Abstract
Posttraumatic stress disorder (PTSD) is a heterogeneous disorder that affects individuals exposed to trauma (e.g., combat, interpersonal violence, and natural disasters). Although its diagnostic features have been recently re-classified with the emergence of the Diagnostic and Statistical Manual for Mental Disorders, Fifth Edition (DSM-5), the disorder remains characterized by hyperarousal, intrusive reminders of the trauma, avoidance of trauma-related cues, and negative cognition and mood. This heterogeneity indicates the presence of multiple neurobiological mechanisms underlying the etiology and maintenance of PTSD. Translational research spanning the past few decades has revealed several potential avenues for the identification of diagnostic biomarkers for PTSD. These include, but are not limited to, monoaminergic transmitter systems, the hypothalamic-pituitary-adrenal (HPA) axis, metabolic hormonal pathways, inflammatory mechanisms, psychophysiological reactivity, and neural circuits. The current review provides an update to the literature with regard to the most promising putative PTSD biomarkers with specific emphasis on the interaction between neurobiological influences on disease risk and symptom progression. Such biomarkers will most likely be identified by multi-dimensional models derived from comprehensive descriptions of molecular, neurobiological, behavioral, and clinical phenotypes.
Keywords: PTSD, biomarkers, neuroendocrinology, psychophysiology, neuroimaging, inflammation
Introduction
Post-traumatic stress disorder (PTSD) is a severe psychiatric disorder that occurs after a psychological traumatic life event and increases individual vulnerability to adverse health outcomes (1). PTSD is heterogeneous, often presenting across different symptom domains, including re-experiencing, avoidance/numbing, and hyper-arousal symptoms (2). While extensive work has successfully identified psychological, genomic, and biological risk factors that are associated with PTSD in trauma survivors (3–5), the identification of discrete diagnostic biomarkers for PTSD remains elusive. The lack of diagnostic biomarkers for PTSD is not due to a lack of intensive study, but rather likely due to the complexity of PTSD and the complex set of rules by which we classify individuals according to the 5th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), as illustrated by the recent description of 636,120 different ways in which an individual can be diagnosed with PTSD (6). Furthermore, PTSD is associated with significant mental health (e.g., major depression, substance and alcohol abuse, panic disorder, suicide) and general medical (e.g., diabetes, cardiovascular disease(7, 8) comorbidities, which can obscure the search for diagnostic biomarkers for PTSD. Given that DSM criteria are not based on the underlying biology, PTSD research could benefit significantly from the new approach to mental health diagnoses using the Research Domain Criteria (RDoC; (9)). One of the tenets of this approach is dimensional analyses of neurobiological metrics and symptoms, rather than diagnostic classification. The putative biomarkers listed in this review are reflective of the extant literature, but can also serve RDoC objectives in future studies by linking PTSD symptoms to relevant biological underpinnings.
The vast heterogeneity inherent in PTSD symptom presentation makes it highly unlikely that a valid, singular biomarker will be identified for PTSD (10, 11). However, comprehensive biological phenotyping of the factors associated with PTSD may yield a parsimonious diagnostic model with which to diagnose PTSD in the future. The current review will highlight several biomarkers associated with PTSD symptomatology and vulnerability, in addition to underscoring how individual factors, such as one’s co-morbid diagnoses and gender, must be considered as they can profoundly influence biology and thus influence our search for true biomarkers of PTSD. Specifically, we will emphasize monoamine, neuroendocrine, inflammatory, genetic, epigenetic, psychophysiologial, neuroanatomical and neuroactivational phenotypes associated with PTSD to illustrate the potential efficacy of using multi-dimensional phenotypic data to characterize unique profiles of PTSD.
Monoamine Systems in PTSD
PTSD is characterized by increased sympathetic nervous system (SNS) tone that is coincident with augmented levels of catecholamine secretion (12). Urinary and central levels of norepinephrine (NE) are heightened in individuals with PTSD (13) and in child trauma victims (14), and peripheral and central levels of NE in response to threatening stimuli are also elevated in PTSD (15, 16). Recent evidence suggests that this increase in NE in PTSD is due to attenuated levels of the NE transporter within the brainstem locus coeruleus (17). PTSD has also been associated with decreased expression of peripheral α2-adrenergic receptors; receptors that underlie an autoreceptor-driven mechanism that serves to inhibit synaptic transmitter release (18). Further, facilitation of NE release via blockade of pre-synaptic α2-adrenergic receptors with the antagonist, yohimbine, can produce panic attacks and an increase in anxiety- and trauma-related symptoms in individuals with PTSD (19, 20). A prospective study of motor vehicle accident survivors indicates that urinary levels of NE were associated with increased development of PTSD one-month following trauma, but only in men (21), indicating that gender may be important for characterizing catecholaminergic biomakers of PTSD. Increased catecholamines, however, are also coincident with panic attacks and other fear-related psychopathology (22), indicating that increased sympathetic activation is not a specific biomarker of PTSD, but rather of a common neurobiological feature of fear- and anxiety-related disorders.
Alterations in the serotonergic system have also been implicated in the pathophysiology of PTSD. Individuals with PTSD show decreased levels of paroxetine binding, suggesting that levels of the serotonin (5-HT) transporter (5-HTT) are attenuated in PTSD (23) and involved in the manifestation of arousal and avoidance symptoms (24). Empirical evidence has shown that 5-HTT expression within the amygdala is attenuated in PTSD, and is significantly associated with higher anxiety and depressive symptoms (25). Brainstem and forebrain levels of the 5-HT1A receptor are higher in individuals with PTSD (26), similar to what has been described in depression (27). Likewise, reductions in central 5-HT1B receptors in trauma-exposed individuals are associated with increased PTSD and depression symptoms (25). Taken together, these data indicate that alterations within serotonergic system could reveal putative biomarkers for depressive symptoms common to both PTSD and major depression (26). The effectiveness of selective serotonin reuptake inhibitors (SSRIs; e.g., sertraline) for reducing the symptoms of PTSD (28–30), major depression, and other psychiatric conditions with which PTSD is highly comorbid (2, 22), further suggest that more careful examination of serotonergic phenotypes is warranted to better disentangle the specificity of biomarkers for PTSD- and depression-specific phenotypes.
One way in which to elucidate the specificity of monoaminergic biomarkers on PTSD symptomology is to concurrently characterize sympathetic and serotonergic function within the same individuals. Using a repeated-measures design, Southwick and colleagues (1997) found that both yohimbine and meta-Chlorophenylpiperazine (m-CPP) treatment increased panic attacks, anxiety, and trauma-related symptoms in veterans diagnosed with PTSD (20) in a manner that suggested at least two different biological sub-types of PTSD; thus, underscoring the need for more robust phenotyping of biological factors including the monoaminergic transmitter systems.
Neuroendocrine Biomarkers of PTSD
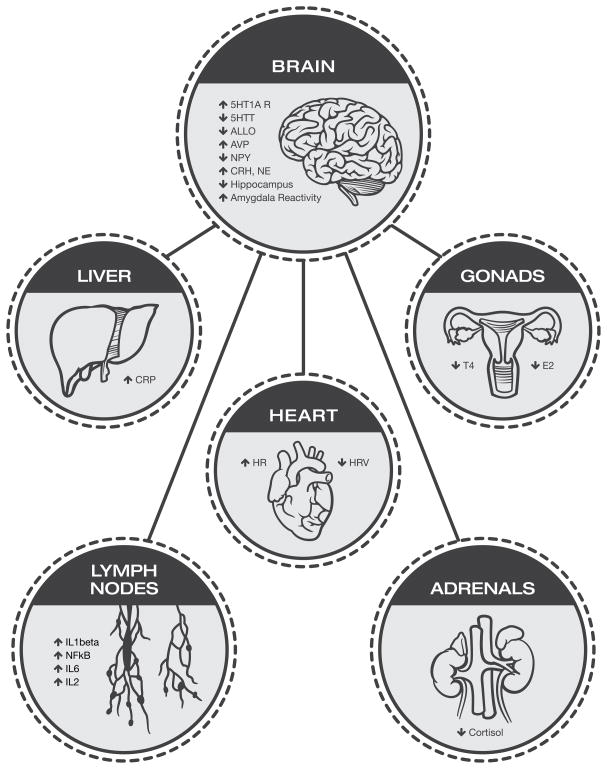
Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis is present in PTSD and has been extensively characterized (Figure 1; for review see (31). Evidence suggests that individuals with PTSD have attenuated levels of basal cortisol (31) and that a low level of cortisol in trauma survivors is associated with increased risk for subsequent development of PTSD (32, 33). However, findings on baseline cortisol levels have been mixed, and a recent meta-analysis concluded that there are no consistent differences between PTSD and controls (34). Similarly, equivocal results exist surrounding the cortisol response to acute cognitive stressors, as reports show heightened or no differences in cortisolresponse to a stressor (35, 36). In part, these discordant HPA results appear to be due to different sampling methods, the diurnal rhythm of cortisol release, and confounding analyses that have disregarded the influence of sex on HPA activity (37).
Figure 1.
A summary of key biomarkers that are associated with PTSD, highlighting the interactions between different biological systems that influence and complicate biological phenotypes within PTSD. Gonadal steroid hormones and the HPA axis modulate neurotransmitter and neuropeptide systems (146), influence amygdala activity (147, 148), and influence inflammatory responses (93). HPA activity, via cortisol and CRH, alters sensitivity to gonadal hormones (149). Inflammation alters HPA activity and has adverse effects on cardiovascular function (150). Taken together, these data indicate that as a field we must begin to study these physiological systems in concert with one another to begin to characterize comprehensive biological phenotypes of PTSD.
Rather than focus on baseline cortisol, a more promising approach is to measure cortisol reactivity to a challenge. Blunted cortisol reactivity to acute stress exposure is associated with increased prospective risk for PTSD (38). Low cortisol levels in PTSD have been coupled to enhanced glucocorticoid negative feedback inhibition of the HPA axis as evidenced by increased suppression of cortisol levels following a dexamethasone suppression test (39). This enhanced HPA negative feedback in PTSD is coincident with: (1) augmented levels of peripheral and central corticotropin-releasing hormone (CRH) (40, 41), (2) elevated glucocorticoid receptor (GR) levels (42), (3) increased glucocorticoid sensitivity (43), and (4) decreased levels of FKBP5 (44), a co-chaperone of GR that inhibits ligand binding and nuclear translocation of GRs. A recent prospective study indicates that augmented baseline GR levels and diminished FKBP5 mRNA levels are associated with increased risk for PTSD symptoms following trauma (45).
While extensive work has alluded to HPA-based biomarkers of PTSD, it is clear that additional neuroendocrine factors influence PTSD vulnerability and symptomology (Table 1; Figure 1). For example, menstrual cycle phase (46, 47) and pregnancy (48) influence PTSD symptom expression profile and psychophysiology in women, suggesting that ovarian steroid hormones are important modulators of PTSD susceptibility and symptom presentation. Indeed, low levels of estradiol are associated with impaired fear extinction in PTSD (49), and high levels of pituitary adenylate cyclase-activating polypeptide (PACAP), a peptide implicated in stress-related behavior and physiology (50–52), are associated with PTSD only in women (53). Furthermore, central levels of the anxiolytic neuroactive steroid allopregnanolone, a potent modulator of GABAergic inhibition, are decreased in women with PTSD (54). Low levels of testosterone in men, on the other hand, have prospectively been associated with increased rates of PTSD (55) and increased risk for PTSD (56). These data, along with epidemiological studies strongly suggesting that female sex is a risk factor for psychopathology (including PTSD; (57) and reinforce the need to better understand the influence of gonadal steroid hormones in men and women with PTSD.
Table 1.
Neuroendocrine biological factors associated with PTSD.
| Neuroendocrine Biomarkers | Relationship to PTSD | References |
|---|---|---|
| HPA-Axis | ||
| Glucocorticoid negative feedback | Augmented in PTSD | (39) |
| Baseline cortisol | Attenuated in PTSD | (31), (32) |
| Acute cortisol following trauma | Lower levels increase risk for PTSD | (33, 38) |
| Pituitary adenylate cyclase-activating polypeptide (PACAP) | Increased in women with PTSD | (53) |
| Steroid Hormones | ||
| Estradiol | Reduced levels increase risk for PTSD and are associated with impaired fear extinction | (49) |
| Allopregnanolone | Decreased in women with PTSD | (54) |
| Dehydroepiandrosterone (DHEA) | Increased in PTSD | (68) |
| Dehydroepiandrosterone sulfate (DHEAS) | Increased in PTSD; High DHEAS increases risk for PTSD | (68) |
| Testosterone | Low levels increase risk for PTSD | (56) |
| Metabolic Hormones | ||
| NPY | Decreased in PTSD | (59) |
| Ghrelin | Increases fear in rodents | (62) |
| Insulin | Increased response to glucose in PTSD | (63) |
| Endocannabinoids | Decreased in PTSD | (64) |
An additional avenue of exploration with regard to PTSD and putative biomarkers is the expression and regulation of metabolic hormones in individuals with PTSD. Neuropeptide Y (NPY) is an orexigenic peptide neurotransmitter (58) that also shows anxiolytic properties via antagonism of CRH and noradrenergic systems (59). Trauma exposure (60) and PTSD (59) are associated with attenuated peripheral levels of NPY and, conversely, resilience to trauma is associated with increased NPY levels (61). Ghrelin, an orexigenic peptide secreted from the stomach (58), displays fear-enhancing effects in rodents (62) and could serve as a biomarker of trauma exposure and PTSD. More recently, individuals with PTSD have shown a hyperinsulinemic response to an oral glucose challenge (63). Finally, peripheral endocannabinoid levels (64) are reduced and central cannabinoid CB1 receptors (65) are increased in PTSD.
In summary, it is clear that significant progress has been made in identifying and characterizing PTSD-related neuroendocrine perturbations. However, the majority of these neuroendocrine factors have been studied in isolation in traumatized populations exhibiting PTSD signs and symptoms and, as such, it is important to characterize multi-level neuroendocrine profiles of PTSD accounting for parallel trauma-related neuroendocrinological changes, their interaction, and the relationship to stress exposure or resilience. For example, increases in dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEAS) have been linked to PTSD symptom expression, but are also associated with decreased levels of affective symptoms and PTSD severity (66, 67). Thus, it has been suggested that the ratio of these adrenal hormones to cortisol might be important for resilience to stress and recovery from PTSD (68, 69). Furthermore, elucidating the complex interaction of neuroendocrine factors (i.e. allopregnanlone/estradiol/NPY effects on cortisol) on the regulation of the HPA axis will likely expand our ability to further describe PTSD-specific and may prove beneficial in characterizing biological sub-profiles of PTSD (Figure 1). For instance, avoidance symptoms in male veterans with PTSD (70) may be related to arginine vasopressin (AVP) levels, and as such, may serve as a biomarker for increased aggression in men with PTSD (71).
Biomarkers of Heightened Inflammation in PTSD
The high comorbidity between PTSD, physical illness (7), and inflammation (spanning cardiovascular (72) and metabolic disease; (73) has led to investigations of the relationship between inflammatory markers and PTSD symptomology (Table 2). Pro-inflammatory cytokines (i.e. proteins), including interleukin (IL)-6 (74), IL-1β (75), and IL-2 (76) are elevated in individuals with PTSD and peripheral levels of inflammatory markers correlate positively with PTSD symptomology (Figure 1) (77). C-reactive protein (CRP) levels are also elevated in individuals with PTSD (78–80). More specifically, increased CRP levels have been reported with exacerbated PTSD symptoms and impaired inhibition of fear-potentiated startle (FPS) in the presence of a safety signal (79); a psychophysiological biomarker for PTSD described in a later section of this review (81).
Table 2.
Immunological factors associated with PTSD.
| Immune Biomarkers | Relationship to PTSD | References |
|---|---|---|
| Interleukin-6 (IL-6) | Increased in PTSD | (74) |
| Interleukin-1β (IL-1β) | Increased in PTSD | (75) |
| Interleukin-2 (IL-2) | Increased in PTSD | (76) |
| C-reactive protein (CRP) | Increased in PTSD; Increases risk for PTSD | (78, 79, 94) |
| Nuclear factor-κB (NF-κB) | Increased in PTSD | (86, 87) |
| Tumor necrosis factor (TNF)-α | Increased in PTSD | (145) |
| Immune cell sensitivity to glucocorticoids | Enhanced in PTSD | (43) |
In addition, individuals with PTSD also show altered immune cell sensitivity to glucocorticoids that results in increased inflammation (82). Lysozyme enzyme activity is more sensitive to dexamethasone in PTSD (43), indicating that innate immune efficiency is higher in individuals with PTSD. Enhanced monocyte sensitivity to glucocorticoids in individuals with PTSD is also coincident with hypocortisolemia and can lead to increased cytokine production (83). The transcriptional factor, nuclear factor-κB (NF-κB), lays upstream of cytokine activation (84) and is activated by exposure to psychosocial stress (85) as well as noradrenergic activity (85), and thus may be critically sensitive to immune changes following trauma exposure. Individuals with PTSD show augmented NF-κB gene expression (86) and NF-κB activity (87).
Overall, the cross-sectional data linking PTSD to a pro-inflammatory state further support the notion that PTSD is associated with chronic inflammation (Table 2), and suggest that inflammation may serve as a possible therapeutic target for alleviating PTSD symptoms. However, increased inflammation is a hallmark of depression (88) and other adverse health outcomes that are comorbid with PTSD (7, 72, 73, 89), thus complicating the view that immune factors may serve as diagnostic biomarkers for PTSD specifically. This point is further highlighted by other reports that have described no differences, or decreases in pro-inflammatory markers, such as CRP, in individuals with PTSD (90–92). Factors such as gender should also be considered in our examination of immunological biomarkers, as there are clear sex differences in immune system function and risk for infection (93). Finally, it is still unclear whether increased inflammation is a consequence of trauma exposure and PTSD, or whether a baseline pro-inflammatory state increases individual vulnerability to PTSD after trauma exposure. As such, baseline inflammation may serve as biomarker of PTSD vulnerability, as recent evidence from a prospective study indicates that pre-deployment levels of CRP significantly predict post-deployment PTSD (94).
Genetic and epigenetic biomarkers of PTSD
Genetic loci within genes critical for the neuroendocrine regulation of the HPA axis and emotional behavior have been associated with increased risk for PTSD (see review (5). However, these genetic loci have been associated with other psychiatric conditions as well, indicating that these genetic polymorphisms are not specific to PTSD, but rather may serve as biomarkers for stress-induced psychopathology in general or common underlying symptoms. There are several recent genomic reviews of PTSD (e.g., (95)) and the disorders with which it is co-morbid and, as such, will not be discussed at length in the current review. We will simply note that the emerging genetic and epigenetic findings related to PTSD risk versus resilience have focused on modulators of HPA axis function (prior to and following trauma - e.g., FKBP5; PACAP.
Psychophysiological biomarkers of PTSD
Hyperarousal symptoms, which include some of the longstanding, hallmark symptoms of PTSD, can be strongly influenced by an individual’s autonomic response following trauma; the output of the autonomic nervous system can be indexed non-invasively via psychophysiological assessments of peripheral targets, such as heart rate (HR), blood pressure (BP), skin conductance (SC), respiration rate (RR), muscle contractions using electromyography (EMG; e.g., startle), and body temperature. However, the use of these psychophysiological measures as biomarkers of PTSD may rely heavily on the timing and context in which they are collected. For instance, while some reports indicate that HR in the immediate aftermath of trauma exposure is predictive of later PTSD development (96), others suggest this is not the case (97, 98). These equivocal findings suggest that a more robust and controlled measurement of psychophysiological data may be necessary (99). Indeed, HR and SC changes in response to a challenge have been repeatedly associated with a diagnosis of PTSD (100–104).
Exaggerated startle response, a hyperarousal symptom that remains central to DSM-based PTSD diagnosis, is readily assessed by psychophysiology. Increased HR (SC and EMG less so) reactivity to startling loud tones has been found to reliably differentiate PTSD from Non-PTSD (105). Heightened HR reactivity to loud tones does not appear to be pre-existing, but rather is acquired with the development of PTSD (106, 107). Whereas heightened HR reactivity to loud tones appears to be an acquired marker, there is accumulating evidence that heightened SC reactivity to loud tones is a pre-trauma risk marker for posttraumatic stress (108, 109). Exaggerated arousal can manifest as sleep disturbances, which are frequently observed in PTSD (110, 111); however the diagnostic specificity of these disturbances are not yet understood.
In order to examine further explore hyper-reactivity following trauma, Pitman and colleagues (112) modified an imagery procedure originally developed to study phobias (113). In this method, psychophysiological data are recorded from participants while listening to a script of their actual traumatic event. This method has been used with divergent PTSD populations, including several combat populations (114–116) and a heavily traumatized civilian population (117). In all trauma survivors, PTSD patients exhibit a stronger HR and SC response to scripts than non-PTSD trauma survivors. In studies using script-driven imagery, SC was found to be the most sensitive measure of hyperarousal in PTSD. In 1998, Keane and colleagues (101) published the results of the largest study (multi-site VA Cooperative Study with Vietnam veterans) to date examining the utility of psychophysiological measures in diagnosing PTSD. The study employed script-driven imagery coupled to psychophysiological recordings. While this study did not find a perfect correlation between interview-based PTSD diagnosis and psychophysiological reactivity, they concluded that psychophysiological data did provide useful and objective assessment of the disorder. Recent re-analyses of script-driven imagery data collected in the 1990s have shown high specificity for PTSD (i.e., 90% of individuals without PTSD classified correctly) (102), and high concordance with subjective distress (118), but sensitivity to PTSD diagnosis remained at approximately 60% (102). Simply talking about autobiographical trauma appears to have similar effects as script-driven imagery in increasing physiological arousal (118). These methods are currently being standardized as common data elements, in order to promote generation of large datasets using the same approach. Technological advances have afforded the opportunity to employ physiological indices that can be easily obtained in most clinical settings and may prove beneficial in the diagnosis and treatment evaluation of PTSD. A recent application of these methods using virtual reality techniques to provide immersive trauma-related imagery during recording of psychophysiological responses showed utility of this approach in tracking treatment outcomes (119).
The findings described previously support the notion that the etiology and maintenance of the fear-related symptoms of PTSD can be characterized according to the principles of fear conditioning (120, 121). Given the richness of the translational literature, the neural underpinnings of fear conditioning are well understood, and PTSD research can capitalize on these findings (122). Fear conditioning is based on a simple Pavlovian conditioning model in which a neutral conditioned stimulus (CS, for example, a light) is paired with an aversive unconditioned stimulus (US, for example, electric shock). After a number of pairings, an association is formed such that the CS alone elicits a conditioned response (CR, for example, a fear response). Following initial acquisition, conditioned fear is subject to consolidation, extinction, and reconsolidation, all of which may be disturbed in PTSD (123–125). Fear conditioned responses can be measured with peripheral outcomes such as SC (123) or EMG startle responses (81). These psychophysiological measures can be used to index both the increase in fear during conditioning, as well as the reduction of fear during extinction, or the repeated presentation of the CS without the US. In addition, these measures can be used in differential conditioning studies using a CS+ cue predicting danger (US), and a CS− predicting safety from the US; these have shown that PTSD, but not depression, is associated with a reduced ability to inhibit fear-potentiated startle responses to safety signals (81). Similarly, retention of the extinction memory has been tested using SC 24 hours after fear extinction, and PTSD subjects have exhibited reduced levels of extinction recall (125). Taken together, these studies indicate the fear responses to traumatic memories may be serve as biomarkers specific for dysregulated fear in PTSD.
Neuroanatomical and neuroactivational biomarkers of PTSD
Neuroimaging data gathered during the last decade demonstrate that PTSD is associated with greater amygdala activation compared to controls (126). Functional magnetic resonance imaging (fMRI) studies have shown that trauma-relevant words increase amygdala activation in PTSD subjects more than in controls (127–130). Exaggerated fear responses observed in PTSD may be due to a weakened inhibitory control of the amygdala by the medial prefrontal cortex (mPFC). A large number of imaging studies have indicated that this inhibitory neurocircuit is dysregulated in patients with PTSD (126, 128, 130). A recent meta-analysis of imaging studies during emotion processing in PTSD, social anxiety, and specific phobia indicated that the rostral anterior cingulate cortex (ACC) is less active in PTSD patients relative to controls; an effect not found in other anxiety disorders (131).
Neuroimaging studies using fear conditioning paradigms demonstrate that fear acquisition and extinction of fear activate the prefrontal cortex (PFC), specifically the ventromedial (vmPFC) (132). For example, activation of the vmPFC (which includes the rostral ACC; rACC) is decreased in PTSD patients during an extinction recall in fMRI task (133). The vmPFC also differs in shape and size in PTSD patients (134). To date, one of the most replicated neuroanatomical findings in PTSD has been reduced hippocampal volume (135, 136). Early studies of twins discordant for trauma exposure suggested that smaller hippocampal volume likely confers individual vulnerability to PTSD(137), however, a recent prospective study found that hippocampal reductions were acquired with trauma exposure (136). Finally, methods using higher resolution imaging techniques have indicated that reductions in specific subregions of the hippocampus, such as the cornu ammonis 3 (CA3) and dentate gyrus, are associated with PTSD symptoms (138). Studies of neural activation have used several fMRI paradigms to activate the mPFC; the simplest and most commonly used tasks involve response inhibition. In such tasks, the participant is presented with a stimulus indicating that a response is required, for example, to press a button when a letter appears on the monitor. This is referred to as a “Go” signal. On a minority of trials, however, the participant is required to withhold a response during a “NoGo” signal (the Go/NoGo task). The Go/NoGo task has been used in subjects with PTSD with functional magnetic resonance imaging (fMRI) and it reliably indicates decreased activation in the rostral vmPFC and rACC in PTSD subjects compared to controls (139, 140). Weakened mPFC control of the amygdala may be a risk factor for trauma-related psychopathology: a recent study of children with depressed parents found a lack of ACC activation to the emotional Stroop, using both fear-relevant words depicting physical threat as well as social threat (141).
Summary and Conclusions
To date, an array of putative biomarkers associated with PTSD risk and symptom progression have been identified across distinct biological domains, including, but not limited to, alterations and differences in monoaminergic systems, neuroendocrinology, inflammation, genomics, psychophysiology, and neuroanatomy. However, the heterogeneity inherent in PTSD symptom presentation, and the common comorbidity with other psychiatric and general medical conditions represent formidable obstacles in the identification of valid biomarkers specifically for PTSD when considered as a diagnostic categorization (10, 11). Indeed, the likelihood of characterizing one biological marker associated with the suggested 636,120 different ways in which an individual can present with PTSD (6) is vanishingly small. Rather, it is more prudent that future studies develop a cross-dimensional, comprehensive biological and psychological phenotypic profile in individuals with PTSD to: (1) characterize biomarkers for specific clusters of symptoms and/or (2) uncover divergent biological profiles of PTSD using more complex statistical techniques (142). In order to be compatible with the RDoC approach, biomarkers should be dimensional as well as transdiagnostic—in effect, not biomarkers specific to PTSD as a DSM disorder, but biomarkers of features associated with PTSD. For example, physiological measures of fear responses would be relevant to other fear-related disorders such as phobias in addition to PTSD. Similarly, deficient prefrontal activity could be associated with PTSD symptoms, as well as addiction, and could clarify common bases for comorbid disorders.
In order to begin collecting comprehensive phenotypes necessary for such analyses, the importance of studying the interaction between biological factors (e.g., cellular, molecular, genetic, neurotransmitter, endocrine; Figure 1) needs to be emphasized; most notably as they relate to physiology and behaviors underlying complex biological phenotypes within PTSD. It is important to note that biology is dynamic. Thus, it is critical for the field to understand that biomarkers might be relevant at one time point (HR immediately following trauma exposure) and not at another (143). Lastly, the implications of characterizing diagnostic biomarkers for PTSD must be carefully considered to ensure that the benefits outweigh the costs (144).
In summary, the available biological and translational data point to promising new horizons for diagnostic biomarkers of PTSD symptoms. It is most likely that such biomarkers will represent a panel of several measures that will combine molecular with behavioral and clinical information to increase specificity and sensitivity of these tools.
Acknowledgments
This work was funded in part by the Brain and Behavior Foundation (formerly NARSAD; S.D.N. and T.J.), the Department of Defense (DOD)/Congressionally Directed Medical Research Program (CDMRP, Award # W81XWH-08-2-0170) (S.D.N.), the Emory University Research Committee, a PHS Grant (UL1 RR025008) from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources (S.D.N.), National Institutes of Mental Health (MH098212 and MH100122, T.J.).
Footnotes
Financial Disclosures
Dr. Michopoulos, Dr. Norrholm, and Dr. Jovanovic reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dedert EA, Calhoun PS, Watkins LL, Sherwood A, Beckham JC. Posttraumatic stress disorder, cardiovascular, and metabolic disease: a review of the evidence. Ann Behav Med. 2010;39(1):61–78. doi: 10.1007/s12160-010-9165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the national comorbidity survey. Archives of General Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 3.Breslau N. The epidemiology of posttraumatic stress disorder: what is the extent of the problem? J Clin Psychiatry. 2001;62(17):16–22. [PubMed] [Google Scholar]
- 4.Roberts AL, Galea S, Austin SB, Cerda M, Wright RJ, Rich-Edwards JW, Koenen KC. Posttraumatic Stress Disorder Across Two Generations: Concordance and Mechanisms in a Population-Based Sample. Biological Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, Liberzon I. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13(11):769–87. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galatzer-Levy IR, Bryant RA. 636,120 Ways to Have Posttraumatic Stress Disorder. Perspectives on Psychological Science. 2013;8(6):651–662. doi: 10.1177/1745691613504115. [DOI] [PubMed] [Google Scholar]
- 7.Boscarino JA. Posttraumatic stress disorder and physical illness: results from clinical and epidemiologic studies. Ann N Y Acad Sci. 2004;1032:141–53. doi: 10.1196/annals.1314.011. [DOI] [PubMed] [Google Scholar]
- 8.Jacobsen LK, Southwick SM, Kosten TR. Substance use disorders in patients with posttraumatic stress disorder: a review of the literature. Am J Psychiatry. 2001;158(8):1184–90. doi: 10.1176/appi.ajp.158.8.1184. [DOI] [PubMed] [Google Scholar]
- 9.Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Medicine. 2013;11:126–126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zoladz PR, Diamond DM. Current status on behavioral and biological markers of PTSD: a search for clarity in a conflicting literature. Neurosci Biobehav Rev. 2013;37(5):860–95. doi: 10.1016/j.neubiorev.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt U, Kaltwasser SF, Wotjak CT. Biomarkers in posttraumatic stress disorder: overview and implications for future research. Dis Markers. 2013;35(1):43–54. doi: 10.1155/2013/835876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Southwick SM, Bremner JD, Rasmusson A, Morgan CA, 3rd, Arnsten A, Charney DS. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biological Psychiatry. 1999;46(9):1192–204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- 13.Geracioti TD, Jr, Baker DG, Ekhator NN, West SA, Hill KK, Bruce AB, Schmidt D, Rounds-Kugler B, Yehuda R, Keck PE, Jr, Kasckow JW. CSF norepinephrine concentrations in posttraumatic stress disorder. American Journal of Psychiatry. 2001;158(8):1227–30. doi: 10.1176/appi.ajp.158.8.1227. [DOI] [PubMed] [Google Scholar]
- 14.Delahanty DL, Nugent NR, Christopher NC, Walsh M. Initial urinary epinephrine and cortisol levels predict acute PTSD symptoms in child trauma victims. Psychoneuroendocrinology. 2005;30(2):121–8. doi: 10.1016/j.psyneuen.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Blanchard EB, Kolb LC, Prins A, Gates S, McCoy GC. Changes in plasma norepinephrine to combat-related stimuli among Vietnam veterans with posttraumatic stress disorder. J Nerv Ment Dis. 1991;179(6):371–3. doi: 10.1097/00005053-199106000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Geracioti TD, Jr, Baker DG, Kasckow JW, Strawn JR, Jeffrey Mulchahey J, Dashevsky BA, Horn PS, Ekhator NN. Effects of trauma-related audiovisual stimulation on cerebrospinal fluid norepinephrine and corticotropin-releasing hormone concentrations in post-traumatic stress disorder. Psychoneuroendocrinology. 2008;33(4):416–24. doi: 10.1016/j.psyneuen.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Pietrzak RH, Gallezot JD, Ding YS, Henry S, Potenza MN, Southwick SM, Krystal JH, Carson RE, Neumeister A. Association of posttraumatic stress disorder with reduced in vivo norepinephrine transporter availability in the locus coeruleus. JAMA Psychiatry. 2013;70(11):1199–205. doi: 10.1001/jamapsychiatry.2013.399. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Perry BD, Giller EL, Jr, Southwick SM. Altered platelet alpha 2-adrenergic binding sites in posttraumatic stress disorder. Am J Psychiatry. 1987;144(11):1511–2. doi: 10.1176/ajp.144.11.1511a. [DOI] [PubMed] [Google Scholar]
- 19.Southwick SM, Krystal JH, Morgan CA, Johnson D, Nagy LM, Nicolaou A, Heninger GR, Charney DS. Abnormal noradrenergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 1993;50(4):266–74. doi: 10.1001/archpsyc.1993.01820160036003. [DOI] [PubMed] [Google Scholar]
- 20.Southwick SM, Krystal JH, Bremner JD, Morgan CA, 3rd, Nicolaou AL, Nagy LM, Johnson DR, Heninger GR, Charney DS. Noradrenergic and serotonergic function in posttraumatic stress disorder. Archives of General Psychiatry. 1997;54(8):749–58. doi: 10.1001/archpsyc.1997.01830200083012. [DOI] [PubMed] [Google Scholar]
- 21.Hawk LW, Dougall AL, Ursano RJ, Baum A. Urinary catecholamines and cortisol in recent-onset posttraumatic stress disorder after motor vehicle accidents. Psychosomatic Medicine. 2000;62(3):423–34. doi: 10.1097/00006842-200005000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Martin EI, Ressler KJ, Binder E, Nemeroff CB. The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. Psychiatr Clin North Am. 2009;32(3):549–75. doi: 10.1016/j.psc.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arora RC, Fichtner CG, O’Connor F, Crayton JW. Paroxetine binding in the blood platelets of post-traumatic stress disorder patients. Life Sci. 1993;53(11):919–28. doi: 10.1016/0024-3205(93)90444-8. [DOI] [PubMed] [Google Scholar]
- 24.Maes M, Lin AH, Verkerk R, Delmeire L, Van Gastel A, Van der Planken M, Scharpe S. Serotonergic and noradrenergic markers of post-traumatic stress disorder with and without major depression. Neuropsychopharmacology. 1999;20(2):188–97. doi: 10.1016/S0893-133X(98)00058-X. [DOI] [PubMed] [Google Scholar]
- 25.Murrough JW, Huang Y, Hu J, Henry S, Williams W, Gallezot JD, Bailey CR, Krystal JH, Carson RE, Neumeister A. Reduced amygdala serotonin transporter binding in posttraumatic stress disorder. Biol Psychiatry. 2011;70(11):1033–8. doi: 10.1016/j.biopsych.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullivan GM, Ogden RT, Huang YY, Oquendo MA, Mann JJ, Parsey RV. Higher in vivo serotonin-1a binding in posttraumatic stress disorder: a PET study with [11C]WAY-100635. Depress Anxiety. 2013;30(3):197–206. doi: 10.1002/da.22019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parsey RV, Oquendo MA, Ogden RT, Olvet DM, Simpson N, Huang YY, Van Heertum RL, Arango V, Mann JJ. Altered serotonin 1A binding in major depression: a [carbonyl-C-11]WAY100635 positron emission tomography study. Biol Psychiatry. 2006;59(2):106–13. doi: 10.1016/j.biopsych.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 28.McDougle CJ, Southwick SM, Charney DS, St James RL. An open trial of fluoxetine in the treatment of posttraumatic stress disorder. J Clin Psychopharmacol. 1991;11(5):325–7. [PubMed] [Google Scholar]
- 29.Nagy LM, Morgan CA, Southwick S, Charney D. Open prospective trial of fluoxetine for posttraumatic stress disorder. Journal of Clinical Psychopharmacology. 1993;13:107–113. [PubMed] [Google Scholar]
- 30.Van der Kolk BA, Dreyfuss D, Michaels M, Shera D, Berkowitz R, Fisler R, Saxe G. Fluoxetine in posttraumatic stress disorder. Journal of Clinical Psychiatry. 1994;55:517–522. [PubMed] [Google Scholar]
- 31.Yehuda R. Neuroendocrine aspects of PTSD. Handb Exp Pharmacol. 2005;(169):371–403. doi: 10.1007/3-540-28082-0_13. [DOI] [PubMed] [Google Scholar]
- 32.Walsh K, Nugent NR, Kotte A, Amstadter AB, Wang S, Guille C, Acierno R, Kilpatrick DG, Resnick HS. Cortisol at the emergency room rape visit as a predictor of PTSD and depression symptoms over time. Psychoneuroendocrinology. 2013;38(11):2520–2528. doi: 10.1016/j.psyneuen.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mouthaan J, Sijbrandij M, Luitse JS, Goslings JC, Gersons BP, Olff M. The role of acute cortisol and DHEAS in predicting acute and chronic PTSD symptoms. Psychoneuroendocrinology. 2014;45:179–86. doi: 10.1016/j.psyneuen.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Meewisse M, Reitsma JB, De Vries GJ, Gersons BPR, Olff M. Cortisol and post-traumatic stress disorder in adults: Systematic review and meta-analysis. The British Journal of Psychiatry. 2007;191(5):387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- 35.Bremner JD, Vythilingam M, Vermetten E, Adil J, Khan S, Nazeer A, Afzal N, McGlashan T, Anderson G, Heninger GR, Southwick SM, Charney DS. Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology. 2003;28:733–750. doi: 10.1016/s0306-4530(02)00067-7. [DOI] [PubMed] [Google Scholar]
- 36.Kolassa IT, Eckart C, Ruf M, Neuner F, de Quervain DJF, Elbert T. Lack of cortisol response in patients with posttraumatic stress disorder (PTSD) undergoing a diagnostic interview. BMC Psychiatry. 2007;7:54–54. doi: 10.1186/1471-244X-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freidenberg BM, Gusmano R, Hickling EJ, Blanchard EB, Bremner JD, Frye C. Women with PTSD have lower basal salivary cortisol levels later in the day than do men with PTSD: A preliminary study. Physiology & Behavior. 2010;99(2):234–236. doi: 10.1016/j.physbeh.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galatzer-Levy IR, Steenkamp MM, Brown AD, Qian M, Inslicht S, Henn-Haase C, Otte C, Yehuda R, Neylan TC, Marmar CR. Cortisol response to an experimental stress paradigm prospectively predicts long-term distress and resilience trajectories in response to active police service. J Psychiatr Res. 2014;56:36–42. doi: 10.1016/j.jpsychires.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yehuda R, Boisoneau D, Lowy MT, Giller EL., Jr Dose-response changes in plasma cortisol and lymphocyte glucocorticoid receptors following dexamethasone administration in combat veterans with and without posttraumatic stress disorder. Arch Gen Psychiatry. 1995;52(7):583–593. doi: 10.1001/archpsyc.1995.03950190065010. [DOI] [PubMed] [Google Scholar]
- 40.de Kloet CS, Vermetten E, Geuze E, Lentjes EG, Heijnen CJ, Stalla GK, Westenberg HG. Elevated plasma corticotrophin-releasing hormone levels in veterans with posttraumatic stress disorder. Prog Brain Res. 2008;167:287–91. doi: 10.1016/S0079-6123(07)67025-3. [DOI] [PubMed] [Google Scholar]
- 41.Baker DG, Ekhator NN, Kasckow JW, Dashevsky B, Horn PS, Bednarik L, Geracioti TD., Jr Higher levels of basal serial CSF cortisol in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 2005;162(5):992–4. doi: 10.1176/appi.ajp.162.5.992. [DOI] [PubMed] [Google Scholar]
- 42.Matic G, Milutinovic DV, Nestorov J, Elakovic I, Jovanovic SM, Perisic T, Dunderski J, Damjanovic S, Knezevic G, Spiric Z, Vermetten E, Savic D. Lymphocyte glucocorticoid receptor expression level and hormone-binding properties differ between war trauma-exposed men with and without PTSD. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:238–45. doi: 10.1016/j.pnpbp.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 43.Yehuda R, Golier JA, Yang RK, Tischler L. Enhanced sensitivity to glucocorticoids in peripheral mononuclear leukocytes in posttraumatic stress disorder. Biol Psychiatry. 2004;55(11):1110–6. doi: 10.1016/j.biopsych.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 44.Yehuda R, Cai G, Golier JA, Sarapas C, Galea S, Ising M, Rein T, Schmeidler J, Muller-Myhsok B, Holsboer F, Buxbaum JD. Gene expression patterns associated with posttraumatic stress disorder following exposure to the World Trade Center attacks. Biol Psychiatry. 2009;66(7):708–11. doi: 10.1016/j.biopsych.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 45.van Zuiden M, Geuze E, Willemen HL, Vermetten E, Maas M, Amarouchi K, Kavelaars A, Heijnen CJ. Glucocorticoid receptor pathway components predict posttraumatic stress disorder symptom development: a prospective study. Biol Psychiatry. 2012;71(4):309–16. doi: 10.1016/j.biopsych.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 46.Bryant RA, Felmingham KL, Silove D, Creamer M, O’Donnell M, McFarlane AC. The association between menstrual cycle and traumatic memories. Journal of Affective Disorders. 2011;131(1):398–401. doi: 10.1016/j.jad.2010.10.049. [DOI] [PubMed] [Google Scholar]
- 47.Glover EM, Mercer KB, Norrholm SD, Davis M, Duncan E, Bradley B, Ressler KJ, Jovanovic T. Inhibition of fear is differentially associated with cycling estrogen levels in women. doi: 10.1503/jpn.120129. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Michopoulos V, Rothbaum AO, Corwin E, Bradley B, Ressler KJ, Jovanovic T. Psychophysiology and posttraumatic stress disorder symptom profile in pregnant African-American women with trauma exposure. Arch Womens Ment Health. 2014 doi: 10.1007/s00737-014-0467-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glover EM, Jovanovic T, Mercer KB, Kerley K, Bradley B, Ressler KJ, Norrholm SD. Estrogen Levels Are Associated with Extinction Deficits in Women with Posttraumatic Stress Disorder. Biological Psychiatry. 2012;72(1):19–24. doi: 10.1016/j.biopsych.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hammack SE, Cheung J, Rhodes KM, Schutz KC, Falls WA, Braas KM, May V. Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology. 2009;34(6):833–43. doi: 10.1016/j.psyneuen.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hashimoto H, Shintani N, Baba A. New insights into the central PACAPergic system from the phenotypes in PACAP- and PACAP receptor-knockout mice. Ann N Y Acad Sci. 2006;1070:75–89. doi: 10.1196/annals.1317.038. [DOI] [PubMed] [Google Scholar]
- 52.Ghzili H, Grumolato L, Thouennon E, Tanguy Y, Turquier V, Vaudry H, Anouar Y. Role of PACAP in the physiology and pathology of the sympathoadrenal system. Front Neuroendocrinol. 2008;29(1):128–41. doi: 10.1016/j.yfrne.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 53.Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470(7335):492–7. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rasmusson AM, Pinna G, Paliwal P, Weisman D, Gottschalk C, Charney D, Krystal J, Guidotti A. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biol Psychiatry. 2006;60(7):704–13. doi: 10.1016/j.biopsych.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 55.Mulchahey JJ, Ekhator NN, Zhang H, Kasckow JW, Baker DG, Geracioti TD., Jr Cerebrospinal fluid and plasma testosterone levels in post-traumatic stress disorder and tobacco dependence. Psychoneuroendocrinology. 2001;26(3):273–85. doi: 10.1016/s0306-4530(00)00052-4. [DOI] [PubMed] [Google Scholar]
- 56.Reijnen A, Geuze E, Vermetten E. The effect of deployment to a combat zone on testosterone levels and the association with the development of posttraumatic stress symptoms: A longitudinal prospective Dutch military cohort study. Psychoneuroendocrinology. 2014 doi: 10.1016/j.psyneuen.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 57.Bromet E, Sonnega A, Kessler RC. Risk Factors for DSM-III-R Posttraumatic Stress Disorder: Findings from the National Comorbidity Survey. Am J Epidemiol. 1998;147(4):353–361. doi: 10.1093/oxfordjournals.aje.a009457. [DOI] [PubMed] [Google Scholar]
- 58.Keen-Rhinehart E, Ondek K, Schneider JE. Neuroendocrine regulation of appetitive ingestive behavior. Front Neurosci. 2013;7:213. doi: 10.3389/fnins.2013.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rasmusson AM, Hauger RL, Morgan CA, Bremner JD, Charney DS, Southwick SM. Low baseline and yohimbine-stimulated plasma neuropeptide Y (NPY) levels in combat-related PTSD. Biol Psychiatry. 2000;47(6):526–39. doi: 10.1016/s0006-3223(99)00185-7. [DOI] [PubMed] [Google Scholar]
- 60.Morgan CA, 3rd, Rasmusson AM, Winters B, Hauger RL, Morgan J, Hazlett G, Southwick S. Trauma exposure rather than posttraumatic stress disorder is associated with reduced baseline plasma neuropeptide-Y levels. Biol Psychiatry. 2003;54(10):1087–91. doi: 10.1016/s0006-3223(03)00433-5. [DOI] [PubMed] [Google Scholar]
- 61.Morgan CA, 3rd, Rasmusson AM, Wang S, Hoyt G, Hauger RL, Hazlett G. Neuropeptide-Y, cortisol, and subjective distress in humans exposed to acute stress: replication and extension of previous report. Biol Psychiatry. 2002;52(2):136–42. doi: 10.1016/s0006-3223(02)01319-7. [DOI] [PubMed] [Google Scholar]
- 62.Meyer RM, Burgos-Robles A, Liu E, Correia SS, Goosens KA. A ghrelin-growth hormone axis drives stress-induced vulnerability to enhanced fear. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rao MN, Chau A, Madden E, Inslicht S, Talbot L, Richards A, O’Donovan A, Ruoff L, Grunfeld C, Neylan TC. Hyperinsulinemic response to oral glucose challenge in individuals with posttraumatic stress disorder. Psychoneuroendocrinology. 2014;49:171–81. doi: 10.1016/j.psyneuen.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hill MN, Bierer LM, Makotkine I, Golier JA, Galea S, McEwen BS, Hillard CJ, Yehuda R. Reductions in circulating endocannabinoid levels in individuals with post-traumatic stress disorder following exposure to the World Trade Center attacks. Psychoneuroendocrinology. 2013;38(12):2952–61. doi: 10.1016/j.psyneuen.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neumeister A, Normandin MD, Pietrzak RH, Piomelli D, Zheng MQ, Gujarro-Anton A, Potenza MN, Bailey CR, Lin SF, Najafzadeh S, Ropchan J, Henry S, Corsi-Travali S, Carson RE, Huang Y. Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: a positron emission tomography study. Mol Psychiatry. 2013;18(9):1034–40. doi: 10.1038/mp.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sondergaard HP, Hansson LO, Theorell T. Elevated blood levels of dehydroepiandrosterone sulphate vary with symptom load in posttraumatic stress disorder: findings from a longitudinal study of refugees in Sweden. Psychother Psychosom. 2002;71(5):298–303. doi: 10.1159/000064806. [DOI] [PubMed] [Google Scholar]
- 67.Spivak B, Maayan R, Kotler M, Mester R, Gil-Ad I, Shtaif B, Weizman A. Elevated circulatory level of GABA(A)--antagonistic neurosteroids in patients with combat-related post-traumatic stress disorder. Psychol Med. 2000;30(5):1227–31. doi: 10.1017/s0033291799002731. [DOI] [PubMed] [Google Scholar]
- 68.Yehuda R, Brand SR, Golier JA, Yang RK. Clinical correlates of DHEA associated with post-traumatic stress disorder. Acta Psychiatr Scand. 2006;114(3):187–93. doi: 10.1111/j.1600-0447.2006.00801.x. [DOI] [PubMed] [Google Scholar]
- 69.Morgan CA, 3rd, Southwick S, Hazlett G, Rasmusson A, Hoyt G, Zimolo Z, Charney D. Relationships among plasma dehydroepiandrosterone sulfate and cortisol levels, symptoms of dissociation, and objective performance in humans exposed to acute stress. Arch Gen Psychiatry. 2004;61(8):819–25. doi: 10.1001/archpsyc.61.8.819. [DOI] [PubMed] [Google Scholar]
- 70.de Kloet CS, Vermetten E, Geuze E, Wiegant VM, Westenberg HG. Elevated plasma arginine vasopressin levels in veterans with posttraumatic stress disorder. J Psychiatr Res. 2008;42(3):192–8. doi: 10.1016/j.jpsychires.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 71.Stappenbeck CA, Hellmuth JC, Simpson T, Jakupcak M. The Effects of Alcohol Problems, PTSD, and Combat Exposure on Nonphysical and Physical Aggression Among Iraq and Afghanistan War Veterans. Psychol Trauma. 2014;6(1):65–72. doi: 10.1037/a0031468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boscarino JA, Chang J. Electrocardiogram abnormalities among men with stress-related psychiatric disorders: implications for coronary heart disease and clinical research. Ann Behav Med. 1999;21(3):227–34. doi: 10.1007/BF02884839. [DOI] [PubMed] [Google Scholar]
- 73.Weiss T, Skelton K, Phifer J, Jovanovic T, Gillespie CF, Smith A, Umpierrez G, Bradley B, Ressler KJ. Posttraumatic stress disorder is a risk factor for metabolic syndrome in an impoverished urban population. General Hospital Psychiatry. 2011;33(2):135–142. doi: 10.1016/j.genhosppsych.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maes M, Lin AH, Delmeire L, Van Gastel A, Kenis G, De Jongh R, Bosmans E. Elevated serum interleukin-6 (IL-6) and IL-6 receptor concentrations in posttraumatic stress disorder following accidental man-made traumatic events. Biol Psychiatry. 1999;45(7):833–9. doi: 10.1016/s0006-3223(98)00131-0. [DOI] [PubMed] [Google Scholar]
- 75.Spivak B, Shohat B, Mester R, Avraham S, Gil-Ad I, Bleich A, Valevski A, Weizman A. Elevated levels of serum interleukin-1 beta in combat-related posttraumatic stress disorder. Biol Psychiatry. 1997;42(5):345–8. doi: 10.1016/S0006-3223(96)00375-7. [DOI] [PubMed] [Google Scholar]
- 76.Smith AK, Conneely KN, Kilaru V, Mercer KB, Weiss TE, Bradley B, Tang Y, Gillespie CF, Cubells JF, Ressler KJ. Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. Am J Med Genet B Neuropsychiatr Genet. 2011;156B(6):700–8. doi: 10.1002/ajmg.b.31212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.von Kanel R, Hepp U, Kraemer B, Traber R, Keel M, Mica L, Schnyder U. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J Psychiatr Res. 2007;41(9):744–52. doi: 10.1016/j.jpsychires.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 78.Miller RJ, Sutherland AG, Hutchison JD, Alexander DA. C-reactive protein and interleukin 6 receptor in post-traumatic stress disorder: a pilot study. Cytokine. 2001;13(4):253–5. doi: 10.1006/cyto.2000.0825. [DOI] [PubMed] [Google Scholar]
- 79.Michopoulos V, Rothbaum AO, Jovanovic T, Almli LM, Bradley B, Rothbaum B, Gillespie CF, Ressler KJ. CRP levels are associated with increased PTSD symptoms and influenced by CRP genetic variation in a highly traumatized civilian population. [Accepted 2014];American Journal of Psychiatry. doi: 10.1176/appi.ajp.2014.14020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Plantinga L, Bremner JD, Miller AH, Jones DP, Veledar E, Goldberg J, Vaccarino V. Association between posttraumatic stress disorder and inflammation: a twin study. Brain Behav Immun. 2013;30:125–32. doi: 10.1016/j.bbi.2013.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, Ressler KJ. Impaired fear inhibition is a biomarker of PTSD but not depression. Depression and Anxiety. 2010;27(3):244–251. doi: 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rohleder N, Wolf JM, Wolf OT. Glucocorticoid sensitivity of cognitive and inflammatory processes in depression and posttraumatic stress disorder. Neurosci Biobehav Rev. 2010;35(1):104–14. doi: 10.1016/j.neubiorev.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 83.Rohleder N, Joksimovic L, Wolf JM, Kirschbaum C. Hypocortisolism and increased glucocorticoid sensitivity of pro-Inflammatory cytokine production in Bosnian war refugees with posttraumatic stress disorder. Biol Psychiatry. 2004;55(7):745–51. doi: 10.1016/j.biopsych.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 84.Liang Y, Zhou Y, Shen P. NF-kappaB and its regulation on the immune system. Cell Mol Immunol. 2004;1(5):343–50. [PubMed] [Google Scholar]
- 85.Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G, Joswig M, Morcos M, Schwaninger M, McEwen B, Kirschbaum C, Nawroth PP. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U S A. 2003;100(4):1920–5. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.O’Donovan A, Sun B, Cole S, Rempel H, Lenoci M, Pulliam L, Neylan T. Transcriptional control of monocyte gene expression in post-traumatic stress disorder. Dis Markers. 2011;30(2–3):123–32. doi: 10.3233/DMA-2011-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pace TW, Wingenfeld K, Schmidt I, Meinlschmidt G, Hellhammer DH, Heim CM. Increased peripheral NF-kappaB pathway activity in women with childhood abuse-related posttraumatic stress disorder. Brain Behav Immun. 2012;26(1):13–7. doi: 10.1016/j.bbi.2011.07.232. [DOI] [PubMed] [Google Scholar]
- 88.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boscarino JA. Diseases among men 20 years after exposure to severe stress: implications for clinical research and medical care. Psychosomatic Medicine. 1997;59:605–615. doi: 10.1097/00006842-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 90.Söndergaard HP, Hansson LO, Theorell T. The inflammatory markers C-reactive protein and serum amyloid A in refugees with and without posttraumatic stress disorder. Clinica Chimica Acta. 2004;342(1–2):93–98. doi: 10.1016/j.cccn.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 91.von Känel R, Hepp U, Kraemer B, Traber R, Keel M, Mica L, Schnyder U. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. Journal of Psychiatric Research. 41(9):744–752. doi: 10.1016/j.jpsychires.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 92.McCanlies EC, Araia SK, Joseph PN, Mnatsakanova A, Andrew ME, Burchfiel CM, Violanti JM. C-reactive protein, Interleukin-6, and posttraumatic stress disorder symptomology in urban police officers. Cytokine. 2011;55(1):74–78. doi: 10.1016/j.cyto.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 93.Klein SL. Hormones and mating system affect sex and species differences in immune function among vertebrates. Behavioural Processes. 2000;51(1–3):149–166. doi: 10.1016/s0376-6357(00)00125-x. [DOI] [PubMed] [Google Scholar]
- 94.Eraly SA, Nievergelt CM, Maihofer AX, Barkauskas DA, Biswas N, Agorastos A, O’Connor DT, Baker DG. Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry. 2014;71(4):423–31. doi: 10.1001/jamapsychiatry.2013.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Skelton K, Ressler KJ, Norrholm SD, Jovanovic T, Bradley-Davino B. PTSD and gene variants: New pathways and new thinking. Neuropharmacology. 2012;62(2):628–37. doi: 10.1016/j.neuropharm.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shalev A, Peri T, Brandes D, Freedman S, Orr S, Pitman R. Auditory startle response in trauma survivors with posttraumatic stress disorder: a prospective study. American Journal of Psychiatry. 2000;157(2):255–261. doi: 10.1176/appi.ajp.157.2.255. [DOI] [PubMed] [Google Scholar]
- 97.Buckley B, Nugent N, Sledjeski E, Raimonde AJ, Spoonster E, Bogart LM, Delahanty DL. Evaluation of initial posttrauma cardiovascular levels in association with acute PTSD symptoms following a serious motor vehicle accident. J Trauma Stress. 2004;17(4):317–324. doi: 10.1023/B:JOTS.0000038480.87290.4a. [DOI] [PubMed] [Google Scholar]
- 98.Blanchard EB, Hickling EJ, Galovski T, Veazey C. Emergency room vital signs and PTSD in a treatment seeking sample of motor vehicle accident survivors. J Trauma Stress. 2002;15(3):199–204. doi: 10.1023/A:1015299126858. [DOI] [PubMed] [Google Scholar]
- 99.Shalev AY, Sahar T, Freedman S, et al. A prospective study of heart rate response following trauma and the subsequent development of posttraumatic stress disorder. Archives of General Psychiatry. 1998;55(6):553–559. doi: 10.1001/archpsyc.55.6.553. [DOI] [PubMed] [Google Scholar]
- 100.Blanchard EB, Kolb LC, Pallmeyer TP, Gerardi RJ. A psychophysiological study of post-traumatic stress disorder in Vietnam veterans. Psychiatric Quarterly. 1982;54:220–229. doi: 10.1007/BF01064817. [DOI] [PubMed] [Google Scholar]
- 101.Keane TM, Kolb LC, Kaloupek D, Blanchard EB, Thomas RG, Hsieh FY, Lavori PW. Utility of psychophysiological measurement in the diagnosis of posttraumatic stress disorder: results from a Department of Veterans Affairs Cooperative Study. Journal of Consulting and Clinical Psychology. 1998;66:914–923. doi: 10.1037//0022-006x.66.6.914. [DOI] [PubMed] [Google Scholar]
- 102.APA. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: APA; 2013. [Google Scholar]
- 103.Orr SP, Metzger LJ, Lasko NB, Macklin ML, Hu FB, Shalev AY, Pitman RK. Physiologic responses to sudden, loud tones in monozygotic twins discordant for combat exposure: association with posttraumatic stress disorder. Arch Gen Psychiatry. 2003;60(3):283–8. doi: 10.1001/archpsyc.60.3.283. [DOI] [PubMed] [Google Scholar]
- 104.Shalev AY, Peri T, Brandes D, Freedman S, Orr SP, Pitman RK. Auditory startle response in trauma survivors with posttraumatic stress disorder: A prospective study. American Journal of Psychiatry. 2000;157:255–261. doi: 10.1176/appi.ajp.157.2.255. [DOI] [PubMed] [Google Scholar]
- 105.Orr SP, Metzger LJ, Miller MW, Kaloupek DG. Psychophysiological assessment of PTSD. In: Wilson JP, Keane TM, editors. Assessing Psychological Trauma and PTSD: A Handbook for Practicioners, 2nd Ed. Guilford Publications; New York: 2004. pp. 289–343. [Google Scholar]
- 106.Griffin MG. A prospective assessment of auditory startle alterations in rape and physical assault survivors. Journal of Traumatic Stress. 2008;21(1):91–99. doi: 10.1002/jts.20300. [DOI] [PubMed] [Google Scholar]
- 107.Orr SP, Roth WT. Psychophysiological assessment: clinical applications for PTSD. Journal of Affective Disorders. 2000;61:225–240. doi: 10.1016/s0165-0327(00)00340-2. [DOI] [PubMed] [Google Scholar]
- 108.Pole N, Neylan TC, Best SR, Orr SP, Marmar CR. Fear-potentiated startle and posttraumatic stress symptoms in urban police officers. J Trauma Stress. 2003;15:471–479. doi: 10.1023/A:1025758411370. [DOI] [PubMed] [Google Scholar]
- 109.Orr SP, Lasko NB, Macklin ML, Pineles SL, Chang Y, Pitman RK. Predicting post-trauma stress symptoms from pre-trauma psychophysiologic reactivity, personality traits and measures of psychopathology. Biol Mood Anxiety Disord. 2012;18(2):8. doi: 10.1186/2045-5380-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Neylan TC, Otte C, Yehuda R, Marmar CR. Neuroendocrine Regulation of Sleep Disturbances in PTSD. Annals of the New York Academy of Sciences. 2006;1071(1):203–215. doi: 10.1196/annals.1364.015. [DOI] [PubMed] [Google Scholar]
- 111.Kobayashi I, Boarts JM, Delahanty DL. Polysomnographically measured sleep abnormalities in PTSD: A meta-analytic review. Psychophysiology. 2007;44(4):660–669. doi: 10.1111/j.1469-8986.2007.537.x. [DOI] [PubMed] [Google Scholar]
- 112.Pitman RK, Orr SP, Forgue DF, de Jong JB, Claiborn JM. Psychophysiologic assessment of posttraumatic stress disorder imagery in Vietnam combat veterans. Arch Gen Psychiatry. 1987;44(11):970–5. doi: 10.1001/archpsyc.1987.01800230050009. [DOI] [PubMed] [Google Scholar]
- 113.Lang PJ, Levin DN, Miller GA, Kozak MJ. Fear behaviour, fear imagery, and the psychophysiology of emotion: The problem of affective response integration. J Abnorm Psychol. 1983;92(3):276–306. doi: 10.1037//0021-843x.92.3.276. [DOI] [PubMed] [Google Scholar]
- 114.Pitman RK, Orr SP, Forgus DF, Altman B, de Jong JB, Herz LR. Psychophysiologic responses to combat imagery of Vietnam veterans with posttraumatic stress disorder versus other anxiety disorders. Journal of Abnormal Psychology. 1990;99:49–54. doi: 10.1037//0021-843x.99.1.49. [DOI] [PubMed] [Google Scholar]
- 115.Orr SP, Pitman RK, Lasko NB, Herz LR. Psychophysiological assessment of posttraumatic stress disorder imagery in World War II and Korean combat veterans. Journal of Abnormal Psychology. 1993;102:152–159. doi: 10.1037//0021-843x.102.1.152. [DOI] [PubMed] [Google Scholar]
- 116.Shalev AY, Orr SP, Pitman RK. Psychophysiologic response during script-driven imagery as an outcome measure in posttraumatic stress disorder. J Clin Psychiatry. 1992;53(9):324–6. [PubMed] [Google Scholar]
- 117.McTeague LM, Lang PJ, Laplante MC, Cuthbert BN, Shumen JR, Bradley MM. Aversive Imagery in Posttraumatic Stress Disorder: Trauma Recurrence, Comorbidity, and Physiological Reactivity. Biological Psychiatry. 2010;67(4):346–356. doi: 10.1016/j.biopsych.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Marx BP, Bovin MJ, Suvak MK, Monson CM, Sloan DM, Fredman SJ, Humphreys KL, Kaloupek DG, Keane TM. Concordance between physiological arousal and subjective distress among vietnam combat veterans undergoing challenge testing for PTSD. Journal of Traumatic Stress. 2012;25(4):416–425. doi: 10.1002/jts.21729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rothbaum BO, Price ML, Jovanovic T, Norrholm SD, Gerardi M, Dunlop B, Davis M, Bradley B, Duncan EJ, Rizzo A, Ressler KJ. A randomized, double-blind evaluation of D-cycloserine or alprazolam combined with virtual reality exposure therapy for posttraumatic stress disorder in Iraq and Afghanistan War veterans. Am J Psychiatry. 2014;171(6):640–648. doi: 10.1176/appi.ajp.2014.13121625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Amstedler AB, Nugent NR, Koenen KC. Genetics of PTSD: Fear conditioning as a model for future research. Psychiatric Annals. 2009;39(6):338–369. doi: 10.3928/00485713-20090526-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Foa EB, Steketee G, Rothbaum BO. Behavioral/cognitive conceptualizations of post-traumatic stress disorder. Behavioral Therapy. 1989;20:155–176. [Google Scholar]
- 122.Jovanovic T, Ressler KJ. How the Neurocircuitry and Genetics of Fear Inhibition May Inform Our Understanding of PTSD. Am J Psychiatry. 2010;167:648–662. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. Journal of Abnormal Psychology. 2000;109(2):290–298. [PubMed] [Google Scholar]
- 124.Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, Ressler KJ. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol Psychiatry. 2011;69(6):556–63. doi: 10.1016/j.biopsych.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NaB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. Neurobiological Basis of Failure to Recall Extinction Memory in Posttraumatic Stress Disorder. Biological Psychiatry. 2009;66(12):1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hughes KC, Shin LM. Functional neuroimaging studies of post-traumatic stress disorder. Expert Review of Neurotherapeutics. 2011;11(2):275–285. doi: 10.1586/ern.10.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldstein M, Engelien W, Epstein J, Yang Y, Gorman J, LeDoux J, Silbersweig D, Stern E. Differential Time Courses and Specificity of Amygdala Activity in Posttraumatic Stress Disorder Subjects and Normal Control Subjects. Biological Psychiatry. 2005;57(5):464–473. doi: 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 128.Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological Psychiatry. 2000;47(9):769–76. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 129.Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62(3):273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 130.Stevens JS, Jovanovic T, Fani N, Ely TD, Glover EM, Bradley B, Ressler KJ. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. Journal of Psychiatric Research. 47(10):1469–1478. doi: 10.1016/j.jpsychires.2013.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Etkin A, Wager T. Functional Neuroimaging of Anxiety: A Meta-Analysis of Emotional Processing in PTSD, Social Anxiety Disorder, and Specific Phobia. Am J Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 133.Rougemont-Bücking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron-Milad K, Rodriguez-Romaguera J, Rauch SL, Pitman RK, Milad MR. Altered Processing of Contextual Information during Fear Extinction in PTSD: An fMRI Study. CNS Neuroscience & Therapeutics. 2010 doi: 10.1111/j.1755-5949.2010.00152.x. no-no. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Corbo V, Clément MH, Armony JL, Pruessner JC, Brunet A. Size Versus Shape Differences: Contrasting Voxel-Based and Volumetric Analyses of the Anterior Cingulate Cortex in Individuals with Acute Posttraumatic Stress Disorder. Biological Psychiatry. 2005;58(2):119–124. doi: 10.1016/j.biopsych.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 135.Bremner JD, Elzinga B, Schmahl C, Vermetten E, Ronald De Kloet MSOE, Eric V. Progress in Brain Research. Elsevier; 2008. Structural and functional plasticity of the human brain in posttraumatic stress disorder; pp. 171–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Admon R, Milad MR, Hendler T. A causal model of post-traumatic stress disorder: disentangling predisposed from acquired neural abnormalities. Trends in Cognitive Sciences. 17(7):337–347. doi: 10.1016/j.tics.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 137.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma.[see comment] Nature Neuroscience. 2002;5(11):1242–7. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Neylan TC, Mueller SG, Wang Z, Metzler TJ, Lenoci M, Truran D, Marmar CR, Weiner MW, Schuff N. Insomnia Severity Is Associated with a Decreased Volume of the CA3/Dentate Gyrus Hippocampal Subfield. Biological Psychiatry. 2010;68(5):494–496. doi: 10.1016/j.biopsych.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Carrion VG, Garrett A, Menon V, Weems CF, Reiss AL. Posttraumatic stress symptoms and brain function during a response-inhibition task: an fMRI study in youth. Depression and Anxiety. 2008;25(6):514–526. doi: 10.1002/da.20346. [DOI] [PubMed] [Google Scholar]
- 140.Jovanovic T, Ely T, Fani N, Glover EM, Gutman D, Tone EB, Norrholm SD, Bradley B, Ressler KJ. Reduced neural activation during an inhibition task is associated with impaired fear inhibition in a traumatized civilian sample. Cortex. 2013;49(7):1884–1891. doi: 10.1016/j.cortex.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mannie ZN, Norbury R, Murphy SE, Inkster B, Harmer CJ, Cowen PJ. Affective modulation of anterior cingulate cortex in young people at increased familial risk of depression. The British Journal of Psychiatry. 2008;192(5):356–361. doi: 10.1192/bjp.bp.107.043398. [DOI] [PubMed] [Google Scholar]
- 142.Neylan TC, Schadt EE, Yehuda R. Biomarkers for combat-related PTSD: focus on molecular networks from high-dimensional data. Eur J Psychotraumatol. 2014:5. doi: 10.3402/ejpt.v5.23938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schmidt U, Kaltwasser SF, Wotjak CT. Biomarkers in Posttraumatic Stress Disorder: Overview and Implications for Future Research. Disease markers. 2013;35(1):43–54. doi: 10.1155/2013/835876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lehrner A, Yehuda R. Biomarkers of PTSD: military applications and considerations. European Journal of Psychotraumatology. 2014;5 doi: 10.3402/ejpt.v5.23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gill J, Vythilingam M, Page GG. Low cortisol, high DHEA, and high levels of stimulated TNF-alpha, and IL-6 in women with PTSD. J Trauma Stress. 2008;21(6):530–9. doi: 10.1002/jts.20372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse Actions of Ovarian Steroids in the Serotonin Neural System. Frontiers in Neuroendocrinology. 2002;23(1):41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
- 147.Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, Goldstein JM, Milad MR. Estradiol Modulates Medial Prefrontal Cortex and Amygdala Activity During Fear Extinction in Women and Female Rats. Biological Psychiatry. 2011;70(10):920–927. doi: 10.1016/j.biopsych.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Goetz SMM, Tang L, Thomason ME, Diamond MP, Hariri AR, Carré JM. Testosterone Rapidly Increases Neural Reactivity to Threat in Healthy Men: A Novel Two-Step Pharmacological Challenge Paradigm. Biological Psychiatry. 76(4):324–331. doi: 10.1016/j.biopsych.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Michopoulos V, Berga SL, Kaplan JR, Wilson ME. Social Subordination and Polymorphisms in the Gene Encoding the Serotonin Transporter Enhance Estradiol Inhibition of Luteinizing Hormone Secretion in Female Rhesus Monkeys. Biology of Reproduction. 2009;81(6):1154–1163. doi: 10.1095/biolreprod.109.079038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nijm J, Jonasson L. Inflammation and cortisol response in coronary artery disease. Annals of Medicine. 2009;41(3):224–233. doi: 10.1080/07853890802508934. [DOI] [PubMed] [Google Scholar]