Abstract
Objective
To examine whether premature infants receiving the maternally administered H-HOPE intervention had more rapid weight gain and growth, improved feeding progression, and reduced length of hospital stay, compared to controls.
Study Design
Premature infants born at 29–34 GA and their mothers with at least 2 social-environmental risk factors, were randomly assigned to H-HOPE intervention (n = 88) or an attention control (n = 94) groups. H-HOPE consists of a 15-minute multisensory intervention (auditory, tactile, visual and vestibular stimuli) performed twice daily prior to feeding plus maternal participatory guidance on preterm infant behavioral cues.
Results
H-HOPE group infants gained weight more rapidly over time than infants in the control group and grew in length more rapidly than control infants, especially during the latter part of the hospital stay.
Conclusions
For healthy preterm infants, the H-HOPE intervention appears to improve weight gain and length over time from birth to hospital discharge.
Keywords: Developmental intervention, preterm infants, length, weight gain
Introduction
Infants born preterm frequently demonstrate extrauterine growth restriction during hospitalization,1, 2, 3, 4 after hospital discharge,5 and in later life.6 Formerly preterm children have lower height, weight, and head circumference than their full-term peers7 and infants born extremely preterm (< 28 weeks of gestation) are persistently shorter than full-term controls through eighteen years.8 For preterm infants, weight gain after birth and before reaching term is critical for neurodevelopmental function.9, 10 For low birth weight (LBW) and extremely LBW (< 1000 g) infants, more rapid weight gain and growth in head circumference were associated with improved mental and psychomotor development at 18 and 22 months corrected age (CA).9, 10
Numerous interventions intended to optimize growth and development of hospitalized preterm infants provide additional stimulation that include touch and massage.11, 12, 13, 14, 15, 16, 17, 18 The Cochrane Review Collaboration concluded that although massage alone interventions improved daily weight gain by 5.1 g and decreased hospital length of stay by 4.5 days, the increased growth was only weakly related to development.19 In contrast the Auditory, Tactile, Visual and Vestibular (ATVV) intervention is a multi-sensory, well-defined intervention that includes social interaction in addition to massage.20 The ATVV intervention improves weight gain and feeding progression, and reduces length of stay.21, 22, 23, 24, 25, 26, 27, 28
In this study, the ATVV intervention was integrated with a parent participatory guidance intervention (Hospital to Home Transition – Optimizing Premature Infant’s Environment, or H-HOPE) as a means to support mothers’ feeding and social interactive skills as well as infant alertness prior to and during oral feeding, to improve hospital progression.29 We examined whether premature infants receiving the H-HOPE intervention had more rapid weight gain and growth, improved feeding progression, and reduced length of hospital stay, compared to infants assigned to an attention control group.
Methods
Design
This study employed a balanced two-group randomized clinical trial (RCT) design to evaluate infant outcomes following the H-HOPE mother-infant intervention. Mother-infant dyads were enrolled shortly after birth and randomized to the H-Hope or Control group. Random assignment was centrally controlled by using two computer-generated lists of random numbers (0 = intervention and 1 = control), one for each site, balanced so that half the infants at each site were in the experimental group. The intervention began the day after the baseline assessment of oral feeding. Data were abstracted from the infants’ medical records and included weight, length and head circumference from date of birth to discharge, daily proportion of oral to gavage intake, and length of hospital stay.
Setting and Sample
The RCT was conducted at two inner city community hospitals, one with a Level II (with extended capabilities) and one with a Level III Special Care Nursery. The Institutional Review Boards at the two clinical sites and the university approved the clinical trial. Written informed consent was obtained from the mother at enrollment into the study. Infants were eligible if they were born between 29 and 34 weeks gestational age and were clinically stable at enrollment. Gestational age at birth was measured via the Ballard.30 Both clinical sites used this method. Exclusion criteria included congenital anomalies, parenchymal brain injury, necrotizing enterocolitis, chronic lung disease, receiving oxygen via nasal canula, HIV, and prenatal drug exposure. The infants’ mothers were eligible if they had at least two social-environmental risk factors which included: self-identified as African-American or Latina, less than high school education, less than 18 years old, history of current mental illness, depression, family income less than 150% of poverty, more than one child under 24 months, 4 or more children under 4 in the household, or residing in a disadvantaged neighborhood. Mother-infant dyads were excluded if the mother was positive for illicit drug use.
One hundred eight-two research participants were enrolled. A total of 32 dyads were excluded from the study; 26 of these mother-infant dyads were deemed ineligible to participate after enrollment. Seventy-nine infants (n = 39 controls and n = 40 H-HOPE) were enrolled from the Level II NICU and one hundred three infants (n = 55 controls and n = 48 H-HOPE) were enrolled from the Level III NICU. The enrolled sample was predominately African American (n = 89) and Latina (n = 93). There were 87 male infants and 95 female infants. Mean infant gestational age at birth was 32.6 weeks (SD = 1.5). Mean birth weight was 1824.8 grams (SD = 357.1). The mean chronological age of the infants at baseline was 9.2 days (SD = 6.5). The mean 5-minute infant APGAR score was 8.3 (SD = 1.0). Infant health status throughout the infant’s hospital stay was calculated via the Problem-Oriented Perinatal Risk Assessment System (POPRAS).31 The mean infant health status score from the POPRAS was 68.1 (SD = 18.8). One hundred twenty-seven mothers began milk expression. At hospital discharge 48 (27% plus 2 missing milk types) mothers continued with breast milk expression. Of the H-HOPE babies, 55.8% were fed any breast milk and 64.6% of controls were fed breast milk (p = 0.23). The standard of care in both NICUs was to fortify all breast milk feedings when 50% of the feedings contained breast milk. There were no significant group differences. See Table I.
Table I.
Sample characteristics by Intervention Group
| Characteristic | Value> | H-HOPE* (n=88) | Control* (n=94) |
|---|---|---|---|
| Sex | Female | 59.1 | 45.7 |
| Male | 40.9 | 54.3 | |
| Race | Latina | 51.1 | 51.1 |
| African American | 48.9 | 48.9 | |
| Plurality | Singleton | 85.2 | 84.0 |
| Twin/Triplet | 14.8 | 16.0 | |
| Small for Gestational Age | Yes | 29.6 | 29.8 |
| No | 70.5 | 70.2 | |
|
| |||
| Characteristic: | Mean (std) | Mean (std) | |
|
| |||
| Gestational Age @ Birth (wks) | 32.5 (1.5) | 32.6 (1.4) | |
| Birth Weight (g) | 1809.5 (330.3) | 1839.1 (381.6) | |
| Birth Length (cm) | 42.7 (2.6) | 42.9 (3.0) | |
| Birth Head Circumference (cm) | 29.4 (1.6) | 29.3 (2.3) | |
| Apgar Score (5-minute) | 8.3 (1.0) | 8.3 (1.1) | |
| Chronological Age at Baseline (days) | 8.8 (6.2) | 9.5 (6.7) | |
| Post Menstrual Age at Baseline (wks) | 34.0 (1.0) | 33.8 (1.0) | |
| Infant morbidity score (POPRAS) | 66.3 (17.8) | 69.9 (19.7) | |
| Calories/kg per Day | 174.2 (50.3) | 174.1 (57.3) | |
Percent
Experimental (H-HOPE) and Control Groups
The infant-directed component of H-HOPE, the ATVV,21, 24, 25, 32, 33, 34 provided 10 minutes of auditory (female voice), tactile (moderate touch massage) and visual (eye to eye) stimulation, followed by 5 min of vestibular stimulation (horizontal rocking).20 The stimuli were presented in a gradual progression and in the following sequence: auditory only, followed by auditory and tactile, with visual added as the infant becomes alert. The vestibular stimuli is added and the tactile component withdrawn for the remaining 5 minutes. The ATVV began after the day after the baseline feeding assessment, when the infant reached 32 weeks postmenstrual age (PMA). The ATVV was administered twice daily during hospitalization by the mother or the nurse from the Nurse-Advocate Team.21, 25, 33
The mother directed component of H-HOPE consisted of maternal education and social support through individualized participatory guidance.29 This was provided by the Nurse-Advocate Team during 2 in-hospital visits. Of the women assigned to the H-HOPE group, 63% participated in both of the visits in the hospital.
The attention control intervention was designed to provide a similar amount of contact and staff attention, but with distinctly different content from the H-HOPE intervention. Infants received the current standard of feeding and nursery care. Additionally, their mothers received educational content that included premature infant care including bathing, sleep positions and sleep habits, holding the baby, safety of infant equipment, and car safety.
Training and Fidelity
Different personnel conducted the Attention Control condition and the H-HOPE intervention. These two intervention teams were trained separately. Fidelity of both the Attention Control Condition (Parent Education Program or PEP) and the H-HOPE intervention was assessed in several ways. All Attention Control Condition contacts were guided by a PEP Script and documented on the PEP checklist. To ensure fidelity of the ATVV portion of the intervention, the Nurse-Advocate Team and the mothers were taught the ATVV intervention and reliability (> 90% agreement with the ATVV checklist) was established prior to initiation of the study.21, 22, 24, 25, 33 Mothers were taught how to administer the ATVV during the infants’ hospitalization and recorded each time they administered it in the ATVV intervention log. Additionally, the fidelity of the mother’s performance of the ATVV was confirmed at the hospital visits by asking the mother to administer the ATVV to the infant while the Nurse- Advocate Team observed the mother. The Nurse-Advocate Team recorded the fidelity of ATVV on the ATVV checklist. Reliability of both the mothers and nurse administration was randomly checked by the principle investigator using the ATVV checklist and was maintained at > 90% agreement.
Members of the evaluation team were trained separately and collected the data at both clinical sites.
Measures
The length of hospital stay was calculated by subtracting the date of birth from the discharge date. Daily progression from partial (oral plus gavage) to full oral feeding was measured for the subsample of infants who were still partially gavage feeding at the time of the baseline oral feeding observation (n = 115), after which the intervention began. Feeding progression was calculated as the number of days from the baseline feeding assessment to 100% oral feeding. Achievement of 100% oral feeding was defined as the first day of which infants received all of their nutrition by mouth (none gavage), as long as complete oral feeding was maintained for at least two additional consecutive days, or the infant was discharged home. Infant weight was measured daily from birth until hospital discharge, while length and head circumference were obtained every 7 days from birth through hospital discharge.
Statistical Analyses
The distribution of infant characteristics, overall and by experimental group, was analyzed using descriptive and bivariate statistics. Chi-square and t-tests compared baseline characteristics of H-HOPE and control infants. Mean values for length of hospital stay, PMA at discharge, and time from first to full oral feeding were compared between H-HOPE and control infants using t-tests. Mixed effects regression models (SAS) were implemented to model weight gain during hospital stay, and change in length and head circumference during hospital stay, comparing the H-HOPE to the control group. Sample characteristics were examined as potential covariates for each of the models. Covariates included infant sex, plurality (singleton or multiple), small for gestational age (SGA) status, gestational age at birth, birthweight, and length and head circumference at birth, 5-minute Apgar score, chronological and PMA at the baseline feeding observation and the POPRAS score,31 and the daily average calories/kg. Time was measured in days since baseline feeding assessment (after which the intervention began) until hospital discharge. Analyses were performed using IBM SPSS statistics software Version 19 and SAS statistics software Version 9.3 with α set at 0.05.
Results
Infants in the H-HOPE group gained weight more rapidly over time than controls (Figure 1, Panel A). The model that best fit the data was a quadratic growth curve, with a group by time effect and repeated time and an autoregressive covariance structure. The main effect for group assignment in the model was not significant, indicating successful randomization and similar weights at baseline for H-HOPE and controls. However, there was a significant group by time interaction (Table II). By day 20, the H-HOPE infants were 46.3 grams heavier on average than controls. The model also indicated that there were significant interactions for calories per day by time and infant morbidity score by time. Additional covariates that were significant in the model were chronological age at baseline, maternal race, and birthweight (Table II).
Figure 1.
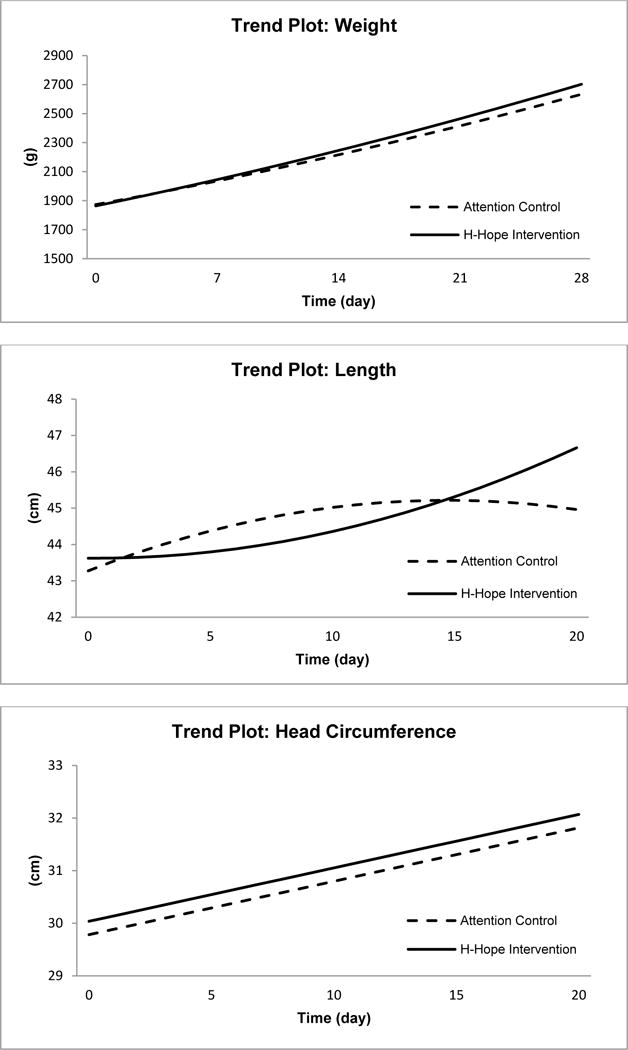
Panels A (Weight), B (Length) and C (Head Circumference)
Table II.
Model Results for Weight, Length and Head Circumference over Time: Parameter Estimates (Standard error) and p-values from mixed effects regression models
| Variable | Weight | p-value | Head Circumference | p-value | Length | p-value |
|---|---|---|---|---|---|---|
| Intercept | 353.46 (69.33) | <0.001 | 18.85 (1.38) | <0.001 | 18.60 (2.16) | <0.001 |
| Group (H-HOPE vs control) | −9.77 (19.36) | 0.615 | 0.25 (0.15) | 0.098 | 0.35 (0.54) | 0.518 |
| Birth Head Circumference, cm | – | – | 0.39 (0.045) | <0.001 | – | – |
| Birth Length, cm | – | – | – | – | 0.54 (0.048) | <0.001 |
| Birthweight, kg | 0.84 (0.028) | <0.001 | – | – | – | – |
| Small for Gestational Age | – | – | −0.40 (0.17) | 0.024 | – | – |
| Chronological age at baseline, day | 21.80 (1.63) | <0.001 | 0.074 (0.014) | <0.001 | 0.090 (0.019) | <0.001 |
| Sex (Male=1) | – | – | – | – | 0.66 (0.23) | 0.005 |
| Race (Latina=1) | −46.65 (17.54) | 0.009 | −0.42 (0.16) | 0.009 | 0.65 (0.23) | 0.005 |
| Infant morbidity score (POPRAS) | −2.63 (0.57) | <0.001 | −0.018 (0.0050) | <0.001 | – | – |
| Daily Calories/Kg | −0.084 (0.023) | <0.001 | 0.0025 (0.00071) | <0.001 | – | – |
| Day | 14.38 (2.82) | <0.001 | 0.10 (0.011) | <0.001 | 0.26 (0.079) | 0.001 |
| Day*Day | 0.19 (0.064) | 0.003 | – | – | −0.0090 (0.0039) | 0.020 |
| Daily Calories/Kg *Day | 0.0054 (0.0019) | 0.004 | – | – | – | – |
| Infant morbidity score (POPRAS)*Day | 0.096 (0.036) | 0.007 | – | – | – | – |
| Group*Day | 2.80 (1.36) | 0.040 | – | – | −0.27 (0.11) | 0.015 |
| Group*Day*Day | – | – | – | – | 0.017 (0.0052) | 0.001 |
For length data, the model that best fit was a quadratic growth curve with repeated time and a heterozygous autoregressive covariance structure. A significant quadratic time by group effect was observed (Table II) indicating that the H-HOPE infants grew in length more rapidly than controls, especially during the latter part of the hospital stay (Table III, Figure 1, Panel B). Significant covariates included chronological age at baseline, race, sex, and birth length.
Table III.
Estimated Mean Difference (Standard Error) between H-HOPE and Control Infants in Weight and Length at Different Time Points during the hospital stay
| n | Weight (grams) | n | Length (cm) | |
|---|---|---|---|---|
| Day 0 (baseline) | 181 | −9.77 (19.36) | 180 | 0.35 (0.54) |
| Day 10 | 103 | 18.26 (17.93) | 100 | −0.66 (0.30)** |
| Day 20 | 33 | 46.30 (25.27)* | 9 | 1.70 (0.51)** |
| Day 28 | 8 | 68.72 (34.04)** | – | – |
p<0.10(marginally significant);
p<0.05
The model that best fit the longitudinal data for head circumference was a linear growth curve with repeated time and a heterozygous compound symmetry covariance structure. While head circumference increased significantly over time for all infants, as indicated by the significant time effect (Table II), no differences were observed by intervention group for head circumference at baseline or change in head circumference over time (Figure 1, panel C). Chronological age at baseline, small for gestational age, birth head circumference, race, POPRAS score, and calories per day were significant covariates in the model (Table II).
Length of hospital stay did not differ for infants in the H-HOPE (mean = 21.3 days, SD = 10.4) and control (mean = 21.4 days, SD = 12.5) groups. Similarly, the mean infant PMA at hospital discharge was 35.6 weeks (SD = 1.1) for H-HOPE and control infants alike. There was no difference in the number of days between the baseline oral feeding measurement and achievement of full oral feeding for H-HOPE (mean = 6.5, SD = 4.2) compared to controls (mean = 6.1, SD = 4.9) (p = 0.67).
Discussion
Infants assigned to the H-HOPE intervention gained weight and grew in length more rapidly than infants in the attention control group. Interventions that support growth during preterm infant hospitalization are critical because extrauterine growth restriction is common in the preterm infant population1, 3, 4 and greater weight gain in preterm infants until term has been associated with improved neurodevelopment.9, 10 Previous research has also shown that weight gain out of proportion to linear growth does not incur additional neurodevelopmental benefits.9
In contrast to interventions that employ massage alone, the Auditory, Tactile, Visual and Vestibular (ATVV) intervention is a multi-sensory, well-defined intervention that includes social interaction in addition to massage.20 In this study, the ATVV intervention was integrated with a parent participatory guidance intervention (Hospital to Home Transition – Optimizing Premature Infant’s Environment, or H-HOPE) to support mothers’ feeding and social interactive skills as well as infant alertness prior to and during oral feeding, improving hospital progression.29
Research employing various modes of sensory stimulation have yielded conflicting results. In previous research, preterm infants receiving the ATVV intervention demonstrated a trend for improved weight gain, but the results did not reach statistical significance.28 However, full term infants living in a Korean orphanage who received the ATVV intervention tended to gain more weight per day and grow faster in length and head circumference when compared with controls.23 Other studies have investigated the effects of various components of sensory stimulation interventions (in isolation) for preterm infants on weight gain. Field and colleagues reported greater weight gain in preterm infants who received one mode of sensory stimuli, moderate pressure massage (tactile stimulation), combined with flexion and extension of the upper and lower extremities (kinesthetic stimulation) for 10 to 15 minutes, 2 to 3 times daily for 5 to 10 days.11, 16, 17, 35, 36, 37, 38 In contrast, a recent study by Moyer-Mileur and colleagues found that a massage alone and range-of-motion intervention administered to preterm infants twice daily for 20 minutes failed to increase weight gain.18 However, the researchers found that male infants who received the intervention had decreased concentrations of circulating adiponection over time leading to lower body fat deposition and consequently, improved growth quality.18 In their study of 75 hospitalized preterm infants, Fucile and Gisel39 found that infants who received oral or tactile/kinesthetic stimulation gained more weight during the study period than control infants. However, infants who received a combination intervention employing both oral and tactile/kinesthetic stimulation did not have significantly increased weight gain compared with oral stimulation alone, tactile/kinesthetic, or controls which the researchers speculate may have been related to the shorter duration of each intervention in the combined protocol (15 minutes of oral and 15 minutes of tactile/kinesthetic stimulation for the combined protocol versus 30 minutes of each modality for the singular intervention groups). In another study, massage combined with kinesthetic stimulation, or range-of-motion exercises, was shown to increase average daily weight gain in preterm infants greater than 1000 g at birth whereas massage alone was not associated with increased weight gain.40 The lack of consistency in these collective findings is likely to be related to the different intervention protocols, types of stimuli, duration of the stimuli, and the number of days the intervention was provided within these studies.12 Further research with standardized intervention is warranted.
In contrast to previous research utilizing the ATVV,21, 24, 25, 26, 27, 28 preterm infants in this study did not demonstrate significant differences in length of hospital stay. We speculate that our lack of significant findings for length of hospital stay may be due to this particular study protocol which delayed the administration of the ATVV intervention until after a baseline oral feeding assessment had been obtained (nine days on average). This change in procedure was employed to evaluate baseline sucking data for another aim in the larger study.41 In previous study protocols that found a significant intervention effect on length of hospital stay, infants began receiving the ATVV weekly beginning at 33 weeks PCA regardless of oral feeding status.21, 26, 27 Therefore, administration of the ATVV during the weeks before initiation of oral feeding may have prepared the infant for oral feeding, resulting in earlier initiation of oral feedings, faster progression to full oral feeds, and a more rapid hospital discharge.
A key component of the H-HOPE intervention is the application of sensory stimuli that are appropriate for the infant’s current development.42 The sensory stimuli provide human social interaction in conjunction with touch at the time when infants are learning social interactive skills and beginning oral feeding. In the past, the ATVV intervention was initiated when the infant reached 33 weeks PMA to support the beginning development of these skills.21, 22 Additionally, This intervention has recently been implemented for infants born less than 29 weeks gestation, however the intervention was not initiated until the infants were stable and weighed at least 1000 grams.43 The H-HOPE intervention also provides guidance to mothers as to how to read and interpret her infant’s behavioral cues.34 Mothers learn to change their behavior during the ATVV to support optimal infant behavior.
What remains unknown is the biologic mechanism that is associated with these various sensory interventions. Comparison of the massage only component of the ATVV (no human social interaction) with the multisensory ATVV revealed that massage only increased cortisol levels and infants demonstrated higher pulse rates, suggestive of increasing stress reactivity.34, 44 For premature infants, massage with kinesthetic stimulation increases vagal activity17, bone strength and biomarkers for bone growth45, and a decrease in plasma cortisol concentration46 Maternally administered massage has also been shown to improve maternal mood while decreasing maternal cortisol.47,48 Since many beneficial effects of maternal proximity, handling, and touch on the development of emotion regulation have been reported in both human and animal models, it has been speculated that neuropeptide systems are linked to the infant’s emotion regulation to influence stress-reactivity and behavior.49,50 In light of these reports, additional research is warranted to better understand the neuroendocrine responses to massage and massage with human social interaction.
Unlike previous research35, 36, we did not find a significant difference in feeding progression in infants who received the ATVV. The sample size for the feeding progression analysis was confined to only those infants who were gavage feeding at the time of the baseline assessment. This resulted in a small sample size with insufficient power for the feeding progression analysis which is a study limitation.
There were a number of limitations to this study. The number of infants decreased over the course of hospitalization and thus the number available for analysis after 20 days was reduced. The change in our protocol does not allow for comparison of these findings with our previous research. Finally, infants in this study were all African American or Latina and predominately low income, limiting generalizability to other groups. Future research with specific subgroups of premature infants, for example those diagnosed with bronchopulmonary dysplasia, and longer term outcomes would advance our understanding of infant response to this intervention. A major strength of the study is the use of a developmentally appropriate intervention with a well-defined protocol that offers mothers a guided approach to interacting with their infants while promoting growth.
Conclusion/Implications
For healthy preterm infants, the H-HOPE intervention appears to improve weight gain and length over time from birth to hospital discharge. This is the first study to evaluate the effects of the H-HOPE intervention on hospitalized preterm infant weight gain and linear growth and adds to the growing body of research demonstrating improved weight gain and growth in hospitalized preterm infants who receive massage. While length of stay and feeding progression are important clinical variables, the procedure employed in this research precluded significant group findings for these indicators.
Acknowledgments
Funded by the National Institute of Child Health and Development and the National Institute of Nursing Research, Grant 1 R01 HD050738-01A2 and the Harris Foundation to the University of Illinois at Chicago, College of Nursing. We would like to sincerely thank all the infants and their mothers who participated in this project. There was no honorarium, grant, or other form of payment given to anyone to produce the manuscript. The author wrote the first draft of this manuscript.
Footnotes
Conflicts of Interest: There is not any competing financial interests in relation to this publication.
No reprints requested.
References
- 1.Funkquist EL, Tuvemo T, Jonsson B, Serenius F, Nyqvist K. Preterm appropriate for gestational age infants: size at birth explains subsequent growth. Acta Paediatr. 2010;99(12):1828–1833. doi: 10.1111/j.1651-2227.2010.01966.x. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia J. Growth curves: how to best measure growth of the preterm infant. J Pediatr. 2013;162(3 Suppl):S2–6. doi: 10.1016/j.jpeds.2012.11.047. [DOI] [PubMed] [Google Scholar]
- 3.Blackwell MT, Eichenwald EC, McAlmon K, Petit K, Linton PT, McCormick MC, et al. Interneonatal intensive care unit variation in growth rates and feeding practices in healthy moderately premature infants. J Perinatol. 2005;25(7):478–485. doi: 10.1038/sj.jp.7211302. [DOI] [PubMed] [Google Scholar]
- 4.Ehrenkranz RA, Younes N, Lemons JA, Fanaroff AA, Donovan EF, Wright LL, et al. Longitudinal growth of hospitalized very low birth weight infants. Pediatrics. 1999;104(2 Pt 1):280–289. doi: 10.1542/peds.104.2.280. [DOI] [PubMed] [Google Scholar]
- 5.Roggero P, Gianni ML, Amato O, Orsi A, Piemontese P, Cosma B, et al. Postnatal growth failure in preterm infants: recovery of growth and body composition after term. Early Hum Dev. 2008;84(8):555–559. doi: 10.1016/j.earlhumdev.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Abou Samra H, Stevens D, Binkley T, Specker B. Determinants of bone mass and size in 7-year-old former term, late-preterm, and preterm boys. Osteoporos Int. 2009;20(11):1903–1910. doi: 10.1007/s00198-009-0896-z. [DOI] [PubMed] [Google Scholar]
- 7.Bocca-Tjeertes IF, van Buuren S, Bos AF, Kerstjens JM, Ten Vergert EM, Reijneveld SA. Growth of preterm and full-term children aged 0–4 years: integrating median growth and variability in growth charts. J Pediatr. 2012;161(3):460–465 e461. doi: 10.1016/j.jpeds.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Roberts G, Cheong J, Opie G, Carse E, Davis N, Duff J, et al. Growth of extremely preterm survivors from birth to 18 years of age compared with term controls. Pediatrics. 2013;131(2):e439–445. doi: 10.1542/peds.2012-1135. [DOI] [PubMed] [Google Scholar]
- 9.Belfort MB, Rifas-Shiman SL, Sullivan T, Collins CT, McPhee AJ, Ryan P, et al. Infant growth before and after term: effects on neurodevelopment in preterm infants. Pediatrics. 2011;128(4):e899–906. doi: 10.1542/peds.2011-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117(4):1253–1261. doi: 10.1542/peds.2005-1368. [DOI] [PubMed] [Google Scholar]
- 11.Field T, Diego M, Hernandez-Reif M. Preterm infant massage therapy research: a review. Infant Behav Dev. 2010;33(2):115–124. doi: 10.1016/j.infbeh.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, He JL, Zhang XH. The Efficacy of Massage on Preterm Infants: A Meta-Analysis. Am J Perinatol. 2013 doi: 10.1055/s-0032-1332801. [DOI] [PubMed] [Google Scholar]
- 13.Field T. Stimulation of preterm infants. Pediatr Rev. 2003;24(1):4–11. doi: 10.1542/pir.24-1-4. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez AP, Vasquez-Mendoza G, Garcia-Vela A, Guzman-Ramirez A, Salazar-Torres M, Romero-Gutierrez G. Weight gain in preterm infants following parent-administered Vimala massage: a randomized controlled trial. Am J Perinatol. 2009;26(4):247–252. doi: 10.1055/s-0028-1103151. [DOI] [PubMed] [Google Scholar]
- 15.Oswalt K, Biasini F. Effects of infant massage on HIV-infected mothers and their infants. J Spec Pediatr Nurs. 2011;16(3):169–178. doi: 10.1111/j.1744-6155.2011.00291.x. [DOI] [PubMed] [Google Scholar]
- 16.Dieter JN, Field T, Hernandez-Reif M, Emory EK, Redzepi M. Stable preterm infants gain more weight and sleep less after five days of massage therapy. J Pediatr Psychol. 2003;28(6):403–411. doi: 10.1093/jpepsy/jsg030. [DOI] [PubMed] [Google Scholar]
- 17.Diego MA, Field T, Hernandez-Reif M. Vagal activity, gastric motility, and weight gain in massaged preterm neonates. J Pediatr. 2005;147(1):50–55. doi: 10.1016/j.jpeds.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 18.Moyer-Mileur LJ, Haley S, Slater H, Beachy J, Smith SL. Massage improves growth quality by decreasing body fat deposition in male preterm infants. J Pediatr. 2013;162(3):490–495. doi: 10.1016/j.jpeds.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vickers A, Ohlsson A, Lacy JB, Horsley A. Massage for promoting growth and development of preterm and/or low birth-weight infants. Cochrane Database Syst Rev. 2004;2:CD000390. doi: 10.1002/14651858.CD000390.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burns K, Cunningham N, White-Traut R, Silvestri J, Nelson MN. Infant stimulation: modification of an intervention based on physiologic and behavioral cues. J Obstet Gynecol Neonatal Nurs. 1994;23(7):581–589. doi: 10.1111/j.1552-6909.1994.tb01924.x. [DOI] [PubMed] [Google Scholar]
- 21.White-Traut RC, Nelson MN, Silvestri JM, Vasan U, Littau S, Meleedy-Rey P, et al. Effect of auditory, tactile, visual, and vestibular intervention on length of stay, alertness, and feeding progression in preterm infants. Dev Med Child Neurol. 2002;44(2):91–97. doi: 10.1017/s0012162201001736. [DOI] [PubMed] [Google Scholar]
- 22.White-Traut RC, Berbaum ML, Lessen B, McFarlin B, Cardenas L. Feeding readiness in preterm infants: the relationship between preterm behavioral state and feeding readiness behaviors and efficiency during transition from gavage to oral feeding. Am J Matern Child Nurs. 2005;30(1):52–59. [PubMed] [Google Scholar]
- 23.Kim TI, Shin YH, White-Traut RC. Multisensory intervention improves physical growth and illness rates in Korean orphaned newborn infants. Res Nurs Health. 2003;26(6):424–433. doi: 10.1002/nur.10105. [DOI] [PubMed] [Google Scholar]
- 24.White-Traut RC, Nelson MN, Silvestri JM, Vasan U, Patel M, Cardenas L. Feeding readiness behaviors and feeding efficiency in response to ATVV intervention. Newborn Infant Nurs Rev. 2002;2(3):166–173. [Google Scholar]
- 25.White-Traut RC, Nelson MN. Maternally administered tactile, auditory, visual, and vestibular stimulation – relationship to later interactions between mothers and premature infants. Res Nurs Health. 1988;11(1):31–39. doi: 10.1002/nur.4770110106. [DOI] [PubMed] [Google Scholar]
- 26.Nelson MN, White-Traut RC, Vasan U, Silvestri JM, Comiskey E, Meleedy-Rey P, et al. One-year outcome of Auditory-Tactile-Visual-Vestibular intervention in the Neonatal Intensive Care Unit: Effects of severe prematurity and central nervous system injury. J Child Neurol. 2001;16:493–498. doi: 10.1177/088307380101600706. [DOI] [PubMed] [Google Scholar]
- 27.White-Traut RC, Nelson MN, Silvestri JM, Patel M, Vasan U, Han BK, et al. Developmental intervention for preterm infants diagnosed with periventricular leukomalacia. Res Nurs Health. 1999;22:131–143. doi: 10.1002/(sici)1098-240x(199904)22:2<131::aid-nur5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 28.White-Traut RC, Tubeszewski KA. Multimodal stimulation of the premature infant. J Pediatr Nurs. 1986;1(2):90–95. [PubMed] [Google Scholar]
- 29.White-Traut RC, Norr KF. An ecological model for premature infant feeding. J Obstet Gynecol Neonatal Nurs. 2009;38(4):478–490. doi: 10.1111/j.1552-6909.2009.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, Lipp R. New Ballard Score, expanded to include extremely premature infants. Journal of Pediatrics. 1991;119:417–423. doi: 10.1016/s0022-3476(05)82056-6. [DOI] [PubMed] [Google Scholar]
- 31.Davidson EC, Hobel CJ. POPRAS: A guide to using the prenatal, intrapartum, postpartum record. South Bay Regional Perinatal Project Professional Staff Association; Torrence, CA: 1978. [Google Scholar]
- 32.White-Traut R, Studer T, Meleedy-Rey P, Murray P, Labovsky S, Kahn J. Pulse rate and behavioral state correlates after auditory, tactile, visual, and vestibular intervention in drug-exposed neonates. J Perinatol. 2002;22(4):291–299. doi: 10.1038/sj.jp.7210695. [DOI] [PubMed] [Google Scholar]
- 33.White-Traut RC, Pate CM. Modulating infant state in premature infants. J Pediatr Nurs. 1987;2(2):96–101. [PubMed] [Google Scholar]
- 34.White-Traut RC, Nelson MN, Silvestri JM, Cunningham N, Patel M. Responses of preterm infants to unimodal and multimodal sensory intervention. Pediatr Nurs. 1997;23(2):169–175. [PubMed] [Google Scholar]
- 35.Field T, Diego MA, Hernandez-Reif M, Deeds O, Figuereido B. Moderate versus light pressure massage therapy leads to greater weight gain in preterm infants. Infant Behav Dev. 2006;29(4):574–578. doi: 10.1016/j.infbeh.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Field T, Schanberg SM, Scafidi F, Bauer CR, Vega-Lahr N, Garcia R, et al. Tactile/kinesthetic stimulation effects on preterm neonates. Pediatrics. 1986;77(5):654–658. [PubMed] [Google Scholar]
- 37.Scafidi FA, Field TM, Schanberg SM, Bauer CR. Massage stimulates growth in preterm infants: A replication. Infant Behav Dev. 1990;13(2):167–188. [Google Scholar]
- 38.Wheeden A, Scafidi FA, Field T, Ironson G, Valdeon C, Bandstra E. Massage effects on cocaine-exposed preterm neonates. J Dev Behav Pediatr. 1993;14(5):318–322. [PubMed] [Google Scholar]
- 39.Fucile S, Gisel EG. Sensorimotor interventions improve growth and motor function in preterm infants. Neonatal Netw. 2010;29(6):359–366. doi: 10.1891/0730-0832.29.6.359. [DOI] [PubMed] [Google Scholar]
- 40.Massaro AN, Hammad TA, Jazzo B, Aly H. Massage with kinesthetic stimulation improves weight gain in preterm infants. J Perinatol. 2009;29(5):352–357. doi: 10.1038/jp.2008.230. [DOI] [PubMed] [Google Scholar]
- 41.Medoff-Cooper B, Rankin K, Li Z, Liu L, White-Traut R. Developmental intervention for preterm infants improves sucking organization. 2013 doi: 10.1097/ANC.0000000000000166. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White-Traut RC, Nelson MN, Burns K, Cunningham N. Environmental influences on the developing premature infant: Theoretical issues and applications to practice. J Obstet Gynecol Neonatal Nurs. 1994;23(5):393–401. doi: 10.1111/j.1552-6909.1994.tb01896.x. [DOI] [PubMed] [Google Scholar]
- 43.Holditch-Davis D, White-Traut R, Levy JA, O’Shea TM, Victoria Geraldo V, David RJ. Maternally administered interventions for preterm infants in the NICU: Effects on maternal psychological distress and mother-infant relationship. Infant Behav Dev. 2014;37(4):695–710. doi: 10.1016/j.infbeh.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White-Traut RC, Schwertz D, McFarlin B, Kogan J. Salivary cortisol and behavioral state responses of healthy newborn infants to tactile-only and multisensory interventions. J Obstet Gynecol Neonatal Nurs. 2009;38(1):22–34. doi: 10.1111/j.1552-6909.2008.00307.x. [DOI] [PubMed] [Google Scholar]
- 45.Haley S, Beachy J, Ivaska K, Slater H, Smith S, Moyer-Mileur L. Tactile/kinesthetic stimulation (TKS) increases tibial speed of sound and urinary osteocalcin (U-MidOC and unOC) in premature infants (29–32 wks PMA) Bone. 2012 doi: 10.1016/j.bone.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Acolet D, Modi N, Giannakoulopoulos X, Bond C, Weg W, Clow A, et al. Changes in plasma cortisol and catecholamine concentrations in response to massage in preterm infants. Arch Dis Child. 1993;68:29–31. doi: 10.1136/adc.68.1_spec_no.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujita M, Endoh Y, Saimon N, Yamaguchi S. Effect of massaging babies on mothers: pilot study on the changes in mood states and salivary cortisol level. Complement Ther Clin Pract. 2006;12(3):181–185. doi: 10.1016/j.ctcp.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 48.Ferber SG, Feldman R, Kohelet D, Kuint J, Dollberg S, Arbel E, et al. Massage therapy facilitates mother–infant interaction in premature infants. Infant Behav Dev. 2005;28(1):74–81. [Google Scholar]
- 49.Weller A, Feldman R. Emotion regulation and touch in infants: the role of cholecystokinin and opioids. Peptides. 2003;24(5):779–788. doi: 10.1016/s0196-9781(03)00118-9. [DOI] [PubMed] [Google Scholar]
- 50.Gordon I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin and the development of parenting in humans. Biol Psychiatry. 2010;68(4):377–382. doi: 10.1016/j.biopsych.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


