Abstract
Gene transfer is an increasingly utilized approach for research and clinical applications involving the central nervous system (CNS). Vectors for gene transfer can be as simple as an unmodified plasmid, but more commonly involve complex modifications to viruses to make them suitable gene delivery vehicles. This chapter will explain how tools for CNS gene transfer have been derived from naturally occurring viruses. The current capabilities of plasmid, retroviral, adeno-associated virus, adenovirus, and herpes simplex virus vectors for CNS gene delivery will be described. These include both focal and global CNS gene transfer strategies, with short- or long-term gene expression. As is described in this chapter, an important aspect of any vector is the cis-acting regulatory elements incorporated into the vector genome that control when, where, and how the transgene is expressed.
1. INTRODUCTION
The purpose of this chapter is to provide a detailed background on vectorology as it relates to central nervous system (CNS) gene transfer. Ever since Hershey and Chase discovered that DNA was the inherited genetic material in 1952 (Hershey & Chase, 1952), and that viruses carried DNA, the notion to utilize viruses to treat genetic conditions became a natural next step. Since that time the use of gene transfer vectors as tools for research and therapeutic purposes has been extensively explored. While this chapter will touch on the use of vectors for therapeutic purposes, it will mostly describe their capabilities as general CNS gene transfer reagents.
To begin with, a few relevant terms will be defined. A vector is a vehicle used to move genetic information into a target cell. While gene transfer refers to the movement of genetic material, transduction more specifically refers to the successful delivery and expression of a foreign nucleic acid within a target cell. Transduction typically refers to virus-mediated gene transfer, while transfection refers to nonviral gene transfer. While gene transfer can refer to any movement of genetic material, it should not be termed gene therapy until the gene transfer results in a positive therapeutic outcome.
The scope of this chapter will cover the most common vectors for CNS gene transfer, including plasmid-containing nanoparticles, and recombinant vectors derived from retroviruses, adeno-associated virus (AAV), adenovirus, and herpes simplex virus (HSV). Retroviral and AAV vectors have become the prominent CNS delivery tools, and these will be covered in the most detail. Each of these vectors has advantages and disadvantages for specific gene transfer needs. For each viral vector, background will be provided on the virus from which the vectors were derived and on the process that was undertaken to engineer a wild-type (wt) virus into a gene transfer vector. Further modifications, such as transcapsidation or pseudo-enveloping, are described that alter the targeting of the vectors based on the needed gene transfer application. The nanoparticle design, viral capsid, or viral envelop dictates what cells are targeted. Within that population of cells, which receive the transgene, expression and vector genome persistence is dictated by the design of the expression cassette. This is an extremely important aspect of the over vector design and key cis-acting regulatory elements and expression systems will be discussed.
There are other vectors that have been used for CNS gene transfer, notably including a large range of strategies to develop antitumor therapies. Many of these approaches utilize replicating lytic viruses to specifically infect and eliminate cancer cells. These strategies and vectors are not covered in this chapter except for some examples provided in the section on HSV vectors, but they are covered well in recent review articles (Assi et al., 2012; Tobias, Ahmed, Moon, & Lesniak, 2013). Similarly, the topics covered will be limited to the delivery of transgene expression constructs. For the purpose of this chapter, this excludes the delivery of small oligonucleotides which by some definitions could be considered gene therapy.
2. VECTORS FOR CNS GENE TRANSFER
2.1 Plasmid DNA/Nanoparticles
Perhaps the simplest, cheapest, and least toxic approach to gene therapy is to utilize naked plasmid DNA to express a foreign transgene. The normally transient nature of gene expression from plasmids is usually seen as a detriment, but it could be a benefit in certain applications where only short-term gene expression is needed or risks of long-term transgene expression are uncertain (such as delivery of growth factors). In contrast to some viral vectors, the use of plasmid DNA minimizes safety complications such as high frequency of insertional mutagenesis and immunogenicity to the viral carrier. Moreover, the plasmid could be injected repeatedly as needed, similar to pharmacologic intervention. Unfortunately, the uptake of naked DNA into cells, subsequent trafficking to the nucleus, and long-term retention of transcriptionally active plasmid is an extremely inefficient process. Moreover, repeated direct injection of vector into the brain parenchyma is not practical. Approaches to deliver plasmids to the brain are mostly focused on packaging the plasmids within nanoparticles that can be administered peripherally. The use of various transfection reagents complexed to the DNA, as well as cis-elements in the plasmid design to promote stability, is the main area of optimization using nonviral DNA vectors.
The field of nanoparticle design is rapidly evolving, and although it is often developed for protein or small molecule delivery to the brain it can often be adapted to carry plasmid cargo. A common nanoparticle design is to use a polyethylene glycol polymer as a basic scaffold of the nanoparticle, to provide stability and basic structure. Specific receptor ligands can be incorporated into the nanoparticle to target them to specific tissues or cells, often termed “Trojan horse liposomes” (reviewed by Boado and Pardridge (2011), Boado (2007), Tosi et al. (2013)). As an example, Zhang and colleagues created polyethylene glycolylated immunoliposome (PIL) that incorporated receptor ligands for blood–brain barrier (BBB) transporters. After intravenous infusion of reporter plasmids contained in these PILs, widespread and efficient neuronal expression of the reporter genes was achieved in mouse and rat brains (Zhang, Boado, & Pardridge, 2003). This approach was applied in a therapeutic preclinical setting for mucopolysaccharidosis (MPS) type VII, wherein a plasmid-containing nanoparticle expressing the GUSB gene was administered to MPS VII mice intravenously and led to thereapeutic levels of GUSB enzyme production in the brain and peripheral organs (Zhang, Wang, Boado, & Pardridge, 2008).
Expression is typically transient, requiring repeated administration of the plasmid-containing nanoparticles. This is due to either silencing of the plasmid DNA and/or failure to replicate with dividing cells. Incorporation of specific cis-acting chromatin modulators into the plasmid can increase the efficiency of plasmid retention and lead to long-term gene expression through the inhibition of heterochromatin formation. The ubiquitous chromatin-opening element and insulator elements such as the chicken beta-globin hypersensitive site 4 (HS4) were identified as optimal elements to enhance gene expression strength and length (Hagedorn, Antoniou, & Lipps, 2013). Another common modification to prolong expression and improve the safety of plasmid vectors is to remove CpG dinucleotides from the plasmid sequence, which are known to promote both heterochromatin formation as well as inflammatory responses (Takahashi, Nishikawa, & Takakura, 2012; Tolmachov, 2009).
In conclusion, plasmid-based vectors have a potential advantage over viral-based vectors due to their reduced immunogenicity and safety. In certain applications, the transient nature of expression is an advantage. Evolving improvements in nanoparticle design and incorporation of cis-DNA elements may increase their equivalence to virus-based approaches for gene transfer, allowing efficient gene transfer and long-term transgene expression after a single dose. A technical challenge is the scalability of increasingly complex nanoparticle designs for large animal and human use.
2.2 Adeno-Associated Virus
2.2.1 Background and Advantages/Disadvantages
AAV has emerged as one of the safest and most commonly used vectors for the delivery of therapeutic genes (reviewed in Lentz, Gray, and Samulski (2012)). AAV belongs to the Parvoviridae family in the Dependovirus genus, which depend on the coinfection of a helper virus (adenovirus or HSV) for replication in host cells (Atchison, Casto, & Hammon, 1965). In the absence of a helper virus, AAV may stably integrate, albeit at relatively low frequency, into the host gene cell and remain quiescent. Consequently, wt AAV and recombinant AAV (rAAV) used for gene therapy do not have any known associated pathologies and cause a very mild immune response. The human population has widespread exposure to a variety of AAV serotypes, however. Preexisiting immunity and the presence of anticapsid neutralizing antibodies to AAV is a serious challenge to overcome in human clinical trials utilizing rAAV.
AAV is a small, non-enveloped virion that is only ~20 nm in diameter and has an icosahedral protein capsid encompassing ~4.7 Kb of linear single-stranded DNA (Cassinotti, Weitz, & Tratschin, 1988; Rose, Maizel, Inman, & Shatkin, 1971; Xie et al., 2002). The benefit of a small genome is that scientists can easily manipulate AAV to package a transgene of interest into rAAV for the purpose of gene delivery. A disadvantage of a small vector genome, however, is that the size of the transgene that can be packaged is limited and large genes are not suitable for use in a standard AAV vectors. Multiple serotypes of AAV exist with distinct tissue selectivity and transduction efficiency due to differences in the capsid protein composition. Extensive research of naturally occurring serotypes and manipulation of these serotypes in the laboratory has provided the knowledge for engineering capsids of rAAV vectors that have a broad selection of specifically targeted tissues with minimal transduction of off-target cells, tissues, and organs. Importantly, the duration of AAV-delivered transgene expression is essentially permanent in nondividing cells following just a single dose. AAV-delivered transgenes express for more than 6 months in the mouse brain (Klein et al., 1999) and can persist in other tissues for at least 6 years in primates (Rivera et al., 2005) and at least 8 years in dogs (Niemeyer et al., 2009; Stieger et al., 2009). Importantly, a recent gene therapy trial has shown that the therapeutic effects of AAV-delivered transgenes can persist for at least 10 years in the human brain (Leone et al., 2012). AAV vector plasmids have been further improved to allow for the large-scale production of highly pure vector necessary for the treatment of humans. Overall, rAAV vectors have emerged as a viable delivery method for human gene therapy as they can be designed to meet the precise treatment needs of a given disease by delivering a gene to specific cell types within the affected tissues with a minimal immune response.
A final concern, similar to other viral vectors, is the possibility of immune clearance of the vector and vector-infected cells (Vandenberghe & Wilson, 2007). An estimated 25–30% of the human population carries neutralizing antibodies against AAV2, the most common serotype for rAAV-mediated clinical trials (Halbert et al., 2006; Hildinger et al., 2001; Xiao et al., 1999). The use of other AAV serotype capsids that are less prevalent in the human population, as well as modification of immunogenic epitopes on the capsid surface, may also circumvent this problem.
2.2.2 Basic Biology and How AAV Is Used as a Vector
The single-stranded DNA AAV genome consists of three open reading frames that are flanked on either side by 145 base-pair inverted terminal repeats (ITRs) (Lusby, Fife, & Berns, 1980; Sonntag, Schmidt, & Kleinschmidt, 2010; Srivastava, Lusby, & Berns, 1983). The ITRs are predicted to form a stem loop structure. ITRs are the only cis-acting elements that are necessary for genome replication, integration, and packing into the capsid (Lusby et al., 1980; Nash, Chen, & Muzyczka, 2008; Straus, Sebring, & Rose, 1976). The wt viral genome encodes four replication proteins (Rep 78, 68, 52, 40) that are critical for many aspects of the viral life cycle, three viral structural proteins (VP1, 2, 3) that form the virion capsid and the assembly activating protein (AAP), which is thought to localize the AAV capsid proteins to the nucleolus and function in capsid assembly (reviewed in R. H. Smith (2008)). The capsid is the protein shell of a virus that protects the genetic material of a virus while interacting with the host environment. As such, the capsid determines the tissue specificity or tropism of a given virus by regulating the immediate cellular response to the virus, mediating pathways for internalization into the cell, and functions in the uncoating process within the nucleus. The capsid is icosahedral and has 20 equilateral triangular faces, with each face consisting of VP1, VP2, and VP3 proteins that are estimated to combine in a ratio of 1:1:10 in wt AAV (Kronenberg, Kleinschmidt, & Bottcher, 2001). VP1 and VP2 are identical to VP3 except that they have an additional N-terminus. Specific regions of the capsid proteins interact with receptors and coreceptors on the host cellular surface to mediate the viral infection process and serotypes can differ with respect to the receptors that they bind to. AAV infects a host cell through receptor-mediated endocytosis via clathrin-coated pits (Bartlett, Wilcher, & Samulski, 2000). Following phagocytosis, the virus must escape from the early endosome and be transported to the nucleus where uncoating occurs to release the viral genome that is then transformed to double-stranded (ds) DNA (Ferrari, Samulski, Shenk, & Samulski, 1996). In the absence of a helper virus, wt AAV DNA can be retained in linear and circular episomal forms (Duan et al., 1998; Schnepp, Jensen, Chen, Johnson, & Clark, 2005) or it can be stably integrated into the host cell genome on human chromosome 19 (Cheung, Hoggan, Hauswirth, & Berns, 1980; Kotin et al., 1990; Samulski et al., 1991). Coinfection with a helper virus or cellular stress triggers a lytic cycle where AAV transcription and DNA replication are reactivated to produce AAV viral particles.
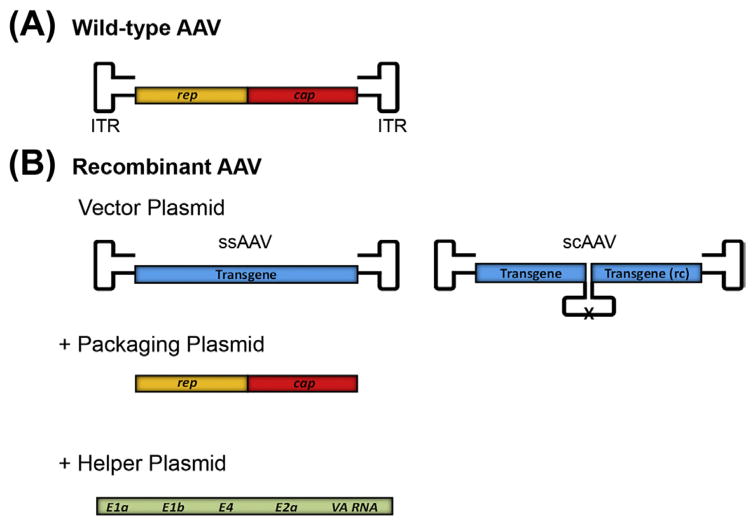
Over the last 30 years, wt AAV plasmids have been drastically transformed to create nonpathogenic, pure rAAV vectors that can be used for human gene therapy (Figure 3.1). rAAV vectors are derived from a wt AAV plasmid construct that retains only the ITRs that flank a transgene cassette that consists of a promoter with a gene of interest. AAV2 was the first extensively used AAV serotype and as a result, the majority of rAAV vectors today contain the ITRs from AAV2. The AAV genome is limited to a packaging capacity of ~4.7 kb, and the genome cannot exceed this size (Dong, Nakai, & Xiao, 2010; Dong, Fan, & Frizzell, 1996; Hirsch, Agbandje-McKenna, & Samulski, 2010; Lai, Yue, & Duan, 2010; Wu, Yang, & Colosi, 2010), so the AAV rep and cap coding sequence are replaced with the transgene cassette in rAAV vectors. The rep and cap genes are then expressed on a separate plasmid, called an AAV packaging plasmid or AAV helper plasmid. The separation of these genes from the vector plasmid DNA is critical to prevent the formation of wt AAV. rAAV also loses the specificity of integration into human chromosome 19 and appears to integrate randomly at an infrequent rate while most genomes are maintained as episomes. To generate rAAV, the vector and packaging constructs must be cotransfected into cells that have been infected with a helper virus, such as adenovirus. Adenovirus functions as a helper virus by supplying the E1a, E1b, E2a, E4orf6 and viral-associated RNA genes for rAAV production (Xiao, Li, & Samulski, 1998).While coinfection of adenovirus and rAAV vectors into producer cells is an effective means of generating rAAV, it also results in the production/contamination of adenovirus particles. To circumvent this issue, an adenovirus helper plasmid, called pXX6, was developed that contains only the essential adenovirus helper genes (Xiao et al., 1998). Using a triple transfection method, the vector DNA plasmid with the transgene cassette flanked by AAV ITRs, the AAV packaging plasmid containing the rep and cap genes of a specific AAV serotype, and the adenovirus helper plasmid are cotransfected into cells, such as HEK 293, for rAAV production (Xiao et al., 1998). Together these optimized plasmids and methods enable the large scale production of pure rAAV with low immunogenicity that can be used for gene transfer, including human gene therapy.
Figure 3.1.
Recombinant AAV vector genome design. (A) The wild-type AAV genome generally consists of the viral rep and cap genes between two inverted terminal repeats (ITRs). (B) Recombinant AAV vector is produced by cotransfection of a vector plasmid containing an ITR-flanked transgene cassette, a packaging plasmid that encodes the rep and cap genes of a specific AAV serotype and a helper plasmid that supplies the essential adenovirus helper genes (E1a, E1b, E2a, E4, and VA RNA). The vector plasmid may be either single-stranded DNA that encodes ~4.5 kb of novel transgene sequence or self-complementary (sc) DNA that can encodes ~2.2 kb of novel transgene sequence in duplex form. The sc AAV genome consists of the forward and reverse complement (rc) transgene sequences with wild-type ITRs at the extremities and a mutated ITR (X) at the axis of symmetry.
2.2.3 Transcapsidation to Change Tropism
Over 100 AAV serotypes and variants have been described (Gao, Vandenberghe, & Wilson, 2005; Wu, Asokan, & Samulski, 2006), each of which differs in amino acid sequence, particularly in the hypervariable regions or looped out domains that are found on the capsid surface (Gao et al., 2003). The most studied serotype is AAV2, which binds the primary receptor heparan sulfate proteoglycan and the coreceptors αvβ5 integrins (Qing et al., 1999; Summerford & Samulski, 1998; Summerford, Bartlett, & Samulski, 1999). AAV3 also binds to heparan sulfate, although with an elution profile that is quite distinct from AAV2, suggesting that these serotypes interact differently (Rabinowitz et al., 2002). In contrast, AAV4 and AAV5 do not interact with heparan sulfate but instead interact with sialic acid moieties, although through different linkages (Kaludov, Brown, Walters, Zabner, & Chiorini, 2001; Walters et al., 2001). See Table 3.1 for an expanded list of the receptor specificity for each serotype. Different cell types have different cell surface receptors, so each AAV serotype transduces multiple cell types with distinct specificity between serotypes and with varying efficiency. This was well exemplified in a study using AAV serotypes 1–9 packaging a luciferase reporter gene that was injected into the tail vein of mice (Zincarelli, Soltys, Rengo, & Rabinowitz, 2008). It is also thought that serotype may determine the particular mechanism of viral trafficking from the cell surface to the nucleus and in the nuclear viral uncoating process, which may in turn regulate the efficiency of transduction (Keiser, Yan, Zhang, Lei-Butters, & Engelhardt, 2011).
Table 3.1.
Primary and Secondary Receptors for Common AAV Serotypes
| Serotype | Primary Receptor | Secondary Receptor(s) |
|---|---|---|
| AAV1 | N-linked α2,3-/α2,6-Sialic acid (Ng et al., 2010; Wu, Miller, Agbandje-McKenna, & Samulski, 2006) | Unknown |
| AAV2 | Heparan sulfate (Summerford & Samulski, 1998) | Integrins αVβ5/α5β1, FGFR1 (fibroblast growth factor receptor 1) (Qing et al., 1999), HGFR (hepatocyte growth factor receptor) (Qing et al., 1999), and laminin receptor (Akache et al., 2006) |
| AAV3 | Heparan sulfate (Rabinowitz et al., 2002) | FGFR1 (Blackburn, Steadman, & Johnson, 2006), HGFR (Ling et al., 2010), laminin receptor (Akache et al., 2006) |
| AAV4 | O-linked α2,3-Sialic acid (Kaludov et al., 2001) | Unknown |
| AAV5 | N-linked α2,3-Sialic acid (Kaludov et al., 2001; Walters et al., 2001) | PDGFR (platelet-derived growth factor receptor) (Di Pasquale et al., 2003) |
| AAV6 | N-linked α2,3-/α2,6-Sialic acid (Wu, Miller, et al., 2006), heparan sulfate (Ng et al., 2010) | EGFR (epidermal growth factor receptor) (Weller et al., 2010) |
| AAV7 | Unknown | Unknown |
| AAV8 | Unknown | Laminin receptor (Akache et al., 2006) |
| AAV9 | N-linked β1,4-Galactose (Shen, Bryant, Brown, Randell, & Asokan, 2011) | Laminin receptor (Akache et al., 2006) |
The driving force behind the manipulation of the AAV capsid is to map and understand the contribution of the capsid amino acids, as well as to exploit this information to alter the tropism and/or transduction efficiency. The simplest way to alter the capsid is through transcapsidation, which is the packaging of a genome containing ITRs from one serotype in the capsid to a different serotype. This technique has been adopted for rAAV vectors so one recombinant genome construct can be easily packaged in multiple capsids, so that gene transfer can be targeted to different tissues. Historically, AAV2 was the most widely used serotype and early vectors that used the ITRs of serotype 2 could only be packaged in the corresponding capsid serotype to obtain sufficient yields. Rabinowitz et al. (2002) developed a cross-packaging system that allowed a vector with serotype-2 ITRs to be transcapsidated with the capsids of other AAV serotypes. In the AAV packaging plasmid, the AAV2 rep sequence downstream of the p19 promoter was replaced with the rep sequence corresponding to the desired capsid serotype. These packaging plasmids were named pXR1-5 to denote replication and packaging of a serotype-2 ITR genome into serotypes 1–5. A benefit of transcapsidation is that serotype tropism can be directly compared in vivo when they package equivalent genomes and the only variable is the AAV capsid (Rabinowitz et al., 2002; Zincarelli et al., 2008). This technique has now been expanded to mosaic capsids that are a packaged mixture of unmodified capsid proteins from different serotypes. To generate mosaic vectors, a mixture of AAV packaging constructs are used that encode capsid proteins from different serotypes or wt and mutant capsid proteins of the same serotype or even from two different capsid subunit of the same serotype (reviewed in Choi, McCarty, and Samulski (2005)). In doing so, features of each source can be handpicked that synergistically enhance transgene expression in addition to altering tropism.
2.2.4 Capsid Engineering: Rational Design, Shuffing, Peptide Insertion
One of the most critical aspects of viral-mediated gene therapy is the specific targeting of tissues that require the gene of interest with minimal transduction of off-target cells, tissues, or organs. Transduction of off-target tissues can have deleterious effects including insufficient delivery of therapeutic genes to target tissue and large therapeutic doses to compensate for this. Tissue tropism is controlled via the viral capsid and extensive work has been done to identify naturally occurring AAV serotypes and to expand their tissue tropism through transcapsidation. Utilizing knowledge gained from naturally occurring serotypes and mosaic capsids, researchers are now able to engineer rAAV capsids to enhance tissue selectivity and specificity as well as to evade host neutralizing antibodies. Second-generation AAV vectors are generated through methods including (1) rational design based on known AAV structure and biology, (2) use of directed evolution through mutagenesis and DNA shuffling, and (3) peptide insertion of ligands into the AAV capsid (reviewed in Gray, Woodard, and Samulski, (2010)). Together, these newly engineered AAV vectors offer a broad range of selection to meet different experimental and therapeutic needs.
The rational design method of vector engineering uses the current body of knowledge of capsid protein structure–function to insert or exchange small epitopes into the capsid shell as a means of retargeting AAV tropism. As the functions of each component of the capsid shell are delineated, this information can be used to select or remove specific components of the viral tropism, until only the desired properties are combined into a single capsid coating. In addition to altering cell specificity, rational design can be used to enhance the efficiency of gene transduction and improve the safety profile of a given vector. Rational design has been used to develop chimeric AAV capsids, which have critical amino acids and/or domains from one serotype incorporated into the capsid of another. The choice and locations of these amino acid modifications are determined by analysis of known capsid function combined with structural information. For example, using the AAV2 capsid, Bowles et al. (2012) used sequence alignment and site-directed mutagenesis to swap five AAV1 amino acids into the AAV2 capsid that were different from the AAV2 sequence and were located in a structurally variable region on the capsid surface to create the AAV2.5 vector. The AAV2.5 vector had improved muscle targeting properties of AAV1, as well as reduced cross-reactivity with antibodies against both AAV1 and AAV2, and was successfully used in Phase 1 clinical trials for Duchenne muscular dystrophy to deliver the minidystrophin gene into the muscles of patients with no adverse effects (Bowles et al., 2012). In another example of capsid mutagenesis, Pulicherla et al. (2011) introduced point mutations into AAV9 to knock down its liver tropism, potentially creating a safer version of AAV9 to deliver intravenously to the CNS. Rational design of AAV capsids can also be accomplished by mutating tyrosine residues on the capsid shell. Phosphorylation of tyrosine residues on the capsid of AAV2 has been shown to negatively impact the intracellular trafficking of virus following uptake and transgene expression in vivo (Zhong et al., 2008). Mutation of surface exposed capsid protein tyrosine residues to phenylalanine results in increased transduction efficiency due to reduced intracellular trafficking to the proteasome and improved intracellular trafficking to the nucleus (Zhong et al., 2008). A similar increase in transduction efficiencies was also found when capsid tyrosine residues on AAV2, AAV8, and AAV9 were mutated to prevent phosphorylation (Petrs-Silva et al., 2009).
Directed, or molecular, evolution is an unbiased method by which investigators randomly mutate and/or shuffle the cap protein coding sequence to develop novel AAV vectors with altered tropism. This approach expands upon the DNA shuffling technique originally developed by Stemmer, which uses random fragmentation of a gene to create pools of selected mutant genes that are then reassembled into full-length sequences using a polymerase chain reaction (PCR)-like process (Stemmer, 1994). In directed evolution, the cap genes from multiple AAV serotypes are fragmented, reannealed using a primerless PCR reaction, and then in a second PCR reaction are assembled into full-length chimeric cap genes. Chimeric viral libraries produced in this manner can then be administered systemically, the target tissue isolated, and viral capsid sequences within this tissue amplified through PCR. Using these recovered library capsids, a new library can be produced to undergo subsequent rounds of genetic diversification and selection. This approach enables the investigator to select a capsid with the desired vector phenotype from a pool of diverse mutants and does not require prior knowledge of AAV’s structure–function biology. Recently, Gray, Blake, et al. (2010) have used DNA shuffling and directed evolution to create novel AAV vectors with the ability to target therapy transgene to sites of seizure damage in the brain of an epileptic rat while being detargeted from the liver and other off-target tissues. Specifically, the capsid DNA from AAV serotypes 1–6, 8, and 9 was fragmented, shuffled, and recombined to create a library of chimeric AAVs that were then injected intravenously into rats following the induction of a seizure. Three days later, seizure-prone brain sites were harvested and viral DNA was isolated from these tissues. Through four cycles of selection, two novel AAV vectors were identified that were able to cross the seizure-compromised BBB and efficiently transduced brain cells. A potential caveat of the directed evolution approach, however, is that vectors designed in cell lines and small animal models may not have fully recapitulated properties in large animals and humans.
Peptide insertion is a method in which known ligands are directly inserted into the AAV cap gene as a means of expanding the cell or tissue tropism of the wt vector. Recently, this approach has been used to target AAV2 vectors to the CNS. For example, in one study, Xu, Ma, Bass, & Terwilliger, 2005 inserted peptides into the AAV2 capsid that were derived from an N-methyl-D-aspartate receptor agonist and a dynein binding motif to increase the axonal retrograde transport of AAV2 to the CNS by 10- to 100-fold. In a second study, Chen, Chang, and Davidson (2009) used phage-display biopanning in two different lysosomal storage diseased mouse models and in wt mice to identify peptides that bound the blood vasculature under diseased and normal conditions. By incorporating these peptides into the AAV2 capsid, AAV2 tropism could be expanded to include selective targeting of virus to the CNS vasculature of a disease model but not wt mice, or to wt mice but not to pathogenic mice (Chen et al., 2009). A major challenge with peptide insertion, however, is the disruption of the stability and function of both the ligand and the transducing vector. Additionally, a given peptide insertion allows the targeting of a single receptor, so that targeting of a new receptor requires additional genetic modification of the capsid proteins.
2.2.5 Self-Complementary AAV
Researchers have created an altered version of AAV termed scAAV that packages two complementary copies of the genome that are linked in cis through a mutated ITR (McCarty, Monahan, & Samulski, 2001). One of the factors affecting the transduction efficiency of rAAV vectors is the conversion of the single-stranded DNA vector genome into dsDNA to achieve gene expression. By avoiding synthesis of a second strand, scAAV can form a stable ds intermediate more quickly, leading to better vector genome retention and faster transgene expression. scAAV vectors are produced by deleting the terminal resolution site from one rAAV ITR, so that replication cannot be initiated from the mutated ITR (McCarty et al., 2003). These constructs result in single-stranded, inverted repeat genomes with a wt ITR at each end and a mutated ITR in the middle. After uncoating, it is thought that intramolecular base pairing begins at the mutant ITR and then proceeds through the vector genome to fold the genome into a ds or self-complementary form. Benefits of using scAAV vectors over traditional single-stranded AAV vectors include the quicker onset of transgene expression and a 10- to 100-fold increase in transduction efficiency (Gray, Matagne, et al., 2011; McCarty et al., 2003, 2001). As a caveat though, scAAV can only encode half of the already limited capacity of AAV. The AAV vector, including the ITRs, is a maximum of ~4.7 kb in length. If carrying single-stranded DNA, AAV vectors can deliver ~4.5 kb of unique transgene sequence; however, scAAV vectors are only able to carry ~2.2kb because the unique transgene sequence is in duplex form. Thus, as part of AAV vector development, much emphasis has been placed on the design of minimal promoters, 3′ and 5′ untranslated regions, and polyadenylation (polyA) signals to increase the remaining amount of available coding sequence for the transgene (reviewed in (Gray (2013)).
2.2.6 Utilizing Specific Serotype Capsids and Routes of Administration to Transduce CNS Targets
Recent advancements in vector design and the use of alternative routes of viral administration place rAAV at the forefront of vectors for gene delivery to the CNS (reviewed in (Gray (2013)). AAV vectors have been extensively used to deliver genes to neurons in both basic and clinical applications due to their ability to infect nondividing cells, high transduction efficiency, long-lasting expression from a single dose, and relatively low host immune response (Kaplitt et al., 1994; McCown, Xiao, Li, Breese, & Samulski, 1996). The most commonly used AAV serotypes in the CNS include AAV1, AAV2, AAV4, AAV5, AAV6, AAV8, and AAV9. The early characterized AAV2 vector has been most extensively used by researchers and in clinical studies, but the spread and transduction in the brain is rather limited compared to more recently characterized serotypes. While AAV2 preferentially transduces neurons after direct injection into the brain parenchyma, AAV1 and AAV5 have been shown to be more efficient at targeting neurons and transduce some glia as well, in multiple regions of rat and nonhuman primate brain regions (Mandel & Burger, 2004). When injected intracerebrally, AAV7, AAV8, and AAV9 primarily transduce neurons and AAV9 vector shows the greatest spread from the site of injection (Cassia N. Cearley & Wolfe, 2006). Viral spread is dependent upon both extracellular and intracellular transport, the latter of which can occur in either the anterograde or retrograde direction along axons (Kaspar, Llado, Sherkat, Rothstein, & Gage, 2003; Kaspar et al., 2002). Axonal transport varies amongst the AAV serotypes and can be exploited to enhance therapeutic efficacy by infecting both the cell types targeted by a vector as well as the projection field of those cells. For example, when injected into the ventral tegmental area, both AAV1 and AAV9 have been shown to disseminate along axonal projections in both directions (Cearley & Wolfe, 2007). AAV1, AAV5, and AAV9 show the greatest spread and highest transgene expression. When administered intrathecally, AAV6, AAV8, and AAV9 infect cells in the spinal cord and dorsal root ganglia, with the affected cell population dependent on the serotype (Snyder et al., 2011; Storek et al., 2008; Towne, Pertin, Beggah, Aebischer, & Decosterd, 2009). AAV4 preferentially targets ependymal cells through intracerebral ventricular injection, and this strategy was successfully employed to treat mice with MPS VII (Liu, Martins, Wemmie, Chiorini, & Davidson, 2005). Given that AAV genomes do not persist in dividing cell populations, the long-term efficacy of this approach is questionable and remains to be tested, since the ependyma has a turnover rate of approximately 130 days (Chauhan & Lewis, 1979). One of the challenges of targeting the brain for gene delivery is identifying vectors that are able to cross the BBB so that, ideally, a gene therapy can be administered peripherally. Work by Duque et al. (2009) and Foust et al. (2009) demonstrated that AAV9 crosses the BBB in mice and cats when injected intravenously in both neonatal and adult animals; AAV8 was also found to cross the BBB in mice, although to a lesser extent than AAV9 (Gray, Matagne, et al., 2011). Importantly, both neurons and astrocytes were transduced by intravenously injected AAV9 vectors, demonstrating that it is possible to deliver gene therapy to a large portion of the brain and spinal cord without having to inject directly into the CNS. The ability of AAV9 vectors to cross the BBB following intravenous injection in mice, rats, cats, and nonhuman primates has been reported by multiple groups (Bevan et al., 2011; Gray, Matagne, et al., 2011; Zhang et al., 2011), and found to be at least 10 times more efficient when performed with scAAV vectors rather than single-stranded AAV vectors (Gray, Matagne, et al., 2011; Wang et al., 2010). In mice, intravenous delivery of AAV9 has been successful in treating disorders of the CNS, including spinal muscular atrophy (SMA) (Foust et al., 2010) and MPS IIIB (Fu, Dirosario, Killedar, Zaraspe, & McCarty, 2011) in mice. Conversely, the use of AAV9 vectors to treat specific CNS disorders may be precluded by altered receptor expression on the cell surface of target tissue as part of the disease phenotype. For example, in a mouse model of lysosomal storage disease, peripherally administered AAV9 failed to transduce CNS tissue due to increased brain levels of sialic acid, which covered the terminal galactose residues used by AAV9 (Chen, Claflin, Geoghegan, & Davidson, 2012). There are several additional challenges in moving gene therapies from small animals to humans, including the presence of anti-AAV9 neutralizing antibodies in the human population, the large amounts of vector that must be produced for therapy when scaled to larger animals and humans, and the off-target distribution of virus to peripheral tissues.
One strategy for translating AAV9 gene therapy from small animals to humans is to utilize alternate viral delivery routes. Though more invasive than an intravenous injection, one approach that researchers are currently exploring is the use of intra-CSF delivery via intrathecal injection into the lumbar cistern or cisterna magna (Bevan et al., 2011; Federici et al., 2012; Gray, Nagabhushan Kalburgi, McCown, & Jude Samulski, 2013; Samaranch et al., 2012; Wang et al., 2014). Intra-CSF delivery could better target cells in the spinal cord, reduce expression in off-target peripheral organs, and lower viral doses compared to those used for intravenous delivery. In contrast to direct intracranial injection to the brain parenchyma, use of the CSF for viral delivery would still allow volume dose scaling across different species. In pigs, AAV9 delivery via intrathecal injection transduced the majority of motor neurons across the entire spinal cord with minimal targeting of virus to peripheral organs (Federici et al., 2012). In a study that compared the use of intravascular versus intracisternal injection of AAV9 in nonhuman primates, greater transduction of the CNS was achieved using intra-CSF vector delivery at a lower dose than the intravascular injections (Samaranch et al., 2012). Interestingly, circulating anti-AAV9 neutralizing antibodies were detected following intra-CSF injection (Samaranch et al., 2012). In a second study by Gray et al. (2013), intra-CSF delivery via intrathecal or intracisternal injections were compared in nonhuman primates. Intra-CSF delivery using a single injection by either method resulted in widespread transduction of AAV9 throughout the entire CNS, particularly in the spinal cord. Biodistribution to peripheral organs was detected, although at much lower levels than seen with intravascular delivery. Low levels of circulating anti-AAV9 neutralizing antibodies did not appear to have inhibitory effects on targeted gene transfer in the CNS by intra-CSF administration, although higher levels did show some inhibition (Gray et al, 2013; Samaranch et al., 2012). Overall, multiple groups have now shown that intra-CSF delivery of AAV9 vectors results in widespread expression of transgenes in large animals and support the use of intra-CSF AAV9 vector delivery for gene therapy in humans (Haurigot et al., 2013).
2.3 Retrovirus/Lentivirus
2.3.1 Introduction
HIV-1 based (lentiviral) vectors are among the most intensely studied vectors utilized for virus-mediated gene transfer. These studies established the foundation of exploiting lentiviral vectors as vehicles for efficient gene delivery into broad range of tissues and organs. The capacity of efficient integration into the host genome, ability to infect nondividing cells and shuttle large genetic payloads, and maintenance of stable, long-term trans-gene expression are attributes that have brought lentiviral vectors to the forefront of gene therapy.
A little more than 30 years after the first retroviral gene transfer experiment demonstrated transfer of a HSV, thymidine kinase (tk) gene into the genome of a mouse cells (Shimotohno & Temin, 1981; Wei, Gibson, Spear, & Scolnick, 1981), a retroviral gene transfer field has reached a stage of great diversity and progress. This development is attributed to better understanding of biology of the Retroviridae family members. As a hallmark, all members of this family are capable of converting single-stranded RNA (ssRNA) of the retrovirus into dscDNA (dsDNA), which can be then stably integrated into the host genome and replicated along with it (Baltimore & Huang, 1970; Mizutani, Boettiger, & Temin, 1970; Temin & Mizutani, 1970). As highly evolved parasites retroviruses act in concert with cellular host factors to ensure delivery of their genetic payload into the nucleus, where they exploit host machineries to fulfill replication and long-term expression.
The Retroviridae family comprises seven major genera: alpha-retroviruses (prototype ALSV), beta-retrovirus (prototype MMTV), delta-retrovirus (prototype HTLV-I, HTLV-II), gamma-retrovirus (prototype MLV), epsilon-retrovirus (prototype WDSV), lenti-retroviruses (prototype HIV-1), and spuma viruses (prototype SFV) (Figure 3.2) (classified in (Coffin (1992)). Many of these retroviruses have been studied and developed for retroviral gene transfer. Although all the aforementioned viruses have potential interest for retroviral gene transfer, so far the focus has almost exclusively been on the two genera: simple gamma-retroviruses (mammalian C-type viruses), exemplified by murine leukemia virus (MLV) (reviewed in Baum, Schambach, Bohne, and Galla (2006)), and complex lentiviruses, exemplified by HIV-1. However, inability of transducing nondividing cells has restricted employment of the gamma-retroviruses primarily for gene transfer into hematopoietic cells (Lewis & Emerman, 1994; Roe, Reynolds, Yu, & Brown, 1993; Suzuki & Craigie, 2007).
Figure 3.2.
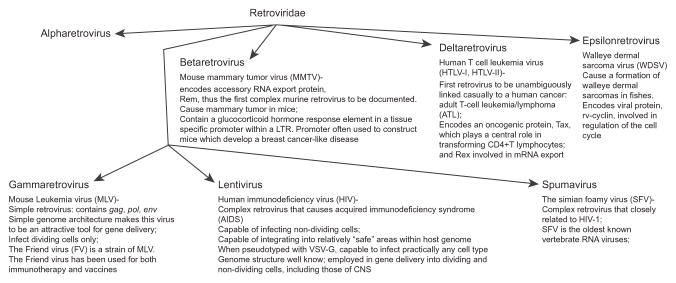
Retroviridae family comprises seven major genera: alpha-retroviruses (prototype ALSV), beta-retrovirus (prototype MMTV), delta-retrovirus (prototype HTLV-I, HTLV-II), gamma-retrovirus (prototype MLV), epsilon-retrovirus (prototype WDSV), lentiretroviruses (prototype HIV-1), and spumaviruses (prototype SFV). Notable characteristics of the viruses are included.
Lentiviral vectors, in contrast, have evolved the ability to transduce non-dividing and slowly dividing cells (Bukrinsky et al., 1993; Lewis, Hensel, & Emerman, 1992), an attribute that significantly broadened the use of the lentiviral vectors for the gene delivery into numerous tissues and organs, including the CNS.
2.3.2 HIV-1: Structure and Life Cycle
HIV-1, as mentioned above is a member of the Lentivirinae genus also including HIV-2, simian immunodeficiency virus (SIV) and nonprimate lentiviruses, such as visna virus, equine infectious anemia virus (EIAV), caprine arthritis-encephalitis virus (CAEV), and the feline and bovine immunodeficiency viruses (FIV and BIV) (classified in (Coffin (1992)) (Figure 3.2). Although, all of the aforementioned lentiviruses were intensively studied and engineered into vectors, the first lentiviral vector that developed, HIV-1 based, is still the most promising among them (Naldini, Blomer, Gage, Trono, & Verma, 1996; Naldini, Blomer, Gallay, et al., 1996). This is attributed to a better understanding of the basic biology and the life cycle of the HIV-1 (for more comprehensive review see Coffin, Hughes, and Varmus (1997)).
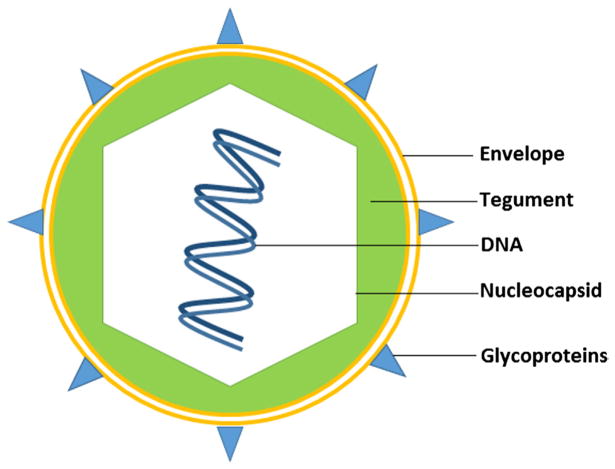
A capsid of the HIV-1 is an enveloped protein shell that is 80–100 nm in diameter and contains the viral genome. The HIV-1 genome is encoded by an approximately 9 kb positive sense single-stranded RNA molecule, which is packaged within lipid-enveloped viral particles. The HIV-1 env gene encodes the envelope glycoprotein of the virus. The native envelope of HIV is a glycoprotein, gp120, that is essential for viral entry into cells as it plays a vital role in attachment to specific cell surface receptors via a specific interaction with the CD4 receptor and coreceptors, which are located on macrophages, dendritic cells, and particularly on helper T-cells. Binding to CD4 induces the start of a cascade of conformational changes in gp120 and its internal part, gp41, that lead to the fusion of the viral and host cell membranes. Interaction between gp120–gp41 and the host receptor and coreceptors is under intensive investigation, since it is hoped that better understanding of this step will be essential for the design of a vaccine against HIV-1 (Julien et al.; Lyumkis et al.; Merk & Subramaniam).
Following attachment and entry into host cells, viral reverse transcription takes place in the host’s cytoplasm. The process of reverse transcription generates a ds linear DNA that serves as a precursor for integration (Figure 3.3). This DNA is collinear with its RNA template, but it contains terminal duplications on both ends: U3 region at the 5′-LTR (long terminal repeat) harbors the promoter sequence, while U5 region at the 3′-LTR carries the poly-A signal of HIV. Viral LTRs are fully restored prior to integration. Other indispensable elements within the HIV genome include the primer binding site (PBS) and polypurine tract (PPT). A PBS is a region proximal to the 5′-LTR where a primer binds to initiate minus-strand synthesis. The primer of HIV is delivered by a tRNA3Lys, although the virus can utilize other tRNAs (Hansen, Schulze, Mellert, & Moelling, 1988; Panganiban & Fiore, 1988).The plus-strand DNA primer is provided by a 15-nucleotide PPT, a purine-rich sequence generated from viral RNA by the RNase H activity of reverse transcriptase (reviewed in Rausch and Le Grice, (2004)). The PPT is highly conserved in most retroviruses and has been shown to be selectively used as the site of plus-strand initiation. Soon after completion of the DNA synthesis, viral integrase protein (Int) recognizes and cleaves within the att sites located on the both ends of the viral DNA, eliminating the terminal two bases from each 3′ end. The resulting recessed 3′-OH group defines the pro-virus attachment sites utilized by the viral cDNA for integrating into the host chromosomes (Colicelli & Goff, 1985, 1988a, 1988b; Bushman & Craigie, 1990; Bushman, Fujiwara, & Craigie, 1990; Craigie, Fujiwara, & Bushman, 1990; Leavitt, Rose, & Varmus, 1992). Following integration, the DNA of the virus replicates along with the host genome and passes on to the cell’s progeny; thus, all descendants of the infected cell will also bear proviruses in their genomes (Buchow, Tschachler, Gallo, & Reitz, 1989; Farnet & Haseltine, 1991; Shin, Taddeo, Haseltine, & Farnet, 1994). Following replication, viral mRNA is transcribed by the host RNA polymerase II.
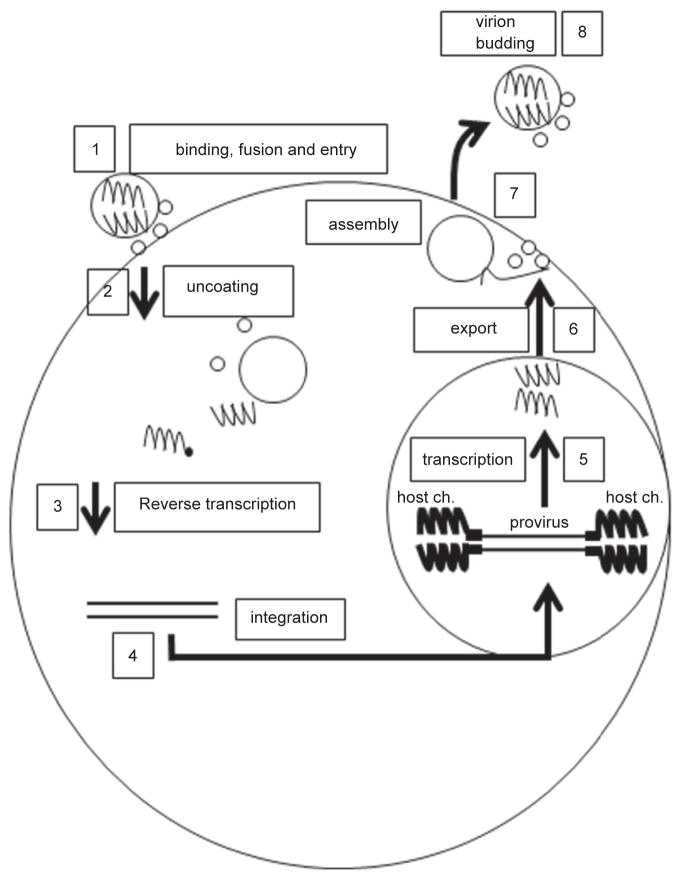
Figure 3.3.
Life cycle of the HIV-1 including binding-fusion, uncoating, reverse transcription, integration, transcription, nuclear export, assembly and virion budding are shown. The star represents reverse transcriptase. Steps of the life cycle labeled from one to eight.
In addition to core proteins encoded by three genes of the HIV-1: gag (encodes viral matrix, capsid, and nucleocapsid proteins), pol (encodes a protease, reverse transcriptase, and integrase), and env (encodes a surface envelope protein), complex retroviruses, such as HIV-1, harbor six additional genes: two regulatory (rev and tat), and four accessory genes (nef, vif, vpr, and vpu), involved in the viral entry, replication, and particle release (reviewed in Coffin et al. (1997)). The accessory genes are dispensable for the vector’s production, and can be deleted, thus creating space for the insertion of transgenic sequences (Blomer et al., 1997; Dull et al., 1998; Kafri, Blomer, Peterson, Gage, & Verma, 1997; Kim, Mitrophanous, Kingsman, & Kingsman, 1998; Zufferey, Nagy, Mandel, Naldini, & Trono, 1997). In contrast, the regulatory protein Rev is essential for exporting full-length and partially spliced RNAs harbored a Rev response element from the nucleus to the cytoplasm (reviewed in Cockrell and Kafri (2007)). When transcription initiates, the host RNA-splicing machinery in the nucleus quickly splices the RNA so that only the regulatory proteins Rev and Tat and the accessory protein Nef are generated. In the presence of Rev protein, RNA is exported from the nucleus before it is spliced, thus affirming transcription of the viral proteins. This mechanism allows a positive feedback loop to allow HIV to overwhelm the host’s defenses, and provides time-dependent regulation of replication (Dayton, Powell, & Dayton, 1989; Emerman, Vazeux, & Peden, 1989; Hadzopoulou-Cladaras et al., 1989). After HIV-1 reassembles in the cytoplasm, it escapes the cell by budding out from the cellular membrane (reviewed in (Gomez & Hope, 2005; Sundquist & Krausslich).
2.3.3 Lentiviral Vectors: Transgenic, Packaging, and Envelope Cassettes
As mentioned above, the first lentiviral vectors were evolved from the HIV-1 virus. In contrast to gamma-retroviral vectors, HIV-1 based vectors retain the ability of transducing, nondividing, and slowly dividing cells. Yet, they share the ability of gamma-retroviral vectors to integrate into the host chromosomes, without triggering a significant inflammatory response (Consiglio et al., 2001; Kordower et al., 2000).
Due to the relative complexity of the HIV-based vector system, production of viral stocks at high titers was a challenge initially. Furthermore, instability of the Env protein further contributed to the problem (Akkina et al., 1996). Nevertheless, it has been found that HIV-based vectors are capable of incorporating heterologous proteins that can replace the native envelope. In fact, early studies have shown that coinfection of the HIV-1 with other viruses may result in phenotypically mixed particles acquiring a broader host range. (Canivet, Hoffman, Hardy, Sernatinger, & Levy, 1990; Chesebro, Wehrly, & Maury, 1990). Following these publications, Page, Landau, and Littman (1990) demonstrated that the wild type HIV-1 was rendered replication defective by replacing the gp120 protein with a guanine-phosphoribosyl transferase (gpt) gene driven by the simian virus 40 (SV40) early promoter. These early observations extended in the experiments demonstrated that the envelope glycoprotein of the vesicular stomatitis virus (VSV-G) is capable of being efficiently incorporated into Moloney murine leukemia virus (MoMLV)-based retroviral vectors encoding the gene for neomycin phosphotransferase (Neo) (Emi, Friedmann, & Yee, 1991) and HIV-1 particles (Akkina et al., 1996; Reiser et al., 1996). Furthermore, VSV-G envelope was found to be significantly more stable allowing vector concentration by ultracentrifugation (Burns, Friedmann, Driever, Burrascano, & Yee, 1993). In addition, pseudotyping with VSV-G dramatically broadened vector tropism, as it has been initially suggested that VSV-G utilizes phosphatidyl serine-contained receptors on target cells (Schlegel, Tralka, Willingham, & Pastan, 1983). However, more recent data has demonstrated that phosphatidylserine is not the cell surface receptor for VSV-G, although it may play role in a postbinding step of virus entry (Coil & Miller, 2004). It also has been shown that VSV-G guides the vector to endocytic pathway, reducing thus the requirements for viral accessory proteins (Aiken, 1997). Nevertheless, works by Croyle et al. (2004); DePolo et al. (2000); Higashikawa and Chang (2001) have demonstrated that transduction of the mammalian cells with lentiviral vector can be hampered by complement- and antibody-mediated immune responses directed against the VSV-G envelope. As an alternative, lentiviral vectors can be successfully pseudotyped with other envelops including simple retroviral vector’s envelope proteins, for example, the glycoprotein of the lymphocytic choriomeningitis virus (LCMV); and the hemagglutinin of the avian influenza virus (reviewed in Cronin, Zhang, and Reiser (2005)) and discussed below.
To reduce risk of replication-competent viruses (RCVs), packaging and envelope cassettes of the vectors are expressed separately from two different plasmids delivering respective proteins in trans (Naldini, Blomer, Gage, et al., 1996) (Figure 3.4). Because the above plasmids share almost no homology, it is very unlikely that they are capable of reconstituting a wt virus. Furthermore, if replication competent virus is inadvertently generated by recombinations between the plasmids, it will lack all of the accessory proteins and the pathogenic properties of the HIV. To avoid transfer of the HIV-gene coding sequences into target cells, the packaging and envelope cassettes of the virus are deleted from viral cis-elements, including a packaging signal and LTRs, yet it retains the Rev binding site, and a parental splice donor site.
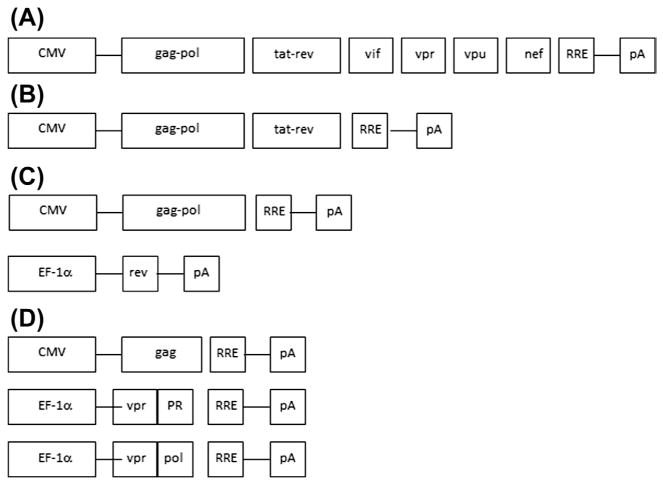
Figure 3.4.
Development of the packaging cassette. Four generations of the packaging cassette are shown. (A) First generation included all four accessory proteins, Vpu, Vpr, Vif, and Nef and the regulatory proteins, tat and rev. RRE stands for rev response element. Expression is driven from the CMV promoter; PolyA signal (pA) is shown. (B) Second generation excluded all four accessory proteins, Vpu, Vpr, Vif, and Nef, but included regulatory proteins, tat and rev. (C) Further split of the packaging cassette defined the third generation. (D) Four generation characterized by a further split of the cassette into three components: gag expressed from the CMV promoter; Vpr-protease expressed from the EF1-α promoter. Vpr-pol transcript is driven from the EF1-α promoter. All three packaging cassettes contain RRE and pA signals. CMV, cytomegalovirus; RRE, Rev response element.
To maximize expression of viral mRNA, heterologous promoters, such as cytomegalovirus (CMV) and the Rous sarcoma virus (RSV) promoters, were successfully incorporated into the packaging cassette in place of the parental LTRs. In addition, insulin or bovine growth hormone poly-adenylation signals replaced the relatively weak endogenous poly-A of the virus, potentiating mRNA stability (Dull et al., 1998; Kafri et al., 1997; Kim et al., 1998; Naldini, Blomer, Gage, et al., 1996; Zufferey et al., 1997). Deletion of the accessory proteins, Vpu, Vpr, Vif, and Nef (Dull et al., 1998; Kafri et al., 1997; Kim et al., 1998; Naldini, Blomer, Gage, et al., 1996; Zufferey et al., 1997), (Figure 3.4) resulted in second-generation packaging cassette harboring only the tat and the rev genes (Zufferey et al., 1997). Further separation of the gag/pol and rev sequences into two different cassettes, combining with tat deletion resulted in third-generation packaging system (Dull et al., 1998). All of these improvements further decreased a likelihood of generating RCVs. Importantly, they neither reduced vector yield nor hampered the ability of lentiviral vectors to transduce nondividing cells, such as terminally differentiated neurons (Dull et al., 1998; Kafri et al., 1997; Kim et al., 1998; Naldini, Blomer, Gage, et al., 1996; Zufferey et al., 1997). In all, the expression (transgenic) cassette had undergone significant changes resulted in improvement of biosafety, yield, and expression of the vectors (Figure 3.5).
Figure 3.5.
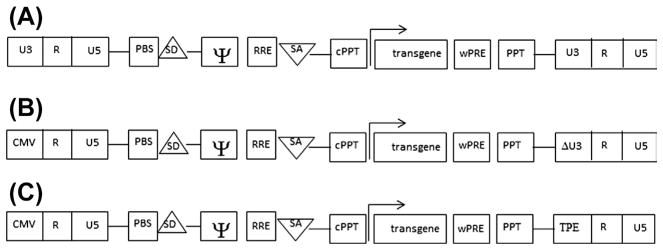
Development of the expression (transgene) cassette. (A) First generation contains wild-typed 5′- and 3′-LTRs, primer-binding site (PBS), splice donor (SD), and splice acceptor (SA), central polypurine tract (cPPT) and polypurine tract (PPT), Rev response element (RRE), woodchuck hepatitis virus posttranscriptional regulatory element (wPRE), and the retroviral vector packaging element, psi (Y) signal. Expression of a transgene is driven from the promoter-of-choice shown by the arrows. (B) SIN vector is devoid of the parental enhancer/promoter sequences, located at the U3′ of the 3 ′-LTR (deletion is shown); thus, lacking the ability to transcribe full-length mRNA. CMV promoter incorporated in the 5′-LTR employed to generate full-length mRNA. (C) Inducible expression cassette drives from the tetracycline response element (TRE) incorporated in the U3 region of the 3′-LTR. This cassette named a conditional SIN cassette, expressing the genome in the presence of the ttA. SIN, self-inactivating; LTR, long terminal repeat; CMV, cytomegalovirus.
By replacing the parental HIV-promoter located in the 5′-LTR with CMV or RSV promoters, the vector acquires independence from the Tat protein, and, therefore, enables the vector to be packaged with a third-generation packaging cassette (Kim et al., 1998; Miyoshi, Blomer,Takahashi, Gage, & Verma, 1998; Zufferey et al., 1998). Subsequently, deletions within the 3′-region of the 3-LTR that included the enhancer/promoter sequence′ and the TATA box, helped to create a self-inactivating (SIN) lentiviral vectors (Iwakuma, Cui, & Chang, 1999; Miyoshi et al., 1998; Zufferey et al., 1998). Since the region relocates to the 5′-LTR, during the reverse transcription, SIN-vectors are completely devoid of HIV-parental enhancer/ promoter sequences; thus, it lacks the ability of generating a full-length RNA that could be packaged into the virions. Significantly, the deletion did not affect vector production, and vector’s yield remains comparable with those of non-SIN vectors. Development of a SIN platform further reduced the likelihood of generating RCVs. Furthermore, it minimized a likelihood of mobilizing the vector’s mRNA by the replication-wt virus. In addition, absence of enhancer/promoter sequences reduced the risk of inadvertent activation of silent host-cell promoters by the provirus.
Finally, incorporation of the woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) (Zufferey, Donello, Trono, & Hope, 1999) and the central polypurine tract (cPPT) (Zennou et al., 2000) into the vector cassette significantly enhanced expression of transgenes delivered by lentiviral vectors and efficiency of transduction, respectively (Figure 3.5). Interestingly, WPRE has also been shown to have a strong positive effect on the vector’s yield and expression when integrated into adeno-associated and gamma-retroviral vectors (Loeb, Cordier, Harris, Weitzman, & Hope, 1999; Zufferey et al., 1999).
2.3.4 Production of the Retroviral Vectors and Lentiviral Vectors; Stable Cell Lines
Conventional method for generating retro- and lentiviral vectors based on a calcium-phosphate and polyethylenimine (PEI) protocol (Figure 3.6) (reviewed in details in Cockrell and Kafri, (2007)). Briefly, highly permissible, human embryonic kidney (HEK) 293T cells are usually utilized for the transfection. These cells express a polyomavirus-derived large-T antigen, which is exploited to enhance a vector’s yield through binding to the origin of replication (Ori) sequence of SV-40 virus harbored in the expression cassette. While efficient, the transient transfection protocol has several disadvantages, including a risk of DNA recombinations, variability in the quality of vector’s stocks, and difficulties in scaling up production process. For this reason, a number of stable packaging cell lines have been recently developed (Cockrell, Ma, Fu, McCown, & Kafri, 2006; Throm et al., 2009) (Figure 3.7).
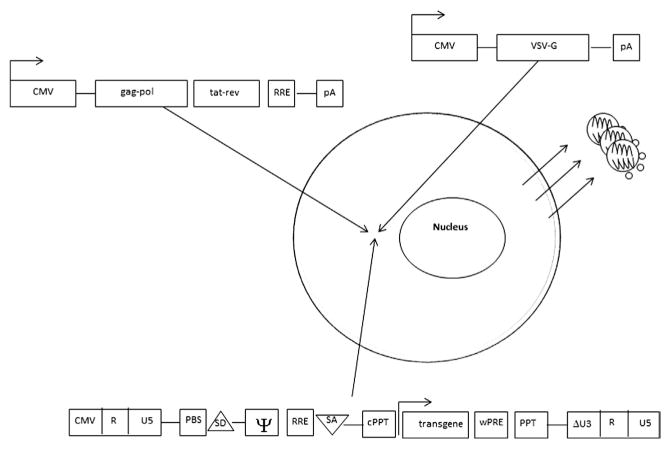
Figure 3.6.
Transient transfection protocol employed to generate lentiviral particles. 293T cells transfected with VSV-G, packaging, and transgene cassettes. Viral particles that bud out from the cell membrane contain full-length RNA of the vector (expressed from the transgene cassette). VSV-G, vesicular stomatitis virus G-protein.
Figure 3.7.
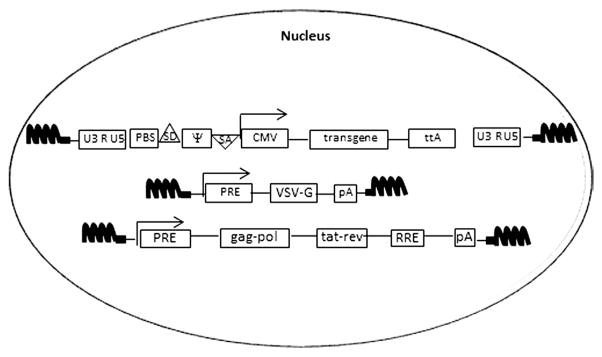
Stable cell lines developed to generate lentiviral vectors. Transgene, envelope, and packaging cassettes introduced into vector’s producer cells by stable transfection following by selection. Tet-inducible system is commonly employed to circumvent the toxicity associated with the viral proteins. Tetracycline transactivator, ttA can be expressed from the same cassette (transgene cassette) as does a transgene.
Development of stable packaging cell lines are impeded by cytotoxic effects associated with constitutive expression of the VSV-G (Ory, Neugeboren, & Mulligan, 1996), protease (Konvalinka et al., 1995), and Vpr (Bartz, Rogel, & Emerman, 1996). The successful development of inducible packaging systems was an important improvement to address the problem (Cockrell et al., 2006; Kafri, van Praag, Gage, & Verma, 2000; Reiser, Lai, Zhang, & Brady, 2000; Xu, Ma, McCown, Verma, & Kafri, 2001).
Commonly employed inducible systems are based on a tetracycline (tet) regulator (Gossen, Bonin, Freundlieb, & Bujard, 1994) (Figure 3.7). To develop the system, producer cells are introduced by transfection with a constitutively expressed tetracycline transactivator (tTA) that is maintained in the “off state” in the presence of tetracycline analog, doxycycline (Dox). Initially, SIN vector platform could not be used, because of its inability to generate full-length mRNA, implying a need for a non-SIN, Tat-dependent system (Farson et al., 2001; Kafri, van Praag, Ouyang, Gage, & Verma, 1999; Kaul, Yu, Ron, & Dougherty, 1998). Development of Tat-independent, CMV promoter-driven vectors (Klages, Zufferey, & Trono, 2000) has been an important step toward establishing a conditional SIN system, included heptameric repeats of a tetracycline response elements (TRE), and CMV-minimal promoter in the LTRs of the vector (Xu et al., 2001). This enables the vector’s production in the packaging cells constitutively expressing the tTA transactivator, while concomitantly maintaining the SIN phenotype in the tTA-negative target cells (Haack et al., 2004; Xu et al., 2001). Similarly, packaging and envelope cassettes can be equipped with the inducible promoters allowing tTA-dependent regulation (Haack et al., 2004).
2.3.5 Risk of Insertional Mutagenesis; Non-integrating Lentiviral Vectors
Employment of gamma-retroviruses for correcting human diseases is hampered by a relatively high risk of insertional mutagenesis associated with these vector systems. Thus, initially successful treatments of ADA-SCID, SCID-X1, and X-linked CGD with retroviral vectors were unfortunately troubled by leukemias developed by several patients. It has been demonstrated that the leukemia’s patients harbored provirus DNA in the vicinity of proto oncogenes deregulating their expression (Cavazzana-Calvo et al., 2000; Hacein-Bey-Abina, von Kalle, Schmidt, Le Deist, et al., 2003; Hacein-Bey-Abina, Von Kalle, Schmidt, McCormack, et al., 2003). Similar to gamma-retroviruses, lentiviral vectors are capable of integrating into the host genome, thus potentially retaining the ability to induce onco- and tumorigenicity. Furthermore, lentiviral vectors are not completely detached from the potential for insertional mutagenesis. In fact, EIAV vectors have been shown to be associated with the formation of tumors in the livers of mice following in utero and neonatal vector administration (Themis et al., 2005). A causal relationship between EIAV vectors and tumorigenesis has yet to be established; nevertheless, it is important to note that in the same study the use of HIV-based vectors were not associated with formation of any detectable tumors (Themis et al., 2005). Despite the evidence from this study, the lack of any precedent for HIV-based vectors to be associated with tumorigenecity and oncogenicity, and presumption that the risk of insertional mutagenesis in nondividing cells is not as immense as in dividing cells, lentiviral vectors that would obviate insertional mutagenesis are most desirable (Bayer et al., 2008; Kantor et al., 2011).
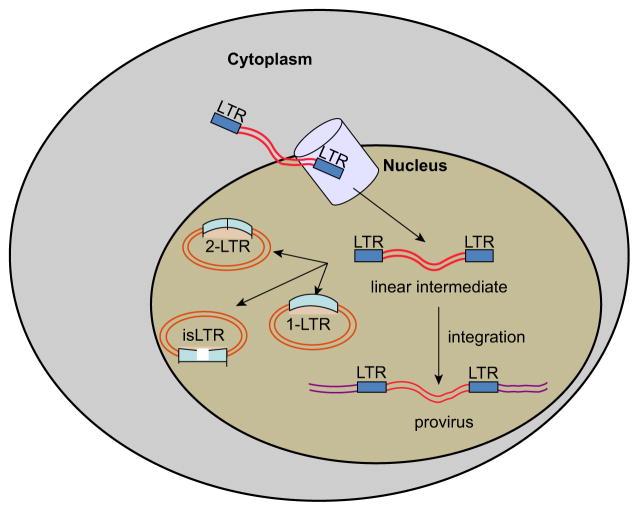
A strategy to modify the lentiviral vector-packaging cassette is being pursued to avert insertional mutagenesis based on developing a nonintegrating vector platform. This approach premises on findings that the HIV-1 and other retroviruses generate extrachromosomal (episomal) genomes over the course of infection. Furthermore, episomal DNA is observed to constitute the vast majority of viral genomes (Chun et al., 1997; Kantor, Ma, Webster-Cyriaque, Monahan, & Kafri, 2009; Pang et al., 1990; Teo et al., 1997), and found to be exceptionally stable in nondividing cells (Bayer et al., 2008; Butler, Johnson, & Bushman, 2002; Kantor et al., 2011; Pierson et al., 2002). Extrachromosomal DNA appears in the four major forms: linear episomes, which are the precursor for integration; 2-LTR (double-LTR) and 1-LTR (single-LTR), are circular forms generated by host-mediated repair mechanisms; and auto-integration episomes (Figure 3.8) (Bayer et al., 2008; Kantor et al., 2011).
Figure 3.8.
Episomal forms generated during the HIV infection. Episomal genomes of the HIV-1 appear in four major forms: linear episomes: precursors for integration and for the rest of the episomes; 2-LTR (double-LTR) and 1-LTR (single-LTR), are aberrant circular forms, generated by the DNA repair machinery of the host, or through the aberrant reverse transcription reaction; the autointegrative forms are product of self-integration mediated by the host repair machinery. LTR, long terminal repeat.
Nonintegrating vectors can be generated by introducing nonpleiotropic mutations within the open reading frame (ORF) of Int protein of the packaging cassette. These mutations have been shown to specifically target the integration process (Engelman, Englund, Orenstein, Martin, & Craigie, 1995; Nakajima, Lu, & Engelman, 2001). Our data demonstrated that an Int-deficient genome is capable of being efficiently transcribed, although the levels of protein expression are significantly lower than that of integrase wt vectors (Bayer et al., 2008; Kantor et al., 2009). Nevertheless, even the reduced expression levels of nonintegrating vectors have been shown to be sufficient for correcting genetic disorders in experimental animals (Philippe et al., 2006; Yanez-Munoz et al., 2006). In regards to the mechanism of gene repression of nonintegrating vectors, the reduced level of episomal expression is attributed to formation of repressive chromatin structure around the episomal DNA (Kantor et al., 2009). Furthermore, this chromatin has been found to be enriched in posttranslational histone modifications typically associated with transcriptionally silenced genes (Kantor et al., 2009). Remarkably, the reduced expression of the episomal genome associated with vector transduction or viral infection can be improved by treatment of transduced or infected cells by histone deacetylase inhibitors (HDACi). Importantly, these HDACi are endogenously generated in the gastrointestinal tract in the forms of short-chain fatty acids by normal microbial flora (Kantor et al., 2009). Depletion of the HDACs could be achieved also by mutating cis-acting elements within the U3′- region of the vector’s LTRs, enriched in HDACs-interacting negative transcriptional regulators. In fact, by mutating these elements a significant improvement in gene expression of nonintegrating vectors can be achieved both in vitro and in vivo (Bayer et al., 2008), (Kantor et al., 2011), and (Suwanmanee et al., 2014). Remarkably, these mutations have not changed the relative abundances of the episomal forms, suggesting that the increase in expression is not attributed to a distinct episomal form appearance (Bayer et al., 2008). Furthermore, the effect of the deletion has been shown to be tissue-specific in the rat’s brain, which is in line with earlier observations demonstrating cell-type-dependent gene expression of the nonintegrating vectors (Bayer et al., 2008; Philippe et al., 2006; Vargas, Gusella, Najfeld, Klotman, & Cara, 2004). These studies altogether suggest that nonintegrating lentiviral vectors may provide an effective means of delivery of therapeutic transgenes to nondividing and slowly dividing cells.
2.3.6 Lentiviral Vector for Use in the CNS
Lentiviral vectors have been used extensively as gene transfer tools for the CNS throughout the past two decades since they transduce most cell types in the brain, resulting in robust and long-lasting transgene expression. In fact, in the very first publication reported the use of the lentiviral vectors for gene transfer in vivo, Naldini and coworkers demonstrated efficient transduction into the neurons of the brain (Naldini, Blomer, Gage, et al., 1996). Following this report, hundreds of publications have demonstrated successful gene transfer utilizing both integrase-competent and integrase-deficient platforms in the CNS (de Almeida, Zala, Aebischer, & Deglon, 2001; Azzouz et al., 2002; Baekelandt et al., 2002; Bayer et al., 2008; Consiglio et al., 2001; Kantor et al., 2011; Perrin et al., 2007; Sergijenko et al., 2013; Wong et al., 2004).
As mentioned above, lentiviral vectors are considered attractive tools for gene transfer into the CNS, due their ability to transduce nondividing and slowly dividing cells. Lentiviral vectors have been demonstrated to be safer in comparison to gamma-retroviruses. Moreover, significant improvements of the packaging and expression cassettes of the vector, described above immensely contributed to reduce the likelihood of generating RCVs (Dull et al., 1998; Zufferey et al., 1997, 1998). Importantly, these modifications have not resulted in reduction of the vector yield nor did they hampered the ability of the vectors to transduce nondividing cells (Dull et al., 1998; Kafri et al., 1997; Naldini, Blomer, Gage, et al., 1996; Naldini, Blomer, Gallay, et al., 1996; Zufferey et al., 1997). Pseudotyping the vector with different envelopes tremendously expanded the range of transduction (Table 3.2). Multiple studies demonstrated that lentiviral vectors are capable of transducing most cell types within the CNS in vivo, including terminally differentiated neurons, dendritic cells, glial cells, astrocytes, and oligodendrocytes (Cheng et al.; Bayer et al., 2008; Blomer et al., 1997; Consiglio et al., 2004; Jakobsson, Ericson, Jansson, Bjork, & Lundberg, 2003; Kafri et al., 1997). Interestingly, it has been demonstrated that although other types of lentiviral vectors, such as SIV, EIAV, and FIV are capable to deliver transgenes into the CNS, the most robust and efficient delivery has been achieved employing the HIV-1-based vectors. This is likely because of species-specific restrictions hampering the transduction of nonhuman lentiviral vectors (Wiznerowicz & Trono, 2005).
Table 3.2.
Heterologous Envelope Proteins Used for Psuedotyping Lentiviral Vector for CNS Applications
| Envelope | Host Range | Cell Type | Stability upon Concentration |
|---|---|---|---|
| VSV-G | Mouse, rat, pig, dog, human | Ubiquitous | The most established protocol; stable, suitable for concentration |
| LCMV | Mouse, rat | Strong preference to astrocytes, glioma cells; neurons to a lesser degree | Stable, suitable for concentration |
| MV | Mouse, rat | Ubiquitous | Stable, suitable for concentration |
| Rabies-G | Mouse, rat | Preference to neurons; excellent axonal transport | Stable, suitable for concentration |
| MoMLV | Mouse, rat | Similar pattern to LCMV; capable of infecting neurons | Stable, suitable for concentration |
| RRV-G | Mouse, rat, human cells in vitro | Glial cells and neurons | Stable, suitable for concentration |
Vesicular stomatitis virus G-protein (VSV-G); lymphocytic choriomeningitis virus protein (LCMV); Mokola virus G-protein (MV); Rabies virus G-protein (Rabies-G); murine leukemia virus envelope protein (MoMLV); Ross River virus G-protein (RRV-G).
2.3.7 Tissue-Specific Promoters Used in Lentiviral Gene Transfer into the CNS
Transduction efficiency and posttransduction gene expression of the lentiviral vectors depend on a promoter type that employed (Table 3.3). At the early days, ubiquitous promoters such as CMV and phosphoglycerate kinase (PGK) were utilized to drive the transgene expression (Jakobsson et al., 2003; Naldini, Blomer, Gage, et al., 1996). These promoters are predominantly active in the neuronal cells, but this could be partially attributed to a greater abundance of the neurons, and the lower rate of transduction in the glial cells. Moreover, transgenic expression driven from the CMV promoter is weakened over the time, likely because of the DNA methylation (Grassi et al., 2003; Mehta, Majumdar, Alam, Gulati, & Brahmachari, 2009). Development of tissue-specific promoters provides an important tool to control lentiviral vector’s gene expression in multiple cell lineages of the CNS (Dittgen et al., 2004; Jakobsson et al., 2003; Lai & Brady, 2002). Moreover, both neuron- and glial-specific promoters have been demonstrated to confer cell-type-specific transgene expression in the desired cell type (Dittgen et al., 2004; Jakobsson et al., 2003; Lai & Brady, 2002) (Table 3.3). These promoters appear to be highly specific, at least in the experiments when reporter GFP transgene was employed. Notwithstanding these findings, expression driven by the glial-specific promoter, glial fibrillary acidic protein (GFAP) was detected not exclusively in the glial cells when the glial cell line-derived neurotropic factor was carried (Jakobsson et al., 2003). It is possible that the gene of interest can affect the specificity of transduction and expression. Alternatively, integration of silenced transgene into active chromatin environment may stimulate the promoter activation via interaction with the surrounding enhancer sequences. To increase specificity of transduction, a combinatory approach should be considered, in which cell-type-specific promoters combined with cell-specific envelopes ensuring thus more specific targeting of the desired cell population.
Table 3.3.
Typical Promoter Types Used to Drive Expression in Lentiviral Vectors in the CNS
| Promoter | Strength | Cell Type |
|---|---|---|
| CMV | Strong but subject to transcriptional silencing over time | Ubiquitous |
| PGK | Strong | Ubiquitous |
| EF1-α | Strong | Ubiquitous |
| TRE | Strong, inducible by ttA or ttS+/−Dox, respectively | Ubiquitous |
| GFAP | Moderate-strong | Astrocytes |
| CaMKII | Strong | Postnatal neurons (strong after week 4–5 in mice and rats) |
| Synapsin I | Moderate-strong | Developing neurons (weaker after week 4–5 in mice and rats) |
| Thy-1,2 | Moderate-strong | Prenatal and postnatal neurons |
Cytomegalovirus (CMV); phosphoglycerate kinase 1 promoter, (PGK); Human elongation factor-1 alpha promoter, (EF1-α); The tetracycline response element-containing promoter, (TRE); Glial fibrillary acidic protein promoter, (GFAP); α-calcium/calmodulin-dependent protein kinase II, (CaMKII); Synapsin type I promoters, (Synapsin I); thymocyte differentiation antigen 1 and 2 promoter, (Thy-1,2).
2.3.8 Envelopes for Gene Delivery into the CNS
As was mentioned before, VSV-G is the most common envelope utilized the vector transduction (Table 3.2). The widespread use of this protein for pseudotyping lentiviral vectors transduction has made it, in effect, the standard against which the effectiveness of other viral envelopes is compared. Other glycoproteins are also capable of governing vector delivery into the CNS. Among them LCMV envelope mentioned above, Mokola virus (MV), Moloney murine leukemia virus (MoMLV), Ross River virus (RRV) and Rabies virus (RV) have been demonstrated to be effective in pseudotyping vectors (reviewed in Cronin et al. (2005)). RV and MV belong to the same genus, Lyssavirus, and are closely related. Glycoproteins derived from these viruses were the first to be incorporated into the HIV-1-based vectors demonstrating robust transduction into the brain (Conzelmann, Cox, Schneider, & Thiel, 1990; Mochizuki, Schwartz, Tanaka, Brady, & Reiser, 1998). Furthermore, Watson and coworkers demonstrated that the lentiviral vector pseudotyped with the glycoprotein of MV injected into the rats’ striatum efficiently expressed the β-gal reporter. In addition, they found that the pattern of transduction governed by the envelope of MV was similar to that of VSV-G-pseudotyped vectors, with efficient delivery of both vectors into neuronal cells (Watson, Kobinger, Passini, Wilson, & Wolfe, 2002) (Table 3.2). In a related study, Desmaris and colleagues tested and compared the ability of the glycoproteins of MV and VSV to govern retrograde transport following reporter transfer with lentiviral vectors (Desmaris et al., 2001). In this study lentiviral vectors pseudotyped with either VSV-G, or MV-GP were injected into the nasal cavity, or the limb muscles of rats. Both vectors efficiently transduced neurons of the olfactory bulb following nasal delivery; however no β-gal expression was detected in the motor neurons in the spine for either vector (Desmaris et al., 2001). The study concluded that lentiviral vectors pseudotyped with VSV-G and MV-GP envelopes are similar in the efficiency of retrograde axonal transport, although both are incapable of infecting via neuromuscular junctions. In contrast, lentiviral vector pseudotyped with the glycoprotein of RV injected into the rat’s striatum was efficiently delivered into thalamus and substantia nigra suggesting both retrograde and anterograde transport (Sacramento, Badrane, Bourhy, & Tordo, 1992) (Table 3.2).
The original report of VSV-pseudotyped lentiviral vectors showed transduction of both neurons and glial cells in the hippocampus and striatum of adult mice (Naldini, Blomer, Gage, et al., 1996), implying the ubiquitous nature of transduction utilizing this envelope. In the related study, Cannon and colleagues demonstrated that a lentiviral vector pseudotyped with the glycoprotein of MV delivered by the intranigral infusion in the rat’s brain provided a similar pattern of expression to that observed after infusion of the glycoprotein of VSV (Cannon, Sew, Montero, Burton, & Greenamyre, 2011). They suggested that because it is straightforward to generate high-titer lentiviral vector stocks pseudotyped with the G-protein of VSV, the lentiviral vectors pseudotyped with the glycoprotein of MV may confer no advantage for gene transfer to the rat substantia nigra (Table 3.2).
A different pattern of transduction was observed when the lentiviral vectors pseudotyped with LCMV and MoMLV envelopes were employed for gene delivery into the mouse brain (Watson et al., 2002). In this study reporter gene expression was detected in the white matter after striatal infusion, showing only limited neuronal transduction after infusion near the hippocampus. In comparison, lentiviral vectors pseudotyped with the glycoprotein of MoMLV efficiently transduced striatal cells of undetermined nature and granule neurons of the hippocampus after hippocampal infusion (Watson et al., 2002). In contrast, Cannon and coworkers have found that both vectors pseudotyped with LCMV and MoMLV envelopes demonstrated selective transduction of astrocytes in the rat substantia nigra (Cannon et al., 2011). Similarly to the envelopes of VSV and MV, the glycoprotein derived from the RRV-governed efficient lentiviral transduction and GFP-expression in both neurons and glial cells of the brain (Jakobsson, Rosenqvist, Marild, D, & Lundberg, 2006). Using two cell-type-specific promoters, neuron-specific enolase, and human GFAP protein, Jakobsson and colleagues demonstrated cell-specific transgene expression in aforementioned cell types (Jakobsson et al., 2006). In the same publication, the glycoprotein protein of the RRV was found capable of transducing human neural progenitor cells in vitro, suggesting that receptors for the envelope of the RRV are present on human neural and glial cells (Jakobsson et al., 2006) (Table 3.2).
2.3.9 Peripheral Administration of Lentivirus to Target the CNS
Systemic route of delivery of retro- and lentiviral vectors would be the most desired option for introducing a transgenic DNA into the CNS. In fact, the necessity of utilizing intracranial route reduces the feasibility of employing vector protocols for clinical applications. Since, the BBB serves as a significant impediment to the vector’s transport, developing a system that will penetrate the CNS after peripheral administration is greatly important. In this regard, vectors that are capable of reaching the brain by retrograde transport are in great demand. As discussed earlier in this chapter, pseudotyping the vectors with glycoproteins that support a retrograde transport, such RV, beneficial for exploring the peripheral route of delivery (Sacramento et al., 1992) (Table 3.2). These vectors already has been successfully implemented for treating animal models of amyotrophic lateral sclerosis and SMA, in which lentiviral vectors expressing either vascular endothelial growth factor or the human survival motor neuron gene were retrogradually transported to motor neurons after intramuscular injection (Azzouz et al., 2004; Wong et al., 2004).
One strategy to target the CNS after peripheral delivery of the gene transfer vector is to exploit the secreted form of the lentivirus regulatory protein, Tat, to invade the CNS (reviewed in (Rapoport & Lorberboum-Galski, 2009; Wang et al., 2009). The Tat protein can destabilize a monolayer of blood–brain covering endothelial cells, thus allowing permeation of high molecular weight cargoes (Cooper et al., 2012). Furthermore, it has been shown that β-gal protein fused to Tat protein was efficiently delivered into the brain by penetrating the BBB (Schwarze, Ho, Vocero-Akbani, & Dowdy, 1999). Subsequently, various groups have used the Tat-based delivery approach to administrate a variety of therapeutically relevant cargoes to the brain (reviewed in Rapoport & Lorberboum-Galski (2009); Wang et al., 2009)). Specifically, fusion of the Tat to the proteins which bind the postsynaptic density protein, PSD-95, was found to be effective in protecting neurons from excitotoxicity (Aarts et al., 2002). Furthermore, studies in rodents in vivo revealed that not only intact Tat protein can cross the BBB, but also Tat derivatives contained the protein transduction domain are capable of penetrating the BBB and accumulate in brain tissue (reviewed in (Rapoport & Lorberboum-Galski, 2009)).
2.3.10 Conclusions on the Use of Lentivirus for CNS Gene Transfer
The ability of lentiviral vectors to transduce nondividing or slowly dividing cells and deliver large genetic payloads throughout maintaining stable and long-term transgene expression are attributes that brought lentiviral vectors to the forefront of the gene delivery field. Clinical trials utilizing lentiviral vectors clearly underlined their efficacy for therapies of hereditary diseases including adrenoleukodystrophy, β-thalassemia, Wiskott–Aldrich syndrome, and metachromatic leukodystrophy (discussed in details in the next chapter). In comparison to gamma-retroviral vectors, which were commonly employed in the first clinical trials, lentiviral vectors own favorable features including a potentially safer integration profile. Nevertheless, stable integration of the retroviral and lentiviral vectors, risk of appearance of RCVs, insertional mutagenesis that may deregulate expression profiles of neighboring genes, aberrant splicing, and germ-line transmission are points of major concern from the gene therapy perspective. Therefore, along with appreciation of the substantial benefits of using lentiviral vectors for gene transfer into the CNS, it is important to consider other vector platforms while considering therapeutic outcomes.
2.4 Adenovirus
Adenovirus had been the traditional workhorse of gene therapy, but it has mostly fallen out of favor for CNS gene transfer in lieu of AAV and retroviral vectors. Adenovirus is a common and pathogenic human dsDNA virus, and the recombinant gene therapy vector can package up to 35 kb of foreign DNA, depending on the version of the vector (Campos & Barry, 2007; Volpers & Kochanek, 2004). Adenovirus vectors have the advantages of a large packaging size, high functional titers, the ability to transduce dividing and nondividing cells, and fast transgene expression (1–2 days postdelivery). Following transduction, the vector DNA does not normally integrate in the host genome and is instead maintained as an episome with minimal risk of insertional mutagenesis. Adenovirus vectors have several disadvantages that have limited their application in CNS gene transfer, including the often transient nature of transgene expression and their higher preponderance to provoke an inflammatory response (Lentz, Gray, & Samulski, 2012).
Adenovirus vector genomes have improved significantly since their original development, such that current vectors have much lower immunogenicity and longer transgene expression (Campos & Barry, 2007; Chen et al., 1997; Schiedner et al., 1998). First-generation recombinant adenovirus vectors have the E1 genes removed, but they still contain and express at low levels the E2, E3, E4, and late genes, which are highly immunogenic (Campos & Barry, 2007). Low-level expression of these viral antigens leads to elimination of transduced cells by the host immune system, thereby limiting the duration of transgene expression in vivo (McConnell & Imperiale, 2004). Second-generation vectors lack the E2, E3, and E4 genes, and third-generation “gutless” vectors do not express any viral proteins (Amalfitano, 1999; Chen et al., 1997). Even with these improvements, third-generation adenovirus vectors still generate significant immune responses against the viral capsids, which creates a patient-specific limit to the amount of vector that can be administered (Muruve, 2004). Switching to canine adenovirus serotype 2 (CAdV2), rather than the commonly used human adenovirus serotype 5, has the advantage of reduced immune responses and prolonged transgene expression (Keriel, Rene, Galer, Zabner, & Kremer, 2006; Perreau et al., 2007). Using CAdV2 vectors, MPS VII mice showed stable expression, reduced pathology, and improved phenotype for at least 16 weeks after a single intracranial vector injection (Ariza et al., 2014).
2.5 Herpes Simplex Virus
2.5.1 Introduction
HSV is a member of Herpesviridae and belongs to the subfamily Alphaherpesvirinae. Depending on the cellular site of latency, herpesviruses are classified as HSV-1 or as HSV-2. HSV-1 is the primary cause of orolabial cold sores caused by the establishment of latency in the trigeminal nerve; whereas genital herpes is caused by the latency of HSV-2 in the sacral ganglia. HSV-1 is acquired during childhood or adolescence by primary infection of the oral mucosa. About 40% of adults are seropositive for HSV-1 in developed countries (Smith & Robinson, 2002). Latency occurs when progeny viruses produced at the site of infection enter sensory nerve terminals and translocate their nucleocapsid to the soma of neurons by microtubule dependent retrograde axonal transport (Tomishima, Smith, & Enquist, 2001).
2.5.2 Structure
The HSV-1 virion is made up of an icosahedral capsid covered by a lipid bilayer viral envelope (Figure 3.9). The envelope is embedded with glycoproteins essential for viral entry into hosts mediated by attachment of these glycoproteins to cellular receptors. Between the envelope and the nucleocapsid lies the tegument which contains structural proteins and regulatory enzymes encoded by the viral genome. The main role of the tegument is thought to be the ability to supply proteins essential in DNA replication and evasion of the immune system (Baron, 1996). The ds viral genome is 152 kb in length and encodes about 90 proteins for replication and cellular attachment (Donald W Kufe et al., 2003). The genome contains unique long (UL) and unique short (US) segments and is flanked on either end by inverted repeat sequences (Osten, Grinevich, & Cetin, 2007).
Figure 3.9.
HSV structure. HSV, Herpes simplex virus.
2.5.3 Life Cycle of HSV
Cellular attachment is mediated by the interaction of the glycoproteins gB and gC of the viral envelope with heparan sulfate of the host-cell membrane. Cellular entry is then mediated by the envelope protein gD by binding to the viral entry receptor, herpesvirus entry mediator. The fusion of the cell membrane and the viral envelope is mediated by gB and the gH–gL complex, resulting in the release of the nucleocapsid into the cytoplasm of the host cell. In neurons, the nucleocapsid is transported to the cell body by retrograde axonal transport mediated by the interaction of the host microtubule network with the tegument proteins of HSV-1 (Diefenbach, Miranda-Saksena, Douglas, & Cunningham, 2008).
Upon cellular entry, HSV-1 can establish either of these two states: lytic or latent. The lytic pathway ultimately results in the death of the host cell whereas latent HSV establishes as an extrachromosomal episome within host nuclei. However, latent HSV is capable of reactivation where the expression of the ICP0 gene is thought to play a key role (Lachmann, 2004).
Replication and transcription of viral DNA is a multistep process requiring the production and coordination of multiple proteins. The lytic pathway is mediated by the sequential expression of three classes of viral gene products: these proteins are classified as α-proteins or immediate-early (IE) proteins, which encode major transcriptional regulatory proteins, β-proteins or early (E) proteins, which encode viral DNA polymerase while altering the intracellular composition to favor viral replication and γ -proteins or late (L) proteins, which primarily encode structural proteins. Among these, the IE genes are most essential for the lytic pathway as these genes are necessary for the synthesis of the β- and γ -proteins (Bernard Roizman, 2001). There are 37 essential genes in the HSV genome out of which 36 are located in the UL region. This allows for the deletion of a large amount of viral DNA containing the nonessential genes in the US region (Ho et al., 1995; Roizman, 1996, 1999), which can then accommodate large amounts of transgene DNA in vectors.
2.5.4 Vectors Derived from HSV
Vector properties required to correct genetic deficiencies are vastly different from the properties of a vector used to target cancer cells. The versatility of HSV-1 vectors cannot only provide long-term gene expression with lack of cytotoxicity but can also provide high levels of expression of toxic products malignant cells. The adaptations that make HSV vectors suitable for neurological and cancer therapy applications are discussed below:
HSV-1 adaptations for neurological applications:
The virus can be disseminated transynaptically from neuron to neuron.
Movement of the virus is possible in retrograde and anterograde directions.
Glycoproteins on the envelope recognize receptors such as nectin that are particularly useful for viral entry into neurons.
Upon entry into the nucleus, the virus is able to persist as an extrachromosomal episome and all lytic genes are suppressed establishing latency in neurons, which are immune privileged.
The properties of HSV-1 that make it an attractive candidate for cancer gene therapy are
HSV vectors efficiently infect a number of human tumor cell lines in vitro.
Several HSV vectors take advantage of underlying defects in molecular pathways of tumor cells.
HSV-1 vectors express the tk gene, which encodes thymidine kinase, which results in apoptosis of host cells (Kuriyama et al., 1995; Vile & Hart, 1993)
Modification of envelope proteins can increase infectivity of tumor cells, which bear the corresponding receptors (Grandi, Spear, Breakefield, & Wang, 2004; Grandi, Wang, et al., 2004)
These various properties can be exploited by engineering various classes of vectors. The three categories of HSV-1-derived vectors are as follows, which are discussed in detail in the subsections below.
Replication-defective vectors
Replication-competent vectors
Amplicon vectors
2.5.4.1 Replication-Defective HSV Vectors
As the name suggests, replication-defective vectors have deletions of the essential genes from the viral genome, which prevents the proliferation of the HSV-1 particles in vitro. These essential genes are IE genes and are required for the E and L cascade responsible and functions such as viral entry, replication, splicing, polyA, and mRNA stability. The first generation of these vectors were mutants, which lacked the expression of the IE gene encoding ICP4 (DeLuca & Schaffer, 1985), which is essential in viral replication and induction of early and late gene expression (DeLuca & Schaffer, 1988). These vectors showed reduced pathogenicity but were found to be cytotoxic in neurons. Several combinations of deletions in five essential genes namely ICP0, ICP4, ICP22, ICP27, and ICP47 produced a variety of replication defective vectors that are highly defective mutants (Krisky et al., 1998; Samaniego, Neiderhiser, & DeLuca, 1998). Deletion of all five IE genes reduced cytotoxicity and allowed the viral genes to persist in the cells for prolonged periods of time (Kaplitt & Makimura, 1997). Deletion of these IE genes allowed for the accommodation of transgene cassettes as well as regulatory elements (Krisky et al., 1998).
Due to their reduced toxicity, replication-defective vectors have a wide range of therapeutic applications in neurological diseases like lysosomal storage disorders (Martino et al., 2005), Alzheimer’s (Hong, Goins, Goss, Burton, & Glorioso, 2006) and chronic pain (discussed in detail below) (Goss et al., 2001). Martino et al. have shown successful clearance of the GM2 ganglioside, the lysosomal storage material that accumulates due to defects in the gene encoding the enzyme hexaminidase A α-subunit, in a murine model of Tay–Sachs disease (Martino et al., 2005). These vectors have also been used in the treatment of motor neuron disease due to its ability to deliver multiple neurotrophic factors to specific cell populations (Marconi et al., 2005; Perez, Hunt, Coffin, & Palmer, 2004).
Attenuated HSV vectors have been used in cancer therapy primarily as anticancer transgene delivery vehicles including suicide genes such as thymidine kinase, immune-stimulatory molecules such as cytokines and anti-angiogenic factors to tumors such as melanomas (Krisky et al., 1998) and glioblastomas (Niranjan et al., 2000).
2.5.4.2 Replication-Competent Vectors
Deletion of certain nonessential genes responsible for HSV-1 immune evasion and virulence gives rise to replication-competent vectors, which proliferate in vitro but are compromised in vivo (Todo, 2008). These vectors are modified such that they have attenuated ability to replicate in healthy cells but do so rapidly in dividing cells present in tumors (Hu & Coffin, 2003) and can be used to deliver therapeutic agents to tumors. Thymidine kinase, ribonucleotide reductase, virion host shut-off, and ICP34.5 protein are nonessential gene products that are responsible for the virulence and pathogenicity of HSV-1 (Samady et al., 2003). Deletion of these genes allows these vectors to take advantage of physiological changes in tumors that allow for the lytic infection of the vector specifically in these tumor cells (Hu & Coffin, 2003; Todo, 2008).
Replication-competent vectors have been used in the treatment of peripheral nervous system disorders. These vectors are allowed to carry out their natural life cycle and establish latency in the cell bodies after retrograde transport from the periphery. Transduction of the dorsal root ganglia by proenkephalin A mediated by these vectors has been used in various pain studies (Goss et al., 2001). These vectors have also shown efficient transduction of primate retinal pigmented epithelial cells and retinal ganglion cells with limited inflammatory and cytotoxic responses, which make them amenable to clinical applications in models of optic nerve degeneration (Liu et al., 1999).
Among the various types of HSV vectors, replication-competent vectors have been one of the most commonly used vectors in cancer therapies at preclinical and clinical trials (Chiocca, 2002; Mullen & Tanabe, 2002).These viruses selectively replicate and lyse cancer cells by using the cancer cell machinery while sparing the nondividing healthy cells. The first generation of oncolytic viruses contained three distinct mutations:
Deletion of the thymidine kinase gene (Martuza, Malick, Markert, Ruffner, & Coen, 1991), which slowed tumor growth and prolonged survival.
Insertion of the lacZ gene in the E gene creating ICP6 (Jeyaretna, Rabkin, & Martuza, 2007), which showed enhanced killing of tumors and improved survival.
Deletion of the nonvirulence factor ICP34.5 (Dambach,Trecki, Martin, & Markovitz, 2006) essential for HSV pathogenicity, showed antiglioma activity.
Among the first-generation replication-competent vectors, only the ICP34.5 mutant is currently in clinical trials (Liu et al., 2003) as the other two cause fatal encephalitis in animals (Martuza et al., 1991). The second-generation replication-competent vector G207 that contained multiple mutations (deletion of the ICP34.5 and insertion of the lacZ gene in ICP6 gene (Mineta, Rabkin, Yazaki, Hunter, & Martuza, 1995) has a higher safety profile and low neurotoxicity which allowed it to move into Phase Ib/II trials for malignant tumors (Markert et al., 2000). Chemotherapy and radiation are required in combination with the oncolytic viruses to increase the success rates in cancer treatment (Post, Fulci, Chiocca, & Van Meir, 2004).
2.5.4.3 Amplicon Vectors
Amplicon vectors are prepared by transfecting cells with the amplicon plasmid and superinfecting the cells with helper HSV-1. This leads to the production of vector particles that are similar to wt HSV-1 structure and immunogenicity but contain a bacterial plasmid DNA instead of the viral genome (Frenkel, Singer, & Kwong, 1994). This concatemeric plasmid is capable of packaging more than one transgene cassette, a origin of replication, and a cleavage/package signal (Epstein, 2005). The advantages of amplicon vectors are numerous: these have a large packaging capacity of about 150 kb and reduced toxicity due to the lack of viral genes. The repetitive nature of the amplicon plasmid ensures multiple copies of transgene are delivered to each cell transduced. However, one disadvantage of these vectors was the inability to produce helper-free vector stocks. Helper-contaminated vectors induced significant inflammatory responses as well as cytotoxicity. To overcome this issue, helper-free vector stocks were produced by supplying the HSV-1 genome in trans through bacterial artificial chromosomes (Saeki et al., 1998) or by employing the Cre/loxP-based site-specific recombination to remove helper virus (Logvinoff & Epstein, 2001).
Helper-free vectors show reduced cytotoxicity in neurons as well as long-term expression of transgenes. The use of these vectors has been multi-faceted in the field of neuroscience. Transgenes expressing neurotransmitters and/or receptors have been useful in behavioral studies involving learning and memory (Adrover et al., 2003; Brooks, Cory-Slechta, Bowers, Murg, & Federoff, 2000). These vectors have also been used as therapeutic agents when packaged with transgenes such as brain-derived neurotrophic factor (Geschwind et al., 1996), nerve growth factor (NGF) (Pakzaban, Geller, & Isacson, 1994), and antiapoptotic genes (Antonawich, Federoff, & Davis, 1999) to confer neuroprotection in experimental settings. These vectors have been essential in experiments studying the treatment of Parkinson’s disease in culture by delivery of tyrosine hydroxylase (Geller, During, Oh, Freese, & O’Malley, 1995; Geller, Yu, Wang, & Fraefel, 1997).
Due to the nonreplicating nature of amplicon vectors, their application in cancer therapy is limited to genes which stunt tumor growth by inducing hypoxia and attenuate angiogenesis (Shah & Breakefield, 2006). The major application of amplicon vectors in cancer gene therapy has been the delivery of the synergistic prodrug-activating enzymes, thymidine kinase and cytosine deaminase (Jacobs et al., 2003, 2007), and the delivery of tumor necrosis factor-related apoptosis-inducing ligand for in vivo imaging and treatment of tumors (Shah, Tang, Breakefield, & Weissleder, 2003; Shah, Tung, Breakefield, & Weissleder, 2005).
2.5.5 High-Impact Application of HSV Vectors in the Treatment of Chronic Pain
Latency, neurospecificity, and neuroinvasiveness are several adaptations of HSV, which make it useful in the treatment of neurological conditions. Retrograde transport allows these vectors to be transported from the periphery to the cell bodies where it remains latent by means of the latency-active promoter system. These mechanisms can be used favorably to obtain long-term transgene expression in neurons of the central and peripheral nervous systems (Chattopadhyay et al., 2005; Goins et al., 1999) and have been used for the development of novel therapies for chronic pain.
Chronic pain is pain that lasts for prolonged periods of time in the absence of injury or stimulus. Chronic pain can result from a number of causes including arthritis, fibromyalgia, peripheral neuropathies, etc., and can last longer than 6 months.The relief obtained from analgesics and opiods is limited due to tolerance and the adverse systemic effects produced. Gene therapy can be used to produce localized transcription of therapeutic genes which have analgesic effects or inhibit pain signaling without producing systemic effects.
HSV vectors have been used for delivery of therapeutic agents to sensory neurons that inhibit pain signaling pathways. Inhibitory tone is increased in phasically active neurons, which are interneurons that transmit signals between the dorsal horn and the brain stem, by replication-defective HSV vector-mediated transfer of endogenous opiod peptide encephalin (ENK) in the dorsal root ganglia. Rodents that received this treatment showed reduced pain response to noxious stimuli or underlying inflammation (Braz et al., 2001; Goss et al., 2001, 2002). In a dose escalation gene therapy clinical trial using an HSV vector expressing ENK, 10 patients reported focal pain relief with no adverse effects (Fink et al., 2011). HSV-mediated gene therapy using GABA and endomorphin has also been successful in treating neuropathic pain, spinal cord injury, and inflammatory pain in animal models (Hao et al., 2005; Hao, Wolfe, Glorioso, Mata, & Fink, 2009; Liu et al., 2004). GABA is the dominant inhibitory neurotransmitter in the spinal cord and exerts tonic inhibitory control over nociceptive transmission and endomorphin is a natural ligand of the mu opioid receptor, which is the major site of action for narcotic drugs such as morphine. HSV vectors have also been used to deliver anti-inflammatory cytokines such as IL-4 and IL-10, which decrease neuroimmune stimulation and provide analgesia in several models of chronic pain due to chronic inflammation (Cunha, Poole, Lorenzetti, Veiga, & Ferreira, 1999; Poole, Cunha, Selkirk, Lorenzetti, & Ferreira, 1995). For a detailed review of the applications of HSV vectors in chronic pain, please refer to (Goins, Cohen, & Glorioso, 2012; Goss, Krisky, Wechuck, & Wolfe, 2014; Srinivasan, Fink, & Glorioso, 2008).
3. GENOME DESIGNS FOR OPTIMAL EXPRESSIONS
In recent years, gene therapy has begun to make the advance from proof of concept to proof of practice. An often underappreciated aspect of vector design is the means and control of transgene expression. Until recently, the majority of constructs have used only ubiquitous viral promoters to drive expression from simple gene expression cassettes lacking introns, matrix attachment regions (MARs), WPRE, and other components of the expression cassette. In fact, these elements have a key role in determining the levels and permanence of gene expression in vivo, regardless of the vector system used. In conjunction with modification of vector tropism and strategies to limit their immunogenicity, vector genome optimization should create superior vectors for highly specific research and clinical applications involving gene transfer.
A myriad of neurological disorders including Parkinson’s disease, Huntington’s disease, epilepsy, Alzheimer’s disease, and many others may be treatable using gene therapy. Each disorder has differing requirements in terms of cell type and level/range of therapeutic protein necessary to fall within the therapeutic window for that particular disease. For example, gene therapy has been identified as a potential therapeutic for Rett Syndrome. Rett syndrome is a neurodevelopmental disorder that is characterized by normal early growth and development followed by a slowing of development, loss of use of the hands, distinctive hand movements, slowed brain and head growth, problems with walking, seizures, and intellectual disability. Delivery of the MeCP2 gene to neurons and glial cells has given positive results to alleviate disease symptoms on a sustained basis (Gadalla et al., 2013; Garg et al., 2013). Although this gene holds therapeutic promise for Rett patients, overexpression of MeCP2 is known to have detrimental effects and in those studies there was toxicity observed in areas such as the liver where the transgene was overexpressed (Gadalla et al., 2013). In this instance, a safe and effective gene therapy approach for Rett syndrome is likely to require tightly controlled transgene expression that is limited to specific cell populations (Gray, 2013).
By creating these custom expression cassettes, expression or down regulation of a particular gene can be controlled in many different dimensions. These dimensions include the strength, time lapse, and modulation of the specific transgene inside the vector. This section aims to highlight some of the principle considerations of gene expression in vitro and in vivo, and the means by which it may most effectively be achieved through modifications of the expression cassette. A list of promoters is provided in Table 3.4 and a summary of other regulatory elements is provided in Table 3.5.
Table 3.4.
Representative Promoters for CNS Gene Transfer
| Promoter | Specificity | Strength | Ref |
|---|---|---|---|
| Viral (CMV-IE, RSV-LTR, MoMLV-LTR, SV40) | Ubiquitous | +++ | (Papadakis, Nicklin, Baker, & White, 2004) |
| Eukaryotic (EF1-α) | Ubiquitous | ++++ | (Xu et al., 2001) |
| Ubiquitin C | Ubiquitous | + | (Qin et al., 2010) |
| GUSB | Ubiquitous | + | (Husain, Passini, Parente, Fraser, & Wolfe, 2009) |
| PPE | All of CNS | +++ | (Xu et al., 2001) |
| SYN1 | Neurons | ++ | (Kugler et al., 2001) |
| NSE | Neurons | ++++ | (Smith-Arica et al., 2000) |
| GFAP | Astrocytes | +++ | (Lee, Messing, Su, & Brenner, 2008; Smith- Arica et al., 2000) |
| MBP | Oligodendrocytes | ++ | (Gow, Friedrich, & Lazzarini, 1992) |
| F4/80 | Microglia | ++ | (Cucchiarini, Ren, Perides, & Terwilliger, 2003) |
| MCH | MCH neurons | ++ | (van den Pol, Acuna- Goycolea, Clark, & Ghosh, 2004) |
| MeCP2 (MeP229) | Neurons | + | (Gray, Foti, et al., 2011) |
CMV, cytomegalovirus; RSV-LTR, Rous sarcoma virus long terminal repeat; SV40, simian virus 40; EF1-α, Human elongation factor-1 alpha promoter; PPE, preproenkephalin; GFAP, Glial fibrillary acidic protein.
Table 3.5.
Regulatory Elements to Modulate Expression
| Element | Role/Effect | Reference |
|---|---|---|
| WPRE | Increases RNA stability, transgene expression | (Xu et al., 2003) |
| Intron | Increases RNA stability, transgene expression | (Wu et al., 2008) |
| PolyA | Affects mRNA stability, transgene expression | (Hager, Frame, Collins, Burns, & Maitland, 2008; Wu et al., 2008) |
| miRNA-binding site | Restricts expression from specific cells | (Xie et al., 2010) |
| Ligand-inducible system | Activate/deactivate transgene expression | (Blesch et al., 2000; Zoltick & Wilson, 2001) |
| Stimulus-inducible system | Regulates transgene expression based on physiological state | (Ruan & Deen, 2001; Ruan et al., 2001) |
| MAR | Increases long-term retention of the delivered DNA | (Girod et al., 2007) |
| Chromatin insulators | Blocks cross-communication between regulator elements; reduces variability of transgene expression | (Recillas-Targa,Valadez- Graham, & Farrell, 2004) |
MAR, matrix attachment region.
3.1 Viral Promoters
The majority of pioneering gene therapy work has utilized viral promoters. A promoter is a sequence of DNA where transcription is initiated. Usually found near the beginning of a gene, the promoter has a binding site for the enzyme used to make a messenger RNA (mRNA) molecule. Their compact size and ability to induce often higher levels of transcriptions than eukaryotic promoters make them an attractive component in vector cassettes. These include the SV40, cytomegalovirus immediate-early promoter, Rous sarcoma virus long terminal repeat, Moloney murine leukemia virus (MoMLV) LTR, and other retroviral LTR promoters. The use of viral promoters has thus been widespread and successful in vitro and for certain applications in vivo.
However, the use of viral promoters has become increasingly limited due to eukaryotic cells’ ability to detect and silence viral transgene expression. Viral promoters have been commonly shown to be transcribed by many eukaryotic constructs in vivo but in many instances been shown to be specifically down regulated by tumor necrosis factor and interferons. It is certainly clear that powerful nonspecific promoters such as CMV are prone to silencing in the CNS (Gray, Foti, et al., 2011), and specific eukaryotic promoters or cis-acting regulatory elements may be used to avoid this effect.
3.2 CNS Promoters
Although endogenous eukaryotic promoters trail viral promoters in terms of expression intensity, ongoing modifications are continuously closing this gap. Eukaryotic genes are highly regulated and it is to be expected that success in their use will depend upon the use of an appropriate promoter and the incorporation of additional elements to maximize its expression, in contrast to the frequently more compact, self-contained viral promoters. The catalog of tissue- and disease-selective eukaryotic promoters employed to drive target-specific transgene expression has expanded considerably over the past few years and reflects attempts to improve the strength and longevity of transgene expression in vivo.
Several diseases similarly require expression restricted to the CNS. Smith-Arica et al. utilized the astrocyte-specific GFAP and neuronal- specific endolase (NSE) promoters to generate inducible neurospecific vectors, and demonstrated selective expression in vivo (Smith-Arica et al., 2000). Xu et al. found several brain-specific promoters superior to CMV in a panel of neuron-specific AAV vectors in vitro and in vivo, including the NSE, GFAP, preproenkephalin (PPE), and nonspecific elongation factor EF1α-promoters (Xu et al., 2001). In primary cell cultures in vitro the EF1-α and NSE promoters were at least 10 times the strength of CMV, whilst the GFAP and PPE promoters induced a comparable level of reporter gene expression. Following in vivo stereotactic injection of these AAV vectors, the NSE promoter was found to induce almost 100-fold greater levels of expression than the CMV promoter, while the EF1α, GFAP, and PPE promoters gave up to several-fold greater levels of expression.
3.3 Modifications to Enhance Expression
3.3.1 WPRE
Addition of the woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) to the NSE and PPE constructs improved expression levels by up to a further 10-fold in vitro and in vivo. WPRE is a DNA sequence that, when transcribed, creates a tertiary structure enhancing transgene expression. Insertion of the WPRE in the 3′ untranslated region of coding sequences carried by either oncoretroviral or lentiviral vectors substantially increased their levels of expression in a transgene, promoter, and vector-independent manner. Using adenovirus vectors, Xu et al. found that the WPRE in the sense orientation cloned between the luciferase gene and the polyA sequence stimulated 2- to 7-fold more luciferase expression in vitro and 2- to 50-fold more in the liver, kidney, and lung of mouse than occurred without the use of the WPRE (Xu, Mizuguchi, Mayumi, & Hayakawa, 2003). The most efficient Ad vector in this study, which contained an improved CMV promoter and the WPRE, showed more than 700-fold luciferase expression in mouse liver than did the Ad vector containing the conventional CMV promoter but no WPRE. These results indicate that inclusion of the WPRE, combined with the optimization of transcriptional regulatory elements in Ad vectors, can permit a given therapeutic goal to be achieved with substantially fewer viral particles. This and other studies utilizing WPRE provided novel information that incorporated this element in CNS gene therapy projects.
3.3.2 MARs and Chromatin Insulators
Gene transfer in eukaryotic cells and organisms suffers from epigenetic effects that result in low or unstable transgene expression and high clonal variability. Use of epigenetic regulators such as MARs is a promising approach to alleviate such unwanted effects. As discussed in Section 2.1, utilization of MARs in nonviral vectors shows an increase in the expression of recombinant proteins from cultured cells as well as mediating high and sustained expression in mice. Though most of these studies used nonviral vectors, the proof of concept shows promise in the use of this element in viral vectors studies.
To overcome chromatin-dependent repressive transgenic states, chromatin regulatory elements can be used to “insulate” transgene expression. Insulators, or chromatin boundaries, separate a gene locus into independent and separately regulated modules. These are able to protect a transgene against chromatin position effects at their genomic integration sites, or the effects of neighboring enhancer/promoter elements, and they are able to maintain transgene expression for long periods of time. Therefore, these elements may be very useful tools in gene therapy applications for ensuring high-level and stable expression of transgenes. This is also of particular importance for integrating vectors, where a strong vector promoter could influence the expression neighboring proto-oncogenes with deleterious consequences. The chicken beta-globin locus control region contains an insulator, HS4, which has been used in gene transfer studies (Li, Peterson, Fang, & Stamatoyannopoulos, 2002). The 1.2 kb HS4 was inserted into the MoMLV LTR without any adverse effects on vector production, and insulated integrated viral DNA from insertional position effects (Rivella, Callegari, May, Tan, & Sadelain, 2000). Steinwaerder et al. inserted an expression cassette driven by a metal (Zn2+) inducible promoter into an adenoviral vector, and observed that the background transgene expression increased significantly as a result of cis-acting viral sequences (Steinwaerder & Lieber, 2000). Flanking the expression cassette by the HS4 sequence increased the induction ratio by 15-fold in vivo. In an AAV vector, flanking the transgene construct with the 250 bp HS4 insulator stabilized expression levels to constant levels regardless of position effects (Inoue et al., 1999).
3.3.3 MicroRNA
One major obstacle in gene therapy trial is peripheral transduction in other tissue types. To circumvent this, Xie et al. (2010) used tissue-specific, endogenous microRNAs (miRNAs) to repress rAAV expression outside the CNS, by engineering perfectly complementary miRNA-binding sites into the rAAV9 genome (). miRNAs are a class of naturally occurring, small noncoding RNA molecules, about 21–25 nucleotides in length. miRNAs are partially complementary to one or more mRNA molecules, and their main function is to downregulate gene expression in a variety of manners, including translational repression, mRNA cleavage, and dead-enylation. This approach allowed simultaneous multitissue regulation and CNS-directed stable transgene expression without perturbing the endogenous miRNA pathway. In this instance, AAV9 was able to be administered systemically, targeting both the liver and the brain. By incorporating liver-specific miRNA-binding sites into the vector genome, expression in the brain was allowed to occur whereas expression in the liver was silenced. Similarly, target sequences of the mir-142-3p incorporated into lentiviral vectors suppressed expression in hematopoietic lineages, whereas expression was maintained in nonhematopoietic cells (Brown, Venneri, Zingale, Sergi Sergi, & Naldini, 2006). This expression profile enabled stable gene transfer into immunocompetent mice, thus overcoming a major hurdle to successful gene therapy (Brown et al., 2006).
3.3.4 PolyA Site
The polyA tail is a long chain of adenine nucleotides that is added to a mRNA molecule during RNA processing. The polyA tail makes the RNA molecule more stable and prevents its degradation and allows the mature mRNA molecule to be exported from the nucleus and translated into a protein by ribosomes in the cytoplasm. A comparison of different polyA signals, including the mouse α-globin polyA, the bovine growth hormone polyA, and a synthetic polyA showed that by utilizing different polyA sequences expression could be modulated over a 7-fold range (Wu et al., 2008).
3.4 Regulatable Expression Systems
3.4.1 Ligand-Inducible Systems
The majority of regulative systems engineered to date are ligand-inducible promoters, which have the distinct advantage of permitting pharmacological control of transgene expression following vector administration in vivo (reviewed in Zoltick & Wilson (2001)).
The most prominent regulative system has been the tetracycline (tet) on/off system (Figure 3.10). In this system, the transactivator (TA) is constitutively expressed, and the transgene is under control of the tet response element (tet operator). The TA consists of the tet repressor fused to a transcription factor activation domain. The same or two separate vectors may encode the TA and transgene in this system, but the latter “dual” vector system has had more success in maintaining regulatory stringency. The original wt repressor requires constant administration of the drug to keep the transactivator (tTA) displaced from the response element and the switch “off ” (tet-OFF system). The most useful tet switches for many gene therapy applications are those that use the mutant reverse tet repressor (rtTA), which binds in the presence of tetracycline or a suitable analogue. This switch is constitutively off and permits the “on” signal to be transmitted upon administration of tetracycline (the tet-ON system), necessitating its administration only when transgene expression is required rather than vice versa. A very large number of studies have made use of this system, which permits regulation of transgene expression by simple oral administration of Dox.
Figure 3.10.
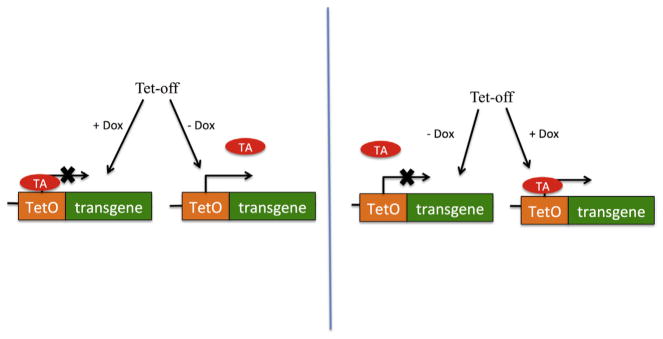
Tetracycline-controlled transcriptional activation. Dox = doxycycline, TA = transactivator, TetO = tetracycline operator.
Blesch et al. used a tetracycline-regulatable gene expression system was generated to determine whether controllable release of NGF and green fluorescent protein (GFP) from primary rat fibroblasts could modulate biological responses (neurite outgrowth) in vitro (Blesch, Uy, Diergardt, & Tuszynski, 2000). Using a tetracycline-repressible construct, it was found that NGF mRNA, NGF protein, and NGF-induced neurite outgrowth could be tightly regulated within a 24-h period, and in a dose-dependent fashion, by exposure to the tetracycline analog Dox. Similarly, levels of green fluorescence could be regulated in GFP-transfected cells. These findings in a neurobiological system lay the framework for future studies using regulated neurotrophin delivery in in vivo models of neurodegenerative diseases and CNS injury.
Steroid response switches using nonhuman analogues have also proved successful because of their suitable pharmacokinetic properties as small fast acting and rapidly metabolized lipophilic molecules (Hoppe, Marban, & Johns, 2000). This system utilizes a modular recombinant receptor consisting of the mutated ligand-binding domain fused to appropriate transcription factor DNA-binding and activation domains. The receptor is constitutively expressed and activates transcription from the target gene when ligand is present and binds to it. One study that evaluated the tet-on and antiprogestin switches in a dual adenoviral vector system in vitro demonstrated up to 1800- and 600-fold induction levels with the two switches, respectively (Molin, Shoshan, Ohman-Forslund, Linder, & Akusjarvi, 1998). Spiga et al. developed a novel glucocorticoid-inducible adenovirus vector that overproduces MMP1 only in the presence of dexamethasone, a synthetic corticosteroid drug (Spiga & Borras, 2010). The availability of this vector sets up the foundation for the development of gene therapy drugs for the potential treatment of ocular hypertension in steroid-responsive patients.
Another ligand-inducible system takes advantage of the dimerizing function of the antibiotic rapamycin, which links the two proteins FK506-binding protein (FKBP) and FKBP12-rapamycin-associated protein (FRAP). FKBP contains the DNA-binding domain while the activation domain of NF-κB p65 is fused to FRAP. The promoter containing the target DNA sequence is only induced when the two are dimerized by the action of rapamycin or (preferably) the use of alternative analogues in vivo (Rivera et al., 2005; Ye et al., 1999). Ye et al. (1999) utilized two AAV vectors, one expressing the dimerizing transcription factors and the other an erythropoietin transgene under transcriptional control of the target promoter. When delivered at an equal ratio by intramuscular injection in mice, plasma levels of erythropoietin were inducible by up to 200-fold upon administration of rapamycin in a dose-responsive fashion at 6 months after injection. Administration of vectors to nonhuman primates permitted 50-fold induction of erythropoietin that returned to baseline in 14 days. This maximal level of induction was comparable to the levels of erythropoietin expressed from a CMV promoter-driven vector.
3.4.2 Stimulus-Inducible Systems
Other types of regulatable promoters are those incorporating elements that are stimuli responsive, such as those inducible by heat, radiation, or metal-responsive elements. Of significant interest to several ischemic and cancer disease therapies are hypoxic response elements (HRE) that are activated in cells via hypoxia-inducible factor-1, which permit the selective induction of gene expression in a hypoxic environment. Many groups have utilized HREs to generate synthetic hypoxia-inducible promoter constructs. In studies by Ruan et al., a rAAV was generated in which the transgene can be regulated by hypoxia in human brain tumors (Ruan & Deen, 2001; Ruan et al., 2001). A DNA fragment containing 9xHRE and the LacZ reporter gene were incorporated into the AAV vector. Under anoxic conditions, this vector produced 79- to 110-fold increase in gene expression. This hypoxia-regulated rAAV vector will provide a useful delivery vehicle for CNS cancer-specific gene therapy.
4. CONCLUSIONS
The biology of different viruses offers unique solutions to the challenges of gene therapy, such as cell targeting, integration rate, transgene expression, and vector production. For example, the AAV platform offers substantial benefits including efficient gene transfer, long-term transgene expression, low-inflammatory responses, minimal integration rate, and scalable manufacture for clinical applications (reviewed in (Grieger & Samulski, 2005), and in this chapter). However, the limited packaging capacity of 4.7 kb is too small for some genes and greatly restricts the use of complex genomic regulatory elements. AAV is also not suitable for prolonged expression in dividing tissues. Lentiviral vectors, both integrating and nonintegrating, are becoming prominent CNS gene transfer tools. Their increased capacity of 8–9 kb of foreign DNA while still maintaining a relatively safe profile and reasonably efficient gene transfer to neural cells gives them a distinct advantage in certain applications. Similarly, adenoviral and HSV vectors have very large packaging capabilities (>25 kb) but adenoviral vectors are limited by their higher propensity to induce inflammation. In certain applications HSV vectors have a strong advantage due to their natural ability for efficient axonal transport, but, in general, they have not undergone the same level of fine-tuned engineering that has been devoted to other vectors to optimize them as safe and effective gene transfer reagents. Overall, it would be safe to predict that it is unlikely that one vector system will be exclusively appropriate for all types of research or therapeutic gene transfer applications into the CNS.
References
- Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, et al. Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science. 2002;298(5594):846–850. doi: 10.1126/science.1072873. [DOI] [PubMed] [Google Scholar]
- Adrover MF, Guyot-Revol V, Cheli VT, Blanco C, Vidal R, Alche L, et al. Hippocampal infection with HSV-1-derived vectors expressing an NMDAR1 antisense modifies behavior. Genes, Brain and Behavior. 2003;2(2):103–113. doi: 10.1034/j.1601-183x.2003.00015.x. [DOI] [PubMed] [Google Scholar]
- Aiken C. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. Journal of Virology. 1997;71(8):5871–5877. doi: 10.1128/jvi.71.8.5871-5877.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akache B, Grimm D, Pandey K, Yant SR, Xu H, Kay MA. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Journal of Virology. 2006;80(19):9831–9836. doi: 10.1128/JVI.00878-06. http://dx.doi.org/10.1128/JVI.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkina RK, Walton RM, Chen ML, Li QX, Planelles V, Chen IS. High-efficiency gene transfer into CD34+ cells with a human immunodeficiency virus type 1-based retroviral vector pseudotyped with vesicular stomatitis virus envelope glycoprotein G. Journal of Virology. 1996;70(4):2581–2585. doi: 10.1128/jvi.70.4.2581-2585.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida LP, Zala D, Aebischer P, Deglon N. Neuroprotective effect of a CNTF-expressing lentiviral vector in the quinolinic acid rat model of Huntington’s disease. Neurobiology of Disease. 2001;8(3):433–446. doi: 10.1006/nbdi.2001.0388. [DOI] [PubMed] [Google Scholar]
- Amalfitano A. Next-generation adenoviral vectors: new and improved. GeneTherapy. 1999;6(10):1643–1645. doi: 10.1038/sj.gt.3301027. [DOI] [PubMed] [Google Scholar]
- Antonawich FJ, Federoff HJ, Davis JN. BCL-2 transduction, using a herpes simplex virus amplicon, protects hippocampal neurons from transient global ischemia. Experimental Neurology. 1999;156(1):130–137. doi: 10.1006/exnr.1998.7004. http://dx.doi.org/10.1006/exnr.1998.7004. [DOI] [PubMed] [Google Scholar]
- Ariza L, Gimenez-Llort L, Cubizolle A, Pages G, Garcia-Lareu B, Serratrice N, Bosch A. Central nervous system delivery of helper-dependent canine adenovirus corrects neuropathology and behavior in mucopolysaccharidosis type VII mice. Human Gene Therapy. 2014;25(3):199–211. doi: 10.1089/hum.2013.152. http://dx.doi.org/10.1089/hum.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assi H, Candolfi M, Baker G, Mineharu Y, Lowenstein PR, Castro MG. Gene therapy for brain tumors: basic developments and clinical implementation. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review] Neuroscience Letters. 2012;527(2):71–77. doi: 10.1016/j.neulet.2012.08.003. http://dx.doi.org/10.1016/j.neulet.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchison RW, Casto BC, Hammon WM. Adenovirus-associated defective virus particles. Science. 1965;149(3685):754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- Azzouz M, Martin-Rendon E, Barber RD, Mitrophanous KA, Carter EE, Rohll JB, et al. Multicistronic lentiviral vector-mediated striatal gene transfer of aromatic L-amino acid decarboxylase, tyrosine hydroxylase, and GTP cyclohydrolase I induces sustained transgene expression, dopamine production, and functional improvement in a rat model of Parkinson’s disease. The Journal of Neuroscience. 2002;22(23):10302–10312. doi: 10.1523/JNEUROSCI.22-23-10302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzouz M, Ralph GS, Storkebaum E, Walmsley LE, Mitrophanous KA, Kingsman SM, et al. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429(6990):413–417. doi: 10.1038/nature02544. http://dx.doi.org/10.1038/nature02544. [DOI] [PubMed] [Google Scholar]
- Baekelandt V, Claeys A, Eggermont K, Lauwers E, De Strooper B, Nuttin B, et al. Characterization of lentiviral vector-mediated gene transfer in adult mouse brain. Human Gene Therapy. 2002;13(7):841–853. doi: 10.1089/10430340252899019. [DOI] [PubMed] [Google Scholar]
- Baltimore D, Huang AS. Interaction of HeLa cell proteins with RNA. Journal of Molecular Biology. 1970;47(3):263–273. doi: 10.1016/0022-2836(70)90301-3. [DOI] [PubMed] [Google Scholar]
- Baron EJ. Classification. In: Baron S, editor. Medical Microbiology. 4. Galveston (TX): 1996. [Google Scholar]
- Bartlett JS, Wilcher R, Samulski RJ. Infectious entry pathway of adeno-associated virus and adeno-associated virus vectors. Journal of Virology. 2000;74(6):2777–2785. doi: 10.1128/jvi.74.6.2777-2785.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz SR, Rogel ME, Emerman M. Human immunodeficiency virus type 1 cell cycle control: Vpr is cytostatic and mediates G2 accumulation by a mechanism which differs from DNA damage checkpoint control. Journal of Virology. 1996;70(4):2324–2331. doi: 10.1128/jvi.70.4.2324-2331.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum C, Schambach A, Bohne J, Galla M. Retrovirus vectors: toward the plentivirus? Molecular Therapy. 2006;13(6):1050–1063. doi: 10.1016/j.ymthe.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Bayer M, Kantor B, Cockrell A, Ma H, Zeithaml B, Li X, et al. A large U3 deletion causes increased in vivo expression from a nonintegrating lentiviral vector. Molecular Therapy. 2008;16(12):1968–1976. doi: 10.1038/mt.2008.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard Roizman DK. Fields Virology. 4. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. Herpes simplex viruses and their replication. [Google Scholar]
- Bevan AK, Duque S, Foust KD, Morales PR, Braun L, Schmelzer L, et al. Systemic gene delivery in large species for targeting spinal cord, brain, and peripheral tissues for pediatric disorders. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Molecular Therapy. 2011;19(11):1971–1980. doi: 10.1038/mt.2011.157. http://dx.doi.org/10.1038/mt.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn SD, Steadman RA, Johnson FB. Attachment of adeno-associated virus type 3H to fibroblast growth factor receptor 1. [In Vitro] Archives ofVirology. 2006;151(3):617–623. doi: 10.1007/s00705-005-0650-6. http://dx.doi.org/10.1007/s00705-005-0650-6. [DOI] [PubMed] [Google Scholar]
- Blesch A, Uy HS, Diergardt N, Tuszynski MH. Neurite outgrowth can be modulated in vitro using a tetracycline-repressible gene therapy vector expressing human nerve growth factor. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.Research Support, U.S. Gov’t, P.H.S.] Journal of Neuroscience Research. 2000;59(3):402–409. doi: 10.1002/(SICI)1097-4547(20000201)59:3<402::AID-JNR14>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Blomer U, Naldini L, Kafri T, Trono D, Verma IM, Gage FH. Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. Journal of Virology. 1997;71(9):6641–6649. doi: 10.1128/jvi.71.9.6641-6649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boado RJ. Blood-brain barrier transport of non-viral gene and RNAi therapeutics [Review] Pharmaceutical Research. 2007;24(9):1772–1787. doi: 10.1007/s11095-007-9321-5. http://dx.doi.org/10.1007/s11095-007-9321-5. [DOI] [PubMed] [Google Scholar]
- Boado RJ, Pardridge WM. The Trojan horse liposome technology for nonviral gene transfer across the blood-brain barrier. Journal of Drug Delivery. 2011;2011:296151. doi: 10.1155/2011/296151. http://dx.doi.org/10.1155/2011/296151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles DE, McPhee SW, Li C, Gray SJ, Samulski JJ, Camp AS, et al. Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector. [Research Support, N.I.H., Extramural Research Support, Non-U.S Gov’t] Molecular Therapy. 2012;20(2):443–455. doi: 10.1038/mt.2011.237. http://dx.doi.org/10.1038/mt.2011.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz J, Beaufour C, Coutaux A, Epstein AL, Cesselin F, Hamon M, et al. Therapeutic efficacy in experimental polyarthritis of viral-driven enkephalin overproduction in sensory neurons. The Journal of Neuroscience. 2001;21(20):7881–7888. doi: 10.1523/JNEUROSCI.21-20-07881.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks AI, Cory-Slechta DA, Bowers WJ, Murg SL, Federoff HJ. Enhanced learning in mice parallels vector-mediated nerve growth factor expression in hippocampus. Human Gene Therapy. 2000;11(17):2341–2352. doi: 10.1089/104303400750038453. http://dx.doi.org/10.1089/104303400750038453. [DOI] [PubMed] [Google Scholar]
- Brown BD, Venneri MA, Zingale A, Sergi Sergi L, Naldini L. Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer. Natural Medicine. 2006;12(5):585–591. doi: 10.1038/nm1398. [DOI] [PubMed] [Google Scholar]
- Buchow HD, Tschachler E, Gallo RC, Reitz M., Jr HIV-I replication requires an intact integrase reading frame. Haematology and Blood Transfusion. 1989;32:402–405. doi: 10.1007/978-3-642-74621-5_68. [DOI] [PubMed] [Google Scholar]
- Bukrinsky MI, Haggerty S, Dempsey MP, Sharova N, Adzhubel A, Spitz L, et al. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature. 1993;365(6447):666–669. doi: 10.1038/365666a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(17):8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushman FD, Craigie R. Sequence requirements for integration of Moloney murine leukemia virus DNA in vitro. Journal of Virology. 1990;64(11):5645–5648. doi: 10.1128/jvi.64.11.5645-5648.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushman FD, Fujiwara T, Craigie R. Retroviral DNA integration directed by HIV integration protein in vitro. Science. 1990;249(4976):1555–1558. doi: 10.1126/science.2171144. [DOI] [PubMed] [Google Scholar]
- Butler SL, Johnson EP, Bushman FD. Human immunodeficiency virus cDNA metabolism: notable stability of two-long terminal repeat circles. Journal ofVirology. 2002;76(8):3739–3747. doi: 10.1128/JVI.76.8.3739-3747.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos SK, Barry MA. Current advances and future challenges in adenoviral vector biology and targeting. Current Gene Therapy. 2007;7(3):189–204. doi: 10.2174/156652307780859062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canivet M, Hoffman AD, Hardy D, Sernatinger J, Levy JA. Replication of HIV-1 in a wide variety of animal cells following phenotypic mixing with murine retroviruses. Virology. 1990;178(2):543–551. doi: 10.1016/0042-6822(90)90352-r. [DOI] [PubMed] [Google Scholar]
- Cannon JR, Sew T, Montero L, Burton EA, Greenamyre JT. Pseudotype-dependent lentiviral transduction of astrocytes or neurons in the rat substantia nigra. [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.] Experimental Neurology. 2011;228(1):41–52. doi: 10.1016/j.expneurol.2010.10.016. http://dx.doi.org/10.1016/j.expneurol.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassinotti P, Weitz M, Tratschin JD. Organization of the adeno-associated virus (AAV) capsid gene: mapping of a minor spliced mRNA coding for virus capsid protein 1. Virology. 1988;167(1):176–184. [PubMed] [Google Scholar]
- Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288(5466):669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- Cearley CN, Wolfe JH. Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Molecular Therapy. 2006;13(3):528–537. doi: 10.1016/j.ymthe.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Cearley CN, Wolfe JH. A single injection of an adeno-associated virus vector into nuclei with divergent connections results in widespread vector distribution in the brain and global correction of a neurogenetic disease. The Journal of Neuroscience. 2007;27(37):9928–9940. doi: 10.1523/JNEUROSCI.2185-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay M, Wolfe D, Mata M, Huang S, Glorioso JC, Fink DJ. Long-term neuroprotection achieved with latency-associated promoter-driven herpes simplex virus gene transfer to the peripheral nervous system. Molecular Therapy. 2005;12(2):307–313. doi: 10.1016/j.ymthe.2005.04.009. http://dx.doi.org/10.1016/j.ymthe.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Chauhan AN, Lewis PD. A quantitative study of cell proliferation in ependyma and choroid plexus in the postnatal rat brain. Neuropathology and Applied Neurobiology. 1979;5(4):303–309. doi: 10.1111/j.1365-2990.1979.tb00629.x. [DOI] [PubMed] [Google Scholar]
- Chen YH, Chang M, Davidson BL. Molecular signatures of disease brain endothelia provide new sites for CNS-directed enzyme therapy. Natural Medicine. 2009;15(10):1215–1218. doi: 10.1038/nm.2025. http://dx.doi.org/10.1038/nm.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Claflin K, Geoghegan JC, Davidson BL. Sialic acid deposition impairs the utility of AAV9, but not peptide-modified AAVs for brain gene therapy in a mouse model of lysosomal storage disease. Molecular Therapy. 2012;20(7):1393–1399. doi: 10.1038/mt.2012.100. http://dx.doi.org/10.1038/mt.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Liu Y, Zhao H, Zhang W, Guo YJ, Nie L. Lentiviral-mediated transfer of CDNF promotes nerve regeneration and functional recovery after sciatic nerve injury in adult rats. Biochemical and Biophysical Research Communications. 440(2):330–335. doi: 10.1016/j.bbrc.2013.09.084. [DOI] [PubMed] [Google Scholar]
- Chen HH, Mack LM, Kelly R, Ontell M, Kochanek S, Clemens PR. Persistence in muscle of an adenoviral vector that lacks all viral genes. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(5):1645–1650. doi: 10.1073/pnas.94.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B, Wehrly K, Maury W. Differential expression in human and mouse cells of human immunodeficiency virus pseudotyped by murine retroviruses. Journal of Virology. 1990;64(9):4553–4557. doi: 10.1128/jvi.64.9.4553-4557.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AK, Hoggan MD, Hauswirth WW, Berns KI. Integration of the adeno-associated virus genome into cellular DNA in latently infected human Detroit 6 cells. Journal of Virology. 1980;33(2):739–748. doi: 10.1128/jvi.33.2.739-748.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiocca EA. Oncolytic viruses. Nature Reviews Cancer. 2002;2(12):938–950. doi: 10.1038/nrc948. http://dx.doi.org/10.1038/nrc948. [DOI] [PubMed] [Google Scholar]
- Choi VW, McCarty DM, Samulski RJ. AAV hybrid serotypes: improved vectors for gene delivery. Current Gene Therapy. 2005;5(3):299–310. doi: 10.2174/1566523054064968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387(6629):183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- Cockrell AS, Kafri T. Gene delivery by lentivirus vectors. Molecular Biotechnology. 2007;36(3):184–204. doi: 10.1007/s12033-007-0010-8. [DOI] [PubMed] [Google Scholar]
- Cockrell AS, Ma H, Fu K, McCown TJ, Kafri T. A trans-lentiviral packaging cell line for high-titer conditional self-inactivating HIV-1 vectors. Molecular Therapy. 2006;14(2):276–284. doi: 10.1016/j.ymthe.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Coffin JM. Genetic diversity and evolution of retroviruses. Current Topics in Microbiology and Immunology. 1992;176:143–164. doi: 10.1007/978-3-642-77011-1_10. [DOI] [PubMed] [Google Scholar]
- Coffin JM, Hughes SH, Varmus HE. The Interactions of Retroviruses and Their Hosts. 1997 [PubMed] [Google Scholar]
- Coil DA, Miller AD. Phosphatidylserine is not the cell surface receptor for vesicular stomatitis virus. [Research Support, U.S. Gov’t, P.H.S.] Journal of Virology. 2004;78(20):10920–10926. doi: 10.1128/JVI.78.20.10920-10926.2004. http://dx.doi.org/10.1128/JVI.78.20.10920-10926.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colicelli J, Goff SP. Mutants and pseudorevertants of Moloney murine leukemia virus with alterations at the integration site. Cell. 1985;42(2):573–580. doi: 10.1016/0092-8674(85)90114-x. [DOI] [PubMed] [Google Scholar]
- Colicelli J, Goff SP. Isolation of an integrated provirus of Moloney murine leukemia virus with long terminal repeats in inverted orientation: integration utilizing two U3 sequences. Journal of Virology. 1988a;62(2):633–636. doi: 10.1128/jvi.62.2.633-636.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colicelli J, Goff SP. Sequence and spacing requirements of a retrovirus integration site. Journal of Molecular Biology. 1988b;199(1):47–59. doi: 10.1016/0022-2836(88)90378-6. [DOI] [PubMed] [Google Scholar]
- Consiglio A, Gritti A, Dolcetta D, Follenzi A, Bordignon C, Gage FH, et al. Robust in vivo gene transfer into adult mammalian neural stem cells by lentiviral vectors. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(41):14835–14840. doi: 10.1073/pnas.0404180101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consiglio A, Quattrini A, Martino S, Bensadoun JC, Dolcetta D, Trojani A, et al. In vivo gene therapy of metachromatic leukodystrophy by lentiviral vectors: correction of neuropathology and protection against learning impairments in affected mice. Natural Medicine. 2001;7(3):310–316. doi: 10.1038/85454. [DOI] [PubMed] [Google Scholar]
- Conzelmann KK, Cox JH, Schneider LG, Thiel HJ. Molecular cloning and complete nucleotide sequence of the attenuated rabies virus SAD B19. Virology. 1990;175(2):485–499. doi: 10.1016/0042-6822(90)90433-r. [DOI] [PubMed] [Google Scholar]
- Cooper I, Sasson K, Teichberg VI, Schnaider-Beeri M, Fridkin M, Shechter Y. Peptide derived from HIV-1 TAT protein destabilizes a monolayer of endothelial cells in an in vitro model of the blood-brain barrier and allows permeation of high molecular weight proteins. [Research Support, Non-U.S. Gov’t] The Journal of Biological Chemistry. 2012;287(53):44676–44683. doi: 10.1074/jbc.M112.395384. http://dx.doi.org/10.1074/jbc.M112.395384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craigie R, Fujiwara T, Bushman F. The IN protein of Moloney murine leukemia virus processes the viral DNA ends and accomplishes their integration in vitro. Cell. 1990;62(4):829–837. doi: 10.1016/0092-8674(90)90126-y. [DOI] [PubMed] [Google Scholar]
- Cronin J, Zhang XY, Reiser J. Altering the tropism of lentiviral vectors through pseudotyping. Current Gene Therapy. 2005;5(4):387–398. doi: 10.2174/1566523054546224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croyle MA, Callahan SM, Auricchio A, Schumer G, Linse KD, Wilson JM, et al. PEGylation of a vesicular stomatitis virus G pseudotyped lentivirus vector prevents inactivation in serum. Journal of Virology. 2004;78(2):912–921. doi: 10.1128/JVI.78.2.912-921.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucchiarini M, Ren XL, Perides G, Terwilliger EF. Selective gene expression in brain microglia mediated via adeno-associated virus type 2 and type 5 vectors. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] GeneTherapy. 2003;10(8):657–667. doi: 10.1038/sj.gt.3301925. http://dx.doi.org/10.1038/sj.gt.3301925. [DOI] [PubMed] [Google Scholar]
- Cunha FQ, Poole S, Lorenzetti BB, Veiga FH, Ferreira SH. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-4. British Journal of Pharmacology. 1999;126(1):45–50. doi: 10.1038/sj.bjp.0702266. http://dx.doi.org/10.1038/sj.bjp.0702266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambach MJ, Trecki J, Martin N, Markovitz NS. Oncolytic viruses derived from the gamma34.5-deleted herpes simplex virus recombinant R3616 encode a truncated UL3 protein. Molecular Therapy. 2006;13(5):891–898. doi: 10.1016/j.ymthe.2006.02.006. http://dx.doi.org/10.1016/j.ymthe.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Dayton ET, Powell DM, Dayton AI. Functional analysis of CAR, the target sequence for the Rev protein of HIV-1. Science. 1989;246(4937):1625–1629. doi: 10.1126/science.2688093. [DOI] [PubMed] [Google Scholar]
- DeLuca NA, Schaffer PA. Activation of immediate-early, early, and late promoters by temperature-sensitive and wild-type forms of herpes simplex virus type 1 protein ICP4. Molecular and Cellular Biology. 1985;5(8):1997–2008. doi: 10.1128/mcb.5.8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca NA, Schaffer PA. Physical and functional domains of the herpes simplex virus transcriptional regulatory protein ICP4. Journal of Virology. 1988;62(3):732–743. doi: 10.1128/jvi.62.3.732-743.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePolo NJ, Reed JD, Sheridan PL, Townsend K, Sauter SL, Jolly DJ, et al. VSV-G pseudotyped lentiviral vector particles produced in human cells are inactivated by human serum. Molecular Therapy. 2000;2(3):218–222. doi: 10.1006/mthe.2000.0116. [DOI] [PubMed] [Google Scholar]
- Desmaris N, Bosch A, Salaun C, Petit C, Prevost MC, Tordo N, et al. Production and neurotropism of lentivirus vectors pseudotyped with lyssavirus envelope glycoproteins. Molecular Therapy. 2001;4(2):149–156. doi: 10.1006/mthe.2001.0431. [DOI] [PubMed] [Google Scholar]
- Di Pasquale G, Davidson BL, Stein CS, Martins I, Scudiero D, Monks A, et al. Identification of PDGFR as a receptor for AAV-5 transduction. Natural Medicine. 2003;9(10):1306–1312. doi: 10.1038/nm929. http://dx.doi.org/10.1038/nm929. [DOI] [PubMed] [Google Scholar]
- Diefenbach RJ, Miranda-Saksena M, Douglas MW, Cunningham AL. Transport and egress of herpes simplex virus in neurons. Reviews in Medical Virology. 2008;18(1):35–51. doi: 10.1002/rmv.560. http://dx.doi.org/10.1002/rmv.560. [DOI] [PubMed] [Google Scholar]
- Dittgen T, Nimmerjahn A, Komai S, Licznerski P, Waters J, Margrie TW, et al. Lentivirus-based genetic manipulations of cortical neurons and their optical and electrophysiological monitoring in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(52):18206–18211. doi: 10.1073/pnas.0407976101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong JY, Fan PD, Frizzell RA. Quantitative analysis of the packaging capacity of recombinant adeno-associated virus. Human Gene Therapy. 1996;7(17):2101–2112. doi: 10.1089/hum.1996.7.17-2101. http://dx.doi.org/10.1089/hum.1996.7.17-2101. [DOI] [PubMed] [Google Scholar]
- Dong B, Nakai H, Xiao W. Characterization of genome integrity for oversized recombinant AAV vector. Molecular Therapy. 2010;18(1):87–92. doi: 10.1038/mt.2009.258. http://dx.doi.org/10.1038/mt.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D, Sharma P, Yang J, Yue Y, Dudus L, Zhang Y, et al. Circular intermediates of recombinant adeno-associated virus have defined structural characteristics responsible for long-term episomal persistence in muscle tissue. Journal of Virology. 1998;72(11):8568–8577. doi: 10.1128/jvi.72.11.8568-8577.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, et al. A third-generation lentivirus vector with a conditional packaging system. Journal of Virology. 1998;72(11):8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque S, Joussemet B, Riviere C, Marais T, Dubreil L, Douar AM, et al. Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Molecular Therapy. 2009;17(7):1187–1196. doi: 10.1038/mt.2009.71. http://dx.doi.org/10.1038/mt.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman M, Vazeux R, Peden K. The rev gene product of the human immunodeficiency virus affects envelope-specific RNA localization. Cell. 1989;57(7):1155–1165. doi: 10.1016/0092-8674(89)90053-6. [DOI] [PubMed] [Google Scholar]
- Emi N, Friedmann T, Yee JK. Pseudotype formation of murine leukemia virus with the G protein of vesicular stomatitis virus. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S. ] Journal of Virology. 1991;65(3):1202–1207. doi: 10.1128/jvi.65.3.1202-1207.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman A, Englund G, Orenstein JM, Martin MA, Craigie R. Multiple effects of mutations in human immunodeficiency virus type 1 integrase on viral replication. Journal of Virology. 1995;69(5):2729–2736. doi: 10.1128/jvi.69.5.2729-2736.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein AL. HSV-1-based amplicon vectors: design and applications. Gene Therapy. 2005;12(Suppl 1):S154–S158. doi: 10.1038/sj.gt.3302617. http://dx.doi.org/10.1038/sj.gt.3302617. [DOI] [PubMed] [Google Scholar]
- Farnet CM, Haseltine WA. Circularization of human immunodeficiency virus type 1 DNA in vitro. Journal of Virology. 1991;65(12):6942–6952. doi: 10.1128/jvi.65.12.6942-6952.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farson D, Witt R, McGuinness R, Dull T, Kelly M, Song J, et al. A new-generation stable inducible packaging cell line for lentiviral vectors. Human Gene Therapy. 2001;12(8):981–997. doi: 10.1089/104303401750195935. [DOI] [PubMed] [Google Scholar]
- Federici T, Taub JS, Baum GR, Gray SJ, Grieger JC, Matthews KA, et al. Robust spinal motor neuron transduction following intrathecal delivery of AAV9 in pigs. Gene Therapy. 2012;19(8):852–859. doi: 10.1038/gt.2011.130. http://dx.doi.org/10.1038/gt.2011.130. [DOI] [PubMed] [Google Scholar]
- Ferrari FK, Samulski T, Shenk T, Samulski RJ. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. Journal of Virology. 1996;70(5):3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink DJ, Wechuck J, Mata M, Glorioso JC, Goss J, Krisky D, et al. Gene therapy for pain: results of a phase I clinical trial. Annals of Neurology. 2011;70(2):207–212. doi: 10.1002/ana.22446. http://dx.doi.org/10.1002/ana.22446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nature Biotechnology. 2009;27(1):59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust KD, Wang X, McGovern VL, Braun L, Bevan AK, Haidet AM, et al. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nature Biotechnology. 2010;28(3):271–274. doi: 10.1038/nbt.1610. http://dx.doi.org/10.1038/nbt.1610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Frenkel N, Singer O, Kwong AD. Minireview: the herpes simplex virus amplicon–a versatile defective virus vector. Gene Therapy. 1994;1(Suppl 1):S40–S46. [PubMed] [Google Scholar]
- Fu H, Dirosario J, Killedar S, Zaraspe K, McCarty DM. Correction of neurological disease of mucopolysaccharidosis IIIB in adult mice by rAAV9 trans-blood-brain barrier gene delivery. Molecular Therapy. 2011;19(6):1025–1033. doi: 10.1038/mt.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadalla KK, Bailey ME, Spike RC, Ross PD, Woodard KT, Kalburgi SN, et al. Improved survival and reduced phenotypic severity following AAV9/MECP2 gene transfer to neonatal and juvenile male Mecp2 knockout mice. [Research Support, Non-U.S. Gov’t] MolecularTherapy. 2013;21(1):18–30. doi: 10.1038/mt.2012.200. http://dx.doi.org/10.1038/mt.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Alvira MR, Somanathan S, Lu Y, Vandenberghe LH, Rux JJ, et al. Adeno-associated viruses undergo substantial evolution in primates during natural infections. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(10):6081–6086. doi: 10.1073/pnas.0937739100. http://dx.doi.org/10.1073/pnas.0937739100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH, Wilson JM. New recombinant serotypes of AAV vectors. Current Gene Therapy. 2005;5(3):285–297. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- Garg SK, Lioy DT, Cheval H, McGann JC, Bissonnette JM, Murtha MJ, et al. Systemic delivery of MeCP2 rescues behavioral and cellular deficits in female mouse models of Rett syndrome. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] The Journal of Neuroscience. 2013;33(34):13612–13620. doi: 10.1523/JNEUROSCI.1854-13.2013. http://dx.doi.org/10.1523/JNEUROSCI.1854-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller AI, During MJ, Oh YJ, Freese A, O’Malley K. An HSV-1 vector expressing tyrosine hydroxylase causes production and release of L-dopa from cultured rat striatal cells. Journal of Neurochemistry. 1995;64(2):487–496. doi: 10.1046/j.1471-4159.1995.64020487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller AI, Yu L, Wang Y, Fraefel C. Helper virus-free herpes simplex virus-1 plasmid vectors for gene therapy of Parkinson’s disease and other neurological disorders. Experimental Neurology. 1997;144(1):98–102. doi: 10.1006/exnr.1996.6394. http://dx.doi.org/10.1006/exnr.1996.6394. [DOI] [PubMed] [Google Scholar]
- Geschwind MD, Hartnick CJ, Liu W, Amat J, Van De Water TR, Federoff HJ. Defective HSV-1 vector expressing BDNF in auditory ganglia elicits neurite outgrowth: model for treatment of neuron loss following cochlear degeneration. Human Gene Therapy. 1996;7(2):173–182. doi: 10.1089/hum.1996.7.2-173. http://dx.doi.org/10.1089/hum.1996.7.2-173. [DOI] [PubMed] [Google Scholar]
- Girod PA, Nguyen DQ, Calabrese D, Puttini S, Grandjean M, Martinet D, et al. Genome-wide prediction of matrix attachment regions that increase gene expression in mammalian cells. [Research Support, Non-U.S. Gov’t] Nature Methods. 2007;4(9):747–753. doi: 10.1038/nmeth1076. http://dx.doi.org/10.1038/nmeth1076. [DOI] [PubMed] [Google Scholar]
- Goins WF, Cohen JB, Glorioso JC. Gene therapy for the treatment of chronic peripheral nervous system pain. Neurobiology of Disease. 2012;48(2):255–270. doi: 10.1016/j.nbd.2012.05.005. http://dx.doi.org/10.1016/j.nbd.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goins WF, Lee KA, Cavalcoli JD, O’Malley ME, DeKosky ST, Fink DJ, et al. Herpes simplex virus type 1 vector-mediated expression of nerve growth factor protects dorsal root ganglion neurons from peroxide toxicity. Journal of Virology. 1999;73(1):519–532. doi: 10.1128/jvi.73.1.519-532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez C, Hope TJ. The ins and outs of HIV replication. Cellular Microbiology. 2005;7(5):621–626. doi: 10.1111/j.1462-5822.2005.00516.x. [DOI] [PubMed] [Google Scholar]
- Gossen M, Bonin AL, Freundlieb S, Bujard H. Inducible gene expression systems for higher eukaryotic cells. Current Opinion in Biotechnology. 1994;5(5):516–520. doi: 10.1016/0958-1669(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Goss JR, Harley CF, Mata M, O’Malley ME, Goins WF, Hu X, et al. Herpes vector-mediated expression of proenkephalin reduces bone cancer pain. Annals of Neurology. 2002;52(5):662–665. doi: 10.1002/ana.10343. http://dx.doi.org/10.1002/ana.10343. [DOI] [PubMed] [Google Scholar]
- Goss JR, Krisky D, Wechuck J, Wolfe D. Herpes simplex virus-based nerve targeting gene therapy in pain management. Journal of Pain Research. 2014;7:71–79. doi: 10.2147/JPR.S36619. http://dx.doi.org/10.2147/JPR.S36619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss JR, Mata M, Goins WF, Wu HH, Glorioso JC, Fink DJ. Anti-nociceptive effect of a genomic herpes simplex virus-based vector expressing human proenkephalin in rat dorsal root ganglion. Gene Therapy. 2001;8(7):551–556. doi: 10.1038/sj.gt.3301430. http://dx.doi.org/10.1038/sj.gt.3301430. [DOI] [PubMed] [Google Scholar]
- Gow A, Friedrich VL, Jr, Lazzarini RA. Myelin basic protein gene contains separate enhancers for oligodendrocyte and Schwann cell expression. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S. ] The Journal of Cell Biology. 1992;119(3):605–616. doi: 10.1083/jcb.119.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandi P, Spear M, Breakefield XO, Wang S. Targeting HSV amplicon vectors. Methods. 2004;33(2):179–186. doi: 10.1016/j.ymeth.2003.11.007. http://dx.doi.org/10.1016/j.ymeth.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Grandi P, Wang S, Schuback D, Krasnykh V, Spear M, Curiel DT, et al. HSV-1 virions engineered for specific binding to cell surface receptors. Molecular Therapy. 2004;9(3):419–427. doi: 10.1016/j.ymthe.2003.12.010. http://dx.doi.org/10.1016/j.ymthe.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Grassi G, Maccaroni P, Meyer R, Kaiser H, D’Ambrosio E, Pascale E, et al. Inhibitors of DNA methylation and histone deacetylation activate cytomegalovirus promoter-controlled reporter gene expression in human glioblastoma cell line U87. Carcinogenesis. 2003;24(10):1625–1635. doi: 10.1093/carcin/bgg118. [DOI] [PubMed] [Google Scholar]
- Gray SJ. Gene therapy and neurodevelopmental disorders. Neuropharmacology. 2013;68:136–142. doi: 10.1016/j.neuropharm.2012.06.024. http://dx.doi.org/10.1016/j.neuropharm.2012.06.024. [DOI] [PubMed] [Google Scholar]
- Gray SJ, Blake BL, Criswell HE, Nicolson SC, Samulski RJ, McCown TJ, et al. Directed evolution of a novel adeno-associated virus (AAV) vector that crosses the seizure-compromised blood–brain barrier (BBB) Molecular Therapy. 2010;18(3):570–578. doi: 10.1038/mt.2009.292. http://dx.doi.org/10.1038/mt.2009.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SJ, Foti SB, Schwartz JW, Bachaboina L, Taylor-Blake B, Coleman J, Samulski RJ. Optimizing promoters for recombinant adeno-associated virus-mediated gene expression in the peripheral and central nervous system using self-complementary vectors. Human Gene Therapy. 2011;22(9):1143–1153. doi: 10.1089/hum.2010.245. http://dx.doi.org/10.1089/hum.2010.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SJ, Matagne V, Bachaboina L, Yadav S, Ojeda SR, Samulski RJ. Pre-clinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Molecular Therapy. 2011;19(6):1058–1069. doi: 10.1038/mt.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SJ, Nagabhushan Kalburgi S, McCown TJ, Jude Samulski R. Global CNS gene delivery and evasion of anti-AAV-neutralizing antibodies by intrathecal AAV administration in non-human primates. Gene Therapy. 2013a;20(4):450–459. doi: 10.1038/gt.2012.101. http://dx.doi.org/10.1038/gt.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SJ, Woodard KT, Samulski RJ. Viral vectors and delivery strategies for CNS gene therapy. Therapeutic Delivery. 2010;1(4):517–534. doi: 10.4155/tde.10.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieger JC, Samulski RJ. Adeno-associated virus as a gene therapy vector: vector development, production and clinical applications. Advances in Biochemical Engineering/Biotechnology. 2005;99:119–145. [PubMed] [Google Scholar]
- Haack K, Cockrell AS, Ma H, Israeli D, Ho SN, McCown TJ, et al. Transactivator and structurally optimized inducible lentiviral vectors. MolecularTherapy. 2004;10(3):585–596. doi: 10.1016/j.ymthe.2004.06.109. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. The New England Journal of Medicine. 2003;348(3):255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302(5644):415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- Hadzopoulou-Cladaras M, Felber BK, Cladaras C, Athanassopoulos A, Tse A, Pavlakis GN. The rev (trs/art) protein of human immunodeficiency virus type 1 affects viral mRNA and protein expression via a cis-acting sequence in the env region. Journal of Virology. 1989;63(3):1265–1274. doi: 10.1128/jvi.63.3.1265-1274.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn C, Antoniou MN, Lipps HJ. Genomic cis-acting sequences improve expression and establishment of a nonviral vector. MolecularTherapy Nucleic Acids. 2013;2:e118. doi: 10.1038/mtna.2013.47. http://dx.doi.org/10.1038/mtna.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager S, Frame FM, Collins AT, Burns JE, Maitland NJ. An internal polyadenylation signal substantially increases expression levels of lentivirus-delivered transgenes but has the potential to reduce viral titer in a promoter-dependent manner. [Research Support, Non-U.S.Gov’t] Human Gene Therapy. 2008;19(8):840–850. doi: 10.1089/hum.2007.165. http://dx.doi.org/10.1089/hum.2007.165. [DOI] [PubMed] [Google Scholar]
- Halbert CL, Miller AD, McNamara S, Emerson J, Gibson RL, Ramsey B, et al. Prevalence of neutralizing antibodies against adeno-associated virus (AAV) types 2, 5, and 6 in cystic fibrosis and normal populations: implications for gene therapy using AAV vectors. Human Gene Therapy. 2006;17(4):440–447. doi: 10.1089/hum.2006.17.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J, Schulze T, Mellert W, Moelling K. Identification and characterization of HIV-specific RNase H by monoclonal antibody. The EMBO Journal. 1988;7(1):239–243. doi: 10.1002/j.1460-2075.1988.tb02805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S, Mata M, Wolfe D, Huang S, Glorioso JC, Fink DJ. Gene transfer of glutamic acid decarboxylase reduces neuropathic pain. Annals of Neurology. 2005;57(6):914–918. doi: 10.1002/ana.20483. http://dx.doi.org/10.1002/ana.20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S, Wolfe D, Glorioso JC, Mata M, Fink DJ. Effects of transgene-mediated endomorphin-2 in inflammatory pain. European Journal of Pain. 2009;13(4):380–386. doi: 10.1016/j.ejpain.2008.05.008. http://dx.doi.org/10.1016/j.ejpain.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haurigot V, Marco S, Ribera A, Garcia M, Ruzo A, Villacampa P, Bosch F. Whole body correction of mucopolysaccharidosis IIIA by intracerebrospinal fluid gene therapy. Journal of Clinical Investigation. 2013;123(8):3254–3271. doi: 10.1172/JCI66778. http://dx.doi.org/10.1172/JCI66778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey AD, Chase M. Independent functions of viral protein and nucleic acid in growth of bacteriophage. The Journal of General Physiology. 1952;36(1):39–56. doi: 10.1085/jgp.36.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashikawa F, Chang L. Kinetic analyses of stability of simple and complex retroviral vectors. Virology. 2001;280(1):124–131. doi: 10.1006/viro.2000.0743. [DOI] [PubMed] [Google Scholar]
- Hildinger M, Auricchio A, Gao G, Wang L, Chirmule N, Wilson JM. Hybrid vectors based on adeno-associated virus serotypes 2 and 5 for muscle-directed gene transfer. Journal of Virology. 2001;75(13):6199–6203. doi: 10.1128/JVI.75.13.6199-6203.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch ML, Agbandje-McKenna M, Samulski RJ. Little vector, big gene transduction: fragmented genome reassembly of adeno-associated virus. Molecular Therapy. 2010;18(1):6–8. doi: 10.1038/mt.2009.280. http://dx.doi.org/10.1038/mt.2009.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho DY, Fink SL, Lawrence MS, Meier TJ, Saydam TC, Dash R, et al. Herpes simplex virus vector system: analysis of its in vivo and in vitro cytopathic effects. Journal of Neuroscience Methods. 1995;57(2):205–215. doi: 10.1016/0165-0270(94)00150-f. [DOI] [PubMed] [Google Scholar]
- Hong CS, Goins WF, Goss JR, Burton EA, Glorioso JC. Herpes simplex virus RNAi and neprilysin gene transfer vectors reduce accumulation of Alzheimer’s disease-related amyloid-beta peptide in vivo. Gene Therapy. 2006;13(14):1068–1079. doi: 10.1038/sj.gt.3302719. http://dx.doi.org/10.1038/sj.gt.3302719. [DOI] [PubMed] [Google Scholar]
- Hoppe UC, Marban E, Johns DC. Adenovirus-mediated inducible gene expression in vivo by a hybrid ecdysone receptor. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] Molecular Therapy. 2000;1(2):159–164. doi: 10.1006/mthe.1999.0023. http://dx.doi.org/10.1006/mthe.1999.0023. [DOI] [PubMed] [Google Scholar]
- Hu JC, Coffin RS. Oncolytic herpes simplex virus for tumor therapy. International Review of Neurobiology. 2003;55:165–184. doi: 10.1016/s0074-7742(03)01007-9. [DOI] [PubMed] [Google Scholar]
- Husain T, Passini MA, Parente MK, Fraser NW, Wolfe JH. Long-term AAV vector gene and protein expression in mouse brain from a small pan-cellular promoter is similar to neural cell promoters. Gene Therapy. 2009;16(7):927–932. doi: 10.1038/gt.2009.52. http://dx.doi.org/10.1038/gt.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Yamaza H, Sakai Y, Mizuno S, Ohno M, Hamasaki N, et al. Position-independent human beta-globin gene expression mediated by a recombinant adeno-associated virus vector carrying the chicken beta-globin insulator. [Research Support, Non-U.S. Gov’t] Journal of Human Genetics. 1999;44(3):152–162. doi: 10.1007/s100380050133. http://dx.doi.org/10.1007/s100380050133. [DOI] [PubMed] [Google Scholar]
- Iwakuma T, Cui Y, Chang LJ. Self-inactivating lentiviral vectors with U3 and U5 modifications. Virology. 1999;261(1):120–132. doi: 10.1006/viro.1999.9850. [DOI] [PubMed] [Google Scholar]
- Jacobs AH, Rueger MA, Winkeler A, Li H, Vollmar S, Waerzeggers Y, et al. Imaging-guided gene therapy of experimental gliomas. Cancer Research. 2007;67(4):1706–1715. doi: 10.1158/0008-5472.CAN-06-2418. http://dx.doi.org/10.1158/0008-5472.CAN-06-2418. [DOI] [PubMed] [Google Scholar]
- Jacobs AH, Winkeler A, Hartung M, Slack M, Dittmar C, Kummer C, et al. Improved herpes simplex virus type 1 amplicon vectors for proportional coexpression of positron emission tomography marker and therapeutic genes. Human Gene Therapy. 2003;14(3):277–297. doi: 10.1089/10430340360535823. http://dx.doi.org/10.1089/10430340360535823. [DOI] [PubMed] [Google Scholar]
- Jakobsson J, Ericson C, Jansson M, Bjork E, Lundberg C. Targeted transgene expression in rat brain using lentiviral vectors. Journal of Neuroscience Research. 2003;73(6):876–885. doi: 10.1002/jnr.10719. [DOI] [PubMed] [Google Scholar]
- Jakobsson J, Rosenqvist N, Marild K, Agoston DV, Lundberg C. Evidence for disease-regulated transgene expression in the brain with use of lentiviral vectors. Journal of Neuroscience Research. 2006;84(1):58–67. doi: 10.1002/jnr.20872. [DOI] [PubMed] [Google Scholar]
- Jeyaretna DS, Rabkin SD, Martuza RL. Oncolytic herpes simplex virus therapy for peripheral nerve tumors. Neurosurgical Focus. 2007;22(6):E4. doi: 10.3171/foc.2007.22.6.5. [DOI] [PubMed] [Google Scholar]
- Julien JP, Cupo A, Sok D, Stanfield RL, Lyumkis D, Deller MC, Wilson IA. Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science. 2013;342(6165):1477–1483. doi: 10.1126/science.1245625. http://dx.doi.org/10.1126/science.1245625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafri T, Blomer U, Peterson DA, Gage FH, Verma IM. Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nature Genetics. 1997;17(3):314–317. doi: 10.1038/ng1197-314. [DOI] [PubMed] [Google Scholar]
- Kafri T, van Praag H, Gage FH, Verma IM. Lentiviral vectors: regulated gene expression. Molecular Therapy. 2000;1(6):516–521. doi: 10.1006/mthe.2000.0083. [DOI] [PubMed] [Google Scholar]
- Kafri T, van Praag H, Ouyang L, Gage FH, Verma IM. A packaging cell line for lentivirus vectors. Journal of Virology. 1999;73(1):576–584. doi: 10.1128/jvi.73.1.576-584.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaludov N, Brown KE, Walters RW, Zabner J, Chiorini JA. Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. Journal of Virology. 2001;75(15):6884–6893. doi: 10.1128/JVI.75.15.6884-6893.2001. http://dx.doi.org/10.1128/JVI.75.15.6884-6893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor B, Bayer M, Ma H, Samulski J, Li C, McCown T, et al. Notable reduction in illegitimate integration mediated by a PPT-deleted, nonintegrating lentiviral vector. Molecular Therapy. 2011;19(3):547–556. doi: 10.1038/mt.2010.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor B, Ma H, Webster-Cyriaque J, Monahan PE, Kafri T. Epigenetic activation of unintegrated HIV-1 genomes by gut-associated short chain fatty acids and its implications for HIV infection. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(44):18786–18791. doi: 10.1073/pnas.0905859106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplitt MG, Leone P, Samulski RJ, Xiao X, Pfaff DW, O’Malley KL, et al. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nature Genetics. 1994;8(2):148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- Kaplitt MG, Makimura H. Defective viral vectors as agents for gene transfer in the nervous system. Journal of Neuroscience Methods. 1997;71(1):125–132. doi: 10.1016/s0165-0270(96)00132-x. [DOI] [PubMed] [Google Scholar]
- Kaspar BK, Erickson D, Schaffer D, Hinh L, Gage FH, Peterson DA. Targeted retrograde gene delivery for neuronal protection. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] Molecular Therapy. 2002;5(1):50–56. doi: 10.1006/mthe.2001.0520. http://dx.doi.org/10.1006/mthe.2001.0520. [DOI] [PubMed] [Google Scholar]
- Kaspar BK, Llado J, Sherkat N, Rothstein JD, Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301(5634):839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- Kaul M, Yu H, Ron Y, Dougherty JP. Regulated lentiviral packaging cell line devoid of most viral cis-acting sequences. Virology. 1998;249(1):167–174. doi: 10.1006/viro.1998.9327. [DOI] [PubMed] [Google Scholar]
- Keiser NW, Yan Z, Zhang Y, Lei-Butters DC, Engelhardt JF. Unique characteristics of AAV1, 2, and 5 viral entry, intracellular trafficking, and nuclear import define transduction efficiency in HeLa cells. Human Gene Therapy. 2011;22(11):1433–1444. doi: 10.1089/hum.2011.044. http://dx.doi.org/10.1089/hum.2011.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keriel A, Rene C, Galer C, Zabner J, Kremer EJ. Canine adenovirus vectors for lung-directed gene transfer: efficacy, immune response, and duration of transgene expression using helper-dependent vectors. [Research Support, Non-U.S. Gov’t] Journal of Virology. 2006;80(3):1487–1496. doi: 10.1128/JVI.80.3.1487-1496.2006. http://dx.doi.org/10.1128/JVI.80.3.1487-1496.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim VN, Mitrophanous K, Kingsman SM, Kingsman AJ. Minimal requirement for a lentivirus vector based on human immunodeficiency virus type 1. Journal of Virology. 1998;72(1):811–816. doi: 10.1128/jvi.72.1.811-816.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klages N, Zufferey R, Trono D. A stable system for the high-titer production of multiply attenuated lentiviral vectors. Molecular Therapy. 2000;2(2):170–176. doi: 10.1006/mthe.2000.0103. [DOI] [PubMed] [Google Scholar]
- Klein RL, Muir D, King MA, Peel AL, Zolotukhin S, Moller JC, et al. Long-term actions of vector-derived nerve growth factor or brain-derived neurotrophic factor on choline acetyltransferase and Trk receptor levels in the adult rat basal forebrain. Neuroscience. 1999;90(3):815–821. doi: 10.1016/s0306-4522(98)00537-5. [DOI] [PubMed] [Google Scholar]
- Konvalinka J, Litterst MA, Welker R, Kottler H, Rippmann F, Heuser AM, et al. An active-site mutation in the human immunodeficiency virus type 1 proteinase (PR) causes reduced PR activity and loss of PR-mediated cytotoxicity without apparent effect on virus maturation and infectivity. Journal of Virology. 1995;69(11):7180–7186. doi: 10.1128/jvi.69.11.7180-7186.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science. 2000;290(5492):767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- Kotin RM, Siniscalco M, Samulski RJ, Zhu XD, Hunter L, Laughlin CA, et al. Site-specific integration by adeno-associated virus. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(6):2211–2215. doi: 10.1073/pnas.87.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisky DM, Marconi PC, Oligino TJ, Rouse RJ, Fink DJ, Cohen JB, et al. Development of herpes simplex virus replication-defective multigene vectors for combination gene therapy applications. Gene Therapy. 1998;5(11):1517–1530. doi: 10.1038/sj.gt.3300755. http://dx.doi.org/10.1038/sj.gt.3300755. [DOI] [PubMed] [Google Scholar]
- Kronenberg S, Kleinschmidt JA, Bottcher B. Electron cryomicroscopy and image reconstruction of adeno-associated virus type 2 empty capsids pii:2/11/997. EMBO Reports. 2001;2(11):997–1002. doi: 10.1093/embo-reports/kve234. http://dx.doi.org/10.1093/embo-reports/kve234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufe DW, Pollock RE, Weichselbaum RR, Bast RC, Jr, Gansler TS, Holland JF, Frei E., III . Cancer Medicine. 7. Hamilton, ON: BC Decker; 2003. [Google Scholar]
- Kugler S, Meyn L, Holzmuller H, Gerhardt E, Isenmann S, Schulz JB, et al. Neuron-specific expression of therapeutic proteins: evaluation of different cellular promoters in recombinant adenoviral vectors. Molecular and Cellular Neuroscience. 2001;17(1):78–96. doi: 10.1006/mcne.2000.0929. [DOI] [PubMed] [Google Scholar]
- Kuriyama S, Nakatani T, Masui K, Sakamoto T, Tominaga K, Yoshikawa M, et al. Bystander effect caused by suicide gene expression indicates the feasibility of gene therapy for hepatocellular carcinoma. Hepatology. 1995;22(6):1838–1846. [PubMed] [Google Scholar]
- Lachmann R. Herpes simplex virus-based vectors. International Journal of Experimental Pathology. 2004;85(4):177–190. doi: 10.1111/j.0959-9673.2004.00383.x. http://dx.doi.org/10.1111/j.0959-9673.2004.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Z, Brady RO. Gene transfer into the central nervous system in vivo using a recombinant lentivirus vector. Journal of Neuroscience Research. 2002;67(3):363–371. doi: 10.1002/jnr.10137. [DOI] [PubMed] [Google Scholar]
- Lai Y, Yue Y, Duan D. Evidence for the failure of adeno-associated virus serotype 5 to package a viral genome > or = 8.2 kb. Molecular Therapy. 2010;18(1):75–79. doi: 10.1038/mt.2009.256. http://dx.doi.org/10.1038/mt.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt AD, Rose RB, Varmus HE. Both substrate and target oligonucleotide sequences affect in vitro integration mediated by human immunodeficiency virus type 1 integrase protein produced in Saccharomyces cerevisiae. Journal ofVirology. 1992;66(4):2359–2368. doi: 10.1128/jvi.66.4.2359-2368.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Messing A, Su M, Brenner M. GFAP promoter elements required for region-specific and astrocyte-specific expression. Glia. 2008;56(5):481–493. doi: 10.1002/glia.20622. [DOI] [PubMed] [Google Scholar]
- Lentz TB, Gray SJ, Samulski RJ. Viral vectors for gene delivery to the central nervous system. Neurobiology of Disease. 2012;48(2):179–188. doi: 10.1016/j.nbd.2011.09.014. http://dx.doi.org/10.1016/j.nbd.2011.09.014.pii:S0969-9961(11)00327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone P, Shera D, McPhee SW, Francis JS, Kolodny EH, Bilaniuk LT, et al. Long-term follow-up after gene therapy for canavan disease. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Science Translational Medicine. 2012;4(165):165ra163. doi: 10.1126/scitranslmed.3003454. http://dx.doi.org/10.1126/scitranslmed.3003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PF, Emerman M. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. Journal of Virology. 1994;68(1):510–516. doi: 10.1128/jvi.68.1.510-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P, Hensel M, Emerman M. Human immunodeficiency virus infection of cells arrested in the cell cycle. The EMBO Journal. 1992;11(8):3053–3058. doi: 10.1002/j.1460-2075.1992.tb05376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling C, Lu Y, Kalsi JK, Jayandharan GR, Li B, Ma W, et al. Human hepatocyte growth factor receptor is a cellular coreceptor for adeno-associated virus serotype 3. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] Human Gene Therapy. 2010;21(12):1741–1747. doi: 10.1089/hum.2010.075. http://dx.doi.org/10.1089/hum.2010.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Peterson KR, Fang X, Stamatoyannopoulos G. Locus control regions. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S Review] Blood. 2002;100(9):3077–3086. doi: 10.1182/blood-2002-04-1104. http://dx.doi.org/10.1182/blood-2002-04-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Brandt CR, Gabelt BT, Bryar PJ, Smith ME, Kaufman PL. Herpes simplex virus mediated gene transfer to primate ocular tissues. Experimental Eye Research. 1999;69(4):385–395. doi: 10.1006/exer.1999.0711. http://dx.doi.org/10.1006/exer.1999.0711. [DOI] [PubMed] [Google Scholar]
- Liu G, Martins I, Wemmie JA, Chiorini JA, Davidson BL. Functional correction of CNS phenotypes in a lysosomal storage disease model using adeno-associated virus type 4 vectors. The Journal of Neuroscience. 2005;25(41):9321–9327. doi: 10.1523/JNEUROSCI.2936-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BL, Robinson M, Han ZQ, Branston RH, English C, Reay P, et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumor properties. Gene Therapy. 2003;10(4):292–303. doi: 10.1038/sj.gt.3301885. http://dx.doi.org/10.1038/sj.gt.3301885. [DOI] [PubMed] [Google Scholar]
- Liu J, Wolfe D, Hao S, Huang S, Glorioso JC, Mata M, et al. Peripherally delivered glutamic acid decarboxylase gene therapy for spinal cord injury pain. Molecular Therapy. 2004;10(1):57–66. doi: 10.1016/j.ymthe.2004.04.017. http://dx.doi.org/10.1016/j.ymthe.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Loeb JE, Cordier WS, Harris ME, Weitzman MD, Hope TJ. Enhanced expression of transgenes from adeno-associated virus vectors with the woodchuck hepatitis virus posttranscriptional regulatory element: implications for gene therapy. Human Gene Therapy. 1999;10(14):2295–2305. doi: 10.1089/10430349950016942. [DOI] [PubMed] [Google Scholar]
- Logvinoff C, Epstein AL. A novel approach for herpes simplex virus type 1 amplicon vector production, using the Cre-loxP recombination system to remove helper virus. Human Gene Therapy. 2001;12(2):161–167. doi: 10.1089/104303401750061221. http://dx.doi.org/10.1089/104303401750061221. [DOI] [PubMed] [Google Scholar]
- Lusby E, Fife KH, Berns KI. Nucleotide sequence of the inverted terminal repetition in adeno-associated virus DNA. Journal of Virology. 1980;34(2):402–409. doi: 10.1128/jvi.34.2.402-409.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyumkis D, Julien JP, de Val N, Cupo A, Potter CS, Klasse PJ, Ward AB. Cryo-EM structure of a fully glycosylated soluble cleaved HIV-1 envelope trimer. Science. 2013;342(6165):1484–1490. doi: 10.1126/science.1245627. http://dx.doi.org/10.1126/science.1245627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel RJ, Burger C. Clinical trials in neurological disorders using AAV vectors: promises and challenges [Review] Current Opinion in Molecular Therapy. 2004;6(5):482–490. [PubMed] [Google Scholar]
- Marconi P, Zucchini S, Berto E, Bozac A, Paradiso B, Bregola G, et al. Effects of defective herpes simplex vectors expressing neurotrophic factors on the proliferation and differentiation of nervous cells in vivo. GeneTherapy. 2005;12(7):559–569. doi: 10.1038/sj.gt.3302438. http://dx.doi.org/10.1038/sj.gt.3302438. [DOI] [PubMed] [Google Scholar]
- Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Therapy. 2000;7(10):867–874. doi: 10.1038/sj.gt.3301205. http://dx.doi.org/10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- Martino S, Marconi P, Tancini B, Dolcetta D, De Angelis MG, et al. A direct gene transfer strategy via brain internal capsule reverses the biochemical defect in Tay-Sachs disease. [Research Support, Non-U.S. Gov’t] Human Molecular Genetics. 2005;14(15):2113–2123. doi: 10.1093/hmg/ddi216. http://dx.doi.org/10.1093/hmg/ddi216. [DOI] [PubMed] [Google Scholar]
- Martuza RL, Malick A, Markert JM, Ruffner KL, Coen DM. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252(5007):854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P, Samulski RJ. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Therapy. 2003;10(26):2112–2118. doi: 10.1038/sj.gt.3302134. [DOI] [PubMed] [Google Scholar]
- McCarty DM, Monahan PE, Samulski RJ. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Therapy. 2001;8(16):1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- McConnell MJ, Imperiale MJ. Biology of adenovirus and its use as a vector for gene therapy. Human Gene Therapy. 2004;15(11):1022–1033. doi: 10.1089/hum.2004.15.1022. [DOI] [PubMed] [Google Scholar]
- McCown TJ, Xiao X, Li J, Breese GR, Samulski RJ. Differential and persistent expression patterns of CNS gene transfer by an adeno-associated virus (AAV) vector. Brain Research. 1996;713(1–2):99–107. doi: 10.1016/0006-8993(95)01488-8. [DOI] [PubMed] [Google Scholar]
- Mehta AK, Majumdar SS, Alam P, Gulati N, Brahmachari V. Epigenetic regulation of cytomegalovirus major immediate-early promoter activity in transgenic mice. Gene. 2009;428(1–2):20–24. doi: 10.1016/j.gene.2008.09.033. [DOI] [PubMed] [Google Scholar]
- Merk A, Subramaniam S. HIV-1 envelope glycoprotein structure. Current Opinion in Structural Biology. 23(2):268–276. doi: 10.1016/j.sbi.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineta T, Rabkin SD, Yazaki T, Hunter WD, Martuza RL. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Natural Medicine. 1995;1(9):938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- Miyoshi H, Blomer U, Takahashi M, Gage FH, Verma IM. Development of a self-inactivating lentivirus vector. Journal of Virology. 1998;72(10):8150–8157. doi: 10.1128/jvi.72.10.8150-8157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani S, Boettiger D, Temin HM. A DNA-dependent DNA polymerase and a DNA endonuclease in virions of Rous sarcoma virus. Nature. 1970;228(5270):424–427. doi: 10.1038/228424a0. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Schwartz JP, Tanaka K, Brady RO, Reiser J. High-titer human immunodeficiency virus type 1-based vector systems for gene delivery into nondividing cells. Journal of Virology. 1998;72(11):8873–8883. doi: 10.1128/jvi.72.11.8873-8883.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molin M, Shoshan MC, Ohman-Forslund K, Linder S, Akusjarvi G. Two novel adenovirus vector systems permitting regulated protein expression in gene transfer experiments. [Research Support, Non-U.S. Gov’t] Journal ofVirology. 1998;72(10):8358–8361. doi: 10.1128/jvi.72.10.8358-8361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen JT, Tanabe KK. Viral oncolysis. Oncologist. 2002;7(2):106–119. doi: 10.1634/theoncologist.7-2-106. [DOI] [PubMed] [Google Scholar]
- Muruve DA. The innate immune response to adenovirus vectors. Human Gene Therapy. 2004;15(12):1157–1166. doi: 10.1089/hum.2004.15.1157. [DOI] [PubMed] [Google Scholar]
- Nakajima N, Lu R, Engelman A. Human immunodeficiency virus type 1 replication in the absence of integrase-mediated DNA recombination: definition of permissive and nonpermissive T-cell lines. Journal of Virology. 2001;75(17):7944–7955. doi: 10.1128/JVI.75.17.7944-7955.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L, Blomer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(21):11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272(5259):263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- Nash K, Chen W, Muzyczka N. Complete in vitro reconstitution of adeno-associated virus DNA replication requires the minichromosome maintenance complex proteins. Journal ofVirology. 2008;82(3):1458–1464. doi: 10.1128/JVI.01968-07. http://dx.doi.org/10.1128/JVI.01968-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng R, Govindasamy L, Gurda BL, McKenna R, Kozyreva OG, Samulski RJ, et al. Structural characterization of the dual glycan binding adeno-associated virus serotype 6. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.] Journal of Virology. 2010;84(24):12945–12957. doi: 10.1128/JVI.01235-10. http://dx.doi.org/10.1128/JVI.01235-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer GP, Herzog RW, Mount J, Arruda VR, Tillson DM, Hathcock J, et al. Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy. Blood. 2009;113(4):797–806. doi: 10.1182/blood-2008-10-181479. http://dx.doi.org/10.1182/blood-2008-10-181479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niranjan A, Moriuchi S, Lunsford LD, Kondziolka D, Flickinger JC, Fellows W, et al. Effective treatment of experimental glioblastoma by HSV vector-mediated TNF alpha and HSV-tk gene transfer in combination with radiosurgery and ganciclovir administration. Molecular Therapy. 2000;2(2):114–120. doi: 10.1006/mthe.2000.0101. http://dx.doi.org/10.1006/mthe.2000.0101. [DOI] [PubMed] [Google Scholar]
- Ory DS, Neugeboren BA, Mulligan RC. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudo-types. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(21):11400–11406. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osten P, Grinevich V, Cetin A. Viral vectors: a wide range of choices and high levels of service. Handbook of Experimental Pharmacology. 2007;178:177–202. doi: 10.1007/978-3-540-35109-2_8. http://dx.doi.org/10.1007/978-3-540-35109-2_8. [DOI] [PubMed] [Google Scholar]
- Page KA, Landau NR, Littman DR. Construction and use of a human immunodeficiency virus vector for analysis of virus infectivity. Journal ofVirology. 1990;64(11):5270–5276. doi: 10.1128/jvi.64.11.5270-5276.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakzaban P, Geller AI, Isacson O. Effect of exogenous nerve growth factor on neurotoxicity of and neuronal gene delivery by a herpes simplex amplicon vector in the rat brain. Human Gene Therapy. 1994;5(8):987–995. doi: 10.1089/hum.1994.5.8-987. http://dx.doi.org/10.1089/hum.1994.5.8-987. [DOI] [PubMed] [Google Scholar]
- Panganiban AT, Fiore D. Ordered interstrand and intrastrand DNA transfer during reverse transcription. Science. 1988;241(4869):1064–1069. doi: 10.1126/science.2457948. [DOI] [PubMed] [Google Scholar]
- Pang S, Koyanagi Y, Miles S, Wiley C, Vinters HV, Chen IS. High levels of unintegrated HIV-1 DNA in brain tissue of AIDS dementia patients. Nature. 1990;343(6253):85–89. doi: 10.1038/343085a0. [DOI] [PubMed] [Google Scholar]
- Papadakis ED, Nicklin SA, Baker AH, White SJ. Promoters and control elements: designing expression cassettes for gene therapy [Review] Current GeneTherapy. 2004;4(1):89–113. doi: 10.2174/1566523044578077. [DOI] [PubMed] [Google Scholar]
- Perez MC, Hunt SP, Coffin RS, Palmer JA. Comparative analysis of genomic HSV vectors for gene delivery to motor neurons following peripheral inoculation in vivo. Gene Therapy. 2004;11(13):1023–1032. doi: 10.1038/sj.gt.3302258. http://dx.doi.org/10.1038/sj.gt.3302258. [DOI] [PubMed] [Google Scholar]
- Perreau M, Mennechet F, Serratrice N, Glasgow JN, Curiel DT, Wodrich H, et al. Contrasting effects of human, canine, and hybrid adenovirus vectors on the phenotypical and functional maturation of human dendritic cells: implications for clinical efficacy. [Research Support, Non-U.S. Gov’t] Journal of Virology. 2007;81(7):3272–3284. doi: 10.1128/JVI.01530-06. http://dx.doi.org/10.1128/JVI.01530-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin V, Regulier E, Abbas-Terki T, Hassig R, Brouillet E, Aebischer P, et al. Neuroprotection by Hsp104 and Hsp27 in lentiviral-based rat models of Huntington’s disease. Molecular Therapy. 2007;15(5):903–911. doi: 10.1038/mt.sj.6300141. [DOI] [PubMed] [Google Scholar]
- Petrs-Silva H, Dinculescu A, Li Q, Min SH, Chiodo V, Pang JJ, et al. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. MolecularTherapy. 2009;17(3):463–471. doi: 10.1038/mt.2008.269. http://dx.doi.org/10.1038/mt.2008.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe S, Sarkis C, Barkats M, Mammeri H, Ladroue C, Petit C, et al. Lentiviral vectors with a defective integrase allow efficient and sustained transgene expression in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(47):17684–17689. doi: 10.1073/pnas.0606197103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson TC, Kieffer TL, Ruff CT, Buck C, Gange SJ, Siliciano RF. Intrinsic stability of episomal circles formed during human immunodeficiency virus type 1 replication. Journal of Virology. 2002;76(8):4138–4144. doi: 10.1128/JVI.76.8.4138-4144.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Acuna-Goycolea C, Clark KR, Ghosh PK. Physiological properties of hypothalamic MCH neurons identified with selective expression of reporter gene after recombinant virus infection. [In Vitro Research Support, U.S. Gov’t, P.H.S. ] Neuron. 2004;42(4):635–652. doi: 10.1016/s0896-6273(04)00251-x. [DOI] [PubMed] [Google Scholar]
- Poole S, Cunha FQ, Selkirk S, Lorenzetti BB, Ferreira SH. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-10. British Journal of Pharmacology. 1995;115(4):684–688. doi: 10.1111/j.1476-5381.1995.tb14987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post DE, Fulci G, Chiocca EA, Van Meir EG. Replicative oncolytic herpes simplex viruses in combination cancer therapies. Current Gene Therapy. 2004;4(1):41–51. doi: 10.2174/1566523044577988. [DOI] [PubMed] [Google Scholar]
- Pulicherla N, Shen S, Yadav S, Debbink K, Govindasamy L, Agbandje-McKenna M, Asokan A. Engineering liver-detargeted AAV9 vectors for cardiac and musculoskeletal gene transfer. Molecular Therapy. 2011;19(6):1070–1078. doi: 10.1038/mt.2011.22. http://dx.doi.org/10.1038/mt.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing K, Mah C, Hansen J, Zhou S, Dwarki V, Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Natural Medicine. 1999;5(1):71–77. doi: 10.1038/4758. http://dx.doi.org/10.1038/4758. [DOI] [PubMed] [Google Scholar]
- Qin JY, Zhang L, Clift KL, Hulur I, Xiang AP, Ren BZ, et al. Systematic comparison of constitutive promoters and the doxycycline-inducible promoter. PLoS One. 2010;5(5):e10611. doi: 10.1371/journal.pone.0010611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, Xiao X, et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. Journal ofVirology. 2002;76(2):791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport M, Lorberboum-Galski H. TAT-based drug delivery system–new directions in protein delivery for new hopes? Expert Opinion on Drug Delivery. 2009;6(5):453–463. doi: 10.1517/17425240902887029. [DOI] [PubMed] [Google Scholar]
- Rausch JW, Le Grice SF. ‘Binding, bending and bonding’: polypurine tract-primed initiation of plus-strand DNA synthesis in human immunodeficiency virus. The International Journal of Biochemistry & Cell Biology. 2004;36(9):1752–1766. doi: 10.1016/j.biocel.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Recillas-Targa F, Valadez-Graham V, Farrell CM. Prospects and implications of using chromatin insulators in gene therapy and transgenesis. [Research Support, Non-U.S. Gov’t Review] Bioessays. 2004;26(7):796–807. doi: 10.1002/bies.20059. http://dx.doi.org/10.1002/bies.20059. [DOI] [PubMed] [Google Scholar]
- Reiser J, Harmison G, Kluepfel-Stahl S, Brady RO, Karlsson S, Schubert M. Transduction of nondividing cells using pseudotyped defective high-titer HIV type 1 particles. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(26):15266–15271. doi: 10.1073/pnas.93.26.15266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser J, Lai Z, Zhang XY, Brady RO. Development of multigene and regulated lentivirus vectors. Journal of Virology. 2000;74(22):10589–10599. doi: 10.1128/jvi.74.22.10589-10599.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivella S, Callegari JA, May C, Tan CW, Sadelain M. The cHS4 insulator increases the probability of retroviral expression at random chromosomal integration sites. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S. ] Journal of Virology. 2000;74(10):4679–4687. doi: 10.1128/jvi.74.10.4679-4687.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera VM, Gao GP, Grant RL, Schnell MA, Zoltick PW, Rozamus LW, et al. Long-term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer. Blood. 2005;105(4):1424–1430. doi: 10.1182/blood-2004-06-2501. 2004-06-2501. [DOI] [PubMed] [Google Scholar]
- Roe T, Reynolds TC, Yu G, Brown PO. Integration of murine leukemia virus DNA depends on mitosis. The EMBO Journal. 1993;12(5):2099–2108. doi: 10.1002/j.1460-2075.1993.tb05858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B. The function of herpes simplex virus genes: a primer for genetic engineering of novel vectors. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(21):11307–11312. doi: 10.1073/pnas.93.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B. HSV gene functions: what have we learned that could be generally applicable to its near and distant cousins? Acta Virologica. 1999;43(2–3):75–80. [PubMed] [Google Scholar]
- Rose JA, Maizel JV, Jr, Inman JK, Shatkin AJ. Structural proteins of adenovirus-associated viruses. Journal of Virology. 1971;8(5):766–770. doi: 10.1128/jvi.8.5.766-770.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan H, Deen DF. Use of hypoxia-regulated gene expression in tumor-specific gene therapy. [Research Support, U.S. Gov’t, P.H.S. Review] Current Opinion in Investigational Drugs. 2001;2(6):839–843. [PubMed] [Google Scholar]
- Ruan H, Su H, Hu L, Lamborn KR, Kan YW, Deen DF. A hypoxia-regulated adeno-associated virus vector for cancer-specific gene therapy. [Comparative Study Research Support, U.S. Gov’t, P.H.S.] Neoplasia. 2001;3(3):255–263. doi: 10.1038/sj.neo.7900157. http://dx.doi.org/10.1038/sj/neo/7900157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacramento D, Badrane H, Bourhy H, Tordo N. Molecular epidemiology of rabies virus in France: comparison with vaccine strains. Journal of General Virology. 1992;73(Pt 5):1149–1158. doi: 10.1099/0022-1317-73-5-1149. [DOI] [PubMed] [Google Scholar]
- Saeki Y, Ichikawa T, Saeki A, Chiocca EA, Tobler K, Ackermann M, et al. Herpes simplex virus type 1 DNA amplified as bacterial artificial chromosome in Escherichia coli: rescue of replication-competent virus progeny and packaging of amplicon vectors. Human Gene Therapy. 1998;9(18):2787–2794. doi: 10.1089/hum.1998.9.18-2787. http://dx.doi.org/10.1089/hum.1998.9.18-2787. [DOI] [PubMed] [Google Scholar]
- Samady L, Costigliola E, MacCormac L, McGrath Y, Cleverley S, Lilley CE, et al. Deletion of the virion host shutoff protein (vhs) from herpes simplex virus (HSV) relieves the viral block to dendritic cell activation: potential of vhs-HSV vectors for dendritic cell-mediated immunotherapy. Journal of Virology. 2003;77(6):3768–3776. doi: 10.1128/JVI.77.6.3768-3776.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaniego LA, Neiderhiser L, DeLuca NA. Persistence and expression of the herpes simplex virus genome in the absence of immediate-early proteins. Journal of Virology. 1998;72(4):3307–3320. doi: 10.1128/jvi.72.4.3307-3320.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaranch L, Salegio EA, San Sebastian W, Kells AP, Foust KD, Bringas JR, et al. Adeno-associated virus serotype 9 transduction in the central nervous system of nonhuman primates. [Research Support, N.I.H., Extramural] Human Gene Therapy. 2012;23(4):382–389. doi: 10.1089/hum.2011.200. http://dx.doi.org/10.1089/hum.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samulski RJ, Zhu X, Xiao X, Brook JD, Housman DE, Epstein N, et al. Targeted integration of adeno-associated virus (AAV) into human chromosome 19. The EMBO Journal. 1991;10(12):3941–3950. doi: 10.1002/j.1460-2075.1991.tb04964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiedner G, Morral N, Parks RJ, Wu Y, Koopmans SC, Langston C, et al. Genomic DNA transfer with a high-capacity adenovirus vector results in improved in vivo gene expression and decreased toxicity. Nature Genetics. 1998;18(2):180–183. doi: 10.1038/ng0298-180. [DOI] [PubMed] [Google Scholar]
- Schlegel R, Tralka TS, Willingham MC, Pastan I. Inhibition of VSV binding and infectivity by phosphatidylserine: is phosphatidylserine a VSV-binding site? Cell. 1983;32(2):639–646. doi: 10.1016/0092-8674(83)90483-x. [DOI] [PubMed] [Google Scholar]
- Schnepp BC, Jensen RL, Chen CL, Johnson PR, Clark KR. Characterization of adeno-associated virus genomes isolated from human tissues. Journal ofVirology. 2005;79(23):14793–14803. doi: 10.1128/JVI.79.23.14793-14803.2005. 79/23/14793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285(5433):1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- Sergijenko A, Langford-Smith A, Liao AY, Pickford CE, McDermott J, Nowinski G, et al. Myeloid/Microglial driven autologous hematopoietic stem cell gene therapy corrects a neuronopathic lysosomal disease. Molecular Therapy. 2013;21(10):1938–1949. doi: 10.1038/mt.2013.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K, Breakefield XO. HSV amplicon vectors for cancer therapy. Current Gene Therapy. 2006;6(3):361–370. doi: 10.2174/156652306777592063. [DOI] [PubMed] [Google Scholar]
- Shah K, Tang Y, Breakefield X, Weissleder R. Real-time imaging of TRAIL-induced apoptosis of glioma tumors in vivo. Oncogene. 2003;22(44):6865–6872. doi: 10.1038/sj.onc.1206748. http://dx.doi.org/10.1038/sj.onc.1206748. [DOI] [PubMed] [Google Scholar]
- Shah K, Tung CH, Breakefield XO, Weissleder R. In vivo imaging of S-TRAIL-mediated tumor regression and apoptosis. Molecular Therapy. 2005;11(6):926–931. doi: 10.1016/j.ymthe.2005.01.017. http://dx.doi.org/10.1016/j.ymthe.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Shen S, Bryant KD, Brown SM, Randell SH, Asokan A. Terminal N-linked galactose is the primary receptor for adeno-associated virus 9. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] The Journal of Biological Chemistry. 2011;286(15):13532–13540. doi: 10.1074/jbc.M110.210922. http://dx.doi.org/10.1074/jbc.M110.210922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K, Temin HM. Formation of infectious progeny virus after insertion of herpes simplex thymidine kinase gene into DNA of an avian retrovirus. Cell. 1981;26(1 Pt 1):67–77. doi: 10.1016/0092-8674(81)90034-9. [DOI] [PubMed] [Google Scholar]
- Shin CG, Taddeo B, Haseltine WA, Farnet CM. Genetic analysis of the human immunodeficiency virus type 1 integrase protein. Journal of Virology. 1994;68(3):1633–1642. doi: 10.1128/jvi.68.3.1633-1642.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RH. Adeno-associated virus integration: virus versus vector. Gene Therapy. 2008;15(11):817–822. doi: 10.1038/gt.2008.55. http://dx.doi.org/10.1038/gt.2008.55. [DOI] [PubMed] [Google Scholar]
- Smith-Arica JR, Morelli AE, Larregina AT, Smith J, Lowenstein PR, Castro MG. Cell-type-specific and regulatable transgenesis in the adult brain: adenovirus-encoded combined transcriptional targeting and inducible transgene expression. [Research Support, Non-U.S. Gov’t] Molecular Therapy. 2000;2(6):579–587. doi: 10.1006/mthe.2000.0215. http://dx.doi.org/10.1006/mthe.2000.0215. [DOI] [PubMed] [Google Scholar]
- Smith JS, Robinson NJ. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. The Journal of Infectious Diseases. 2002;186(Suppl 1):S3–S28. doi: 10.1086/343739. http://dx.doi.org/10.1086/343739. [DOI] [PubMed] [Google Scholar]
- Snyder BR, Gray SJ, Quach ET, Huang JW, Leung CH, Samulski RJ, et al. Comparison of adeno-associated viral vector serotypes for spinal cord and motor neuron gene delivery. [Comparative Study Research Support, Non-U.S. Gov’t] Human Gene Therapy. 2011;22(9):1129–1135. doi: 10.1089/hum.2011.008. http://dx.doi.org/10.1089/hum.2011.008. [DOI] [PubMed] [Google Scholar]
- Sonntag F, Schmidt K, Kleinschmidt JA. A viral assembly factor promotes AAV2 capsid formation in the nucleolus. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(22):10220–10225. doi: 10.1073/pnas.1001673107. http://dx.doi.org/10.1073/pnas.1001673107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiga MG, Borras T. Development of a gene therapy virus with a glucocorticoid-inducible MMP1 for the treatment of steroid glaucoma. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Investigative Ophthalmology & Visual Science. 2010;51(6):3029–3041. doi: 10.1167/iovs.09-4918. http://dx.doi.org/10.1167/iovs.09-4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Fink DJ, Glorioso JC. HSV vectors for gene therapy of chronic pain. Current Opinion in Molecular Therapeutics. 2008;10(5):449–455. [PubMed] [Google Scholar]
- Srivastava A, Lusby EW, Berns KI. Nucleotide sequence and organization of the adeno-associated virus 2 genome. Journal of Virology. 1983;45(2):555–564. doi: 10.1128/jvi.45.2.555-564.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinwaerder DS, Lieber A. Insulation from viral transcriptional regulatory elements improves inducible transgene expression from adenovirus vectors in vitro and in vivo. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] Gene Therapy. 2000;7(7):556–567. doi: 10.1038/sj.gt.3301139. http://dx.doi.org/10.1038/sj.gt.3301139. [DOI] [PubMed] [Google Scholar]
- Stemmer WP. Rapid evolution of a protein in vitro by DNA shuffling. Nature. 1994;370(6488):389–391. doi: 10.1038/370389a0. [DOI] [PubMed] [Google Scholar]
- Stieger K, Schroeder J, Provost N, Mendes-Madeira A, Belbellaa B, Le Meur G, et al. Detection of intact rAAV particles up to 6 years after successful gene transfer in the retina of dogs and primates. Molecular Therapy. 2009;17(3):516–523. doi: 10.1038/mt.2008.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storek B, Reinhardt M, Wang C, Janssen WG, Harder NM, Banck MS, et al. Sensory neuron targeting by self-complementary AAV8 via lumbar puncture for chronic pain. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(3):1055–1060. doi: 10.1073/pnas.0708003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus SE, Sebring ED, Rose JA. Concatemers of alternating plus and minus strands are intermediates in adenovirus-associated virus DNA synthesis. Proceedings of the National Academy of Sciences of the United States of America. 1976;73(3):742–746. doi: 10.1073/pnas.73.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerford C, Bartlett JS, Samulski RJ. AlphaVbeta5 integrin: a co-receptor for adeno-associated virus type 2 infection. Natural Medicine. 1999;5(1):78–82. doi: 10.1038/4768. [DOI] [PubMed] [Google Scholar]
- Summerford C, Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. Journal ofVirology. 1998;72(2):1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundquist WI, Krausslich HG. HIV-1 assembly, budding, and maturation. Cold Spring Harbor Perspectives in Medicine. 2(7):a006924. doi: 10.1101/cshperspect.a006924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanmanee T, Hu G, Gui T, Bartholomae CC, Kutschera I, von Kalle C, Kafri T. Integration-deficient lentiviral vectors expressing codon-optimized R338L human FIX restore normal hemostasis in Hemophilia B mice. Molecular Therapy. 2014;22(3):567–574. doi: 10.1038/mt.2013.188. http://dx.doi.org/10.1038/mt.2013.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Craigie R. The road to chromatin - nuclear entry of retroviruses. Nature Reviews Microbiology. 2007;5(3):187–196. doi: 10.1038/nrmicro1579. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Nishikawa M, Takakura Y. Development of safe and effective non-viral gene therapy by eliminating CpG motifs from plasmid DNA vector. [Research Support, Non-U.S. Gov’t Review] Frontiers in Bioscience (Scholar Edition) 2012;4:133–141. doi: 10.2741/256. [DOI] [PubMed] [Google Scholar]
- Temin HM, Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- Teo I, Veryard C, Barnes H, An SF, Jones M, Lantos PL, et al. Circular forms of unintegrated human immunodeficiency virus type 1 DNA and high levels of viral protein expression: association with dementia and multinucleated giant cells in the brains of patients with AIDS. Journal of Virology. 1997;71(4):2928–2933. doi: 10.1128/jvi.71.4.2928-2933.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Themis M, Waddington SN, Schmidt M, von Kalle C, Wang Y, Al-Allaf F, et al. Oncogenesis following delivery of a nonprimate lentiviral gene therapy vector to fetal and neonatal mice. Molecular Therapy. 2005;12(4):763–771. doi: 10.1016/j.ymthe.2005.07.358. [DOI] [PubMed] [Google Scholar]
- Throm RE, Ouma AA, Zhou S, Chandrasekaran A, Lockey T, Greene M, et al. Efficient construction of producer cell lines for a SIN lentiviral vector for SCID-X1 gene therapy by concatemeric array transfection. Blood. 2009;113(21):5104–5110. doi: 10.1182/blood-2008-11-191049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias A, Ahmed A, Moon KS, Lesniak MS. The art of gene therapy for glioma: a review of the challenging road to the bedside. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review] Journal of Neurology, Neurosurgery & Psychiatry. 2013;84(2):213–222. doi: 10.1136/jnnp-2012-302946. http://dx.doi.org/10.1136/jnnp-2012-302946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todo T. Oncolytic virus therapy using genetically engineered herpes simplex viruses. Frontiers in Bioscience. 2008;13:2060–2064. doi: 10.2741/2823. [DOI] [PubMed] [Google Scholar]
- Tolmachov O. Designing plasmid vectors [Review] Methods in Molecular Biology. 2009;542:117–129. doi: 10.1007/978-1-59745-561-9_6. http://dx.doi.org/10.1007/978-1-59745-561-9_6. [DOI] [PubMed] [Google Scholar]
- Tomishima MJ, Smith GA, Enquist LW. Sorting and transport of alpha herpesviruses in axons. Traffic. 2001;2(7):429–436. doi: 10.1034/j.1600-0854.2001.020701.x. [DOI] [PubMed] [Google Scholar]
- Tosi G, Bortot B, Ruozi B, Dolcetta D, Vandelli MA, Forni F, et al. Potential use of polymeric nanoparticles for drug delivery across the blood–brain barrier [Review] Current Medicinal Chemistry. 2013;20(17):2212–2225. doi: 10.2174/0929867311320170006. [DOI] [PubMed] [Google Scholar]
- Towne C, Pertin M, Beggah AT, Aebischer P, Decosterd I. Recombinant adeno-associated virus serotype 6 (rAAV2/6)-mediated gene transfer to nociceptive neurons through different routes of delivery. Molecular Pain. 2009;5:52. doi: 10.1186/1744-8069-5-52. 1744-8069-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe LH, Wilson JM. AAV as an immunogen. Current Gene Therapy. 2007;7(5):325–333. doi: 10.2174/156652307782151416. [DOI] [PubMed] [Google Scholar]
- Vargas J, Jr, Gusella GL, Najfeld V, Klotman ME, Cara A. Novel integrase-defective lentiviral episomal vectors for gene transfer. Human Gene Therapy. 2004;15(4):361–372. doi: 10.1089/104303404322959515. [DOI] [PubMed] [Google Scholar]
- Vile RG, Hart IR. Use of tissue-specific expression of the herpes simplex virus thymidine kinase gene to inhibit growth of established murine melanomas following direct intratumoral injection of DNA. Cancer Research. 1993;53(17):3860–3864. [PubMed] [Google Scholar]
- Volpers C, Kochanek S. Adenoviral vectors for gene transfer and therapy. The Journal of Gene Medicine. 2004;6(Suppl 1):S164–S171. doi: 10.1002/jgm.496. [DOI] [PubMed] [Google Scholar]
- Walters RW, Yi SM, Keshavjee S, Brown KE, Welsh MJ, Chiorini JA, et al. Binding of adeno-associated virus type 5 to 2,3-linked sialic acid is required for gene transfer. The Journal of Biological Chemistry. 2001;276(23):20610–20616. doi: 10.1074/jbc.M101559200. http://dx.doi.org/10.1074/jbc.M101559200. [DOI] [PubMed] [Google Scholar]
- Wang DB, Dayton RD, Henning PP, Cain CD, Zhao LR, Schrott LM, et al. Expansive gene transfer in the rat CNS rapidly produces amyotrophic lateral sclerosis relevant sequelae when TDP-43 is overexpressed. Molecular Therapy. 2010;18(12):2064–2074. doi: 10.1038/mt.2010.191. http://dx.doi.org/10.1038/mt.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lin H, Lin S, Qu J, Xiao J, Huang Y, et al. Cell-penetrating peptide TAT-mediated delivery of acidic FGF to retina and protection against ischemia-reperfusion injury in rats. Journal of Cellular and Molecular Medicine. 2009;14(7):1998–2005. doi: 10.1111/j.1582-4934.2009.00786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yang B, Qiu L, Yang C, Kramer J, Su Q, et al. Widespread spinal cord transduction by intrathecal injection of rAAV delivers efficacious RNAi therapy for amyotrophic lateral sclerosis. Human Molecular Genetics. 2014;23(3):668–681. doi: 10.1093/hmg/ddt454. http://dx.doi.org/10.1093/hmg/ddt454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson DJ, Kobinger GP, Passini MA, Wilson JM, Wolfe JH. Targeted transduction patterns in the mouse brain by lentivirus vectors pseudotyped with VSV, Ebola, Mokola, LCMV, or MuLV envelope proteins. MolecularTherapy. 2002;5(5 Pt 1):528–537. doi: 10.1006/mthe.2002.0584. [DOI] [PubMed] [Google Scholar]
- Wei CM, Gibson M, Spear PG, Scolnick EM. Construction and isolation of a transmissible retrovirus containing the src gene of Harvey murine sarcoma virus and the thymidine kinase gene of herpes simplex virus type 1. Journal of Virology. 1981;39(3):935–944. doi: 10.1128/jvi.39.3.935-944.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller ML, Amornphimoltham P, Schmidt M, Wilson PA, Gutkind JS, Chiorini JA. Epidermal growth factor receptor is a co-receptor for adeno-associated virus serotype 6. Nature Medicine. 2010;16(6):662–664. doi: 10.1038/nm.2145. http://dx.doi.org/10.1038/nm.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiznerowicz M, Trono D. Harnessing HIV for therapy, basic research and biotechnology. Trends in Biotechnology. 2005;23(1):42–47. doi: 10.1016/j.tibtech.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Wong LF, Azzouz M, Walmsley LE, Askham Z, Wilkes FJ, Mitrophanous KA, et al. Transduction patterns of pseudotyped lentiviral vectors in the nervous system. Molecular Therapy. 2004;9(1):101–111. doi: 10.1016/j.ymthe.2003.09.017. S152500160300337X. [DOI] [PubMed] [Google Scholar]
- Wu Z, Asokan A, Samulski RJ. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Molecular Therapy. 2006;14(3):316–327. doi: 10.1016/j.ymthe.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Wu Z, Miller E, Agbandje-McKenna M, Samulski RJ. Alpha2,3 and alpha2,6 N-linked sialic acids facilitate efficient binding and transduction by adeno-associated virus types 1 and 6. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Journal of Virology. 2006;80(18):9093–9103. doi: 10.1128/JVI.00895-06. http://dx.doi.org/10.1128/JVI.00895-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Sun J, Zhang T, Yin C, Yin F, Van Dyke T, et al. Optimization of self-complementary AAV vectors for liver-directed expression results in sustained correction of hemophilia B at low vector dose. Molecular Therapy. 2008;16(2):280–289. doi: 10.1038/sj.mt.6300355. http://dx.doi.org/10.1038/sj.mt.6300355. [DOI] [PubMed] [Google Scholar]
- Wu Z, Yang H, Colosi P. Effect of genome size on AAV vector packaging. Molecular Therapy. 2010;18(1):80–86. doi: 10.1038/mt.2009.255. http://dx.doi.org/10.1038/mt.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Chirmule N, Berta SC, McCullough B, Gao G, Wilson JM. Gene therapy vectors based on adeno-associated virus type 1. Journal of Virology. 1999;73(5):3994–4003. doi: 10.1128/jvi.73.5.3994-4003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. Journal of Virology. 1998;72(3):2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Bu W, Bhatia S, Hare J, Somasundaram T, Azzi A, et al. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(16):10405–10410. doi: 10.1073/pnas.162250899. http://dx.doi.org/10.1073/pnas.162250899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Xie Q, Zhang H, Ameres SL, Hung JH, Su Q, et al. MicroRNA-regulated, systemically delivered rAAV9: a step closer to CNS-restricted transgene expression. Molecular Therapy. 2010;19(3):526–535. doi: 10.1038/mt.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Janson CG, Mastakov M, Lawlor P, Young D, Mouravlev A, et al. Quantitative comparison of expression with adeno-associated virus (AAV-2) brain-specific gene cassettes. Gene Therapy. 2001;8(17):1323–1332. doi: 10.1038/sj.gt.3301529. http://dx.doi.org/10.1038/sj.gt.3301529. [DOI] [PubMed] [Google Scholar]
- Xu J, Ma C, Bass C, Terwilliger EF. A combination of mutations enhances the neurotropism of AAV-2. Virology. 2005;341(2):203–214. doi: 10.1016/j.virol.2005.06.051. [DOI] [PubMed] [Google Scholar]
- Xu K, Ma H, McCown TJ, Verma IM, Kafri T. Generation of a stable cell line producing high-titer self-inactivating lentiviral vectors. Molecular Therapy. 2001;3(1):97–104. doi: 10.1006/mthe.2000.0238. [DOI] [PubMed] [Google Scholar]
- Xu ZL, Mizuguchi H, Mayumi T, Hayakawa T. Woodchuck hepatitis virus post-transcriptional regulation element enhances transgene expression from adenovirus vectors. [Research Support, Non-U.S. Gov’t] Biochimica et Biophysica Acta. 2003;1621(3):266–271. doi: 10.1016/s0304-4165(03)00078-3. [DOI] [PubMed] [Google Scholar]
- Yanez-Munoz RJ, Balaggan KS, MacNeil A, Howe SJ, Schmidt M, Smith AJ, et al. Effective gene therapy with nonintegrating lentiviral vectors. Natural Medicine. 2006;12(3):348–353. doi: 10.1038/nm1365. http://dx.doi.org/10.1038/nm1365.pii:nm1365. [DOI] [PubMed] [Google Scholar]
- Ye X, Rivera VM, Zoltick P, Cerasoli F, Jr, Schnell MA, Gao G, et al. Regulated delivery of therapeutic proteins after in vivo somatic cell gene transfer. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S ] Science. 1999;283(5398):88–91. doi: 10.1126/science.283.5398.88. [DOI] [PubMed] [Google Scholar]
- Zennou V, Petit C, Guetard D, Nerhbass U, Montagnier L, Charneau P. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell. 2000;101(2):173–185. doi: 10.1016/S0092-8674(00)80828-4. [DOI] [PubMed] [Google Scholar]
- Zhang YF, Boado RJ, Pardridge WM. Absence of toxicity of chronic weekly intravenous gene therapy with pegylated immunoliposomes. Pharmaceutical Research. 2003;20(11):1779–1785. doi: 10.1023/b:pham.0000003375.13655.f9. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang Y, Boado RJ, Pardridge WM. Lysosomal enzyme replacement of the brain with intravenous non-viral gene transfer. Pharmaceutical Research. 2008;25(2):400–406. doi: 10.1007/s11095-007-9357-6. http://dx.doi.org/10.1007/s11095-007-9357-6. [DOI] [PubMed] [Google Scholar]
- Zhang H, Yang B, Mu X, Ahmed SS, Su Q, He R, et al. Several rAAV vectors efficiently cross the blood–brain barrier and transduce neurons and astrocytes in the neonatal mouse central nervous system. Molecular Therapy. 2011;19(8):1440–1448. doi: 10.1038/mt.2011.98. http://dx.doi.org/10.1038/mt.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Li B, Jayandharan G, Mah CS, Govindasamy L, Agbandje-McKenna M, et al. Tyrosine-phosphorylation of AAV2 vectors and its consequences on viral intracellular trafficking and transgene expression. Virology. 2008;381(2):194–202. doi: 10.1016/j.virol.2008.08.027. http://dx.doi.org/10.1016/j.virol.2008.08.027.pii:S0042-6822(08)00529-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Molecular Therapy. 2008;16(6):1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- Zoltick PW, Wilson JM. Regulated gene expression in gene therapy [Review] Annals of New York Academy of Sciences. 2001;953:53–63. doi: 10.1111/j.1749-6632.2001.tb11360.x. [DOI] [PubMed] [Google Scholar]
- Zufferey R, Donello JE, Trono D, Hope TJ. Woodchuck hepatitis virus post-transcriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. Journal of Virology. 1999;73(4):2886–2892. doi: 10.1128/jvi.73.4.2886-2892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L, et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. Journal of Virology. 1998;72(12):9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nature Biotechnology. 1997;15(9):871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]