Abstract
Fungal infections are responsible for millions of human deaths annually. Copper, an essential but toxic trace element, plays an important role at the host-pathogen axis during infection. In this review, we describe how the host uses either Cu compartmentalization within innate immune cells or Cu sequestration in other infected host niches such as in the brain to combat fungal infections. We explore Cu toxicity mechanisms and the Cu homeostasis machinery that fungal pathogens bring into play to succeed in establishing an infection. Finally, we address recent approaches that manipulate Cu-dependent processes at the host-pathogen axis for antifungal drug development.
Keywords: copper, fungi, host-pathogen interaction, innate immunity, metal homeostasis, microbial pathogenesis, fungal pathogenesis, copper homeostasis, nutritional immunity
Introduction
Of the ∼5 million fungal species predicted to exist on Earth, only a few are currently known to infect humans and cause disease (Table 1) (1, 2). However, infection by fungal pathogens can often lead to serious disease and lethality in humans. For example, the toll on humans as a consequence of infection by the fungal pathogen Cryptococcus neoformans exceeds that of infection with Mycobacterium tuberculosis, the causative agent of tuberculosis (3). Fungal pathogens are represented by diverse members of the fungal kingdom that have distinct lifestyles outside of the host and cause infections in different niches within the human host. Consequently, fungal pathogens must be able to cope with a range of conditions both in their environmental reservoir and in distinct tissues within the host. For example, the ability of fungal pathogens to thrive at different temperatures and distinct pH, to colonize different surfaces, and to thrive under varying conditions of nutritional limitation is key to their virulence. The immune system is typically capable of controlling commensal fungi or those acquired from the environment. However, the rise in the number of immunocompromised individuals due to HIV-AIDS, or in an immunosuppressed state due to diabetes or as an organ transplant recipient, has placed an increasing number of individuals at risk for life-threatening fungal infections (4).
TABLE 1.
Human fungal pathogens, corresponding disease pathology, and primary treatment therapies
| Organisma | Diseasea | Description of the diseasea | Primary therapya |
|---|---|---|---|
| Aspergillus spp. | Chronic pulmonary aspergillosis | Long-term condition, mostly in patients with underlying lung disease in which Aspergillus can cause cavities in the lungs | Itraconazole or voriconazole |
| Invasive aspergillosis | Infection of immunocompromised people; most commonly lungs affected, but can disseminate to periphery | Voriconazole | |
| Blastomyces dermatitidis | Blastomycosis | Lung infection in healthy or immunocompromised people | Itraconazole; amphotericin B for severe infections |
| Candida spp. | Oral candidiasis | Oral Candida overgrowth in immunocompromised people | Antifungal topical treatments |
| Genital candidiasis | Genital Candida overgrowth | Antifungal vaginal suppositories or creams | |
| Invasive candidiasis | Bloodstream infection of Candida yeast in immunocompromised people | Fluconazole or an echinocandin | |
| Coccidioides spp. | Coccidioidomycosis or valley fever | Most commonly lung infection in healthy or immunocompromised people, which can disseminate to other tissues, including CNS | Fluconazole |
| Cryptococcus spp. | Pulmonary cryptococcosis | Lung infection in healthy (C. gattii) or immunocompromised people (C. neoformans) | Fluconazole |
| Cryptococcal meningitis | CNS infection after C. neoformans and C. gattii spread from the lungs | Amphotericin B and flucytosine | |
| Histoplasma capsulatum | Histoplasmosis | Primary lung infection can be latent or disseminate, especially in immunocompromised individuals | Itraconazole; amphotericin B for severe infections |
| Rhizopus arrhizus and other Mucoromycotina | Mucormycosis | Infection of immunocompromised people; five major clinical forms, from which infection of sinuses and the brain and lung are the most common; rapid onset of tissue necrosis | Amphotericin B; in cutaneous disease, wound resections |
| Pneumocystis jirovecii | Pneumocystis pneumonia | Lung infection particularly in immunocompromised people, high incidence in HIV/AIDS patients; can be fatal if untreated | Trimethoprim sulfamethoxazole |
| Sporothrix schenckii | Sporotrichosis | Predominantly skin infections; disseminated infections may occur in immunocompromised people | Itraconazole; amphotericin B for severe infections |
| Talaromyces marneffei | Penicilliosis marneffei | Infects immunocompromised people, initially in the lungs and then by hematogenous dissemination to a systemic mycosis | Amphotericin B |
| Dermatophytesb: Trichophyton, Microsporum, Epidermophyton | Skin and nail infections | Infection of the skin and nails of healthy and immunocompromised individuals | Variety of antifungals, the most common are azoles and terbinafine |
| Malassezia spp.b | Dandruff and seborrheic dermatitis | Healthy and immunocompromised individuals; the etiology of the disease still not well understood | Zinc pyrithione and other antifungals in shampoos |
| Atopic dermatitis | Chronic inflammatory skin disease associated with allergic rhinitis, asthma, and immunoglobulin E-mediated food reactions | Steroids | |
| Pityriasis versicolor | Chronic superficial skin disease with lesions hypo- or hyper-pigmented | Several topical antifungals |
a Unless otherwise indicated, the source of information for this table is the website from the Centers for Disease Control and Prevention (www.cdc.gov).
b Ref. 88.
Unfortunately, current therapies used to combat fungal infections are quite limited in scope and efficacy (5). There are three major classes of antifungal drugs in use in the clinic: the polyenes, which weaken the fungal membrane by binding to ergosterol (6); the azoles, which target the ERG11 gene product, an intermediate step in the ergosterol biosynthetic pathway (7); and the echinocandins, which inhibit the enzyme β-1,3-glucan synthase and consequently perturb the synthesis of glucan in the cell wall (8) (Table 1). The efficacy of these compounds is limited; some fungi are intrinsically resistant or have developed resistance over time. Moreover, although antifungals target components that are uniquely fungal, host toxicity is an issue, principally with the polyene class of antifungals. Although azoles are better tolerated, they are fungistatic and are known to interfere with the metabolism of other drugs by inhibiting specific host cytochrome P450 enzymes. Although echinocandins have broad spectrum antifungal activity against Candida spp., they are of limited utility for other fungi such as Cryptococcus spp. The development of novel drugs to treat fungal infections has been challenging, particularly because fungal pathogens are eukaryotes that share many of the same biological processes, enzymes, and structural components with their mammalian hosts (9). However, a detailed understanding of the molecular mechanisms underlying the interactions between fungal pathogens and the host during infection can lead to new approaches for antifungal drug development. Here we discuss the roles of the essential trace element Cu at the host-pathogen axis, and how the manipulation of the chemistry of Cu may lead to more potent antifungal strategies. Previous reviews in the field have comprehensively summarized the mechanisms for Cu homeostasis in fungi and mammals (10–13), as well as the role of Cu in bacterial pathogenesis (14–17).
Based on the ability of Cu to cycle between reduced (Cu+) and oxidized (Cu2+) states, Cu is an essential trace element for virtually all organisms. Cu serves as a cofactor for enzymes that generate ATP and mature hormones, function in neurotransmitter biogenesis and disproportionation of superoxide anion, and pump Fe across membranes into the periphery (18). However, Cu accumulation beyond homeostatic levels is highly toxic in bacterial cells, fungi, and mammals. Indeed, Cu in one form or another has been used through the ages as a potent antimicrobial agent. Early civilizations used Cu to sterilize water and treat wounds, and in more recent times, workers in Cu smelting plants were protected from 19th century cholera epidemics (19, 20). Cu is the active ingredient of Bordeaux and Burgundy mixtures, used as a vineyard fungicide in the late 1800s (21). Currently, Cu is used as an antimicrobial surface in veterinary and healthcare settings, where studies have shown a reduction in nosocomial infection in hospitals that have implemented the use of Cu surfaces on doorknobs, handrails, and other surfaces (22, 23).
In general, with respect to nutrients that are essential for the growth and proliferation of microbial pathogens, the term “nutritional immunity” has been coined to indicate the diverse mechanisms by which the host restricts nutrient availability to limit microbial proliferation (24). The host restriction of metals such as Fe, Zn, and Ca, away from microbial pathogens, has been clearly established, and these mechanisms are under investigation. In turn, microbial pathogens have mounted sophisticated responses to host metal restriction to counter these measures and successfully compete for metals (24, 25). In contrast to other trace elements, work over the past decade demonstrates that host innate immune cells concentrate Cu in the vicinity of invading pathogens, as a means to exploit Cu toxicity and enhance microbial killing (16, 26). Moreover, for C. neoformans, and perhaps other fungal pathogens, hosts use both Cu compartmentalization and Cu sequestration, in distinct infectious niches, to subjugate pathogenesis (27, 28).
Cu Homeostasis in Fungal Pathogens: An Overview
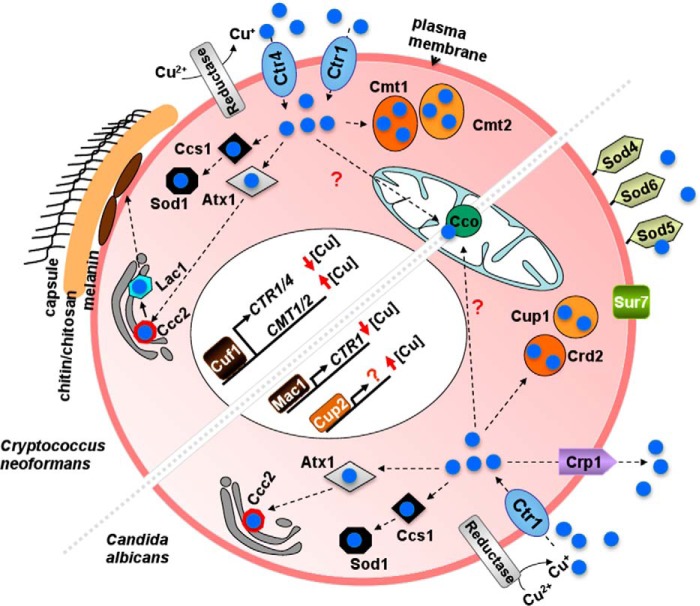
As Cu is an essential trace element with toxic properties, eukaryotic organisms from fungi to humans possess highly orchestrated mechanisms to acquire, distribute, sequester, export, and regulate Cu to provide sufficient Cu for biological processes while preventing Cu toxicity that occurs beyond homeostatic regulatory capabilities. Drawing from mechanisms garnered from studies of Cu homeostasis in model non-pathogenic fungal systems such as Saccharomyces cerevisiae and Schizosaccharomyces pombe, and more recent experimental work in C. neoformans and Candida albicans, we present a current picture of the Cu homeostasis mechanisms in the human pathogens C. neoformans and C. albicans (Fig. 1). Extracellular Cu is imported into C. neoformans as Cu+, via the concerted action of cell surface Cu2+ metalloreductases, and the functionally redundant, plasma membrane high affinity Cu+ importers, Ctr1 and Ctr4 (29). Intracellular Cu+ is bound to Cu chaperones and putative small ligands, which through protein-protein interactions or protein-ligand interactions drive a thermodynamic affinity gradient that allows Cu to be distributed to distinct and specific compartments and proteins where it is required, thereby minimizing the presence of potentially toxic labile Cu ions (30). These duties are carried out by CCS (Cu chaperone for Cu,Zn-superoxide dismutase) (31) or Atx1 (which carries Cu to the Cu+-transporting ATPases that pump Cu into the secretory compartment where it is loaded onto proteins such as laccase (melanin biosynthesis) or the high affinity Fe uptake oxidase-permease complex) (32, 33). Other, as yet undefined cytoplasmic ligands are thought to function in the delivery of Cu to a mitochondrial Cu importer, Pic2 (34), where Cu is ultimately mobilized to cytochrome oxidase through the action of the Sco1/Sco2 proteins on the inner mitochondrial membrane (35). When environmental Cu concentrations are high, Cu toxicity is prevented by one or more of several mechanisms including Cu binding to metallothioneins, small cysteine-rich proteins that bind Cu with high stoichiometry through cysteine-thiolate bonds (36), the import and storage of Cu in vacuoles (37), and Cu extrusion from cells via a P-type ATPase at the plasma membrane, such as Crp1 found for C. albicans (Fig. 1), but not in C. neoformans, S. cerevisiae, or S. pombe (38).
FIGURE 1.
Model for Cu homeostasis mechanisms in two fungal pathogens, Cryptococcus neoformans and in Candida albicans. Before import into C. neoformans cells, Cu2+ is reduced to Cu+ (blue) by cell membrane metalloreductases. Cu+ is transported into cells by the Ctr1 and Ctr4 high affinity Cu+ transporters. The cytosolic Cu chaperone Atx1 delivers Cu to the Ccc2 pump, and then to laccase, Lac1, which drives the synthesis of the cell surface pigment melanin. The Cu chaperone Ccs1 provides Cu to Cu,Zn-Sod1, and by an unknown mechanism, Cu also reaches cytochrome c oxidase (Cco) in the mitochondria. The metallothioneins Cmt1 and Cmt2 bind Cu with an extraordinarily high stoichiometry, protecting C. neoformans from Cu toxicity. The transcription factor Cuf1 induces the expression of Cu acquisition, or Cu detoxification genes, respectively, in response to low or high Cu concentrations. In C. albicans, Cu2+ is reduced to Cu+ (blue) by cell membrane metalloreductases. Cu+ is transported into cells by the Ctr1 high affinity Cu+ transporter. The cytosolic Cu chaperone Atx1 delivers Cu to the Ccc2 pump. The Cu chaperone Ccs1 provides Cu to the Cu,Zn-Sod1, and by an unknown mechanism, Cu also reaches cytochrome c oxidase in the mitochondria. The metallothioneins Cup1 and Crd2 bind Cu and protect C. albicans from Cu toxicity; however, the major mechanism for Cu detoxification is achieved by Cu extrusion via the P-type ATPase Crp1. The transcription factor Mac1 induces the expression of Cu acquisition genes in response to low Cu concentrations, and it has been proposed, by gene homology, that the transcription factor Cup2 induces the expression of the Cu detoxification genes in response to high Cu concentrations (86). The extracellular superoxide dismutases Sod4, Sod5, and Sod6 are GPI-linked to the cell membrane. Sur7 is a tetra-spanning domain protein important for cell wall morphogenesis, also involved in Cu homeostasis, as Sur7 deletion renders cells more sensitive to high Cu than isogenic wild-type cells (87).
When S. cerevisiae cells encounter low or high Cu concentrations, dedicated Cu-sensing transcription factors, Mac1 or Ace1, activate transcription of genes encoding the Cu acquisition or the Cu-detoxifying metallothioneins, respectively, to re-establish homeostasis (39). In vivo footprinting and in vitro biochemical experiments demonstrate that low Cu activates Mac1 binding to target promoter-binding sites (40), whereas high Cu levels allosterically activate Ace1 through the formation of a DNA binding-competent tetra-Cu+ cluster in the DNA-binding domain (41). The presence of Cu status-specific transcription factors in S. cerevisiae is in contrast to that found in C. neoformans, where a single Cu metalloregulatory factor, Cuf1, is critical for potently activating the Cu import genes in response to low Cu levels and the metallothionein genes in response to high Cu levels (29) through as yet poorly understood mechanisms (Fig. 1).
How Is Cu Toxic?
The accumulation of Cu beyond homeostatic needs leads to toxicity in diverse situations that include Wilson disease in humans, resulting in hepatic Cu hyper-accumulation, the antifungal activity of Bordeaux mixture in vineyards, and the antibacterial activity of copper salts. Given the redox active nature of Cu, as well as its ability to oxidize lipids, nucleic acids, and proteins via the generation of hydroxyl radical, these macromolecular insults have long been considered the primary basis for Cu cytotoxicity. However, recent biochemical and genetic experiments bring this view into question. Elegant experiments from Imlay and co-workers (42) demonstrate that, despite a dramatic Cu over-accumulation in Escherichia coli cells, it does not cause oxidative DNA damage, and most of the redox-active Cu accumulates in the periplasmic space. In subsequent work, these investigators suggest that intracellular Cu+ ligates to solvent-accessible sulfur atoms that would otherwise coordinate Fe-sulfur clusters essential for enzyme catalysis, such as for isopropylmalate dehydratase (43). Because a number of proteins involved in amino acid biosynthesis, DNA replication and repair, telomere maintenance, and other critical cellular functions are Fe-S cluster proteins (44), these proteins may represent important targets in Cu toxicity due to loss of catalytic function. Further studies, perhaps guided by structural information, may elucidate the hierarchy of their sensitivity to Cu and importance in Cu toxicity, particularly with respect to fungal pathogens.
Host Cu: A Weapon Against Fungal Pathogens
Although humans have increased complexity at the level of systemic Cu homeostasis and its regulation, at the cellular level, there is great conservation between fungi and humans in the proteins that carry out Cu balance, as well as their mechanisms of action (45–48). In contrast to the nutritional immunity imposed by host Fe-, Zn-, and Ca-withholding mechanisms, accumulating evidence demonstrates that host innate immune cells, such as macrophages, actively accumulate and compartmentalize Cu as a potent weapon against microbial pathogens. For example, early studies using x-ray microprobe analysis demonstrated that Cu concentrations increase dramatically in the phagolysosome of peritoneal macrophages upon infection with Mycobacterium species (49). These studies suggested an active process for host Cu compartmentalization, which was further supported by the observation that activated macrophages increase expression of both the high affinity Cu transporter Ctr1 at the plasma membrane and the Cu+-transporting P-type ATPase ATP7A at the phagolysosomal membrane, as compared with naive macrophages (50). Consistent with active Cu compartmentalization, E. coli, M. tuberculosis, and Salmonella typhimurium mutants lacking Cu+-exporting pumps required for Cu detoxification were more susceptible to macrophage killing and hypo-virulent in animal infection models (50–52), and E. coli Cu-sensitive mutants exhibited increased survival in macrophages in which expression of the ATP7A pump was silenced (50). Moreover, perhaps the acidic environment of the phagolysosome, combined with the elaboration of reactive oxygen and nitrogen species, also exacerbates Cu toxicity due to the labile nature of Cu at low pH and its chemical reactivity with these species.
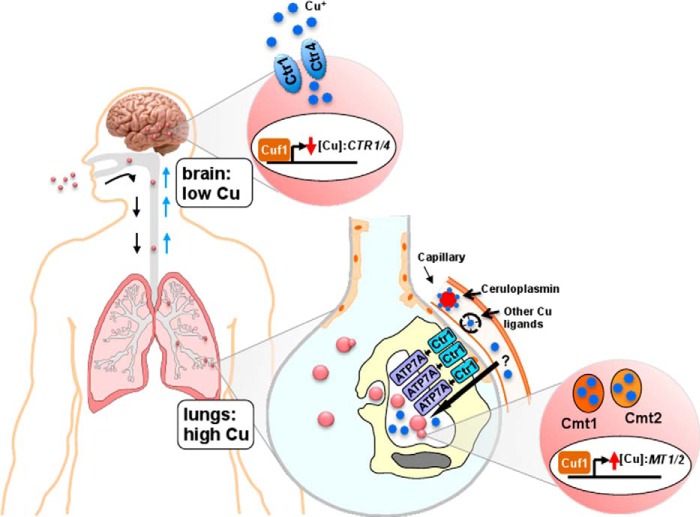
Although most bacterial pathogens have no known requirement for Cu within their cytoplasm, fungal pathogens and humans have Cu-dependent enzymes in many compartments and have common Cu homeostasis mechanisms. Live animal imaging studies demonstrate that C. neoformans senses exposure to high levels of Cu within the lung (Fig. 2), the natural route of infection and initial infectious niche in mammals (27). Accordingly, C. neoformans copes with elevated pulmonary Cu concentrations during infection by robustly inducing the expression of two metallothionein genes, CMT1 and CMT2, in a time-dependent manner. Disruption of both MT2 genes results in a dramatic decrease of the virulence in an intranasal mouse model of cryptococcosis (27). Cmt1 and Cmt2 are primary and redundant defensive mechanisms that protect C. neoformans from otherwise toxic Cu concentrations. Not only is MT1/2 expression induced in a manner dependent on both high Cu and the single C. neoformans Cu regulatory transcription factor Cuf1, but the unusually long primary sequence of the MT1 and MT2 proteins, consisting of tandem modules of cysteine-rich Cu-binding blocks, confers these proteins with the ability to bind up to 16 and 24 Cu+ ions, respectively, an unprecedented Cu binding stoichiometry (53). In contrast to the transcription of mammalian MT genes that are activated by many distinct metals, oxidative stress, and hormones, elevated Cu is the only condition currently known to activate MT1 and MT2 in C. neoformans (27). Interestingly, genes encoding metallothionein homologues are found in the genomes of other fungal pathogens (54, 55), yet in contrast to the MTs of C. neoformans, the Cu-exporting pump, CRP1, in C. albicans has been shown to play a dominant role in Cu detoxification and Cu-related virulence in this fungal pathogen (Fig. 1) (38).
FIGURE 2.
Cu homeostasis mechanisms at the host-pathogen axis during C. neoformans infection. C. neoformans is a fungal pathogen that is acquired through the respiratory route and establishes in the lungs of immunocompromised individuals. If C. neoformans is not cleared at this stage of infection, it disseminates through the bloodstream to the brain where it causes lethal meningitis, and it is responsible for ∼650,000 deaths/year (3). In the lungs, the primary site of the infection, innate immune cells use Cu as a weapon to fight against C. neoformans. In this niche, C. neoformans metallothioneins are highly expressed in a Cuf1-dependent manner as a means to cope with the high Cu concentrations in the phagolysosome that are compartmentalized via the action of the host plasma membrane Ctr1 Cu+ importer and the Cu+ transporter ATP7A at the phagolysosomal membrane. The source of innate immune cell Cu could be derived from ceruloplasmin or other circulating Cu ligands. When C. neoformans reaches the brain, it encounters an environment deprived from Cu, as demonstrated by the Cuf1-dependent expression of the C. neoformans high affinity Cu transporters Ctr1 and Ctr4 at the plasma membrane, as well as the requirement for the Cu acquisition machinery in colonization of the brain and for virulence.
Although Cu compartmentalization within the phagolysosome is emerging as an important mechanism with respect to innate immunity against fungal pathogens, one unresolved question is: what is the source of Cu used by the macrophages for this process? The Cu accessed by cell surface Ctr1 could be mobilized from Cu-binding proteins in the circulation such as ceruloplasmin, an acute phase protein elevated during inflammation and infection (56). Although ∼90% of the Cu in plasma is bound to ceruloplasmin, little information is available regarding a potential role for ceruloplasmin providing Cu to macrophages. Additional plasma Cu-binding proteins that could serve as a source of macrophage Cu include albumin, extracellular Cu,Zn-superoxide dismutase, plasma metallothioneins, or low molecular weight Cu complexes, of an undetermined nature, recently described (57, 58). Alternatively, macrophages may mobilize their own intracellular Cu stores such as through the action of the ATP7A Cu+ pump on the phagolysosome. Alternatively, Cu could be provided through the fusion of Cu-enriched endosomal compartments found in cells in which the Ctr1 high affinity Cu+ transporter, localized to both the plasma membrane and the endosomes, fails to be efficiently cleaved through stimulation by the Ctr2 protein (59). Perhaps in some scenarios, extracellular sources of Cu directly exert antifungal activity, without entering cells.
Host Cu Deprivation in Fungal Infection
Although the Cu detoxification machinery is important for C. neoformans to gain ground on the immune system during initial stages of pulmonary infection, possibly leading to pneumonia, later stages of C. neoformans infection involve bloodstream dissemination and colonization of the CNS, causing lethal meningitis. During brain colonization, C. neoformans activates transcription of the plasma membrane high affinity Cu importers Ctr1 and Ctr4, but not MT1 or MT2, demonstrating that in this niche, C. neoformans senses Cu limitation (Fig. 2) (28). Furthermore, deletion of the two C. neoformans Cu importers causes a significant reduction in fungal burden in an intracranial mouse model of meningitis. In C. neoformans, the expression of Ctr1 and Ctr4 is induced by the single Cu regulatory transcription factor Cuf1 under Cu-deficient conditions (29). Collectively, these results suggest that C. neoformans encounters Cu limitation during the brain stage of infection. More experiments are needed to ascertain whether the host is deliberately creating this local Cu-deprived environment aimed at preventing the growth of this fungal pathogen in the brain, which would constitute a new metal subject to nutritional immunity. However, it is also possible that there is no such Cu-deficient environment in the brain, but there is a dramatic increase in the cryptococcal Cu requirements. As we discuss below, it is in the brain where the major substrate for the C. neoformans melanin biosynthetic pathway is found.
How might the host limit Cu in specific infectious niches? It has been observed that mammalian metallothioneins play an important role in neuroprotection during brain inflammatory processes, by mechanisms that include metal sequestration and reactive oxygen species neutralization (60). Fungal infection might very well be another process where metallothioneins play an important neuroprotective role and might constitute a mechanism for Cu sequestration during C. neoformans brain colonization. Mammals express four metallothionein isoforms, MT1 to MT4. Although MT1 and MT2 are ubiquitously expressed in all tissues, including the brain, MT3 is almost exclusively expressed in the CNS. MT1 and MT2 are able to bind up to 12 Cu+ atoms, and their expression is induced during inflammation in astrocytes or activated microglia, immune cells derived from the monocyte/macrophage lineage (60). MTs have been predominantly localized intracellularly, but they have also been reported to be released from astrocytes to the extracellular environment, suggesting a potential role for MTs in host Cu withholding in the brain (61). The splicing variant of the Cu-binding protein glycosylphosphatidylinositol (GPI)-anchored ceruloplasmin, which is expressed on the surface of astrocytes and which regulates iron levels in both astrocytes and neurons, could also contribute to a host metal sequestration mechanism during fungal infection (62). Although little is known regarding intracellular Cu redistribution in microglia or astrocytes during brain inflammation, alternative mechanisms for Cu sequestration could involve the ATP7A/B pumps or alterations in the regulation of high affinity Cu transport proteins, such as regulation of Ctr1 function by cleavage of its extracellular ecto-domain at vesicular compartments within microglia or astrocytes, which could lead to the accumulation of biologically unavailable Cu in stored endosomes (59).
Fungal Cu-dependent Enzymes in Virulence
Laccases and the Melanin Biosynthetic Pathway
Melanins are highly insoluble negatively charged polymers of phenolic and indolic compounds that, in fungi, are strongly associated with virulence. Melanin provides fungi a defense mechanism against oxidative and nitrosative stress, protects cell wall integrity, supports immune evasion, and confers resistance to killing by antifungal drugs. Fungi have two different pathways for melanin synthesis: the 1,8-dihydroxynaphtalene pathway, where the endogenously synthesized molecules, acetyl CoA and malonyl CoA, are used by the fungi as melanin precursors, and the l-3,4-dihydroxyphenylalanine (l-DOPA) pathway, where either exogenously acquired l-DOPA or tyrosine are the precursors used by fungi to synthesize melanin (63).
Melanin is an established virulence determinant in animal models of infection for numerous fungal pathogens including Wangiella dermatitidis (64), Paracoccidioides brasiliensis (65), Sporothrix schenckii (66), or Cryptococcus gattii (67). This is also the case for C. neoformans (68), which synthesizes melanin exclusively from the l-DOPA pathway, and importantly, the melanin precursor l-DOPA is very abundant in host brain. Melanin allows C. neoformans to survive macrophage-mediated killing by preventing antibody-mediated phagocytosis (69), and increases the intensity of host tissue damage in relation with non-melanized C. neoformans cells. Melanized C. neoformans cells have been found in lungs and brains of infected mice (70) and in brain tissue from human patients with AIDS-associated meningoencephalitis (71), and melanin is a virulence factor in a murine model of cryptococcosis (68). The cell wall-associated Cu-dependent diphenol oxidase laccase, Lac1, is a central enzyme in the melanization pathway in C. neoformans (72). LAC1 mRNA transcription is active in cerebrospinal fluid of C. neoformans-infected rabbits and is an important virulence factor in in vivo models for cryptococcosis (72). Moreover, during brain infection, there is a strong localization of a Lac1-GFP fusion protein at the cryptococcal cell wall, as opposed to the lungs where this enzyme is localized in the cytosol, suggesting a predominant role of the enzyme during the brain infection (73). Several other C. neoformans proteins are also required for melanin biogenesis, such as the Ccc2 Cu+ pump that facilitates Cu metallation in the secretory compartment and is known to be a virulence factor in murine infection models (33). Collectively, these results highlight the relevance of sufficient Cu sources when the organism is colonizing the brain, which might explain the up-regulation of the Cu importers during cryptococcal meningitis.
Invasive pulmonary aspergillosis, the most common manifestation of Aspergillus fumigatus infection in immunosuppressed individuals, results from the germination of respiratory acquired dormant conidia, ubiquitously present in the environment, to hyphae in the nutrient-rich pulmonary alveoli. In A. fumigatus, which uses the dihydroxynaphthalene pathway for melanin biosynthesis, melanization is strongly related to conidia development. Expression of the laccases ABR1 and ABR2, as well as a putative Cu transporter CTPA, likely involved in providing Cu to the laccases, is induced at the hyphal competence phase, prior to conidiation (74). Improperly melanized conidia loose their echinulate morphology, resulting in conidia with a smooth surface, which provokes a switch from a non-immunogenic status to an immunoreactive phenotype (75). Moreover, the melanin conidia coat inhibits acidification of phagolysosomes of innate immune cells, which favors survival of the pathogen (76). Consequently, A. fumigatus mutants defective in melanization are less virulent and cleared faster than wild-type isogenic strains in murine models of infection.
Cu,Zn-Superoxide Dismutases: Reactive Oxygen Species Scavenging
Reactive oxygen species, including superoxide anion, are relevant weapons used by host innate immune cells to fight infections. Therefore, it is not surprising that classical cytosolic Cu,Zn-SODs are virulence factors in animal models of fungal infection, as has been demonstrated for C. neoformans (77) and C. albicans (78). Remarkably, it has recently been reported that some fungal pathogens are able to secrete other types of Cu-dependent SODs, which, similar to the cytosolic Cu,Zn-SODs, are required for virulence. Some examples are the C. albicans Sod4, Sod5, and Sod6 proteins (Fig. 1) (79), and the Histoplasma capsulatum Sod3 protein (80). The roles of CaSod4 and CaSod6 are not well established, but SOD5 has been demonstrated as a virulence factor in C. albicans; it is transcriptionally induced during the yeast to hyphal transitions, during osmotic and oxidative stress, and in non-fermentable carbon sources (79). The recent elucidation of the Sod5 three-dimensional structure (PDB 4N3T (CuI) and PDB 4N3U (CuII)) has revealed fascinating features regarding the mechanism of action of this protein (81). Sod5 is a monomer that lacks both the typical ligands for Zn and the electrostatic loop region, which are both required for superoxide guidance to the catalytic site in the classical cytosolic Cu,Zn-SODs. As a result, the Sod5 Cu-binding site remains solvent-accessible, potentially allowing Sod5 to capture Cu from the environment without the involvement of an Cu chaperone and to reach almost diffusion rate-limited kinetics in the reaction with superoxide anion. The H. capsulatum Sod3, which is in part liberated from the cell, and in part anchored to the cell wall through a GPI link, functions in scavenging extracellular peroxides. Sod3 is important for H. capsulatum viability when incubated with polymorphonuclear leukocytes or macrophages and is a virulence factor inf mice. Interestingly, deletion of the H. capsulatum cytosolic Cu,Zn-Sod1 does not affect virulence, suggesting that this extremely high ability to survive as an intracellular fungus in immune cells directly relies in the ability of the organism to defeat the host oxidative burden at the extracellular site of attack (80).
Cu Ionophores as Potential Antifungal Agents
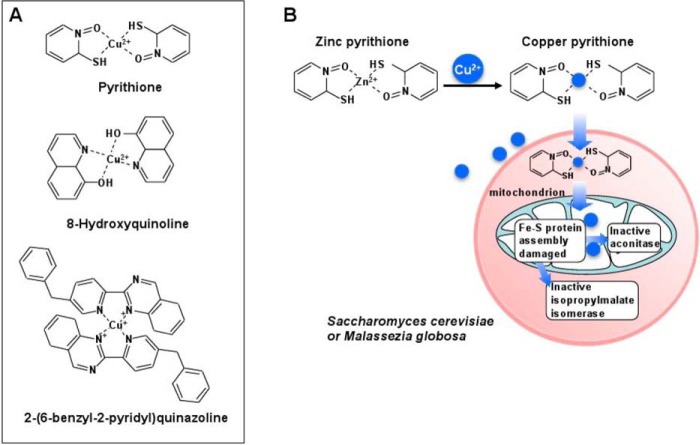
Neutral lipophilic compounds, able to coordinate and shuttle Cu from the extracellular environment to the intracellular milieu (Cu ionophores), offer a way to increase intracellular Cu concentrations, independently of dedicated Cu transporters, and have served as alternative approaches to study the targets for Cu toxicity in yeast (Fig. 3A). Zinc pyrithione (ZPT) is an antifungal molecule present in shampoos commonly used for the treatment of dandruff, a skin condition exacerbated by fungi of the genus Malassezia (82). The mechanism of action of ZPT as an antifungal agent was investigated using S. cerevisiae as a model (83) where micromolar concentrations of ZPT caused a dramatic increase in intracellular Cu concentrations, together with decreased expression of the high affinity Cu transporter Ctr1. Transcriptome analysis demonstrated that ZPT treatment up-regulated the expression of genes coding for the iron import machinery and down-regulated genes coding for heme biosynthesis. Further, using an S. cerevisiae collection of deletion mutants, inactivation of several genes related to the mitochondrial iron-sulfur cluster assembly machinery sensitized cells to ZPT. A closer analysis of iron-sulfur cluster-containing proteins showed decreased specific activities of aconitase, isopropylmalate isomerase (Leu1), and glutamate synthase, whereas little loss of activity was observed for non Fe-S cluster-containing enzymes (Fig. 3B) (83). Similar results have been obtained for 2-(6-benzyl-2-pyridyl)quinazoline (BPQ) (Fig. 3A), another agriculturally important antifungal Cu ionophore that, if used in combination with micromolar concentrations of Cu, potentiates the toxicity of the latter ∼50-fold (84).
FIGURE 3.
Cu ionophores as antifungal agents. A, structures of three well characterized Cu ionophores in coordination with copper. B, proposed antifungal mechanism of ZPT in S. cerevisiae and Malassezia globosa. Some molecules of ZPT exchange Zn2+ for Cu2+, and the complex shuttles into fungal cells independently of the high affinity Cu+ importers. A proposed mechanism of action involves the inactivation of the mitochondrial Fe-S protein assembly machinery, leading to the inactivation of Fe-S cluster-containing proteins such as those found in the mitochondria (aconitase) or in the cytoplasm (isopropylmalate isomerase).
Recent work using conditionally activated Cu ionophores demonstrated how the host Cu compartmentalization within the phagolysosome can be exploited for the design of small molecules that are harmful for the fungal pathogen, yet innocuous for the host (85). To achieve this goal QBP, which is a protected (inactive) version of a well characterized Cu ionophore 8-hydroxyquinoline (8HQ), was used (Fig. 3A). As QBP is converted into 8HQ upon treatment with H2O2 or peroxynitrite, agents typically found in the phagolysosome of activated macrophages, the converted 8HQ can then coordinate Cu (also present at high concentrations in this environment) and shuttle the metal into fungi within the phagolysosome, facilitating immune cell clearance of the fungal pathogen at the site of infection, without conversion to 8HQ in other host compartments. This strategy was shown to work in an intranasal model of infection, where mice infected with C. neoformans showed significantly less C. neoformans burden in the lungs of mice treated with QBP than those treated with vehicle alone, suggesting the potential for further development of conditionally activated Cu ionophores for treating systemic fungal infections (85).
Looking Ahead
At present there is a limited repertoire of effective antifungal agents and a lag in the development pipeline of agents that engage fungi-specific targets. Given the ability of mammalian hosts to mount antifungal responses through the accumulation of toxic Cu within the phagolysosome of innate immune cells, and by Cu limitation in other infectious niches, it is important to understand the underlying host mechanisms and corresponding fungal responses to changes in bioavailable Cu. Chemical biology approaches to manipulate, and perhaps overwhelm, the fungal Cu homeostasis machinery may provide a promising new avenue for the development of novel antifungal therapies.
Acknowledgment
We gratefully acknowledge Richard A. Festa for critical review of this manuscript.
This work was supported by National Institutes of Health Grants GM041840, DK074192, and AI106013 (to D. J. T.). This is the first article in the Thematic Minireview series “Metals at the Host-Pathogen Interface.” The authors declare that they have no conflicts of interest with the contents of this article.
- MT
- metallothionein protein
- GPI
- glycosylphosphatidylinositol
- SOD
- superoxide dismutase
- ZPT
- zinc pyrithione
- 8HQ
- 8-hydroxyquinoline
- QBP
- quinoline boronic acid pinanediol ester
- l-DOPA
- l-3,4-dihydroxyphenylalanine.
References
- 1. O'Brien H. E., Parrent J. L., Jackson J. A., Moncalvo J. M., Vilgalys R. (2005) Fungal community analysis by large-scale sequencing of environmental samples. Appl. Environ. Microbiol. 71, 5544–5550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown G. D., Denning D. W., Gow N. A., Levitz S. M., Netea M. G., White T. C. (2012) Hidden killers: human fungal infections. Sci. Transl. Med. 4, 165rv13. [DOI] [PubMed] [Google Scholar]
- 3. Park B. J., Wannemuehler K. A., Marston B. J., Govender N., Pappas P. G., Chiller T. M. (2009) Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. Aids 23, 525–530 [DOI] [PubMed] [Google Scholar]
- 4. Richardson M. D. (2005) Changing patterns and trends in systemic fungal infections. J. Antimicrob. Chemother. 56, Suppl. 1, i5–i11, 10.1093/jac/dki218 [DOI] [PubMed] [Google Scholar]
- 5. Brown G. D., Denning D. W., Levitz S. M. (2012) Tackling human fungal infections. Science 336, 647. [DOI] [PubMed] [Google Scholar]
- 6. Gray K. C., Palacios D. S., Dailey I., Endo M. M., Uno B. E., Wilcock B. C., Burke M. D. (2012) Amphotericin primarily kills yeast by simply binding ergosterol. Proc. Natl. Acad. Sci. U.S.A. 109, 2234–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lass-Flörl C. (2011) Triazole antifungal agents in invasive fungal infections: a comparative review. Drugs 71, 2405–2419 [DOI] [PubMed] [Google Scholar]
- 8. Mukherjee P. K., Sheehan D., Puzniak L., Schlamm H., Ghannoum M. A. (2011) Echinocandins: are they all the same? J. Chemother. 23, 319–325 [DOI] [PubMed] [Google Scholar]
- 9. Roemer T., Krysan D. J. (2014) Antifungal drug development: challenges, unmet clinical needs, and new approaches. Cold Spring Harb. Perspect. Med. 4, a019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim B. E., Nevitt T., Thiele D. J. (2008) Mechanisms for copper acquisition, distribution and regulation. Nat. Chem. Biol. 4, 176–185 [DOI] [PubMed] [Google Scholar]
- 11. Lutsenko S. (2010) Human copper homeostasis: a network of interconnected pathways. Curr. Opin. Chem. Biol. 14, 211–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ding C., Festa R. A., Sun T. S., Wang Z. Y. (2014) Iron and copper as virulence modulators in human fungal pathogens. Mol. Microbiol. 93, 10–23 [DOI] [PubMed] [Google Scholar]
- 13. Nevitt T., Ohrvik H., Thiele D. J. (2012) Charting the travels of copper in eukaryotes from yeast to mammals. Biochim. Biophys. Acta 1823, 1580–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chaturvedi K. S., Henderson J. P. (2014) Pathogenic adaptations to host-derived antibacterial copper. Front. Cell Infect. Microbiol. 4, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dupont C. L., Grass G., Rensing C. (2011) Copper toxicity and the origin of bacterial resistance: new insights and applications. Metallomics 3, 1109–1118 [DOI] [PubMed] [Google Scholar]
- 16. Ladomersky E., Petris M. J. (2015) Copper tolerance and virulence in bacteria. Metallomics 7, 957–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Samanovic M. I., Ding C., Thiele D. J., Darwin K. H. (2012) Copper in microbial pathogenesis: meddling with the metal. Cell Host. Microbe. 11, 106–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Festa R. A., Thiele D. J. (2011) Copper: an essential metal in biology. Curr. Biol. 21, R877-R883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burq V. (1867) Choléra; De l'immunité Acquise par les Ouvriers en Cuivre: Par Rapport au Choléra Enquêtes Faites à ce Sujet en France et en Italie; Préservation et Traitement par les Armatures et les Sels de Cuivre, Observations et Expériences Depuis, G. Baillière, Paris [Google Scholar]
- 20. Dollwet H. H. A., Sorenson J. R. J. (1985) Historic uses of copper compounds in medicine. Trace Elem. Med. 2, 80–87 [Google Scholar]
- 21. Millardet P. M. A. (1933) The Discovery of Bordeaux Mixture: Three Papers, American Phytopathological Society, Ithaca, NY [Google Scholar]
- 22. Lazary A., Weinberg I., Vatine J. J., Jefidoff A., Bardenstein R., Borkow G., Ohana N. (2014) Reduction of healthcare-associated infections in a long-term care brain injury ward by replacing regular linens with biocidal copper oxide impregnated linens. Int. J. Infect. Dis. 24, 23–29 [DOI] [PubMed] [Google Scholar]
- 23. Casey A. L., Adams D., Karpanen T. J., Lambert P. A., Cookson B. D., Nightingale P., Miruszenko L., Shillam R., Christian P., Elliott T. S. (2010) Role of copper in reducing hospital environment contamination. J. Hosp. Infect. 74, 72–77 [DOI] [PubMed] [Google Scholar]
- 24. Hood M. I., Skaar E. P. (2012) Nutritional immunity: transition metals at the pathogen-host interface. Nat. Rev. Microbiol. 10, 525–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Potrykus J., Ballou E. R., Childers D. S., Brown A. J. (2014) Conflicting interests in the pathogen-host tug of war: fungal micronutrient scavenging versus mammalian nutritional immunity. PLoS Pathog. 10, e1003910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hodgkinson V., Petris M. J. (2012) Copper homeostasis at the host-pathogen interface. J. Biol. Chem. 287, 13549–13555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ding C., Festa R. A., Chen Y. L., Espart A., Palacios Ò., Espín J., Capdevila M., Atrian S., Heitman J., Thiele D. J. (2013) Cryptococcus neoformans copper detoxification machinery is critical for fungal virulence. Cell Host. Microbe. 13, 265–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sun T. S., Ju X., Gao H. L., Wang T., Thiele D. J., Li J. Y., Wang Z. Y., Ding C. (2014) Reciprocal functions of Cryptococcus neoformans copper homeostasis machinery during pulmonary infection and meningoencephalitis. Nat. Commun. 5, 5550. [DOI] [PubMed] [Google Scholar]
- 29. Ding C., Yin J., Tovar E. M., Fitzpatrick D. A., Higgins D. G., Thiele D. J. (2011) The copper regulon of the human fungal pathogen Cryptococcus neoformans H99. Mol. Microbiol. 81, 1560–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Banci L., Bertini I., Ciofi-Baffoni S., Kozyreva T., Zovo K., Palumaa P. (2010) Affinity gradients drive copper to cellular destinations. Nature 465, 645–648 [DOI] [PubMed] [Google Scholar]
- 31. Culotta V. C., Klomp L. W., Strain J., Casareno R. L., Krems B., Gitlin J. D. (1997) The copper chaperone for superoxide dismutase. J. Biol. Chem. 272, 23469–23472 [DOI] [PubMed] [Google Scholar]
- 32. Lin S. J., Pufahl R. A., Dancis A., O'Halloran T. V., Culotta V. C. (1997) A role for the Saccharomyces cerevisiae ATX1 gene in copper trafficking and iron transport. J. Biol. Chem. 272, 9215–9220 [PubMed] [Google Scholar]
- 33. Walton F. J., Idnurm A., Heitman J. (2005) Novel gene functions required for melanization of the human pathogen Cryptococcus neoformans. Mol. Microbiol. 57, 1381–1396 [DOI] [PubMed] [Google Scholar]
- 34. Vest K. E., Leary S. C., Winge D. R., Cobine P. A. (2013) Copper import into the mitochondrial matrix in Saccharomyces cerevisiae is mediated by Pic2, a mitochondrial carrier family protein. J. Biol. Chem. 288, 23884–23892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dickinson E. K., Adams D. L., Schon E. A., Glerum D. M. (2000) A human SCO2 mutation helps define the role of Sco1p in the cytochrome oxidase assembly pathway. J. Biol. Chem. 275, 26780–26785 [DOI] [PubMed] [Google Scholar]
- 36. Thrower A. R., Byrd J., Tarbet E. B., Mehra R. K., Hamer D. H., Winge D. R. (1988) Effect of mutation of cysteinyl residues in yeast Cu-metallothionein. J. Biol. Chem. 263, 7037–7042 [PubMed] [Google Scholar]
- 37. Rees E. M., Lee J., Thiele D. J. (2004) Mobilization of intracellular copper stores by the Ctr2 vacuolar copper transporter. J. Biol. Chem. 279, 54221–54229 [DOI] [PubMed] [Google Scholar]
- 38. Weissman Z., Berdicevsky I., Cavari B. Z., Kornitzer D. (2000) The high copper tolerance of Candida albicans is mediated by a P-type ATPase. Proc. Natl. Acad. Sci. U.S.A. 97, 3520–3525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Keller G., Bird A., Winge D. R. (2005) Independent metalloregulation of Ace1 and Mac1 in Saccharomyces cerevisiae. Eukaryot. Cell 4, 1863–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yamaguchi-Iwai Y., Serpe M., Haile D., Yang W., Kosman D. J., Klausner R. D., Dancis A. (1997) Homeostatic regulation of copper uptake in yeast via direct binding of MAC1 protein to upstream regulatory sequences of FRE1 and CTR1. J. Biol. Chem. 272, 17711–17718 [DOI] [PubMed] [Google Scholar]
- 41. Dameron C. T., Winge D. R., George G. N., Sansone M., Hu S., Hamer D. (1991) A copper-thiolate polynuclear cluster in the ACE1 transcription factor. Proc. Natl. Acad. Sci. U.S.A. 88, 6127–6131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Macomber L., Rensing C., Imlay J. A. (2007) Intracellular copper does not catalyze the formation of oxidative DNA damage in Escherichia coli. J. Bacteriol. 189, 1616–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Macomber L., Imlay J. A. (2009) The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. U.S.A. 106, 8344–8349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stehling O., Vashisht A. A., Mascarenhas J., Jonsson Z. O., Sharma T., Netz D. J., Pierik A. J., Wohlschlegel J. A., Lill R. (2012) MMS19 assembles iron-sulfur proteins required for DNA metabolism and genomic integrity. Science 337, 195–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhou B., Gitschier J. (1997) hCTR1: a human gene for copper uptake identified by complementation in yeast. Proc. Natl. Acad. Sci. U.S.A. 94, 7481–7486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rae T. D., Torres A. S., Pufahl R. A., O'Halloran T. V. (2001) Mechanism of Cu,Zn-superoxide dismutase activation by the human metallochaperone hCCS. J. Biol. Chem. 276, 5166–5176 [DOI] [PubMed] [Google Scholar]
- 47. Hung I. H., Suzuki M., Yamaguchi Y., Yuan D. S., Klausner R. D., Gitlin J. D. (1997) Biochemical characterization of the Wilson disease protein and functional expression in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 272, 21461–21466 [DOI] [PubMed] [Google Scholar]
- 48. Klomp L. W., Lin S. J., Yuan D. S., Klausner R. D., Culotta V. C., Gitlin J. D. (1997) Identification and functional expression of HAH1, a novel human gene involved in copper homeostasis. J. Biol. Chem. 272, 9221–9226 [DOI] [PubMed] [Google Scholar]
- 49. Wagner D., Maser J., Lai B., Cai Z., Barry C. E., 3rd, Höner Zu Bentrup K., Russell D. G., Bermudez L. E. (2005) Elemental analysis of Mycobacterium avium-, Mycobacterium tuberculosis-, and Mycobacterium smegmatis-containing phagosomes indicates pathogen-induced microenvironments within the host cell's endosomal system. J. Immunol. 174, 1491–1500 [DOI] [PubMed] [Google Scholar]
- 50. White C., Lee J., Kambe T., Fritsche K., Petris M. J. (2009) A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J. Biol. Chem. 284, 33949–33956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wolschendorf F., Ackart D., Shrestha T. B., Hascall-Dove L., Nolan S., Lamichhane G., Wang Y., Bossmann S. H., Basaraba R. J., Niederweis M. (2011) Copper resistance is essential for virulence of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U.S.A. 108, 1621–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Achard M. E., Tree J. J., Holden J. A., Simpfendorfer K. R., Wijburg O. L., Strugnell R. A., Schembri M. A., Sweet M. J., Jennings M. P., McEwan A. G. (2010) The multi-copper-ion oxidase CueO of Salmonella enterica serovar Typhimurium is required for systemic virulence. Infect. Immun. 78, 2312–2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Palacios Ò., Espart A., Espín J., Ding C., Thiele D. J., Atrian S., Capdevila M. (2014) Full characterization of the Cu-, Zn-, and Cd-binding properties of CnMT1 and CnMT2, two metallothioneins of the pathogenic fungus Cryptococcus neoformans acting as virulence factors. Metallomics 6, 279–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mehra R. K., Garey J. R., Butt T. R., Gray W. R., Winge D. R. (1989) Candida glabrata metallothioneins: cloning and sequence of the genes and characterization of proteins. J. Biol. Chem. 264, 19747–19753 [PubMed] [Google Scholar]
- 55. Mehra R. K., Thorvaldsen J. L., Macreadie I. G., Winge D. R. (1992) Disruption analysis of metallothionein-encoding genes in Candida glabrata. Gene. 114, 75–80 [DOI] [PubMed] [Google Scholar]
- 56. Gitlin J. D. (1988) Transcriptional regulation of ceruloplasmin gene expression during inflammation. J. Biol. Chem. 263, 6281–6287 [PubMed] [Google Scholar]
- 57. Cabrera A., Alonzo E., Sauble E., Chu Y. L., Nguyen D., Linder M. C., Sato D. S., Mason A. Z. (2008) Copper binding components of blood plasma and organs, and their responses to influx of large doses of 65Cu, in the mouse. Biometals 21, 525–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Linder M. C. (2001) Copper and genomic stability in mammals. Mutat. Res. 475, 141–152 [DOI] [PubMed] [Google Scholar]
- 59. Öhrvik H., Nose Y., Wood L. K., Kim B. E., Gleber S. C., Ralle M., Thiele D. J. (2013) Ctr2 regulates biogenesis of a cleaved form of mammalian Ctr1 metal transporter lacking the copper- and cisplatin-binding ecto-domain. Proc. Natl. Acad. Sci. U.S.A. 110, E4279-E4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Manso Y., Adlard P. A., Carrasco J., Vašák M., Hidalgo J. (2011) Metallothionein and brain inflammation. J. Biol. Inorg. Chem. 16, 1103–1113 [DOI] [PubMed] [Google Scholar]
- 61. Chung R. S., Penkowa M., Dittmann J., King C. E., Bartlett C., Asmussen J. W., Hidalgo J., Carrasco J., Leung Y. K., Walker A. K., Fung S. J., Dunlop S. A., Fitzgerald M., Beazley L. D., Chuah M. I., Vickers J. C., West A. K. (2008) Redefining the role of metallothionein within the injured brain: extracellular metallothioneins play an important role in the astrocyte-neuron response to injury. J. Biol. Chem. 283, 15349–15358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Patel B. N., David S. (1997) A novel glycosylphosphatidylinositol-anchored form of ceruloplasmin is expressed by mammalian astrocytes. J. Biol. Chem. 272, 20185–20190 [DOI] [PubMed] [Google Scholar]
- 63. Butler M. J., Day A. W. (1998) Fungal melanins: a review. Can. J. Microbiol. 44, 1115–1136 [Google Scholar]
- 64. Dixon D. M., Polak A., Szaniszlo P. J. (1987) Pathogenicity and virulence of wild-type and melanin-deficient Wangiella dermatitidis. J. Med. Vet. Mycol. 25, 97–106 [DOI] [PubMed] [Google Scholar]
- 65. Silva M. B., Thomaz L., Marques A. F., Svidzinski A. E., Nosanchuk J. D., Casadevall A., Travassos L. R., Taborda C. P. (2009) Resistance of melanized yeast cells of Paracoccidioides brasiliensis to antimicrobial oxidants and inhibition of phagocytosis using carbohydrates and monoclonal antibody to CD18. Mem. Inst. Oswaldo Cruz 104, 644–648 [DOI] [PubMed] [Google Scholar]
- 66. Morris-Jones R., Youngchim S., Gomez B. L., Aisen P., Hay R. J., Nosanchuk J. D., Casadevall A., Hamilton A. J. (2003) Synthesis of melanin-like pigments by Sporothrix schenckii in vitro and during mammalian infection. Infect. Immun. 71, 4026–4033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ngamskulrungroj P., Price J., Sorrell T., Perfect J. R., Meyer W. (2011) Cryptococcus gattii virulence composite: candidate genes revealed by microarray analysis of high and less virulent Vancouver island outbreak strains. PLoS One 6, e16076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kwon-Chung K. J., Polacheck I., Popkin T. J. (1982) Melanin-lacking mutants of Cryptococcus neoformans and their virulence for mice. J. Bacteriol. 150, 1414–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang Y., Aisen P., Casadevall A. (1995) Cryptococcus neoformans melanin and virulence: mechanisms of action. Infect. Immun. 63, 3131–3136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nosanchuk J. D., Valadon P., Feldmesser M., Casadevall A. (1999) Melanization of Cryptococcus neoformans in murine infection. Mol. Cell Biol. 19, 745–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nosanchuk J. D., Rosas A. L., Lee S. C., Casadevall A. (2000) Melanisation of Cryptococcus neoformans in human brain tissue. Lancet 355, 2049–2050 [DOI] [PubMed] [Google Scholar]
- 72. Salas S. D., Bennett J. E., Kwon-Chung K. J., Perfect J. R., Williamson P. R. (1996) Effect of the laccase gene CNLAC1 on virulence of Cryptococcus neoformans. J. Exp. Med. 184, 377–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Waterman S. R., Hacham M., Panepinto J., Hu G., Shin S., Williamson P. R. (2007) Cell wall targeting of laccase of Cryptococcus neoformans during infection of mice. Infect. Immun. 75, 714–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Upadhyay S., Torres G., Lin X. (2013) Laccases involved in 1,8-dihydroxynaphthalene melanin biosynthesis in Aspergillus fumigatus are regulated by developmental factors and copper homeostasis. Eukaryot. Cell 12, 1641–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pihet M., Vandeputte P., Tronchin G., Renier G., Saulnier P., Georgeault S., Mallet R., Chabasse D., Symoens F., Bouchara J. P. (2009) Melanin is an essential component for the integrity of the cell wall of Aspergillus fumigatus conidia. BMC Microbiol. 9, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Thywißen A., Heinekamp T., Dahse H. M., Schmaler-Ripcke J., Nietzsche S., Zipfel P. F., Brakhage A. A. (2011) Conidial dihydroxynapththalene melanin of the human pthogenic fungus Aspergillus fumigatus interferes with the host endocytosis pathway. Front. Microbiol. 2, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cox G. M., Harrison T. S., McDade H. C., Taborda C. P., Heinrich G., Casadevall A., Perfect J. R. (2003) Superoxide dismutase influences the virulence of Cryptococcus neoformans by affecting growth within macrophages. Infect. Immun. 71, 173–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hwang C. S., Rhie G. E., Oh J. H., Huh W. K., Yim H. S., Kang S. O. (2002) Copper- and zinc-containing superoxide dismutase (Cu/ZnSOD) is required for the protection of Candida albicans against oxidative stresses and the expression of its full virulence. Microbiology 148, 3705–3713 [DOI] [PubMed] [Google Scholar]
- 79. Martchenko M., Alarco A. M., Harcus D., Whiteway M. (2004) Superoxide dismutases in Candida albicans: transcriptional regulation and functional characterization of the hyphal-induced SOD5 gene. Mol. Biol. Cell 15, 456–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Youseff B. H., Holbrook E. D., Smolnycki K. A., Rappleye C. A. (2012) Extracellular superoxide dismutase protects Histoplasma yeast from host-derived oxidative stress. PLoS Pathog. 8, e1002713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gleason J. E., Galaleldeen A., Peterson R. L., Taylor A. B., Holloway S. P., Waninger-Saroni J., Cormack B. P., Cabelli D. E., Hart P. J., Culotta V. C. (2014) Candida albicans SOD5 represents the prototype of an unprecedented class of Cu-only superoxide dismutases required for pathogen defense. Proc. Natl. Acad. Sci. U.S.A. 111, 5866–5871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Boekhout T., Guého-Kellermann E., Mayser P., Velegraki A., eds (2010) Malassezia and the Skin, pp. 6–11, Springer, Heidelberg [Google Scholar]
- 83. Reeder N. L., Kaplan J., Xu J., Youngquist R. S., Wallace J., Hu P., Juhlin K. D., Schwartz J. R., Grant R. A., Fieno A., Nemeth S., Reichling T., Tiesman J. P., Mills T., Steinke M., Wang S. L., Saunders C. W. (2011) Zinc pyrithione inhibits yeast growth through copper influx and inactivation of iron-sulfur proteins. Antimicrob. Agents Chemother. 55, 5753–5760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Foster A. W., Dainty S. J., Patterson C. J., Pohl E., Blackburn H., Wilson C., Hess C. R., Rutherford J. C., Quaranta L., Corran A., Robinson N. J. (2014) A chemical potentiator of copper-accumulation used to investigate the iron-regulons of Saccharomyces cerevisiae. Mol. Microbiol. 93, 317–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Festa R. A., Helsel M. E., Franz K. J., Thiele D. J. (2014) Exploiting innate immune cell activation of a copper-dependent antimicrobial agent during infection. Chem. Biol. 21, 977–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Homann O. R., Dea J., Noble S. M., Johnson A. D. (2009) A phenotypic profile of the Candida albicans regulatory network. PLoS Genet. 5, e1000783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Douglas L. M., Wang H. X., Keppler-Ross S., Dean N., Konopka J. B. (2012) Sur7 promotes plasma membrane organization and is needed for resistance to stressful conditions and to the invasive growth and virulence of Candida albicans. MBio 3, e00254–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. White T. C., Findley K., Dawson T. L., Jr., Scheynius A., Boekhout T., Cuomo C. A., Xu J., Saunders C. W. (2014) Fungi on the skin: dermatophytes and Malassezia. Cold Spring Harb. Perspect. Med. 4, a019802. [DOI] [PMC free article] [PubMed] [Google Scholar]