Abstract
Purpose of review
This review summarizes the phenotype and function of macrophages in the context of solid organ transplantation and will focus on fundamental insights into their paradoxical pro-inflammatory versus suppressive function. We will also discuss the therapeutic potential of regulatory macrophages in tolerance induction.
Recent findings
Macrophages are emerging as an essential element of solid organ transplantation. Macrophages are involved in the pathogenesis of ischemia reperfusion injury, as well as both acute and chronic rejection, exacerbating injury through secretion of inflammatory effectors and by amplifying adaptive immune responses. Notably, not all responses associated with macrophages are deleterious to the graft, and graft protection can in fact be conferred by macrophages. This has been attributed to the presence of macrophages with tissue-repair capabilities, as well as the effects of regulatory macrophages.
Summary
The explosion of new information on the role of macrophages in solid organ transplantation has opened up new avenues of research and the possibility of therapeutic intervention. However, the role of myeloid cells in graft rejection, resolution of rejection and tissue repair remains poorly understood. A better understanding of plasticity and regulation of monocyte polarization is vital for the development of new therapies for the treatment of acute and chronic transplant rejection.
Keywords: Macrophage, allograft rejection, acute, chronic, regulatory macrophages
Introduction
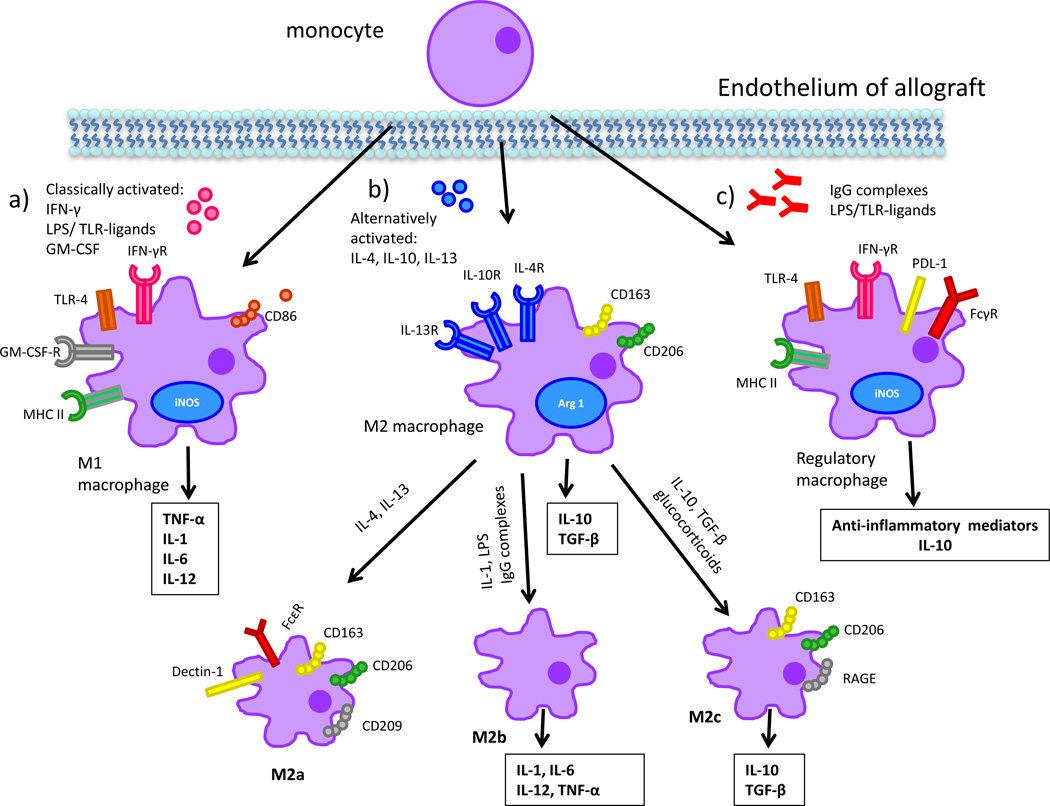
Macrophages and their precursors, monocytes, constitute an essential component of the innate immune system and form the first line of defense against pathogens [1]. Macrophages have the capacity to differentiate into a variety of phenotypes in response to cues from the microenvironment, and it is this notable phenotypic plasticity that governs the expression of the broad range of inducible effectors [2]. In the transplant setting, macrophages can cause allograft injury, tissue remodeling or have immunoregulatory/suppressive effects depending on their state of activation [2–4]. In response to stimuli, infiltrating macrophages differentiate preferentially into “classically activated” or “alternatively-activated” subsets with markedly different functions [3, 4]. Classically activated macrophages, also referred to as M1 macrophages, develop in response to IFN-γ and engagement of Toll-like receptors (TLRs) by microbial products [2, 5]. They generally display a proinflammatory phenotype expressing high levels of CD86, iNOS, TNF-α, IL-1 and IL-6 (Figure 1a) [4]. In contrast, exposure to IL-4 or IL-13 leads to the development of “alternatively-activated” or “wound-healing” macrophages, also referred to as M2 macrophages, that display markers of alternative activation including CD206, the scavenger protein CD163, arginase-1, and IL-10 (Figure 1b) [6–8]. The M2 subset of macrophages is not a uniform population and is further subdivided into M2a, M2b, and M2c. Within this subset, M2a macrophages, generally referred to as alternatively activated macrophages, are induced by IL-4 and IL-13, with surface expression of CD163, CD206, CD209, IL-4, FcεR, and Dectin-1. Ligation of macrophage FcRs by IgG complexes coupled with TLR or CD40/CD44 engagement induces a Type II activation [2, 3, 9], which corresponds with an M2b phenotype. M2b macrophages are immunoregulatory and produce high levels of IL10, IL-1, IL-6 and TNF-α. M2c macrophages are referred to as deactivated macrophages given their role in down-regulation of pro-inflammatory cytokines, as well as tissue repair and remodeling. This macrophage subset is induced by IL-10, TGF-β, and glucocorticoids and in turn produces large amounts of IL-10 and TGFβ with surface expression of CD163, CD206, RAGE and other scavenger receptors [3, 10]. While these M2 variants have been explored in a variety of disease models [11, 12], they have yet to be characterized in the setting of solid organ transplantation. Regulatory macrophages (M regs), a less well-characterized subtype of macrophages, can suppress T cell function and have been utilized as therapeutic agents in transplantation (Figure 1c) [13, 14]. Regulatory macrophages express iNOS, MHC class II, and PD-L1, though little CD40 or CD86 [15]. M regs are fundamentally distinct and do not express most markers found on M1 or M2 macrophages and have been shown to mitigate acute and chronic inflammation in different disease models [16]. Though regulatory macrophages modulate inflammatory immune responses, these cells do not actively participate in wound healing [15]. Notably, peripheral blood monocytes have been divided into two subsets with distinct function and phenotype. The pro-inflammatory CD14+CD16+ subset exhibits high expression of proinflammatory cytokines [17], while the immunosuppressive monocytes are CD14+CD163+ and exhibit immunosuppressive mechanisms including IL-10 production [18]. The role of peripheral blood monocyte subsets in transplantation has been minimally studied, with contradictory findings, and requires further investigation [18, 19].
Figure 1. Macrophage plasticity and function in the context of allograft rejection.
a) M1 macrophages are classically activated, damage graft endothelium, recruit additional leukocytes, and mediate tissue injury. They are the dominant phenotype in acute rejection and their activity can be modulated by blockade of their activation or the factors they produce. b) M2 macrophages are alternatively activated, mediate tissue repair and injury resolution, and promote fibrosis. This subset is predominantly found in chronically-damaged allografts. c) Mregs are activated in a fashion distinct from the other two subsets. They modulate anti-inflammatory response, have T cell suppressive capacity, and are being investigated for use in cell-based therapy.
Macrophages in Ischemia Reperfusion Injury
Ischemia reperfusion injury (IRI) is a multifactorial process, involving both innate and adaptive immunity, which impacts early and late graft dysfunction [20]. Cells of the innate immune system, particularly macrophages, are key potentiators of IRI, participating in both the early stages of injury and in late stage repair [21–25]. Animal models of IRI show that injury is associated with an influx of macrophages, implicating these innate immune cells in augmentation of ischemic injury [26]. One study demonstrated that knocking out CCR2, a receptor for monocyte chemo-attractant protein 1 (MCP-1), protected mice from kidney IRI, correlating with reduced macrophage infiltration [27]. In a murine model of liver IRI, blockade of TIM-1 on CD4 cells inhibited T cell mediated activation of macrophages and mitigated injury [28]. In a clinical study, using the selectin antagonist (rPSGL-1) reduced liver IRI with improved liver function and augmented cytoprotective IL-10, with a reduction in MCP-1, suggesting inhibition of macrophage infiltration [20]. Notably, while inflammatory macrophages contribute to the initial damage during IRI [21], alternatively activated macrophages promote repair following the injury. As such, Huen et al. showed that macrophages in the setting of kidney IRI can be skewed toward a distinct reparative phenotype which supports tubular proliferation and repair in response to GM-CSF [29, 30]. Similarly, a myeloid-specific PTEN knockout conferred protection from liver IRI by promoting development of M2 macrophages in response to TLR engagement. PTEN deficiency resulted in constitutive activation of the pro-survival PI3K pathway, which regulates macrophage differentiation by upregulating miR155. This M2 differentiation correlates with a decrease in expression of certain pro-inflammatory mediators and a marked increase in the anti-inflammatory cytokine IL-10 [31]. Moreover, over-expression of macrophage heme-oxygenase-1, an enzyme with anti-inflammatory properties, imposed an anti-inflammatory or M2 phenotype, selectively inhibiting M1 polarization. When adoptively transferred into mice, these macrophages mitigated injury and inflammation caused by ischemia reperfusion [32]. Collectively, these findings point to an instrumental role for macrophages in the pathophysiology of IRI depending on the nature of the macrophage subset during the time course of injury, as M1 macrophages can mediate the inflammatory process at the onset of ischemic injury, while M2 macrophages are involved in post-injury resolution. As IRI is an antigen independent event, macrophages involved in this process are activated through cytokines and/or engagement of TLRs or other pattern recognition receptors by endogenous ligands generated through cellular damage [33]. Consequently, mice deficient in TLR4 demonstrated reduced IRI after liver transplantation [34], while donor TLR4 was shown to contribute to renal allograft inflammation in humans [35]. A recent study revealed lipocalin2 (Lcn2), a defense mediator expressed in response to TLR activation, plays a crucial role in cardiac IRI, and neutralization of Lcn2 suppressed M1 macrophage polarization and instead mediated skewing of macrophages toward an M2 phenotype. Additionally, Lcn2 treatment suppressed infiltration of macrophages further limiting IRI [36].
Macrophages in Acute Allograft Rejection
Macrophages were first implicated in rejecting renal allografts over fifty years ago [37]. Macrophage accumulation in the allograft is associated with both acute antibody-mediated rejection (AMR), and acute cell mediated rejection [38, 39]. In instances of acute and chronic injury [39] in animal models as well as humans [40, 41], macrophages account for 38–60% of infiltrating leukocytes in rejecting organs [42–45]. Notably, a murine model of pancreatic islet grafts provided evidence of direct destruction of islet tissue by macrophages [46]. The presence of CD68+ macrophage infiltrates is associated with diagnosis of acute rejection in human renal allografts [39, 47–49]. Macrophage depletion has led to amelioration of graft injury and a reduction in pathological features of acute rejection in experimental models [40, 44, 50, 51]. Similarly, inhibition of macrophage accumulation and activation in murine cardiac allografts results in abrogation of graft injury and rejection [43, 52].
Macrophages propagate injury in the setting of AMR [53, 54] and are a distinguishing feature of graft pathology in AMR lesions [49]. In fact, one of the most important diagnostic criteria for AMR in cardiac transplantation is the presence of intravascular macrophages in the capillaries of endomyocardial biopsies [55]. In a clinical study, Kirk and colleagues found that there was a high incidence of AMR associated with infiltrating macrophages in renal transplant patients treated with Campath, a T cell-depleting drug [56, 57]. Similar detrimental effects were observed in a lymphocyte-deficient RAG−/− cardiac murine model of acute AMR [53, 54], adding support to the claim that macrophages are sufficient to induce allograft injury. In this study, passive transfer of anti-donor HLA antibodies induced accumulation of intravascular macrophages in heterotopic cardiac allografts, demonstrating pathological features of injury. In vitro, P-selectin blockade was shown to prevent antibody-mediated monocyte recruitment to endothelial cells, conferring protection from antibody induced damage. This was recapitulated in the above-mentioned murine model of acute AMR [53] and has had promising results in IRI [20], with potential for use in AMR in solid organ transplantation. In the setting of AMR, donor specific HLA IgG antibodies have been shown to recruit monocytes via an FcγR-dependent mechanism [9, 58]. Consequently, eliminating these antibody-FcγR interactions using EndoS, an endoglycosidase that modifies protein glycosylation, and IdeS, an IgG-degrading enzyme, was shown to significantly lessen monocyte recruitment to cardiac endothelium in vitro [58].
These combined findings implicate macrophages as an essential determinant in the induction of acute rejection. Though the exact mechanism by which macrophages mediate injury is not fully understood, in vitro and in vivo studies implicate the production of inflammatory mediators as a central mechanism whereby macrophages contribute to allograft injury [5]. Inside the graft, macrophages release inflammatory mediators such as nitric oxide (iNOS), IL-2, IL-6, IL-12, MCP-1, and TNF-α [40, 44], which activate and damage the microvasculature, recruit leukocytes, and induce donor-specific cytotoxic responses [1]. Studies where macrophages have been depleted, or receptors for leukocyte recruitment antagonized, confirmed the role of macrophage cytokine production and other pro-inflammatory mediators in graft rejection. For instance, chemical macrophage depletion results in a reduction in the severity of acute allograft rejection in rodent models of small bowel transplantation [44, 59]. The reduction in small bowel injury was attributed, in part, to lower expression of inflammatory genes including iNOS, MCP-1 and IL-6, factors associated with M1 macrophages. Blockade of inflammatory cytokines such as TNF-α and iNOS was shown to extend cardiac graft survival, underscoring the importance of macrophage-mediated-inflammation in heart transplant rejection [60, 61]. Similarly, administration of the chemokine receptor antagonist, Met-Rantes, inhibited monocyte adhesion to inflamed endothelium in a rat model of acute cellular renal injury in which monocytes constitute the majority of the infiltrating cells. Correspondingly, the treated animals displayed a decrease in the expression level of several pro-inflammatory cytokines [62, 63]. While M1 macrophages mediate injury, M2 macrophages are generally implicated in injury resolution and tissue remodeling, and therefore, they may promote allograft damage repair; though currently, their role in acute injury remains speculative. Histological studies of murine corneal allografts exhibiting acute rejection revealed the presence of M1 macrophages secreting proinflammatory mediators, while M2 macrophages were detected in the animals that did not reject the transplants [64]. An M1-dominant response was also observed in a rat model of acute renal AMR and in clinical biopsy samples of acutely rejecting kidney allograft recipients [65].
In light of these findings, selective depletion of macrophage subpopulations may be exploited to provide additional insight into the myriad functions of macrophages in the context of acute allograft injury and repair, more specifically targeting M1 macrophages as a therapeutic tactic. Albeit, it might be more prudent to target destructive macrophage subsets for manipulation, such as those skewed toward the M1 phenotype, for manipulation, rather than depletion, as studies suggest that macrophages are plastic and do not remain committed to a single phenotype/activation state [2, 3].
Macrophages in Chronic Allograft Rejection
Chronic rejection is the leading cause of long-term graft failure. The manifestations of chronic allograft rejection include vasculopathy and chronic vascular lesions, often accompanied by sub-endothelial leukocytes, and proliferation of vascular endothelial and smooth muscle cells [66]. Histological sections of chronically rejecting tissues stain positive for macrophage infiltrates, and macrophage labeling has been explored as a means of detecting chronic rejection prior to the onset of graft dysfunction [67]. Intragraft macrophages are associated with worse outcome in renal, liver, and cardiac transplantation in humans as well as animal models [68–70], and macrophages have been shown to directly cause tissue injury and fibrosis. Case studies focusing on the development of chronic allograft nephropathy have emphasized the pivotal role of macrophages in human biopsies culminating in end-stage renal failure [69, 71, 72]. Interestingly, monocytes have been shown to have altered activation levels, exhibited by enhanced TNF-α production, in patients undergoing chronic renal rejection [73].
As in the case of acute rejection, the current view is that macrophages promote worse graft outcome through the release of inflammatory mediators and regulation of cytokine dynamics. Studies conducted during the course of chronic rejection found up-regulation of MCP-1, RANTES, TNF-α, IFN-γ and iNOS among others, correlating with macrophage activation [74]. Yang et al. used a previously established rat renal allograft model to target a variety of macrophage-derived and macrophage-activating soluble mediators implicated in chronic graft rejection. Blocking the actions of TNF-α, IL-12, and IFN-γ reduced macrophage-mediated chronic injury [75]. Macrophage participation in chronically rejecting vascularized grafts can be further modulated by blockade of chemokine-chemokine receptor interactions, as administration of Met-RANTES, an agonist to the chemokine receptor CCR5, to transplant recipients has been successful in significantly lessening chronic injury in cardiac and renal grafts [76, 77]. A macrophage-specific inhibitor, gamma lactone, was successfully used to prevent murine chronic renal allograft nephropathy [68] with a correlative reduction in the levels of macrophage-produced inflammatory mediators. As in acute injury, the impact of macrophages in models of chronic rejection has been assessed through depletion strategies, demonstrating attenuation of chronic lesions and vasculopathy [78].
In patients presenting with chronic allograft nephropathy, mRNA levels of PAI-1, a glycoprotein which promotes fibrosis by inhibiting degradation of the extracellular matrix, were found to be increased in macrophages infiltrating the kidney [72]. These findings identify an additional mechanism where macrophages incite chronic rejection by promoting fibrosis. Fibrosis precedes clinical dysfunction of the allograft and the development of progressive fibrosis in turn has been attributed to M2 macrophages in the context of dysregulated inflammation [48]. Though the majority of M2 macrophages, including M2a and M2c macrophages, are generally considered to demonstrate beneficial reparative characteristics, with regard to ongoing injury, sustained activity may result in the continuous production of various wound-healing growth factors, ultimately becoming a pathological process leading to fibrosis [79]. Consequently, M2 macrophages were identified as the dominant macrophage subset found in chronic lesions [6]. Steroids and calcineurin inhibitors, used routinely in transplantation therapy, have been shown to induce CD163+ M2 macrophage polarization, with a correlative increase in mRNA levels of pro-fibrotic cytokines such as TGFβ-1 and connective tissue growth factor, thus promoting development of fibrosis and at times exacerbating rejection [6, 80]. These recent findings link progression of fibrosis to this subset of macrophages, suggesting that they may serve as a predictive biomarker of chronic rejection and that restricting their activity would serve as a potential therapeutic strategy to protect against macrophage-dependent mechanisms related to fibrosis. Fully understanding the function of the M2 macrophage subset in the setting of chronic rejection requires additional studies.
Macrophages as a therapeutic agent
Though much attention has been given to the detrimental role of macrophages in organ transplantation, limited studies have ascertained that regulatory macrophages have the potential to prolong allograft survival. M regs have been used in immunodeficient mice [81], and in non-immunosuppressed recipients of a mismatched heterotopic heart allografts, to ameliorate symptoms of rejection and prolong allograft survival [15]. Furthermore, administration of M regs to porcine recipients of single lung allografts improved graft prognosis [82].
Presently, it is not fully understood how M regs exert their immunosuppressive effects in vivo, though it is assumed it is controlled by multiple mechanisms. In principle, M regs could directly regulate and suppress polyclonal T cell proliferation and mediate T cell elimination through an iNOS-dependent mechanism and their ability to down-regulate L-selectin levels on T cells, which ultimately prevents T cell activation [15, 83]. Alternatively, M regs may secrete anti-inflammatory mediators, which help promote tissue repair. Consistent with this idea, the suppressive capacity of M regs has been attributed to IFN-γ-induced iNOS [15, 84], which has recently been implicated in macrophage-mediated immune suppression [15, 85].
From a therapeutic viewpoint, regulatory macrophages with the capacity to quell an aberrant inflammatory response could be used as a pharmacological agent for tolerance induction. A recent study showed that M regs can be generated from peripheral blood monocytes for potential use in solid organ transplantation [86]. Two human recipients of kidney allografts were adoptively transferred with donor-derived infusions of M regs and weaned to monotherapy [13]. No incidence of acute or chronic rejection has been observed at 5 years. The absence of acute rejection and lack of signs indicative of subclinical rejection suggested a lack of or attenuation of anti-donor reactivity [87]. In these studies, M regs demonstrated graft protective functions and pre-operative administration of M reg-based therapy was shown to mediate tolerance of the donor allograft. Donor M regs are used instead of recipient M regs, as a study by Riquelme et al. established that the graft-protective effect of M regs is specific to donor cells [15]. The described findings suggest there is a benefit to distinguishing between macrophage subsets present in allograft settings, as depletion of certain subsets of macrophages may prove more beneficial than total macrophage depletion.
Several key clinical concerns remain to be addressed regarding the translation of M reg therapy to clinical transplantation, such as the stability and safety of M regs in vivo and the efficacy of M reg usage in a wide and variable population. Some of these questions are now being addressed in the ONE Study consortium in Europe, aimed at determining the efficacy and safety of administering donor-derived M reg preparations to living-donor solid organ transplant recipients as a cellular immunotherapy, with the ultimate goal of reducing the need for conventional immunosuppression (NCT02085629).
The Effects of Immunosuppressives and therapeutics on Macrophages
Immunosuppressive drugs used routinely for the prevention of allograft rejection have been shown to affect the phenotype and function of macrophages. Macrophages treated with rapamycin, an inhibitor of the serine/threonine kinase mTOR, were impaired in their ability to present antigens and displayed a notable reduction in the expression of CD80 [88]. Rapamycin has also been shown to inhibit production of the inflammatory mediator iNOS in macrophage cell lines [89]. Bortezomib is a protease inhibitor mainly used in the treatment of AMR [90] and has also been found to block T-cell mediated responses [91, 92]. In a murine model of contact hypersensitivity, an inflammatory immune reaction mediated by T cells, Bortezomib treatment resulted in a noted reduction in macrophage infiltration [91]. Furthermore, Bortezomib has been shown to reduce inflammatory cytokine production in macrophages stimulated with LPS in vitro [91, 93]. Use of the calcineurin inhibitors CsA and FK506 has been shown to regulate TLR mediated pathways in myeloid cells and lead to macrophage activation by inhibiting the calcineurin/NFAT pathway. Blocking NFAT leads to activation of the downstream NF-κB and MAPK pathways, and to subsequent production of inflammatory mediators including IL-12 and TNF-α [94, 95]. As mentioned previously, calcineurin inhibitors have also been implicated in the promotion of M2 macrophage differentiation, as identified by the marker CD163 [6]. Butyric acid is used for treatment of auto-immune disorders and has been investigated for tolerance induction in allografts [96]. Butyrate treatment of monocytes in vitro was found to decrease their phagocytic capabilities and to reduce expression of markers including CD14, CD86 and MHCII [97]. In a separate study, butyrate prevented IL-12 production in human monocytes and promoted production of IL-10 [98], suggesting that it might play a role in the development of anti-inflammatory macrophages.
Conclusion
Modulation of graft homeostasis involves the interplay between the various subpopulations of macrophages, which can contribute allograft-destructive or protective mechanisms based on their phenotype and function. Though major advances have been made with regard to an improved understanding of the contribution of macrophages to graft outcome, there is a paucity of clinical data and further studies are warranted to establish a comprehensive understanding of their contribution to graft injury, repair and graft acceptance.
Key points.
Based on cues from their microenvironment, macrophages differentiate into inflammatory (M1), wound healing (M2), or regulatory macrophages all with distinct functions and phenotypes.
Macrophages generate inflammatory mediators that contribute to ischemia reperfusion injury and acute and chronic allograft rejection.
Regulatory macrophages are an attractive candidate for use as an adjunct cell-based therapy to suppress allograft rejection in human transplantation.
Acknowledgements
The authors thank Nicole M. Valenzuela for critical review of this manuscript.
Financial support and sponsorship
This work was supported by the NRSA Vascular Biology training grant (S.S.) and RO1AI042819 (E.F.R).
Footnotes
Disclosure: none
Conflicts of interest
None
References
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Murphy K. Janeway's Immunobiology. Garland Science, Taylor & Francis Group, LLC; 2012. [Google Scholar]
- 2.Gordon S. Alternative Activation of Macrophages. Nature Reviews Immunology. 2003:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 3.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000prime reports. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. Journal of Clinical Investigation. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mosser DM. The many faces of macrophage activation. Journal of Leukocyte Biology. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 6. Ikezumi Y, Suzuki T, Yamada T, et al. Alternatively activated macrophages in the pathogenesis of chronic kidney allograft injury. In: University M, editor. Pediatric Nephrology. 2014. This study explored the localization of M2 macrophages and their contribution to intersitial fibrosis in the setting of chronic kidney allograft injury.
- 7.Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Reviews in Molecular Medicine. 2011;13:12. doi: 10.1017/S1462399411001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barros MHM, Hauck F, Dreyer JH, et al. Macrophage Polarisation: an Immunohistochemical Approach for Identifying M1 and M2 Macrophages. Plos One. 2013;8:11. doi: 10.1371/journal.pone.0080908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valenzuela NM, Mulder A, Reed EF. HLA Class I Antibodies Trigger Increased Adherence of Monocytes to Endothelial Cells by Eliciting an Increase in Endothelial P-Selectin and, Depending on Subclass, by Engaging Fc gamma Rs. Journal of Immunology. 2013;190:6635–6650. doi: 10.4049/jimmunol.1201434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mantovani A, Sozzani S, Locati M, et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends in Immunology. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 11.Grugan KD, McCabe FL, Kinder M, et al. Tumor-Associated Macrophages Promote Invasion while Retaining Fc- Dependent Anti-Tumor Function. Journal of Immunology. 2012;189:5457–5466. doi: 10.4049/jimmunol.1201889. [DOI] [PubMed] [Google Scholar]
- 12.Lugo-Villarino G, Verollet C, Maridonneau-Parini I, et al. Macrophage polarization: convergence point targeted by mycobacterium tuberculosis and HIV. Frontiers in immunology. 2011;2:43. doi: 10.3389/fimmu.2011.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broichhausen C, Riquelme P, Geissler EK, et al. Regulatory macrophages as therapeutic targets and therapeutic agents in solid organ transplantation. Current Opinion in Organ Transplantation. 2012;17:332–342. doi: 10.1097/MOT.0b013e328355a979. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki T, Arumugam P, Sakagami T, et al. Pulmonary macrophage transplantation therapy. Nature. 2014;514:450-+. doi: 10.1038/nature13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riquelme P, Tomiuk S, Kammler A, et al. IFN-gamma-induced iNOS Expression in Mouse Regulatory Macrophages Prolongs Allograft Survival in Fully Immunocompetent Recipients. Molecular Therapy. 2013;21:409–422. doi: 10.1038/mt.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleming BD, Mosser DM. Regulatory macrophages: Setting the Threshold for Therapy. European Journal of Immunology. 2011;41:2498–2502. doi: 10.1002/eji.201141717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ziegler-Heitbrock L. The CD14+CD16+blood monocytes: their role in infection and inflammation. Journal of Leukocyte Biology. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 18.Sekerkova A, Krepsova E, Brabcova E, et al. CD14+CD16+ and CD14+CD163+ monocyte subpopulations in kidney allograft transplantation. Bmc Immunology. 2014;15:10. doi: 10.1186/1471-2172-15-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vereyken EJF, Kraaij MD, Baan CC, et al. A Shift towards Pro-Inflammatory CD16+Monocyte Subsets with Preserved Cytokine Production Potential after Kidney Transplantation. Plos One. 2013;8:10. doi: 10.1371/journal.pone.0070152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Busuttil RW, Lipshutz GS, Kupiec-Weglinski JW, et al. rPSGL-Ig for Improvement of Early Liver Allograft Function: A Double-Blind, Placebo-Controlled, Single-Center Phase II Study. American Journal of Transplantation. 2011;11:786–797. doi: 10.1111/j.1600-6143.2011.03441.x. [DOI] [PubMed] [Google Scholar]
- 21.Ysebaert DK, De Greef KE, Vercauteren SR, et al. Identification and kinetics of leukocytes after severe ischaemia/reperfusion renal injury. Nephrology Dialysis Transplantation. 2000;15:1562–1574. doi: 10.1093/ndt/15.10.1562. [DOI] [PubMed] [Google Scholar]
- 22.Takada M, Nadeau KC, Shaw GD, et al. The cytokine-adhesion molecule cascade in ischemia/reperfusion injury of the rat kidney - Inhibition by a soluble P-selectin ligand. Journal of Clinical Investigation. 1997;99:2682–2690. doi: 10.1172/JCI119457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Persy VP, Verhulst A, Ysebaert DK, et al. Reduced postischemic macrophage infiltration and interstitial fibrosis in osteopontin knockout mice. Kidney International. 2003;63:543–553. doi: 10.1046/j.1523-1755.2003.00767.x. [DOI] [PubMed] [Google Scholar]
- 24.Day YJ, Huang LP, McDuffie MJ, et al. Renal protection from ischemia mediated by A(2A) adenosine receptors on bone marrow-derived cells. Journal of Clinical Investigation. 2003;112:883–891. doi: 10.1172/JCI15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemay S, Rabb H, Postler G, et al. Prominent and sustained up-regulation of gp130-signaling cytokines and of the chemokine MIP-2 in murine renal ischemia-reperfusion injury. Transplantation. 2000;69:959–963. doi: 10.1097/00007890-200003150-00049. [DOI] [PubMed] [Google Scholar]
- 26.Jo SK, Sung SA, Cho WY, et al. Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrology Dialysis Transplantation. 2006;21:1231–1239. doi: 10.1093/ndt/gfk047. [DOI] [PubMed] [Google Scholar]
- 27.Furuichi K, Wada T, Iwata Y, et al. CCR2 signaling contributes to ischemia-reperfusion injury in kidney. Journal of the American Society of Nephrology. 2003;14:2503–2515. doi: 10.1097/01.asn.0000089563.63641.a8. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Ji H, Shen X, et al. Targeting TIM-1 on CD4 T Cells Depresses Macrophage Activation and Overcomes Ischemia-Reperfusion Injury in Mouse Orthotopic Liver Transplantation. American Journal of Transplantation. 2013;13:56–66. doi: 10.1111/j.1600-6143.2012.04316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huen SC, Huynh L, Marlier A, et al. GM-CSF Promotes Macrophage Alternative Activation after Renal Ischemia/Reperfusion Injury. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2014060612. This study identifies GM-CSF as a novel factor produced by renal tubular cells that can skew macrophages toward a reparative phenotype that supports tubular proliferation after renal ischemic reperfusion injury.
- 30.Huen SC, Cantley LG. Macrophage-mediated injury and repair after ischemic kidney injury. Pediatric Nephrology. 2015;30:199–209. doi: 10.1007/s00467-013-2726-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yue S, Rao JH, Zhu JJ, et al. Myeloid PTEN Deficiency Protects Livers from Ischemia Reperfusion Injury by Facilitating M2 Macrophage Differentiation. Journal of Immunology. 2014;192:5343–5353. doi: 10.4049/jimmunol.1400280. The novel findings from this study highlight the important role of PTEN in regulating macrophage polarization and the tissue inflammatory immune response in heptic ischemia reperfusion injury.
- 32.Huang J, Shen X-D, Yue S, et al. Adoptive transfer of heme oxygenase-1 (HO-1)-modified macrophages rescues the nuclear factor erythroid 2-related factor (Nrf2) antiinflammatory phenotype in liver ischemia/reperfusion injury. Molecular medicine (Cambridge, Mass.) 2014;20:448–455. doi: 10.2119/molmed.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhai Y, Petrowsky H, Hong JC, et al. Ischaemia-reperfusion injury, in liver transplantation-from bench to bedside. Nature Reviews Gastroenterology & Hepatology. 2013;10:79–89. doi: 10.1038/nrgastro.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen XD, Ke B, Zhai Y, et al. Absence of toll-like receptor 4 (TLR4) signaling in the donor organ reduces ischemia and reperfusion injury in a murine liver transplantation model. Liver Transplantation. 2007;13:1435–1443. doi: 10.1002/lt.21251. [DOI] [PubMed] [Google Scholar]
- 35.Kruger B, Krick S, Dhillon N, et al. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3390–3395. doi: 10.1073/pnas.0810169106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng L, Xing H, Mao X, et al. Lipocalin-2 Promotes M1 Macrophages Polarization in a Mouse Cardiac Ischaemia Reperfusion Injury Model. Scandinavian Journal of Immunology. 2015;81:31–38. doi: 10.1111/sji.12245. [DOI] [PubMed] [Google Scholar]
- 37.Brent L, Brown J, Medawar PB. Skin transplantation immunity in relation to hypersensitivity. Lancet. 1958;2:561564. doi: 10.1016/s0140-6736(58)90202-2. [DOI] [PubMed] [Google Scholar]
- 38.Fishbein MC, Kobashigawa J. Biopsy-negative cardiac transplant rejection: etiology, diagnosis, and therapy. Current Opinion in Cardiology. 2004;19:166–169. doi: 10.1097/00001573-200403000-00018. [DOI] [PubMed] [Google Scholar]
- 39.Tinckam KJ, Djurdjev O, Magil AB. Glomerular monocytes predict worse outcomes after acute renal allograft rejection independent of C4d status. Kidney International. 2005;68:1866–1874. doi: 10.1111/j.1523-1755.2005.00606.x. [DOI] [PubMed] [Google Scholar]
- 40.Wyburn KR, Jose MD, Wu HL, et al. The role of macrophages in allograft rejection. Transplantation. 2005;80:16411647. doi: 10.1097/01.tp.0000173903.26886.20. [DOI] [PubMed] [Google Scholar]
- 41.Strom TB, Tilney NL, Carpenter CB, et al. Identity and Cytotoxic Capacity of Cells Infiltrating Renal Allograft. New England Journal of Medicine. 1975;292:1257–1263. doi: 10.1056/NEJM197506122922402. [DOI] [PubMed] [Google Scholar]
- 42.Hancock WW, Thomson NM, Atkins RC. Composition of interstitial cellular infiltrate identified by monoclocal antibodies in renal biopsies of rejecting human renal allografts. Transplantation. 1983;35:458–463. doi: 10.1097/00007890-198305000-00013. [DOI] [PubMed] [Google Scholar]
- 43. Abe T, Su CA, Iida S, et al. Graft-Derived CCL2 Increases Graft Injury During Antibody-Mediated Rejection of Cardiac Allografts. American Journal of Transplantation. 2014;14:1753–1764. doi: 10.1111/ajt.12780. This study explores the role of graft derived monocyte chemoattractant protein-1 in AMR and the contribution of intragraft macrophages to cardiac AMR.
- 44.Schafer N, Tahara K, von Websky M, et al. Role of resident macrophages in the immunologic response and smooth muscle dysfunction during acute allograft rejection after intestinal transplantation. Transplant International. 2008;21:778–791. doi: 10.1111/j.1432-2277.2008.00676.x. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt A, Sucke J, Fuchs-Moll G, et al. Macrophages in experimental rat lung isografts and allografts: infiltration and proliferation in situ. Journal of Leukocyte Biology. 2007;81:186–194. doi: 10.1189/jlb.0606377. [DOI] [PubMed] [Google Scholar]
- 46.Yi SN, Hawthorne WJ, Lehnert AM, et al. T cell-activated macrophages are capable of both recognition and rejection of pancreatic islet xenografts. Journal of Immunology. 2003;170:2750–2758. doi: 10.4049/jimmunol.170.5.2750. [DOI] [PubMed] [Google Scholar]
- 47.Girlanda R, Kleiner DE, Duan Z, et al. Monocyte infiltration and kidney allograft dysfunction during acute rejection. American Journal of Transplantation. 2008;8:600–607. doi: 10.1111/j.1600-6143.2007.02109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Toki D, Zhang W, Hor KLM, et al. The Role of Macrophages in the Development of Human Renal Allograft Fibrosis in the First Year After Transplantation. American Journal of Transplantation. 2014;14:2126–2136. doi: 10.1111/ajt.12803. This study investigated the role of macrophages in human renal allograft fibrosis. Human renal biopsies with macrophage infiltrates showed subclinical alloimmune inflammation, tubular injury and fibrosis.
- 49.Fishbein GA, Fishbein MC. Morphologic and immunohistochemical findings in antibody-mediated rejection of the cardiac allograft. Human Immunology. 2012;73:1213–1217. doi: 10.1016/j.humimm.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 50.Qi F, Adair A, Ferenbach D, et al. Depletion of Cells of Monocyte Lineage Prevents Loss of Renal Microvasculature in Murine Kidney Transplantation. Transplantation. 2008;86:1267–1274. doi: 10.1097/TP.0b013e318188d433. [DOI] [PubMed] [Google Scholar]
- 51.Jose MD, Ikezumi Y, van Rooijen N, et al. Macrophages act as effectors of tissue damage in acute renal allograft rejection. Transplantation. 2003;76:1015–1022. doi: 10.1097/01.TP.0000083507.67995.13. [DOI] [PubMed] [Google Scholar]
- 52.Takeiri M, Tachibana M, Kaneda A, et al. Inhibition of macrophage activation and suppression of graft rejection by DTCM-glutarimide, a novel piperidine derived from the antibiotic 9-methylstreptimidone. Inflammation Research. 2011;60:879–888. doi: 10.1007/s00011-011-0348-z. [DOI] [PubMed] [Google Scholar]
- 53.Valenzuela NM, Hong L, Shen XD, et al. Blockade of P-Selectin Is Sufficient to Reduce MHC I Antibody-Elicited Monocyte Recruitment In Vitro and In Vivo. American Journal of Transplantation. 2013;13:299–311. doi: 10.1111/ajt.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jindra PT, Hsueh A, Hong L, et al. Anti-MHC class I antibody activation of proliferation and survival signaling in murine cardiac allografts. Journal of Immunology. 2008;180:2214–2224. doi: 10.4049/jimmunol.180.4.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berry GJ, Burke MM, Andersen C, et al. The 2013 International Society for Heart and Lung Transplantation Working Formulation for the standardization of nomenclature in the pathologic diagnosis of antibody-mediated rejection in heart transplantation. Journal of Heart and Lung Transplantation. 2013;32:1147–1162. doi: 10.1016/j.healun.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 56.Agarwal A, Shen LY, Kirk AD. The role of alemtuzumab in facilitating maintenance immunosuppression minimization following solid organ transplantation. Transplant Immunology. 2008;20:6–11. doi: 10.1016/j.trim.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 57.Kirk AD, Mannon RB, Kleiner DE, et al. Results from a human renal allograft tolerance trial evaluating T-cell depletion with alemtuzumab combined with deoxyspergualin. Transplantation. 2005;80:1051–1059. doi: 10.1097/01.tp.0000174341.49741.8f. [DOI] [PubMed] [Google Scholar]
- 58. Valenzuela NM, Trinh KR, Mulder A, et al. Monocyte Recruitment by HLA IgG-Activated Endothelium: The Relationship Between IgG Subclass and FcgammaRIIa Polymorphisms. American Journal of Transplantation. 2015:17. doi: 10.1111/ajt.13174. This is the first study to explore the effects of HLA IgG modulation on FcγR-mediated monocyte recruitment. The findings from this study highlight HLA IgG antibodies as a target for therapy in AMR rejection.
- 59.Fryer J, Grant D, Jiang JF, et al. Influence of macrophage depletion on bacterial translocation and rejection in small bowel transplantation. Transplantation. 1996;62:553–559. doi: 10.1097/00007890-199609150-00002. [DOI] [PubMed] [Google Scholar]
- 60.Imagawa DK, Millis JM, Seu P, et al. The role of tumor necrosis factor in allograft rejection. Evidence that anti-TNF antibody therapy prolongs allograft survival in rats with acute rejection. Transplantation. 1991;51:57–62. doi: 10.1097/00007890-199101000-00008. [DOI] [PubMed] [Google Scholar]
- 61.Roza AM, Cooper M, Pieper G, et al. NOX 100, a nitric oxide scavenger, enhances cardiac allograft survival and promotes long-term graft acceptance. Transplantation. 2000;69:227–231. doi: 10.1097/00007890-200001270-00006. [DOI] [PubMed] [Google Scholar]
- 62.Grone HJ, Weber C, Weber KSC, et al. Met-RANTES reduces vascular and tubular damage during acute renal transplant rejection: blocking monocyte arrest and recruitment. Faseb Journal. 1999;13:1371–1383. [PubMed] [Google Scholar]
- 63.Pattison J, Nelson PJ, Huie P, et al. RANTES chemokine expression in cell-mediated transplant rejection of the kidney. Lancet. 1994;343:209–211. doi: 10.1016/s0140-6736(94)90992-x. [DOI] [PubMed] [Google Scholar]
- 64.Oh JY, Lee HJ, Ko AY, et al. Analysis of Macrophage Phenotype in Rejected Corneal Allografts. Investigative Ophthalmology & Visual Science. 2013;54:7779–7784. doi: 10.1167/iovs.13-12650. [DOI] [PubMed] [Google Scholar]
- 65.Huang G, Wilson NA, Reese SR, et al. Characterization of Transfusion- Elicited Acute Antibody-Mediated Rejection in a Rat Model of Kidney Transplantation. American Journal of Transplantation. 2014;14:1061–1072. doi: 10.1111/ajt.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hutchinson IV. Cardiac allograft vasculopathy - the cellular attack. Zeitschrift Fur Kardiologie. 2000;89:16–20. doi: 10.1007/s003920070020. [DOI] [PubMed] [Google Scholar]
- 67.Beckmann N, Cannet C, Fringeli-Tanner M, et al. Macrophage labeling by SPIO as an early marker of allograft chronic rejection in a rat model of kidney transplantation. Magnetic Resonance in Medicine. 2003;49:459–467. doi: 10.1002/mrm.10387. [DOI] [PubMed] [Google Scholar]
- 68.Azuma H, Nadeau KC, Ishibashi M, et al. Prevention of functional, structural, and molecular changes of chronic rejection of rat renal allografts by a specific macrophage inhibitor. Transplantation. 1995;60:1577–1582. doi: 10.1097/00007890-199560120-00034. [DOI] [PubMed] [Google Scholar]
- 69.Pilmore HL, Painter DM, Bishop GA, et al. Early up-regulation of macrophages and myofibroblasts - A new marker for development of chronic renal allograft rejection. Transplantation. 2000;69:2658–2662. doi: 10.1097/00007890-200006270-00028. [DOI] [PubMed] [Google Scholar]
- 70.Miyagawa-Hayashino A, Tsuruyama T, Haga H, et al. Arteriopathy in chronic allograft rejection in liver transplantation. Liver Transplantation. 2004;10:513–519. doi: 10.1002/lt.20081. [DOI] [PubMed] [Google Scholar]
- 71.Croker BP, Clapp WL, Shamat A, et al. Macrophages and chronic renal allograft nephropathy. Kidney International. 1996;50:S42–S49. [PubMed] [Google Scholar]
- 72.Revelo MP, Federspiel C, Helderman H, et al. Chronic allograft nephropathy: expression and localization of PAI-1 and PPAR-gamma. Nephrology Dialysis Transplantation. 2005;20:2812–2819. doi: 10.1093/ndt/gfi172. [DOI] [PubMed] [Google Scholar]
- 73.Heidenreich S, Lang D, Tepel M, et al. Monocyte activation for enhanced tumour necrosis factor-alpha and interleukin 6 production during chronic renal allograft rejection. Transplant immunology. 1994;2:35–40. doi: 10.1016/0966-3274(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 74.Nadeau KC, Azuma H, Tilney NL. Sequential cytokine dynamics in chronic rejection of rat renal allografts: role for cytokines RANTES and MCP-1. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:8729–8733. doi: 10.1073/pnas.92.19.8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang J, Reutzel-Selke A, Steier C, et al. Targeting of macrophage activity by adenovirus-mediated intragraft overexpression of TNFRp55-Ig, IL-12p40, and vIL-10 ameliorates adenovirus-mediated chronic graft injury, whereas stimulation of macrophages by overexpression of IFN-gamma accelerates chronic graft injury in a rat renal allograft model. Journal of the American Society of Nephrology. 2003;14:214–225. doi: 10.1097/01.asn.0000037703.73850.72. [DOI] [PubMed] [Google Scholar]
- 76.Yun JJ, Whiting D, Fischbein MP, et al. Combined blockade of the chemokine receptors CCR1 and CCR5 attenuates chronic rejection. Circulation. 2004;109:932–937. doi: 10.1161/01.CIR.0000112595.65972.8A. [DOI] [PubMed] [Google Scholar]
- 77.Song EW, Zou HQ, Yao YS, et al. Early application of Met-RANTES ameliorates chronic allograft nephropathy. Kidney International. 2002;61:676–685. doi: 10.1046/j.1523-1755.2002.00148.x. [DOI] [PubMed] [Google Scholar]
- 78.Kitchens WH, Chase CM, Uehara S, et al. Macrophage depletion suppresses cardiac allograft vasculopathy in mice. American Journal of Transplantation. 2007;7:2675–2682. doi: 10.1111/j.1600-6143.2007.01997.x. [DOI] [PubMed] [Google Scholar]
- 79.Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. Journal of Clinical Investigation. 2008;118:3522–3530. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ikezumi Y, Suzuki T, Karasawa T, et al. Identification of alternatively activated macrophages in new-onset paediatric and adult immunoglobulin A nephropathy: potential role in mesangial matrix expansion. Histopathology. 2011;58:198210. doi: 10.1111/j.1365-2559.2011.03742.x. [DOI] [PubMed] [Google Scholar]
- 81. Broichhausen C, Riquelme P, Ahrens N, et al. In question: the scientific value of preclinical safety pharmacology and toxicology studies with cell-based therapies. Molecular Therapy — Methods & Clinical Development. 2014 doi: 10.1038/mtm.2014.26. This study explores the pharmacokinetics and safety of M regs in a murine model and questions the justification of using M regs in human patients.
- 82.Warnecke G, Hutchinson JA, Riquelme P, et al. Postoperative intravenous infusion of donor-derived transplant acceptance-inducing cells as an adjunct immunosuppressive therapy in a porcine pulmonary allograft model. Transplant International. 2009;22:332–341. doi: 10.1111/j.1432-2277.2008.00778.x. [DOI] [PubMed] [Google Scholar]
- 83.Hanson EM, Clements VK, Sinha P, et al. Myeloid-Derived Suppressor Cells Down-Regulate L-Selectin Expression on CD4(+) and CD8(+) T Cells. Journal of Immunology. 2009;183:937–944. doi: 10.4049/jimmunol.0804253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brem-Exner BG, Sattler C, Hutchinson JA, et al. Macrophages driven to a novel state of activation have antiinflammatory properties in mice. Journal of Immunology. 2008;180:335–349. doi: 10.4049/jimmunol.180.1.335. [DOI] [PubMed] [Google Scholar]
- 85.Arakawa Y, Qin J, Chou HS, et al. Cotransplantation With Myeloid-Derived Suppressor Cells Protects Cell Transplants: A Crucial Role of Inducible Nitric Oxide Synthase. Transplantation. 2014;97:740–747. doi: 10.1097/01.TP.0000442504.23885.f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hutchinson JA, Riquelme P, Geissler EK, et al. Human Regulatory Macrophages. In: Cuturi MC, Anegon I, editors. Suppression and Regulation of Immune Responses: Methods and Protocols. Totowa: Humana Press Inc; 2011. pp. 181–192. [Google Scholar]
- 87.Hutchinson JA, Riquelme P, Tomiuk S, et al. Immunological consequences and trafficking of human regulatory macrophages administered to renal transplant recipients. Transplant International. 2011;24:219–219. doi: 10.4049/jimmunol.1100762. [DOI] [PubMed] [Google Scholar]
- 88.Shen H, Li L, Zhao Y. Effects of immunosuppressive drugs on phenotypes and function of differentiated macrophages. Jiepou Xuebao. 2011;42:345–349. [Google Scholar]
- 89.Attur MG, Patel R, Thakker G, et al. Differential anti-inflammatory effects of immunosuppressive drugs: Cyclosporin, rapamycin and FK-506 on inducible nitric oxide synthase, nitric oxide, cyclooxygenase-2 and PGE(2) production. Inflammation Research. 2000;49:20–26. doi: 10.1007/PL00000199. [DOI] [PubMed] [Google Scholar]
- 90.Ejaz NS, Alloway RR, Halleck F, et al. Review of Bortezomib Treatment of Antibody-Mediated Rejection in Renal Transplantation. Antioxidants & Redox Signaling. 2014;21:2401–2418. doi: 10.1089/ars.2014.5892. [DOI] [PubMed] [Google Scholar]
- 91.Yanaba K, Yoshizaki A, Muroi E, et al. The proteasome inhibitor bortezomib inhibits T cell-dependent inflammatory responses. Journal of Leukocyte Biology. 2010;88:117–122. doi: 10.1189/jlb.1009666. [DOI] [PubMed] [Google Scholar]
- 92.Everly MJ, Everly JJ, Susskind B, et al. Bortezomib Provides Effective Therapy for Antibody- and Cell-Mediated Acute Rejection. Transplantation. 2008;86:1754–1761. doi: 10.1097/TP.0b013e318190af83. [DOI] [PubMed] [Google Scholar]
- 93. Han SH, Kim JS, Woo JH, et al. The Effect of Bortezomib on Expression of Inflammatory Cytokines and Survival in a Murine Sepsis Model Induced by Cecal Ligation and Puncture. Yonsei Medical Journal. 2015;56:112–123. doi: 10.3349/ymj.2015.56.1.112. This is the first study to determine that Bortezomib treatment reduces production of inflammatory mediators by macrophages. This finding provides additional insight into the mechanism of Bortezomib and indicates a novel application for its use in allograft rejection.
- 94.Kang YJ, Kusler B, Otsuka M, et al. Calcineurin negatively regulates TLR-Mediated activation pathways. Journal of Immunology. 2007;179:4598–4607. doi: 10.4049/jimmunol.179.7.4598. [DOI] [PubMed] [Google Scholar]
- 95.Fric J, Zelante T, Wong AYW, et al. NFAT control of innate immunity. Blood. 2012;120:1380–1389. doi: 10.1182/blood-2012-02-404475. [DOI] [PubMed] [Google Scholar]
- 96.Bohmig GA, Krieger PM, Saemann MD, et al. Stable prodrugs of n-butyric acid: suppression of T cell alloresponses in vitro and prolongation of heart allograft survival in a fully allogeneic rat strain combination. Transplant Immunology. 1999;7:221–227. doi: 10.1016/s0966-3274(99)80006-9. [DOI] [PubMed] [Google Scholar]
- 97.Millard AL, Mertes PM, Ittelet D, et al. Butyrate affects differentiation, maturation and function of human monocyte derived dendritic cells and macrophages. Clinical and Experimental Immunology. 2002;130:245–255. doi: 10.1046/j.0009-9104.2002.01977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Saemann MD, Bohmig GA, Osterreicher CH, et al. Anti-inflammatory effects of sodium butyrate on human monocytes: potent inhibition of IL-12 and up-regulation of IL-10 production. Faseb Journal. 2000;14:2380–2382. doi: 10.1096/fj.00-0359fje. [DOI] [PubMed] [Google Scholar]