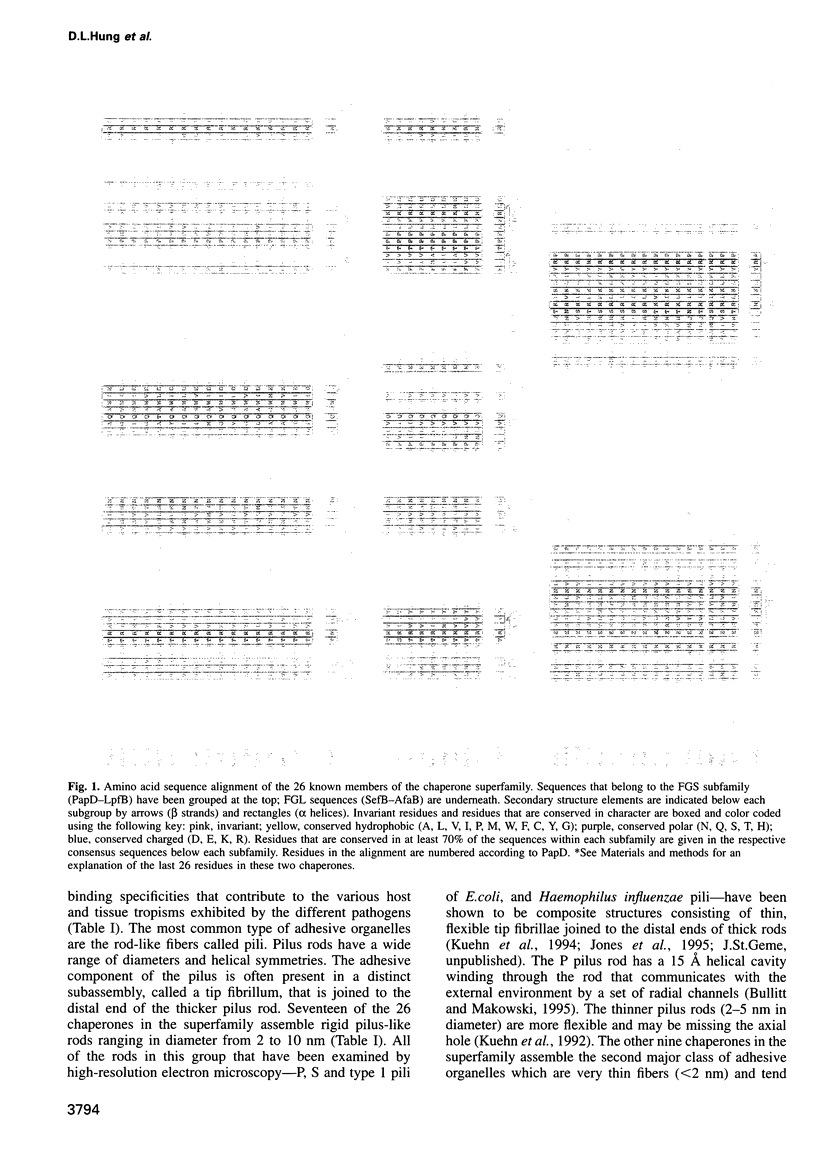
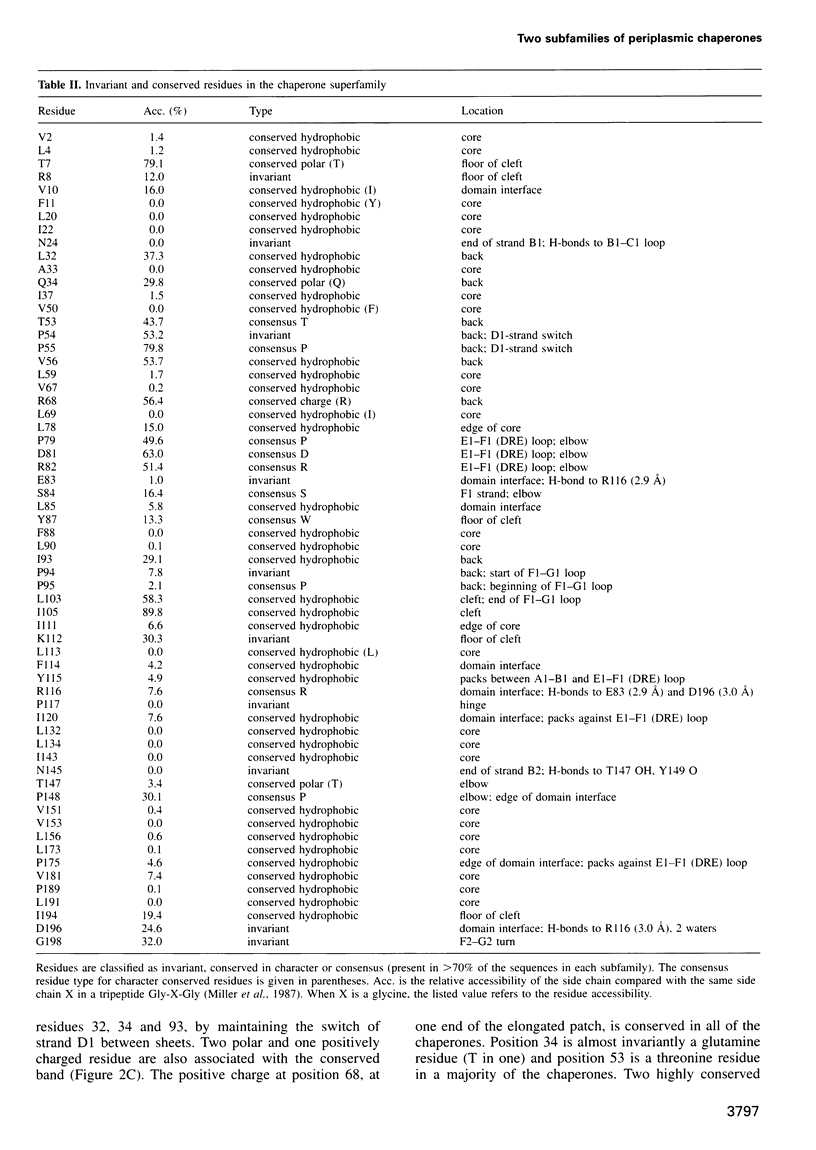
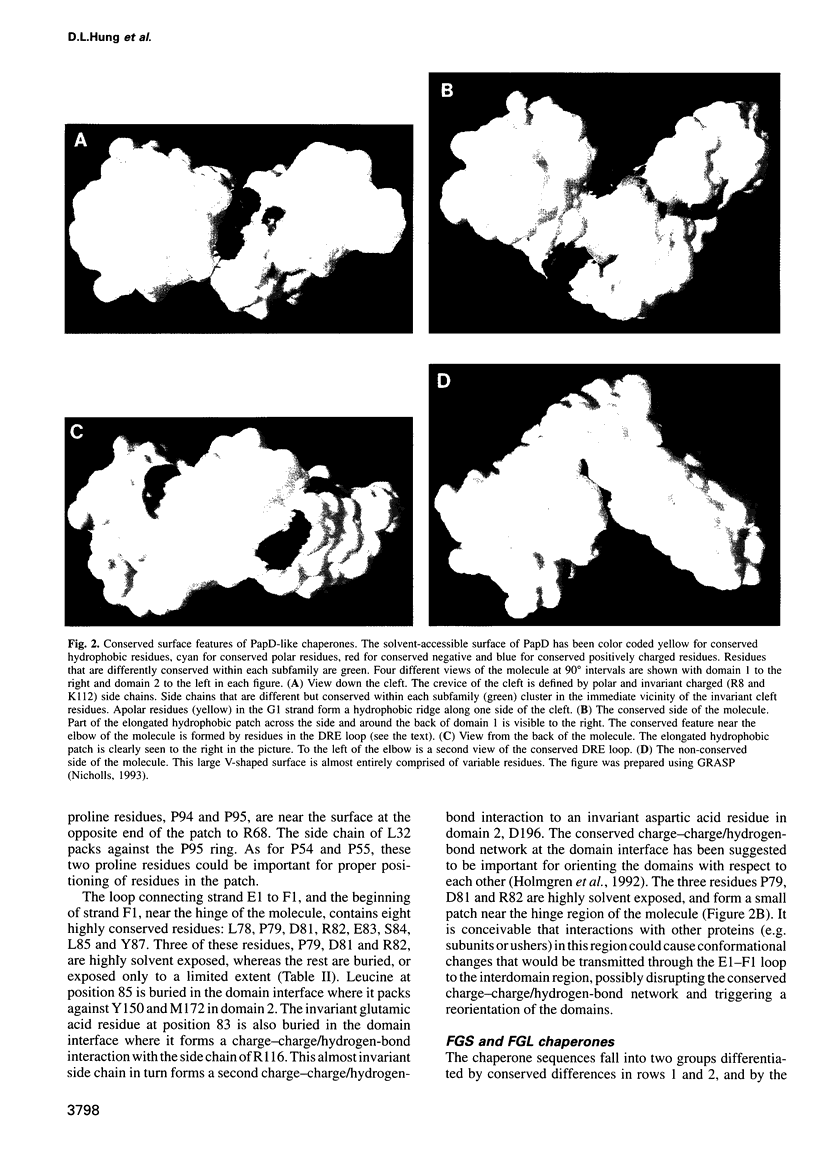
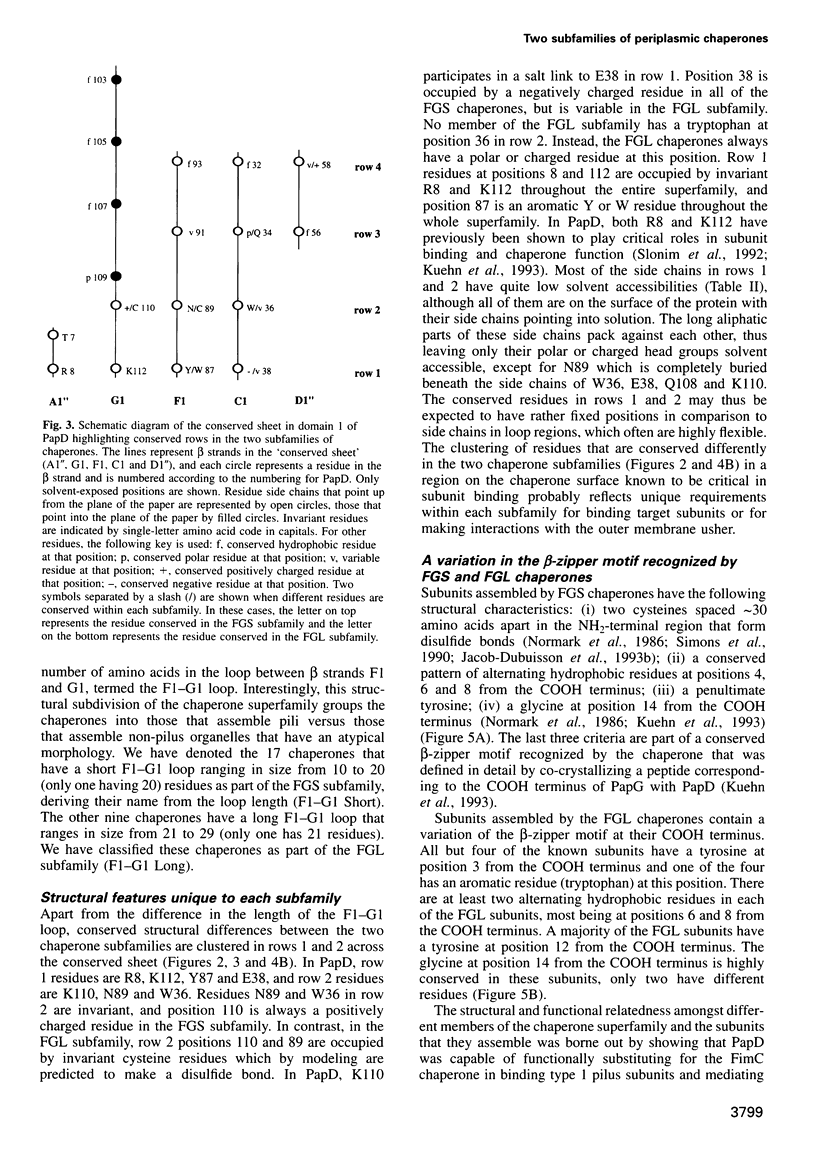
Abstract
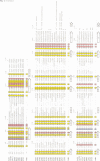
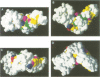
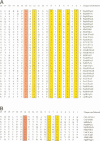
The initial encounter of a microbial pathogen with the host often involves the recognition of host receptors by different kinds of bacterial adhesive organelles called pili, fimbriae, fibrillae or afimbrial adhesins. The development of over 26 of these architecturally diverse adhesive organelles in various Gram-negative pathogens depends on periplasmic chaperones that are comprised of two immunoglobulin-like domains. All of the chaperones possess a highly conserved sheet in domain 1 and a conserved interdomain hydrogen-bonding network. Chaperone-subunit complex formation depends on the anchoring of the carboxylate group of the subunit into the conserved crevice of the chaperone cleft and the subsequent positioning of the COOH terminus of subunits along the exposed edge of the conserved sheet of the chaperone. We discovered that the chaperones can be divided into two distinct subfamilies based upon conserved structural differences that occur in the conserved sheet. Interestingly, a subdivision of the chaperones based upon whether they assemble rod-like pili or non-pilus organelles that have an atypical morphology defines the same two subgroups. The molecular dissection of the two chaperone subfamilies and the adhesive fibers that they assemble has advanced our understanding of the development of virulence-associated organelles in pathogenic bacteria.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahrens R., Ott M., Ritter A., Hoschützky H., Bühler T., Lottspeich F., Boulnois G. J., Jann K., Hacker J. Genetic analysis of the gene cluster encoding nonfimbrial adhesin I from an Escherichia coli uropathogen. Infect Immun. 1993 Jun;61(6):2505–2512. doi: 10.1128/iai.61.6.2505-2512.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen B. L., Gerlach G. F., Clegg S. Nucleotide sequence and functions of mrk determinants necessary for expression of type 3 fimbriae in Klebsiella pneumoniae. J Bacteriol. 1991 Jan;173(2):916–920. doi: 10.1128/jb.173.2.916-920.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amit A. G., Mariuzza R. A., Phillips S. E., Poljak R. J. Three-dimensional structure of an antigen-antibody complex at 2.8 A resolution. Science. 1986 Aug 15;233(4765):747–753. doi: 10.1126/science.2426778. [DOI] [PubMed] [Google Scholar]
- Arnqvist A., Olsén A., Pfeifer J., Russell D. G., Normark S. The Crl protein activates cryptic genes for curli formation and fibronectin binding in Escherichia coli HB101. Mol Microbiol. 1992 Sep;6(17):2443–2452. doi: 10.1111/j.1365-2958.1992.tb01420.x. [DOI] [PubMed] [Google Scholar]
- Bahrani F. K., Cook S., Hull R. A., Massad G., Mobley H. L. Proteus mirabilis fimbriae: N-terminal amino acid sequence of a major fimbrial subunit and nucleotide sequences of the genes from two strains. Infect Immun. 1993 Mar;61(3):884–891. doi: 10.1128/iai.61.3.884-891.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrani F. K., Johnson D. E., Robbins D., Mobley H. L. Proteus mirabilis flagella and MR/P fimbriae: isolation, purification, N-terminal analysis, and serum antibody response following experimental urinary tract infection. Infect Immun. 1991 Oct;59(10):3574–3580. doi: 10.1128/iai.59.10.3574-3580.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrani F. K., Mobley H. L. Proteus mirabilis MR/P fimbrial operon: genetic organization, nucleotide sequence, and conditions for expression. J Bacteriol. 1994 Jun;176(11):3412–3419. doi: 10.1128/jb.176.11.3412-3419.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker D., Vader C. E., Roosendaal B., Mooi F. R., Oudega B., de Graaf F. K. Structure and function of periplasmic chaperone-like proteins involved in the biosynthesis of K88 and K99 fimbriae in enterotoxigenic Escherichia coli. Mol Microbiol. 1991 Apr;5(4):875–886. doi: 10.1111/j.1365-2958.1991.tb00761.x. [DOI] [PubMed] [Google Scholar]
- Bertin Y., Girardeau J. P., Der Vartanian M., Martin C. The ClpE protein involved in biogenesis of the CS31A capsule-like antigen is a member of a periplasmic chaperone family in gram-negative bacteria. FEMS Microbiol Lett. 1993 Mar 15;108(1):59–67. doi: 10.1016/0378-1097(93)90488-n. [DOI] [PubMed] [Google Scholar]
- Bullitt E., Makowski L. Structural polymorphism of bacterial adhesion pili. Nature. 1995 Jan 12;373(6510):164–167. doi: 10.1038/373164a0. [DOI] [PubMed] [Google Scholar]
- Bäumler A. J., Heffron F. Identification and sequence analysis of lpfABCDE, a putative fimbrial operon of Salmonella typhimurium. J Bacteriol. 1995 Apr;177(8):2087–2097. doi: 10.1128/jb.177.8.2087-2097.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouthier S. C., Müller K. H., Doran J. L., Collinson S. K., Kay W. W. Characterization of three fimbrial genes, sefABC, of Salmonella enteritidis. J Bacteriol. 1993 May;175(9):2523–2533. doi: 10.1128/jb.175.9.2523-2533.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson K. W., Jacob-Dubuisson F., Striker R. T., Hultgren S. J. Outer-membrane PapC molecular usher discriminately recognizes periplasmic chaperone-pilus subunit complexes. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3670–3674. doi: 10.1073/pnas.90.8.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchet-Suchaux M., Bertin A., Dubray G. Morphological description of surface structures on strain B41 of bovine enterotoxigenic Escherichia coli bearing both K99 and F41 antigens. J Gen Microbiol. 1988 Apr;134(4):983–995. doi: 10.1099/00221287-134-4-983. [DOI] [PubMed] [Google Scholar]
- Foged N. T., Klemm P., Elling F., Jorsal S. E., Zeuthen J. Monoclonal antibodies to K88ab, K88ac and K88ad fimbriae from enterotoxigenic Escherichia coli. Microb Pathog. 1986 Feb;1(1):57–69. doi: 10.1016/0882-4010(86)90032-x. [DOI] [PubMed] [Google Scholar]
- Friedrich M. J., Kinsey N. E., Vila J., Kadner R. J. Nucleotide sequence of a 13.9 kb segment of the 90 kb virulence plasmid of Salmonella typhimurium: the presence of fimbrial biosynthetic genes. Mol Microbiol. 1993 May;8(3):543–558. doi: 10.1111/j.1365-2958.1993.tb01599.x. [DOI] [PubMed] [Google Scholar]
- Galyov E. E., Karlishev A. V., Chernovskaya T. V., Dolgikh D. A., Smirnov OYu, Volkovoy K. I., Abramov V. M., Zav'yalov V. P. Expression of the envelope antigen F1 of Yersinia pestis is mediated by the product of caf1M gene having homology with the chaperone protein PapD of Escherichia coli. FEBS Lett. 1991 Jul 29;286(1-2):79–82. doi: 10.1016/0014-5793(91)80945-y. [DOI] [PubMed] [Google Scholar]
- Garcia M. I., Labigne A., Le Bouguenec C. Nucleotide sequence of the afimbrial-adhesin-encoding afa-3 gene cluster and its translocation via flanking IS1 insertion sequences. J Bacteriol. 1994 Dec;176(24):7601–7613. doi: 10.1128/jb.176.24.7601-7613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardeau J. P., Der Vartanian M., Ollier J. L., Contrepois M. CS31A, a new K88-related fimbrial antigen on bovine enterotoxigenic and septicemic Escherichia coli strains. Infect Immun. 1988 Aug;56(8):2180–2188. doi: 10.1128/iai.56.8.2180-2188.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldhar J., Perry R., Golecki J. R., Hoschutzky H., Jann B., Jann K. Nonfimbrial, mannose-resistant adhesins from uropathogenic Escherichia coli O83:K1:H4 and O14:K?:H11. Infect Immun. 1987 Aug;55(8):1837–1842. doi: 10.1128/iai.55.8.1837-1842.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A., Bränden C. I. Crystal structure of chaperone protein PapD reveals an immunoglobulin fold. Nature. 1989 Nov 16;342(6247):248–251. doi: 10.1038/342248a0. [DOI] [PubMed] [Google Scholar]
- Holmgren A., Kuehn M. J., Brändén C. I., Hultgren S. J. Conserved immunoglobulin-like features in a family of periplasmic pilus chaperones in bacteria. EMBO J. 1992 Apr;11(4):1617–1622. doi: 10.1002/j.1460-2075.1992.tb05207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultgren S. J., Abraham S., Caparon M., Falk P., St Geme J. W., 3rd, Normark S. Pilus and nonpilus bacterial adhesins: assembly and function in cell recognition. Cell. 1993 Jun 4;73(5):887–901. doi: 10.1016/0092-8674(93)90269-v. [DOI] [PubMed] [Google Scholar]
- Hultgren S. J., Jacob-Dubuisson F., Jones C. H., Bränden C. I. PapD and superfamily of periplasmic immunoglobulin-like pilus chaperones. Adv Protein Chem. 1993;44:99–123. doi: 10.1016/s0065-3233(08)60565-3. [DOI] [PubMed] [Google Scholar]
- Hultgren S. J., Lindberg F., Magnusson G., Kihlberg J., Tennent J. M., Normark S. The PapG adhesin of uropathogenic Escherichia coli contains separate regions for receptor binding and for the incorporation into the pilus. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4357–4361. doi: 10.1073/pnas.86.12.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultgren S. J., Normark S., Abraham S. N. Chaperone-assisted assembly and molecular architecture of adhesive pili. Annu Rev Microbiol. 1991;45:383–415. doi: 10.1146/annurev.mi.45.100191.002123. [DOI] [PubMed] [Google Scholar]
- Iriarte M., Vanooteghem J. C., Delor I., Díaz R., Knutton S., Cornelis G. R. The Myf fibrillae of Yersinia enterocolitica. Mol Microbiol. 1993 Aug;9(3):507–520. doi: 10.1111/j.1365-2958.1993.tb01712.x. [DOI] [PubMed] [Google Scholar]
- Jacob-Dubuisson F., Heuser J., Dodson K., Normark S., Hultgren S. Initiation of assembly and association of the structural elements of a bacterial pilus depend on two specialized tip proteins. EMBO J. 1993 Mar;12(3):837–847. doi: 10.1002/j.1460-2075.1993.tb05724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob-Dubuisson F., Kuehn M., Hultgren S. J. A novel secretion apparatus for the assembly of adhesive bacterial pili. Trends Microbiol. 1993 May;1(2):50–55. doi: 10.1016/0966-842x(93)90032-m. [DOI] [PubMed] [Google Scholar]
- Jalajakumari M. B., Thomas C. J., Halter R., Manning P. A. Genes for biosynthesis and assembly of CS3 pili of CFA/II enterotoxigenic Escherichia coli: novel regulation of pilus production by bypassing an amber codon. Mol Microbiol. 1989 Dec;3(12):1685–1695. doi: 10.1111/j.1365-2958.1989.tb00154.x. [DOI] [PubMed] [Google Scholar]
- Jones C. H., Pinkner J. S., Nicholes A. V., Slonim L. N., Abraham S. N., Hultgren S. J. FimC is a periplasmic PapD-like chaperone that directs assembly of type 1 pili in bacteria. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8397–8401. doi: 10.1073/pnas.90.18.8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. H., Pinkner J. S., Roth R., Heuser J., Nicholes A. V., Abraham S. N., Hultgren S. J. FimH adhesin of type 1 pili is assembled into a fibrillar tip structure in the Enterobacteriaceae. Proc Natl Acad Sci U S A. 1995 Mar 14;92(6):2081–2085. doi: 10.1073/pnas.92.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991 Mar 1;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Karlyshev A. V., Galyov E. E., Smirnov OYu, Guzayev A. P., Abramov V. M., Zav'yalov V. P. A new gene of the f1 operon of Y. pestis involved in the capsule biogenesis. FEBS Lett. 1992 Feb 3;297(1-2):77–80. doi: 10.1016/0014-5793(92)80331-a. [DOI] [PubMed] [Google Scholar]
- Klemm P., Christiansen G., Kreft B., Marre R., Bergmans H. Reciprocal exchange of minor components of type 1 and F1C fimbriae results in hybrid organelles with changed receptor specificities. J Bacteriol. 1994 Apr;176(8):2227–2234. doi: 10.1128/jb.176.8.2227-2234.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P., Jørgensen B. J., Kreft B., Christiansen G. The export systems of type 1 and F1C fimbriae are interchangeable but work in parental pairs. J Bacteriol. 1995 Feb;177(3):621–627. doi: 10.1128/jb.177.3.621-627.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., McConnell M. M., Rowe B., McNeish A. S. Adhesion and ultrastructural properties of human enterotoxigenic Escherichia coli producing colonization factor antigens III and IV. Infect Immun. 1989 Nov;57(11):3364–3371. doi: 10.1128/iai.57.11.3364-3371.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn M. J., Heuser J., Normark S., Hultgren S. J. P pili in uropathogenic E. coli are composite fibres with distinct fibrillar adhesive tips. Nature. 1992 Mar 19;356(6366):252–255. doi: 10.1038/356252a0. [DOI] [PubMed] [Google Scholar]
- Kuehn M. J., Normark S., Hultgren S. J. Immunoglobulin-like PapD chaperone caps and uncaps interactive surfaces of nascently translocated pilus subunits. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10586–10590. doi: 10.1073/pnas.88.23.10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn M. J., Ogg D. J., Kihlberg J., Slonim L. N., Flemmer K., Bergfors T., Hultgren S. J. Structural basis of pilus subunit recognition by the PapD chaperone. Science. 1993 Nov 19;262(5137):1234–1241. doi: 10.1126/science.7901913. [DOI] [PubMed] [Google Scholar]
- Le Bouguenec C., Garcia M. I., Ouin V., Desperrier J. M., Gounon P., Labigne A. Characterization of plasmid-borne afa-3 gene clusters encoding afimbrial adhesins expressed by Escherichia coli strains associated with intestinal or urinary tract infections. Infect Immun. 1993 Dec;61(12):5106–5114. doi: 10.1128/iai.61.12.5106-5114.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Ristaino P., Marley G., Smyth C., Knutton S., Boedeker E., Black R., Young C., Clements M. L., Cheney C. Coli surface antigens 1 and 3 of colonization factor antigen II-positive enterotoxigenic Escherichia coli: morphology, purification, and immune responses in humans. Infect Immun. 1984 May;44(2):409–420. doi: 10.1128/iai.44.2.409-420.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F., Tennent J. M., Hultgren S. J., Lund B., Normark S. PapD, a periplasmic transport protein in P-pilus biogenesis. J Bacteriol. 1989 Nov;171(11):6052–6058. doi: 10.1128/jb.171.11.6052-6058.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindler L. E., Tall B. D. Yersinia pestis pH 6 antigen forms fimbriae and is induced by intracellular association with macrophages. Mol Microbiol. 1993 Apr;8(2):311–324. doi: 10.1111/j.1365-2958.1993.tb01575.x. [DOI] [PubMed] [Google Scholar]
- Lintermans P. F., Pohl P., Bertels A., Charlier G., Vandekerckhove J., Van Damme J., Schoup J., Schlicker C., Korhonen T., De Greve H. Characterization and purification of the F17 adhesin on the surface of bovine enteropathogenic and septicemic Escherichia coli. Am J Vet Res. 1988 Nov;49(11):1794–1799. [PubMed] [Google Scholar]
- Massad G., Mobley H. L. Genetic organization and complete sequence of the Proteus mirabilis pmf fimbrial operon. Gene. 1994 Dec 2;150(1):101–104. doi: 10.1016/0378-1119(94)90866-4. [DOI] [PubMed] [Google Scholar]
- Miller S., Janin J., Lesk A. M., Chothia C. Interior and surface of monomeric proteins. J Mol Biol. 1987 Aug 5;196(3):641–656. doi: 10.1016/0022-2836(87)90038-6. [DOI] [PubMed] [Google Scholar]
- Müller K. H., Collinson S. K., Trust T. J., Kay W. W. Type 1 fimbriae of Salmonella enteritidis. J Bacteriol. 1991 Aug;173(15):4765–4772. doi: 10.1128/jb.173.15.4765-4772.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993 Mar;57(1):50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina S., Missiakas D., Baird L., Kumar S., Georgopoulos C. Identification and transcriptional analysis of the Escherichia coli htrE operon which is homologous to pap and related pilin operons. J Bacteriol. 1993 Aug;175(16):5009–5021. doi: 10.1128/jb.175.16.5009-5021.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. A., Marklund B. I., Ilver D., Haslam D., Kaack M. B., Baskin G., Louis M., Möllby R., Winberg J., Normark S. The Gal(alpha 1-4)Gal-specific tip adhesin of Escherichia coli P-fimbriae is needed for pyelonephritis to occur in the normal urinary tract. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):11889–11893. doi: 10.1073/pnas.91.25.11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarino S. J., Fox P., Deng Y., Nataro J. P. Identification and characterization of a gene cluster mediating enteroaggregative Escherichia coli aggregative adherence fimbria I biogenesis. J Bacteriol. 1994 Aug;176(16):4949–4957. doi: 10.1128/jb.176.16.4949-4957.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmoll T., Morschhäuser J., Ott M., Ludwig B., van Die I., Hacker J. Complete genetic organization and functional aspects of the Escherichia coli S fimbrial adhesion determinant: nucleotide sequence of the genes sfa B, C, D, E, F. Microb Pathog. 1990 Nov;9(5):331–343. doi: 10.1016/0882-4010(90)90067-z. [DOI] [PubMed] [Google Scholar]
- Shapiro L., Fannon A. M., Kwong P. D., Thompson A., Lehmann M. S., Grübel G., Legrand J. F., Als-Nielsen J., Colman D. R., Hendrickson W. A. Structural basis of cell-cell adhesion by cadherins. Nature. 1995 Mar 23;374(6520):327–337. doi: 10.1038/374327a0. [DOI] [PubMed] [Google Scholar]
- Simons B. L., Rathman P., Malij C. R., Oudega B., de Graaf F. K. The penultimate tyrosine residue of the K99 fibrillar subunit is essential for stability of the protein and its interaction with the periplasmic carrier protein. FEMS Microbiol Lett. 1990 Jan 15;55(1-2):107–112. doi: 10.1016/0378-1097(90)90177-r. [DOI] [PubMed] [Google Scholar]
- Slonim L. N., Pinkner J. S., Brändén C. I., Hultgren S. J. Interactive surface in the PapD chaperone cleft is conserved in pilus chaperone superfamily and essential in subunit recognition and assembly. EMBO J. 1992 Dec;11(13):4747–4756. doi: 10.1002/j.1460-2075.1992.tb05580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven A. C., Bisher M. E., Trus B. L., Thomas D., Zhang J. M., Cowell J. L. Helical structure of Bordetella pertussis fimbriae. J Bacteriol. 1986 Sep;167(3):968–974. doi: 10.1128/jb.167.3.968-974.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirm S., Orskov F., Orskov I., Birch-Andersen A. Episome-carried surface antigen K88 of Escherichia coli. 3. Morphology. J Bacteriol. 1967 Feb;93(2):740–748. doi: 10.1128/jb.93.2.740-748.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stull T. L., Mendelman P. M., Haas J. E., Schoenborn M. A., Mack K. D., Smith A. L. Characterization of Haemophilus influenzae type b fimbriae. Infect Immun. 1984 Dec;46(3):787–796. doi: 10.1128/iai.46.3.787-796.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems R. J., van der Heide H. G., Mooi F. R. Characterization of a Bordetella pertussis fimbrial gene cluster which is located directly downstream of the filamentous haemagglutinin gene. Mol Microbiol. 1992 Sep;6(18):2661–2671. doi: 10.1111/j.1365-2958.1992.tb01443.x. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N. The immunoglobulin superfamily--domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- Wolf M. K., Andrews G. P., Tall B. D., McConnell M. M., Levine M. M., Boedeker E. C. Characterization of CS4 and CS6 antigenic components of PCF8775, a putative colonization factor complex from enterotoxigenic Escherichia coli E8775. Infect Immun. 1989 Jan;57(1):164–173. doi: 10.1128/iai.57.1.164-173.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]