and rat specific primers with total RNA of F-11 cells we could detect transcripts of TASK-1, TASK-2 and TREK-2, respectively but not TRESK (Fig. 4A). This lack of TRESK channel expression in combination with the ability of firing action potentials renders these cells suitable to investigate the impact of TRESK currents on neuronal excitability.
Figure 4. Transfection of F-11 cells with TRESK reduces cellular excitability.
(A) Expression profile of K2P channels in F-11 cells. PCR-primers specific for transcripts of mouse and rat (as indicated) were used to cover the hybrid genome of F-11 cells. Note that TRESK channels are not expressed. (B) Untransfected F-11 cells displayed typical voltage-gated potassium and sodium channels in ramp recordings and in depolarising step recordings (inset, left panel) whereas TRESK transfected F-11 cells displayed a shift of reversal potential to more negative values in ramp recordings and large outward currents upon depolarising steps (inset, right panel). (C) Current injection (40 pA) leads to a single action potential in non-transfected F-11 cells (left panel) whereas even high amplitude current injection (340 pA) failed to elicit an action potential in TRESK-transfected cells (right panel). Insets display current steps from −60 to 300 pA.
Ramp-recordings and depolarising pulses evoked inward and outward currents in F-11 cells, which are typical for voltage-gated K+ and Na+ channels (Fig. 4B). In the current-clamp mode depolarising pulses (40 pA) elicited single action potentials in every recorded cell (Fig. 4C; n = 18). Upon heterologous expression of TRESK in F-11 cells the resting membrane potential showed no significant alteration. However, compared with naive F-11 cells, TRESK-transfected F-11 cells exhibited a larger outward current and a loss of voltage-gated Na+ currents. Accordingly, depolarising pulses up to 340 pA failed to elicit an action potential (Fig. 4C; n = 18). Thus, transfection of F-11 cells with TRESK dampens cellular excitability to a large extent even after depolarising these cells with high pulses.
DRG neurons
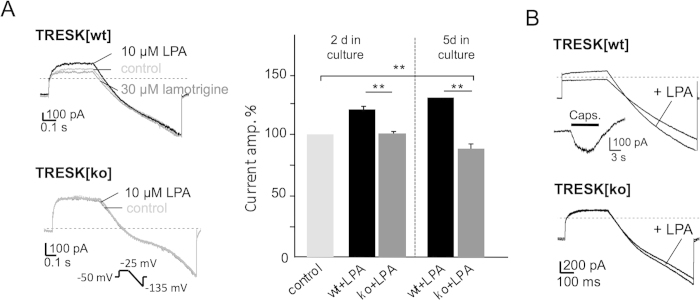
Previous patch-clamp recordings from cultured DRG neurons revealed that about 25% of the standing outward current (IKSO) is due to TRESK current8. To explore the cooperation of TRESK and depolarising currents such as TRPV1 in native DRG neurons we analysed electrophysiological effects of LPA under voltage-clamp and current-clamp conditions. To avoid clamp artefacts we first recorded currents from neurons that were grown for only 2-3 days, thus having only short outgrowing neurites. IKSO currents of DRG neurons from normal C3H mice (TRESK[wt]) increased from 492.3 to 567.6 nA (19.8 ± 2.9%; n = 11, two-tailed paired t-test, p < 0.0001) after application of 10 μM LPA. This LPA-induced activation was completely reversed by lamotrigine (30 μM; Fig 5A, upper panel). To further corroborate this finding with respect to its specificity for TRESK channels, DRG neurons from functional TRESK[G339R] knockout mice (TRESK[ko])8 were cultured and analysed. Under these knockout conditions IKSO was virtually unchanged upon LPA application (437.2 to 442.8 nA; n = 12; Fig. 5A, lower panel). After 5-6 days in culture LPA augmentation of IKSO became even more prominent. Here, the current increased by 28 ± 4.9% (n = 14, two-tailed paired t-test, p = 0.0028) whereas IKSO from TRESK[ko] decreased moderately by 12.95 ± 2.4% (n = 19, two-tailed paired t-test, p = 0.0002; Fig. 5A, right panel) after application of LPA. Most probably the inverse effect in TRESK[ko] DRG neurons resulted from TREK-1 and TASK-1 K2P channels, which also contribute to IKSO in DRG7 and were found to be inhibited by LPA20.
Figure 5. LPA receptors activate IKSO currents in DRG neurons.
(A) Patch-clamp recordings from TRESK[wt] neurons displayed an increase in current amplitude of IKSO standing-outward currents after application of 10 μM LPA (upper panel, black trace) reversed by lamotrigine (upper panel, grey trace). In neurons from TRESK[ko] mice LPA application had no effect on IKSO (lower panel). Bar graph summarises data from left panel and from neurons 5 days in culture as indicated. (B) Recordings from capsaicin positive TRESK[wt] neurons display augmentation of either outward and inward currents upon LPA application, respectively whereas TRESK[ko] neurons display only augmentation of inward currents.
DRG neurons from TRESK[wt] mice that responded to capsaicin application with depolarising currents showed augmentation of both IKSO and inward (TRPV1) currents when LPA was applied, whereas neurons from TRESK[ko] mice only displayed an augmentation of inward currents (Fig. 5B).
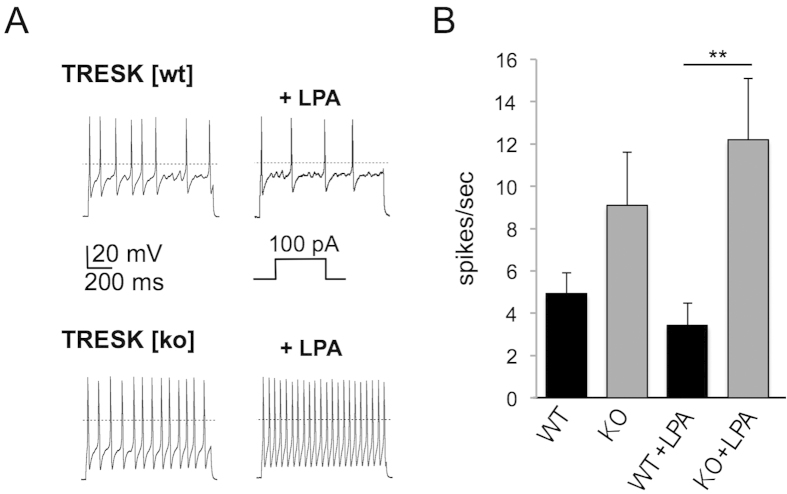
To monitor the excitability of DRG neurons from TRESK[wt] and TRESK[ko] mice spike frequencies were compared under current-clamp conditions. To further characterise this effect we paced small diameter neurons from TRESK[wt] and TRESK[ko] mice with depolarising current injections of 100 pA. More precisely, we calculated the current density by monitoring the capacity of each cell we were recording from. TRESK[wt] cells were depolarised with 7 pA/pF and TRESK[ko] cells with 5 pA/pF, respectively, to induce action potential firing. Spike frequency was 4.9 ± 1 spikes/second in TRESK[wt] neurons (n = 14) and 9.1 ± 2.6 spikes/second in TRESK[ko] neurons (n = 10; Fig 6A, left recordings and Fig. 6B). This emphasises the augmented excitability of neurons lacking TRESK currents. Remarkably, under the same conditions LPA application displayed opposing effects. Spike frequencies were attenuated to 3.4 ± 1 spikes/second in TRESK[wt] neurons and augmented to 12.2 ± 2.9 spikes/second in TRESK[ko] neurons (Mann-Whitney U test, p = 0.009; Fig. 6A, right recordings and Fig. 6B). We hypothesise that this effect is due to the activation of TRESK channels in neurons from wildtype animals thereby dampening LPA induced excitability. In neurons from knockout animals LPA only activated depolarising currents (e.g. TRPV1, TRPA1) or slightly reduced hyperpolarising currents (TREK-1 or TASK-1) leading to enhanced excitability.
Figure 6. Enhanced excitability of DRG neurons by LPA.
(A) Current-clamp recordings displayed spike trains from TRESK[wt] and TRESK[ko] neurons upon LPA application. LPA application after depolarising pulses (100 pA) reduced spike frequency in TRESK[wt] neurons (upper trace) and increased spike frequency in TRESK[ko] neurons. (B) Bar graph quantifies data shown in (A).
Discussion
Pain sensation and the adequate reaction to noxious stimuli are essential for survival. However, the normal physiological function gets lost when pain exaggerates to hyperalgesia during inflammation or chronic pain. Emerging evidence arises that ion channels regulating excitability of afferent nociceptors may constitute an appropriate target for the treatment of pain32. Cationic TRPV and voltage-gated sodium channels are the major current components for initial depolarisation after heat stimulation and propagation of action potentials. TRPV1 is predominantly expressed in small-diameter nociceptive neurons from dorsal root ganglia together with other ion channels modulating cellular excitability. Among these, tandem-pore potassium background channels have been identified to be the key players in stabilising the membrane potential and the threshold for action potential firing1. As revealed by RT-PCR and means of electrophysiology the K2P channels TREK-2 and TRESK are most abundantly expressed in DRG neurons7,8. Using immunocytochemistry we could confirm the expression profile with our newly developed, highly specific TRESK antibody. In addition we demonstrate that TRESK appears in neurites and thus is properly targeted to take over sensory function in the periphery. Within the K2P channel family TREK-1, TREK-2 and TRAAK are also significantly expressed in DRG neurons7,8,24,33. Both TREK and TRESK channels are regulated by G-protein-coupled receptors34,35. Upon stimulation of Gαs and Gαq-coupled receptors TREK-1 and TREK-2 are downmodulated by phosphorylation either by Gαs-activated PKA36,37 or Gαq-activated PKC38,39. In contrast TRESK channels are activated by Gαq-coupled signalling mediated by elevated intracellular Ca2+ and calcineurin-dependent dephosphorylation of the channel. In this former study muscarinic M1 receptors were used to potently deplete intracellular calcium stores followed by augmentation of TRESK currents up to 10-fold when expressed together with the receptor in Xenopus oocytes35,28.
TRESK has been suggested to play a significant role in nociception during inflammation as its transcript levels decreased over time after CFA (complete Freund’s adjuvans)-induced inflammation correlating with spontaneous pain behaviour in rats24. On the protein level our experiments with Gαq-coupled receptors identify the LPA receptor as the most effective activator of TRESK currents compared with B1, 5HT2c or H1 receptors, respectively. A significant increase in TRESK single channel activity was observed in DRG neurons after application of acetylcholine, glutamate and histamine27, which is in accordance to our data in recombinant systems.
Apart from well-known mediators that contribute to inflammatory hyperalgesia, oxidised lipids such as LPA have been identified to modulate nociception. During UVB-induced skin inflammation levels of LPA increase and induce peripheral hyperalgesia resistant to treatment with cyclooxygenase inhibitors40. TRPV1 channels substantially expressed in pain sensing neurons are activated by direct interaction with LPA21. In addition we clearly identify Gαq-coupled LPA receptors to be responsible for TRESK channel activation, because PLC-inhibitors and inhibition of calcineurin interaction with TRESK35 completely blocked K+ current augmentation by LPA. However, signalling by LPA receptors may also lead to inhibition of K2P channels as TREK-1 currents were inhibited by LPA when recombinantly expressed in Xenopus oocytes20.
According to the above mentioned channels their level of expression and their mode of regulation determine the excitability of a neuron. In a first step the consequences were demonstrated by co-expression of TRPV1 and TRESK channels in Xenopus oocytes. Activation of both channels by LPA is supposed to result in a limited excitatory effect of TRPV1 currents due to hyperpolarising TRESK outward currents. On the other hand an inhibitory effect of LPA on TREK-1 channels would promote the excitation by TRPV1. Considering noxious heat stimuli TRPV1 and TREK-1 are both activated and thus influence excitation to the contrary22,41, which led to the concept of noxious excitation balanced by hyperpolarising K+ currents42.
Transfection of DRG-like F-11 cells with TRESK cDNA results in large outwardly rectifying potassium currents that normally stabilise the membrane resting potential. TRESK has a minor impact on the resting potential itself as it is unchanged in non-transfected compared to transfected cells (see also8,43). Excitability can be altered without any change in resting membrane potential. For example DRG neurons display a higher excitability after sciatic nerve axotomy without significant differences in resting membrane potential10. However, TRESK expression inhibits depolarising inputs and depresses the initiation of an action potential. This clearly demonstrates the importance of TRESK channels to cellular excitability. Similarly in cultured trigeminal neurons heterologous expression of TRESK channels significantly reduced the number of evoked action potentials after depolarising pulses43. In contrast, transfection of trigeminal neurons with an inactive TRESK mutant showed a dominant negative effect on endogenous TRESK currents and thus increased cellular excitability12. In DRG neurons responses to painful mechanical stimuli were significantly increased upon in vivo silencing of TRESK channels by intrathecal injection of siRNA9. Thus different lines of evidence demonstrate the pain-alleviating effect of TRESK channel activity.
LPA is generated by the action of different enzymes like phospholipase A1 or autotaxin44 and is present in a couple of biological fluids like blood plasma and serum as well as in several organs including the nervous system17. In DRGs gene expression of LPA receptors has been demonstrated before45. In addition to LPA1 we could also detect LPA3 and exclude the expression of LPA2, LPA4 and LPA5. Although PCR approaches do not differentiate between cell populations co-expression of LPA receptors with TRESK is most likely, as the channel is localised in the majority of DRG neurons8. LPA is able to induce peripheral nociception after intraplantar injection into mouse limbs (for review see 17). Direct activation of TRPV1 channels has been considered as a molecular mechanism leading to depolarisation and spontaneous firing of DRG neurons21. We could show that simultaneous co-activation of TRESK channels by LPA could down regulate depolarisation-induced spike activity of DRG neurons from normal C3H mice (TRESK[wt]). In contrast, DRG neurons from TRESK[ko] mice lack this compensatory mechanism and depolarisation-induced activity was increased. Analgesic compounds like local anaesthetics normally suppress depolarising currents to avoid the generation of sensory potentials or propagation of action potentials in the periphery. Our findings suggest the alternative possibility to amplify hyperpolarising currents and thereby counterbalance excitation. This complements the concept proposed for TRPV1 and TREK-1 by Eric Honoré42 (see above). Whereas TREK-1 inhibition by inflammatory mediators promotes excitation of pain sensing neurons46, the activation of TRESK under such conditions reduces the excitatory influence of TRPV1 channels and thus attenuates nociception. The latter effect is assessed to be dominant as TRESK, compared to TREK-1, is the predominant K2P channel present in DRG neurons8.
A cognate TRP channel, TRPA1, is also substantially expressed in DRG neurons and may also contribute to this balanced system of opposing currents activated by inflammatory substances47. Oocytes injected with TRPA1 displayed a large inward current upon activation by allylisothiocyanate (AITC, 50 μM) that was increased upon co-application of LPA (5 μM) by a factor of 1.35 ± 0.086 (Kollert et al., unpublished).
Another line of evidence arises from TRESK mutational studies in familial migraine with aura, which show that a frameshift mutation in the TRESK gene co-segregates with a hereditary form of migraine11,48. This mutation leads to loss of function with a dominant negative effect on TRESK current amplitude11. Less current density of the mutant channel results from improper membrane targeting of the protein, at least in part11.
Our study supports the notion that TRESK channels are essentially involved in pain perception in general especially under inflammatory conditions. Recently diverse drug screening approaches identified new compounds that specifically bind and modulate the activity of TRESK channels49,50,51 suggesting TRESK to represent a promising target molecule for the treatment of severe pain disorders such as migraine.
Methods
Ethical approval
All experiments were conducted in accordance with the German legislation on protection of animals and were approved by the local animal care committee (Regierung von Unterfranken, Germany).
Molecular cloning
All experiments with recombinant TRESK channels were performed with the mouse orthologue (GenBank Acc. No. NM_207261) unless indicated otherwise. Plasmid vectors for heterologous expression of TRESK channels in Xenopus oocytes were generated as previously described23. Accordingly cDNAs of G-protein-coupled receptors LPA2 (NM_020028), serotonin 5-HT2c (NM_012765), histamine H1 (NM_008285), bradykinin B1 (BC_120684), and bradykinin B2 (L_26047) were amplified from total RNA of neuronal tissue by RT-PCR and cloned into polyadenylating transcription vector pSGEM. For recombinant protein expression in mammalian cell lines TRESK cDNA was subcloned into pcDNA3 vector. TRESK channel mutant defective of calcineurin binding (TRESK[PQAVAD]; 28) was synthesised by QuikChange mutagenesis from Aligent Technologies (Santa Clara, CA) according to the manufacturer’s instructions. Nucleotide sequences of engineered constructs were checked by sequencing service (Eurofin MWG Operon, München, Germany) and ApE analysis software (edited by M. Wayn Davis, UT).
Gene expression profiling by RT-PCR
Total RNA from DRG of wildtype male C3H mice (8–10 weeks old) and F-11 cell line was extracted with RNeasy mini kit following the manufacturer’s instructions. To remove contaminations of genomic DNA either gDNA eliminator spin columns or RNase-free DNase was applied (Qiagen). The quantity and quality (ratio OD 260/280 > 2.0) of RNA was assessed using NanoDrop UV-spectrophotometer (Peqlab Biotechnologies, Erlangen, Germany). As resolved by agarose gel electrophoresis distinct bands of 28S and 18S ribosomal RNA indicate good quality of the nucleic acid. Only intact RNA samples were used for gene expression analysis.
To verify the expression profile of K2P channels and G-protein-coupled receptors 1 μg total RNA from DRG neurons and F-11 cells was reversely transcribed with iScript (Biorad, CA) in a final volume of 20 μl. Gene specific and intron-spanning primers were applied to selectively amplify cDNA fragments of rat K2P channels (rTRAAK forward 5′-ggagcagcctcatgagcagc-3′, reverse 5′-ggtagtgatgatggtccccg-3′ [231 bp]; rTREK-1 forward 5′-gcagggattatccccttagg-3′, reverse 5′-gatccccaacccagccagtag-3′ [203 bp]; rTREK-2 forward 5′-gcaggggtcagcccggtagg-3′, reverse 5′-gaaaccgaagagcgggatccc-3′ [183 bp]; rTASK1 forward 5′-agctggagcgcgtcgtgctgc-3′, reverse 5′-cccaggctctggaacatgac-3′ [204 bp]; rTASK2 forward 5′-ggatcagtgccctgggcaag-3′, reverse 5′-gttccactcttccgtcaccatg-3′ [182 bp]; rTRESK forward 5′-cagcagctcaagccccagtgg-3′, reverse 5′-cggccaggatgtccccgatgtc-3′ [208 bp]), mouse K2P channels (mTREK-2 forward 5′-tgtggatgtatttttcctacataggtt-3′, reverse 5′-ctcttgggctggcacact-3′[91 bp]; mTASK-1 forward 5′-ctatgccttctacctcct-3, reverse 5′-cccttctgttgtcctggttt-3′ [92 bp]; mTRESK forward 5′-ggggaaggccaggggatgc-3′, reverse 5′-agagcgctcaggaaggaccagt-3′ [303 bp]; mTRESK, QT00168189 [QuantiTect Primer Assay, Qiagen]; mTREK-1, QT00250229; mTRAAK, QT00102445; mTASK-2, QT01047739), mouse LPA receptor isoforms (mLPA1-1 forward 5′-ttcgccagaggactatgaggatgt-3′, reverse 5′-tcggccaggaggaggaagaa CT-3′ [208 bp]; mLPA1-2 forward 5′-ttcgccagaggactatgaggatgtct-3′, reverse 5′-tttgtcgcggtaggagtagatga-3′ [253 bp]; mLPA2 forward 5′-cagcctgcttgtcttcctactcatg-3′, reverse 5′-gtccagcacaccacaaatg-3′ [177 bp]; mLPA3 forward 5′-caacctcctg gccttcttcatca-3′, reverse 5′-gcgcctctcggtattgctgtcctg-3′ [378 bp]; mLPA4 forward 5′-tgcggcagcccagagtc-3′; reverse 5′-tcaatgaattttctggaggca-3′ [243 bp]; mLPA5 forward 5′-tgcctgtggtagaaaggagc-3′, reverse 5′-tagggaacaacaa ggtcagagc-3′ [287 bp]) and three Gαq-coupled receptors involved in inflammation (histamin H1 forward 5′-ggagatccaggcaagggggt-3′, reverse 5′-ccacggtgtgtagcttgcgc-3′ [253 bp]; serotonin 5HT2c forward 5′-cgattgcagccgagtccgtttct-3′, reverse 5′-ccgcagtgcccaggttca-3′ [223 bp]; bradykinin B2, forward 5′gcgtccaaatgccctgctcctg-3′; reverse 5′-aaagttattggcgatggtgatgg-3′ [374 bp]).
Using 1 μl cDNA and Taq Polymerase (Qiagen) PCRs were run on a standard thermo cycler (model T3; Biometra, Germany) with the following conditions (total volume 25 μl): initial denaturation step 4 min 94 °C; 30 cycles: 1 min 94 °C, 45 s 58-63 °C, 45 s 72 °C; final elongation step 4 min. 72 °C. Samples were tested for GAPDH expression by PCR (25 cycles) with gene specific primers producing a cDNA fragment of 424 bp (forward 5′-cggcaaattcaacggcacagtcaa-3′, reverse 5′-ctttccagaggggccatccacag-3′) or 100 bp (QT00309099). Fractions of PCR samples were analysed on a 2% agarose gel.
Cell culture and transfection
With some modifications DRGs were isolated as described previously52. Briefly, adult mice anaesthetised with isoflurane were decapitated and DRGs from all levels were dissected. The ganglia were incubated in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen) containing collagenase Type 2 (10 units/ml) for 120 min at 37 °C with threefold replacement of medium. Individual cells were obtained by treatment with trypsin (10000 U/ml PBS) for 10 min and subsequently triturated with a fire-polished siliconised glass pipette. The cell suspension was centrifuged through a layer of 20% Percoll (Amersham Bioscience, Freiburg, Germany) in PBS to result the neuronal cell fraction in the precipitate. Dispersed cells were plated on poly-L-lysine-coated cover slips and cultured in DMEM supplemented with 10% horse serum and 100 units/ml penicillin-streptomycin (Pen/Strep) at 37 °C with 5% CO2. For immunocytochemical analysis DRG neurons from E13.5 mouse embryos were cultured in the presence of 5 ng/ml BDNF, NGF and NT-3 in accordance to the protocol of Jablonka and colleagues with minor modifications53.
HEK-293 cells (ATCC CRL-1573) and F-11 cells54 were cultured in DMEM supplemented with 10% fetal bovine serum and Pen/Strep at 37 °C with 5% CO2. Cells were transfected with polyethylenimine (PEI, Sigma-Aldrich, Germany) at a working concentration of 1 μg/ml. Briefly, cells were plated with a density of 1 × 105 per 35 mm dish and cultured until the following day. For transfection a vigorously stirred mixture of 12 μl PEI, 3 μg plasmid-DNA and 150 μl DMEM w/o serum was incubated for 10 min at room temperature, complemented with 850 μl full culture medium and entirely used to treat pre-cultured cells for 2–3 hours. Supplied with fresh culture medium cells were ready for experiments after 2–3 days.
Immunocytochemistry
To generate an antibody recognising mouse TRESK channels, a peptide sequence corresponding to amino acids 197–264 (RKQPD…VERSNS) of the mouse TRESK subunit was subcloned into pGEX vector resulting in a fusion construct of the partial intracellular TRESK loop and Glutathion S-transferase (GST). The fusion protein was heterologously expressed in E. coli BL21[DE3] and subsequently purified by several steps of column chromatography finished with GST-affinity purification.
Within a period of 18 weeks an adult rabbit was immunised by 4 injections of this antigen (150 μg each). Fourteen days after the final immunisation, a 50 ml serum aliquot was affinity purified on an automated liquid chromatography system using a tandem array of two GST and GST-TRESK affinity cartridges, respectively. The flow-through of the first purification cycle was subjected to five additional purification cycles, each consisting of a loading step and three washing steps followed by elution. The six eluates were pooled and dialysed against PBS. The resulting anti-TRESK antibody was concentrated through ultrafiltration and subsequently sterile filtered. Manufacturing of the antibody was conducted by immunoGlobe GmbH (Himmelstadt, Germany).
For immunocytochemistry HEK-293 cells and DRG neurons were seeded on coverslips and either transfected with mTRESK-eGFP fusion construct (HEK-293) or cultured under normal conditions (DRG). After 2–3 days HEK-293 cells were fixed with 4% paraformaldehyde (PFA) in phosphate buffered saline (PBS) and incubated with blocking buffer (PBS with 10% normal goat serum (NGS) and 0.2% TritonX-100) for 30 min at room temperature. Thereafter coverslips were incubated with rabbit polyclonal TRESK antibody (1:500) in buffer (PBS with 1% NGS and 0.2% TritonX-100) at 4 °C overnight. Following 4 washes with buffer coverslips were incubated with Alexa Fluor 555-conjugated goat anti rabbit antibody (1:1000, Invitrogen) for 2 h and then washed again 4 times with buffer and finally with H2O. The coverslips were mounted with Roti-Mount FluorCareDAPI (Roth, Germany) which in addition counterstained nuclei of the cells. Transfected HEK-293 cells were identified by GFP fluorescence. After 1 day in culture embryonic DRG neurons were fixed with 4% PFA, optionally permeabilized with 0.3% TritonX, and washed three times with TBS-T (0.1% Tween20). Prior to primary antibody incubation (rabbit polyclonal TRESK antibody, 1:500; chicken polyclonal neurofilament antibody (heavy chain), 1:5000, Millipore, Badford, MA, AB5539; goat polyclonal TRPV1 antibody, 1:300, Santa Cruz, CA, USA, sc-12498) overnight at 4 °C cells were incubated with 10% BSA (bovine serum albumin) for 1 h at RT. The following day DRG neurons were washed three times with TBS-T (0.1%), each for 5 min, and incubated with appropriate secondary antibodies (Cy3-conjugated donkey anti-rabbit, 1:700; Cy5-conjugated bovine anti-goat, 1:500; DyLight649 donkey anti-chicken, 1:500; purchased from Jackson Immunoresearch, West Grove, PA) for 1 h at RT. Finally, cells were washed three times with TBS-T (0.1%), counterstained with DAPI and embedded in heated (65 °C) mowiol. Imaging was acquired with an Olympus Fluo View FV1000 (HEK-293) or the Leica SP2 (DRG) confocal microscope. Membrane fluorescence of transfected HEK-293 cells was documented by single z-layer pictures whereas images of DRG neurons were presented as average projection stacks with a step size of 1 μm comprising 3 layers. Linear enhancement of images for visual presentation was carried out with MacBiophotonics ImageJ software processing corresponding images consistently.
Protein preparation and immunoblotting
For protein extraction transfected and non-transfected HEK-293 cells were washed with PBS and harvested by scraping the cell layer and subsequent low speed centrifugation of the cell suspension. Equal amounts of protein extracts (estimated using the BioRad protein assay) were separated on 10% polyacrylamide gels and electroblotted onto Immobilon P membranes (Millipore). Western blots were probed with our new polyclonal rabbit anti-TRESK antibody (1:1000) or monoclonal mouse anti-Actin antibody (1:1000, clone C4, Millipore, Temecula, CA). For detection HRP-conjugated goat anti-rabbit or anti-mouse immunoglobulins (1:10000; Jackson ImmunoResearch Laboratories) were applied and after washing developed with selfmade enhanced chemiluminescence detection reagent (0.1 M Tris pH 8.6, 1.25 mM Luminol, 0.6 mM p-Cumaric acid, 0.01% H2O2).
Electrophysiology
For heterologous gene expression in Xenopus laevis oocytes, capped run-off poly(A+) cRNA transcripts from linearised cDNA of receptors and channels were synthesised and injected into defolliculated oocytes. Cells were incubated at 19 °C in ND96 solution (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 5 mM HEPES, pH 7.4) supplemented with 100 μg/ml gentamicin and 2.5 mM sodium pyruvate. 48-72 hours after injection two electrode voltage-clamp measurements were performed with a TURBO TEC-10 C amplifier (npi, Tamm, Germany). Stimulation and data acquisition were controlled by Pulse software (HEKA, Gemany). Oocytes were placed in a small volume perfusion chamber with a constant flow of ND96 with 0.1% BSA or ND96 with 0.1% BSA supplemented with different concentrations of LPA. For inhibition with U73122 (Sigma Aldrich) oocytes were incubated in ND96 supplemented with 10 μM U73122 for 20 min before performing voltage-clamp measurements.
In addition, primary cultures from adult DRG neurons and F-11 cells were grown on glass cover slips. Whole-cell recordings55 were performed 2–7 days after isolation at room temperature in a bath solution consisting of 135 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 10 mM glucose, 5 mM HEPES, pH 7.4. Patch pipettes were pulled from borosilicate glass capillaries (Kimble Products, UK), and heat-polished to give input resistances of 3–7 MΩ (whole-cell). The pipette recording solution contained 120 mM potassium methansulfonate (CH3KO3S), 4 mM NaCl, 1 mM MgCl2, 0.5 mM CaCl2, 10 mM ethylene-bis(oxyethylenenitrilo) tetraacetate (EGTA), 3 mM ATP-Mg, 0.3 mM GTP-TRIS and 10 mM HEPES (pH 7.2). Currents were recorded with an EPC9 (HEKA) patch-clamp amplifier and low pass-filtered at 1-2 kHz. Stimulation and data acquisition were controlled by the PULSE/PULSEFIT software package (HEKA) on a Macintosh computer, and data analysis was performed with IGOR software (WaveMetrics, Lake Oswego, OR).
Statistics
Data are presented as mean ± SEM (number of cells). Statistical analysis was performed with Prism 6 (GraphPad Software Inc., La Jolla, CA) by using two-tailed paired Student’s t-test after testing for Gaussian distribution with the Kolmogorov-Smirnov test. For non-Gaussian distribution Wilcoxon’s rank test and Mann-Whitney U test were used, respectively. P-values ≤ 0.05 were considered to be significant.
Additional Information
How to cite this article: Kollert, S. et al. Activation of TRESK channels by the inflammatory mediator lysophosphatidic acid balances nociceptive signalling. Sci. Rep. 5, 12548; doi: 10.1038/srep12548 (2015).
Acknowledgments
Plasmid vectors for mouse TRPV1 and human TRPA1 were kindly provided by C. Nau (Anaesthesiologie, Univesitätsklinik Erlangen). We are grateful to Dr. Jürgen Solinski and Prof. Michael Mederos y Schnitzler (Walther-Straub-Institut für Pharmakologie und Toxikologie, LMU München) and Prof. Tim Hucho (Experimentelle Anaesthesiologie und Schmerzforschung, Uniklinik Köln) for providing F-11 cells. We thank Stepan Gambaryan for his generous support in TRESK antigen production, Katharina Gerber for technical assistance, Frank Krieger for help with confocal imaging and Prof. Michael Sendtner for useful comments on the manuscript. We are also grateful to Prof. Jürgen Deckert for generous support. Funding: S.K. was supported by the DFG, RTG 1253/2 and by the IZKF, Project Z-4/104, University of Wuerzburg.
Footnotes
Author Contributions S.K., B.D., F.D. and E.W. performed and analysed the experiments. F.D. and E.W. are both senior authors and contributed equally to design, concept and writing of the manuscript.
References
- Lotshaw D. P. Biophysical, pharmacological, and functional characteristics of cloned and native mammalian two-pore domain K+ channels. Cell. Biochem. Biophys. 47, 209–56 (2007). [DOI] [PubMed] [Google Scholar]
- Mathie A. Neuronal two-pore domain potassium channesl and their regulation by G protein coupled receptors. J. Physiol. 578, 377–385 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirois J. E., Lynch C. 3rd & Bayliss D. A. Convergent and reciprocal modulation of a leak K+ current and I(h) by an inhalational anaesthetic and neurotransmitters in rat brainstem motoneurons. J. Physiol. 15, 717–29 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. et al. Inhibition of a background potassium channel by Gq protein α-subunits. Proc. Natl. Acad. Sci. 103, 3422–3427 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke B. U. et al. Diacylglycerol mediates regulation of TASK potassium channels by Gq-coupled receptors. Nat Commun. 5, 1–11 (2014). [DOI] [PubMed] [Google Scholar]
- Bista P. et al. Differential phospholipase C-dependent modulation of TASK and TREK two-pore domain K+ channels in rat thalamocortical relay neurons. J Physiol. 593, 127–44 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D. & Kim D. TREK-2 (K2P10.1) and TRESK (K2P18.1) are major background K+ channels in dorsal root ganglion neurons. Am. J. Physiol. Cell Physiol. 291, C138–C146 (2006). [DOI] [PubMed] [Google Scholar]
- Dobler T. et al. TRESK two-pore-domain K+ channels constitute a significant component of background potassium currents in murine dorsal root ganglion neurons. J. Physiol. 585, 867–879 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Yang C. X., Zhong J. Y. & Wang H. B. Intrathecal TRESK gene recombinant adenovirus attenuates spared nerve injury-induced neuropathic pain in rats. Neuroreport 24, 131–136 (2013). [DOI] [PubMed] [Google Scholar]
- Tulleuda A. et al. TRESK channel contribution to nociceptive sensory neurons excitability: modulation by nerve injury. Mol. Pain 7, 1–17 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafrenière R. G. et al. A dominant-negative mutation in the TRESK potassium channel is linked to familial migraine with aura. Nat. Med. 16,1157–1160 (2010). [DOI] [PubMed] [Google Scholar]
- Liu P. et al. Functional analysis of a migraine-associated TRESK K+ Channel Mutation. J. Neurosci. 33, 12810–24 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyedi P., Braun G. & Czirják G. TRESK: the lone ranger of two-pore domain potassium channels. Mol. Cell. Endocrinol. 353, 75–81 (2012). [DOI] [PubMed] [Google Scholar]
- Basbaum A., Bautista D. M., Scherrer G. & Julius D. Cellular and molecular mechanisms of pain. Cell 139, 267–84 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevastou I., Kaffe E., Mouratis M. A. & Aidinis V. Lysoglycerophospholipids in chronic inflammatory disorders: The PLA2/LPC and ATX/LPA axes. BBA-Mol. Cell. Biol. 1831, 42–60 (2013). [DOI] [PubMed] [Google Scholar]
- Julius D. & Basbaum A. Molecular mechanisms of nociception. Nature 13, 203–210 (2001). [DOI] [PubMed] [Google Scholar]
- Ueda H. Molecular mechanisms of neuropathic pain-phenotypic switch and initiation mechanisms. Pharmacol. Ther. 109, 57–77 (2006). [DOI] [PubMed] [Google Scholar]
- Noguchi K., Herr D., Mutoh T. & Chun J. Lysophophatidic acid (LPA) and its receptors. Curr. Opin. Pharmacol. 9, 15–23 (2009). [DOI] [PubMed] [Google Scholar]
- Choi J. W. et al. LPA receptors: subtypes and biological actions. Annu. Rev. Pharmacol. Toxicol. 50, 157–186 (2010). [DOI] [PubMed] [Google Scholar]
- Cohen A., Sagron R., Somech E., Segal-Hayoun Y. & Zilberberg N. Pain-associated signals, acidosis and lysophosphatidic acid, modulate the neuronal K(2P)2.1 channel. Mol. Cell. Neurosci. 40, 382–9 (2008). [DOI] [PubMed] [Google Scholar]
- Nieto-Posadas A. et al. Lysophosphatidic acid directly activates TRPV1 through a C-terminal binding site. Nat. Chem. Biol. 20, 78–85 (2011). [DOI] [PubMed] [Google Scholar]
- Catarina M. J. et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824 (1997). [DOI] [PubMed] [Google Scholar]
- Egenberger B., Polleichtner G., Wischmeyer E. & Döring F. N-linked glycosylation determines cell surface expression of two-pore-domain K+ channel TRESK. Biochem. Biophys. Res. Commun. 391, 1262–1267 (2010). [DOI] [PubMed] [Google Scholar]
- Marsh B., Acosta C., Djouhri L. & Lawson S. N. Leak K+ channel mRNAs in dorsal root ganglia: relation to inflammation and spontaneous pain behaviour. Mol. Cell. Neurosci. 49, 375–86 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callejo C., Giblin P. G. & Gasull X. Modulation of TRESK background K+ channel by membrane stretch. PLoS ONE 8, e64471 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y. et al. Two novel Xenopus homologues of mammalian LPa11/EDG-2 function as lysophosphatidic acid receptors in Xenopus oocytes and mammalian cells. J. Biol. Chem. 276, 15208–15251 (2001). [DOI] [PubMed] [Google Scholar]
- Kang D. et al. Lamotrigine inhibits TRESK regulated by G-protein coupled receptor agonists. Biochem. Biophys. Res. Comm. 14, 609–615 (2008). [DOI] [PubMed] [Google Scholar]
- Czirják G. & Enyedi P. Targeting of calcineurin to an NFAT-like docking site is required for the calcium-dependent activation of the background K+ channel, TRESK. J. Biol. Chem. 281, 14677–14682 (2006). [DOI] [PubMed] [Google Scholar]
- Chiesa N., Rosati B., Arcangeli A., Olivotto M. & Wanke E. A novel role for HERG K+ channels: spike-frequency adaptation. J. Physiol. 501, 313–318 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruse K., McGehee D. S. & Oxford G. S. Differential responses of Ca-activated K channels to bradykinin in sensory neurons and F-11 cells. Am. J. Physiol. 262, C453–60 (1992). [DOI] [PubMed] [Google Scholar]
- Bender F. L. et al. The temperature-sensitive ion channel TRPV2 is endogenously expressed and functional in the primary sensory cell line F-11. Cell. Physiol. Biochem. 15, 183–94 (2005). [DOI] [PubMed] [Google Scholar]
- Waxmann S. & Zamponie G. W. Regulating excitability of peripheral afferents: emerging ion channel targets. Nat. Neurosci. 17, 153–163 (2014). [DOI] [PubMed] [Google Scholar]
- Kang D., Choe C. & Kim D. Thermosensitivity of the two-pore domain K+ channels TREK-2 and TRAAK. J. Physiol. 564, 103–116 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink M. et al. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J. 15, 6854–6862 (1996). [PMC free article] [PubMed] [Google Scholar]
- Czirják G., Tóth Z. E. & Enyedi P. The two-pore domain K+ channel, TRESK, is activated by the cytoplasmic calcium signal through calcineurin. J. Biol. Chem. 279, 18550–18558 (2004). [DOI] [PubMed] [Google Scholar]
- Patel A. et al. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 17, 4283–4290 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage F., Terrenoire C., Romey G. & Lazdunski M. Human TREK2, a 2P domain mechano-sensitive K+ channel with multiple regulations by polyunsaturated fatty acids, lysophospholipids, and Gs, Gi, and Gq protein-coupled receptors. J. Biol. Chem. 275, 28398–28405 (2000). [DOI] [PubMed] [Google Scholar]
- Marbatian J., Qiubo L., Sando J. J. & Bayliss D. A. Sequential phosphorylation mediates receptor- and kinase-induces inhibition of TREK-1 background potassium channels. J. Biol. Chem. 280, 30175–30184 (2005). [DOI] [PubMed] [Google Scholar]
- Kang D., Han J. & Kim D. Mechanism of inhibition of TREK2 (K2P10.1) by Gq-coupled M3 muscarinic receptor. Am. J. Physiol. 291, C649–C656 (2006). [DOI] [PubMed] [Google Scholar]
- Sisignano M. et al. Synthesis of lipid mediators during UVB-induced inflammatory hyperagesia in rats and mice. PLoS One 8, e81228 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F. et al. TREK-1 is a heat-activated background K+ channel. EMBO J. 19, 2483–2491 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré E. The neuronal background K2P channels: focus on TREK1. Nat. Rev. Neurosci. 8, 251–261 (2007). [DOI] [PubMed] [Google Scholar]
- Guo Z. & Cao Y. Q. Over-expression of TRESK K(+) channels reduces the excitability of trigeminal ganglion nociceptors. PLoS One 9, e87029 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlden S. & Georas S. N. The autotaxin—LPA axis emerges as a novel regulator of lymphocyte homing and inflammation. J. Immunol. 192, 851–857 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M. et al. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat. Med. 10, 712–718 (2004). [DOI] [PubMed] [Google Scholar]
- Alloui A. et al. TREK-1, a K+ channel involved in polymodal pain perception. EMBO J. 7, 2368–2376 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson D. A., Gentry C., Moss S. & Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J. Neurosci. 28, 2485–94 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafrenière R. G. & Rouleau G. A. Migraine: Role of the TRESK two-pore potassium channel. Int. J. Biochem. Cell Biol. 43, 1533–1536 (2011). [DOI] [PubMed] [Google Scholar]
- Sehgal S. A., Hassan M. & Rashid S. Pharmacoinformatics elucidation of potential drug targets against migraine to target ion channel protein KCNK18. Drug. Des. Devel. Ther. 8, 571–581 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P. D. et al. Cloxyquin (5-Chloroquinolin-8-ol) is an activator of the two-pore domain potassium channel TRESK. Biochem. Biophys. Res. Comm. 441, 463–468 (2013). [PubMed] [Google Scholar]
- Bruner, J. K. et al. Identification of novel small molecule modulators of K2P18.1 two-pore potassium channel. Eur J Pharmacol. 740, 603–10 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen M., Lamotte R. H., Klusch A. & Kniffki K. D. Multiple capsaicin-evoked currents in isolated rat sensory neurones. Neuroscience 75, 495–505 (1996). [DOI] [PubMed] [Google Scholar]
- Jablonka S. et al. Distinct and overlapping alterations in motor and sensory neurons in a mouse model of spinal muscular atrophy. Hum. Mol. Genet. 15, 511–518 (2006). [DOI] [PubMed] [Google Scholar]
- Platika D., Boulos M. H., Baizer L. & Fishman M. C. Neuronal traits of clonal cell lines derived by fusion of dorsal root ganglia neurons with neuroblastoma cells. Proc. Natl. Acad. Sci. 82, 3499–3503 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B. & Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 391, 85–100 (1981). [DOI] [PubMed] [Google Scholar]