Abstract
The existence of the Trypanosoma brucei 5' splice site on a small RNA of uniform sequence (the spliced leader or SL RNA) has allowed us to characterize the RNAs with which it interacts in vivo by psoralen crosslinking treatment. Analysis of the most abundant crosslinks formed by the SL RNA allowed us previously to identify the spliced leader-associated (SLA) RNA. The role of this RNA in trans-splicing, as well as the possible existence of an analogous RNA interaction in cis-splicing, is unknown. We show here that the 5' splice site region of the SL RNA is also crosslinked in vivo to a second small RNA. Although it is very small and lacks a 5' trimethylguanosine (TMG) cap, the SLA2RNA possesses counterparts of the conserved U5 snRNA stem-loop 1 and internal loop 1 sequence elements, as well as a potential trypanosome snRNA core protein binding site; these combined features meet the phylogenetic definition of U5 snRNA. Like U5, the SLA2 RNA forms an RNP complex with the U4 and U6 RNAs, and interacts with the 5' splice site region via its putative loop 1 sequence. In a final analogy with U5, the SLA2 RNA is found crosslinked to a molecule identical to the free 5' exon splicing intermediate. These data present a compelling case for the SLA2 RNA not only as an active trans-spliceosomal component, but also for its identification as the trypanosome U5 structural homolog. The presence of a U5-like RNA in this ancient eukaryote establishes the universality of the spliceosomal RNA core components.
Full text
PDF



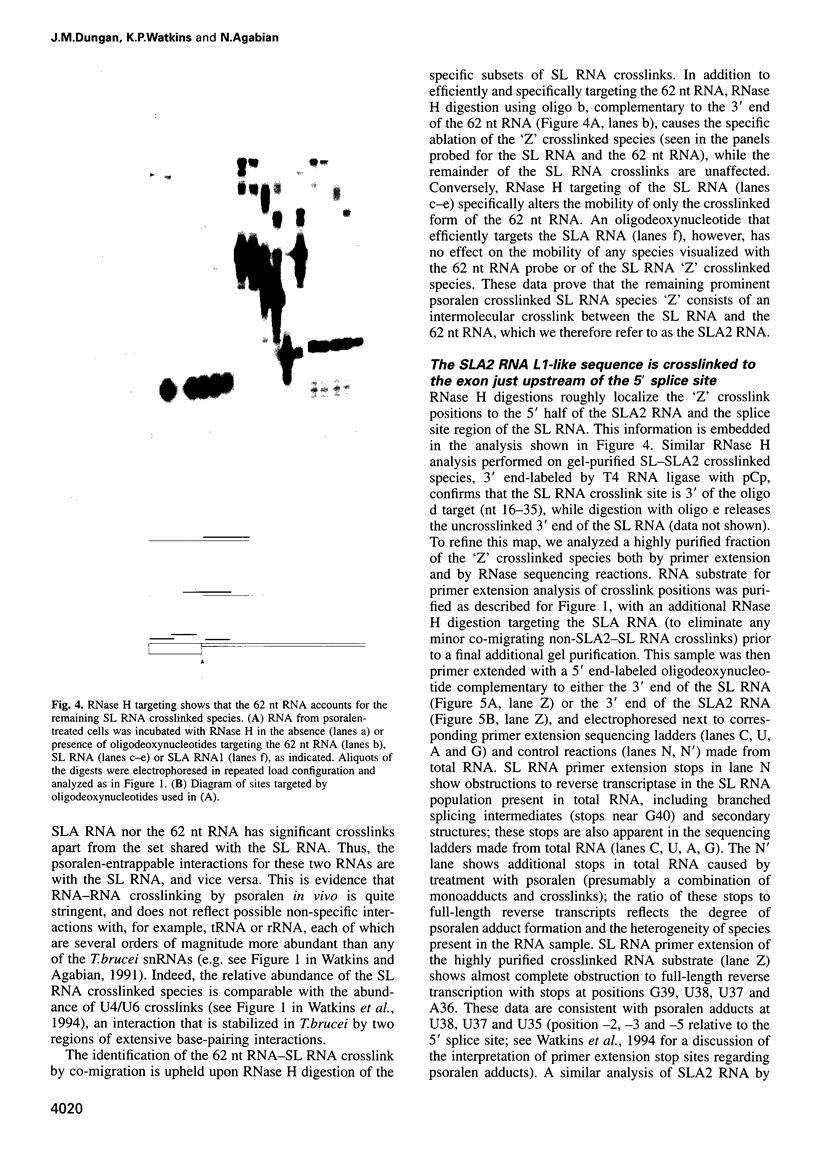
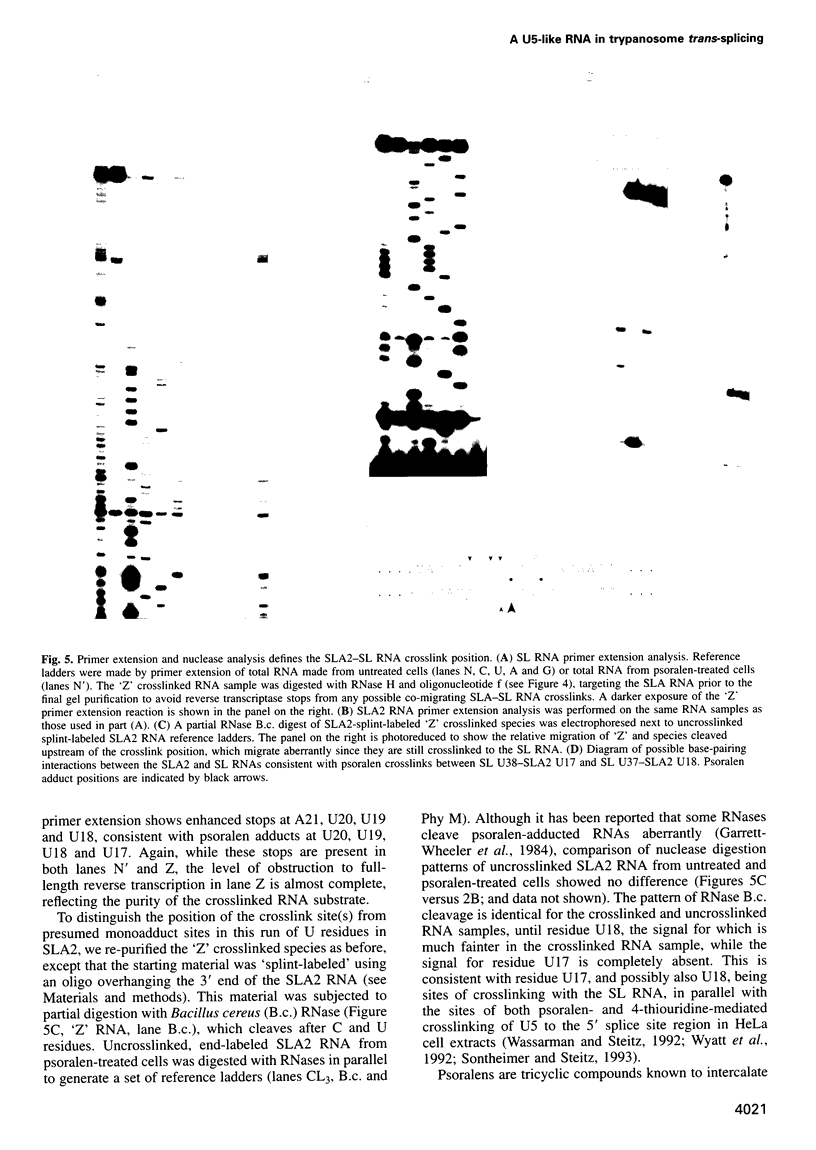




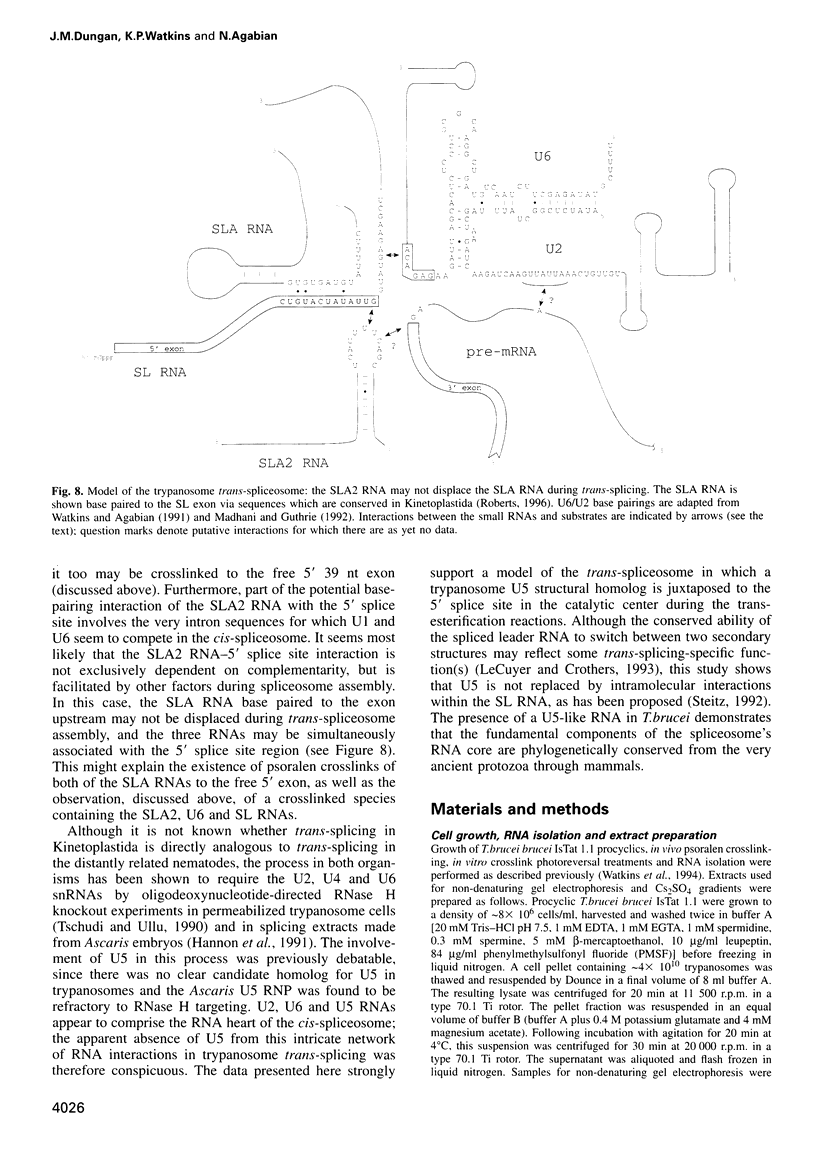



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach M., Lührmann R. Protein-RNA interactions in 20S U5 snRNPs. Biochim Biophys Acta. 1991 Jan 17;1088(1):139–143. doi: 10.1016/0167-4781(91)90164-h. [DOI] [PubMed] [Google Scholar]
- Bangs J. D., Crain P. F., Hashizume T., McCloskey J. A., Boothroyd J. C. Mass spectrometry of mRNA cap 4 from trypanosomatids reveals two novel nucleosides. J Biol Chem. 1992 May 15;267(14):9805–9815. [PubMed] [Google Scholar]
- Black D. L., Pinto A. L. U5 small nuclear ribonucleoprotein: RNA structure analysis and ATP-dependent interaction with U4/U6. Mol Cell Biol. 1989 Aug;9(8):3350–3359. doi: 10.1128/mcb.9.8.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonen L. Trans-splicing of pre-mRNA in plants, animals, and protists. FASEB J. 1993 Jan;7(1):40–46. doi: 10.1096/fasebj.7.1.8422973. [DOI] [PubMed] [Google Scholar]
- Bruzik J. P., Van Doren K., Hirsh D., Steitz J. A. Trans splicing involves a novel form of small nuclear ribonucleoprotein particles. Nature. 1988 Oct 6;335(6190):559–562. doi: 10.1038/335559a0. [DOI] [PubMed] [Google Scholar]
- Cimino G. D., Gamper H. B., Isaacs S. T., Hearst J. E. Psoralens as photoactive probes of nucleic acid structure and function: organic chemistry, photochemistry, and biochemistry. Annu Rev Biochem. 1985;54:1151–1193. doi: 10.1146/annurev.bi.54.070185.005443. [DOI] [PubMed] [Google Scholar]
- Cooper M., Johnston L. H., Beggs J. D. Identification and characterization of Uss1p (Sdb23p): a novel U6 snRNA-associated protein with significant similarity to core proteins of small nuclear ribonucleoproteins. EMBO J. 1995 May 1;14(9):2066–2075. doi: 10.1002/j.1460-2075.1995.tb07198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes J. J., Sontheimer E. J., Seiwert S. D., Steitz J. A. Mutations in the conserved loop of human U5 snRNA generate use of novel cryptic 5' splice sites in vivo. EMBO J. 1993 Dec 15;12(13):5181–5189. doi: 10.1002/j.1460-2075.1993.tb06213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispino J. D., Blencowe B. J., Sharp P. A. Complementation by SR proteins of pre-mRNA splicing reactions depleted of U1 snRNP. Science. 1994 Sep 23;265(5180):1866–1869. doi: 10.1126/science.8091213. [DOI] [PubMed] [Google Scholar]
- Crispino J. D., Sharp P. A. A U6 snRNA:pre-mRNA interaction can be rate-limiting for U1-independent splicing. Genes Dev. 1995 Sep 15;9(18):2314–2323. doi: 10.1101/gad.9.18.2314. [DOI] [PubMed] [Google Scholar]
- Cross M., Günzl A., Palfi Z., Bindereif A. Analysis of small nuclear ribonucleoproteins (RNPs) in Trypanosoma brucei: structural organization and protein components of the spliced leader RNP. Mol Cell Biol. 1991 Nov;11(11):5516–5526. doi: 10.1128/mcb.11.11.5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H. Phy M: an RNase activity specific for U and A residues useful in RNA sequence analysis. Nucleic Acids Res. 1980 Jul 25;8(14):3133–3142. doi: 10.1093/nar/8.14.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England T. E., Bruce A. G., Uhlenbeck O. C. Specific labeling of 3' termini of RNA with T4 RNA ligase. Methods Enzymol. 1980;65(1):65–74. doi: 10.1016/s0076-6879(80)65011-3. [DOI] [PubMed] [Google Scholar]
- Fantoni A., Dare A. O., Tschudi C. RNA polymerase III-mediated transcription of the trypanosome U2 small nuclear RNA gene is controlled by both intragenic and extragenic regulatory elements. Mol Cell Biol. 1994 Mar;14(3):2021–2028. doi: 10.1128/mcb.14.3.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D. N., Roiha H., Guthrie C. Architecture of the U5 small nuclear RNA. Mol Cell Biol. 1994 Mar;14(3):2180–2190. doi: 10.1128/mcb.14.3.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett-Wheeler E., Lockard R. E., Kumar A. Mapping of psoralen cross-linked nucleotides in RNA. Nucleic Acids Res. 1984 Apr 11;12(7):3405–3423. doi: 10.1093/nar/12.7.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. R., Burn V. E. Proteins associated with rabbit reticulocyte mRNA caps during translation as investigated by photocrosslinking. Nucleic Acids Res. 1988 Apr 25;16(8):3437–3454. doi: 10.1093/nar/16.8.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günzl A., Cross M., Bindereif A. Domain structure of U2 and U4/U6 small nuclear ribonucleoprotein particles from Trypanosoma brucei: identification of trans-spliceosomal specific RNA-protein interactions. Mol Cell Biol. 1992 Feb;12(2):468–479. doi: 10.1128/mcb.12.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon G. J., Maroney P. A., Nilsen T. W. U small nuclear ribonucleoprotein requirements for nematode cis- and trans-splicing in vitro. J Biol Chem. 1991 Dec 5;266(34):22792–22795. [PubMed] [Google Scholar]
- Hausner T. P., Giglio L. M., Weiner A. M. Evidence for base-pairing between mammalian U2 and U6 small nuclear ribonucleoprotein particles. Genes Dev. 1990 Dec;4(12A):2146–2156. doi: 10.1101/gad.4.12a.2146. [DOI] [PubMed] [Google Scholar]
- Hermann H., Fabrizio P., Raker V. A., Foulaki K., Hornig H., Brahms H., Lührmann R. snRNP Sm proteins share two evolutionarily conserved sequence motifs which are involved in Sm protein-protein interactions. EMBO J. 1995 May 1;14(9):2076–2088. doi: 10.1002/j.1460-2075.1995.tb07199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges P. E., Jackson S. P., Brown J. D., Beggs J. D. Extraordinary sequence conservation of the PRP8 splicing factor. Yeast. 1995 Apr 15;11(4):337–342. doi: 10.1002/yea.320110406. [DOI] [PubMed] [Google Scholar]
- Izaurralde E., Mattaj I. W. Transport of RNA between nucleus and cytoplasm. Semin Cell Biol. 1992 Aug;3(4):279–288. doi: 10.1016/1043-4682(92)90029-u. [DOI] [PubMed] [Google Scholar]
- Jarmolowski A., Mattaj I. W. The determinants for Sm protein binding to Xenopus U1 and U5 snRNAs are complex and non-identical. EMBO J. 1993 Jan;12(1):223–232. doi: 10.1002/j.1460-2075.1993.tb05648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarska M. M. Analysis of splicing complexes and small nuclear ribonucleoprotein particles by native gel electrophoresis. Methods Enzymol. 1989;180:442–453. doi: 10.1016/0076-6879(89)80116-8. [DOI] [PubMed] [Google Scholar]
- Konforti B. B., Konarska M. M. A short 5' splice site RNA oligo can participate in both steps of splicing in mammalian extracts. RNA. 1995 Oct;1(8):815–827. [PMC free article] [PubMed] [Google Scholar]
- Konforti B. B., Koziolkiewicz M. J., Konarska M. M. Disruption of base pairing between the 5' splice site and the 5' end of U1 snRNA is required for spliceosome assembly. Cell. 1993 Dec 3;75(5):863–873. doi: 10.1016/0092-8674(93)90531-t. [DOI] [PubMed] [Google Scholar]
- LeCuyer K. A., Crothers D. M. The Leptomonas collosoma spliced leader RNA can switch between two alternate structural forms. Biochemistry. 1993 May 25;32(20):5301–5311. doi: 10.1021/bi00071a004. [DOI] [PubMed] [Google Scholar]
- Lelay-Taha M. N., Reveillaud I., Sri-Widada J., Brunel C., Jeanteur P. RNA-protein organization of U1, U5 and U4-U6 small nuclear ribonucleoproteins in HeLa cells. J Mol Biol. 1986 Jun 5;189(3):519–532. doi: 10.1016/0022-2836(86)90321-9. [DOI] [PubMed] [Google Scholar]
- MacMillan A. M., Query C. C., Allerson C. R., Chen S., Verdine G. L., Sharp P. A. Dynamic association of proteins with the pre-mRNA branch region. Genes Dev. 1994 Dec 15;8(24):3008–3020. doi: 10.1101/gad.8.24.3008. [DOI] [PubMed] [Google Scholar]
- Madhani H. D., Guthrie C. A novel base-pairing interaction between U2 and U6 snRNAs suggests a mechanism for the catalytic activation of the spliceosome. Cell. 1992 Nov 27;71(5):803–817. doi: 10.1016/0092-8674(92)90556-r. [DOI] [PubMed] [Google Scholar]
- McNally K. P., Agabian N. Trypanosoma brucei spliced-leader RNA methylations are required for trans splicing in vivo. Mol Cell Biol. 1992 Nov;12(11):4844–4851. doi: 10.1128/mcb.12.11.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzenberg S., Agabian N. Mitochondrial minicircle DNA supports plasmid replication and maintenance in nuclei of Trypanosoma brucei. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5962–5966. doi: 10.1073/pnas.91.13.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaeli S., Roberts T. G., Watkins K. P., Agabian N. Isolation of distinct small ribonucleoprotein particles containing the spliced leader and U2 RNAs of Trypanosoma brucei. J Biol Chem. 1990 Jun 25;265(18):10582–10588. [PubMed] [Google Scholar]
- Mottram J., Perry K. L., Lizardi P. M., Lührmann R., Agabian N., Nelson R. G. Isolation and sequence of four small nuclear U RNA genes of Trypanosoma brucei subsp. brucei: identification of the U2, U4, and U6 RNA analogs. Mol Cell Biol. 1989 Mar;9(3):1212–1223. doi: 10.1128/mcb.9.3.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman A. J., Norman C. U5 snRNA interacts with exon sequences at 5' and 3' splice sites. Cell. 1992 Feb 21;68(4):743–754. doi: 10.1016/0092-8674(92)90149-7. [DOI] [PubMed] [Google Scholar]
- Newman A. J., Teigelkamp S., Beggs J. D. snRNA interactions at 5' and 3' splice sites monitored by photoactivated crosslinking in yeast spliceosomes. RNA. 1995 Nov;1(9):968–980. [PMC free article] [PubMed] [Google Scholar]
- Newman A., Norman C. Mutations in yeast U5 snRNA alter the specificity of 5' splice-site cleavage. Cell. 1991 Apr 5;65(1):115–123. doi: 10.1016/0092-8674(91)90413-s. [DOI] [PubMed] [Google Scholar]
- Nilsen T. W. Trans-splicing of nematode premessenger RNA. Annu Rev Microbiol. 1993;47:413–440. doi: 10.1146/annurev.mi.47.100193.002213. [DOI] [PubMed] [Google Scholar]
- Palfi Z., Bindereif A. Immunological characterization and intracellular localization of trans-spliceosomal small nuclear ribonucleoproteins in Trypanosoma brucei. J Biol Chem. 1992 Oct 5;267(28):20159–20163. [PubMed] [Google Scholar]
- Palfi Z., Günzl A., Cross M., Bindereif A. Affinity purification of Trypanosoma brucei small nuclear ribonucleoproteins reveals common and specific protein components. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9097–9101. doi: 10.1073/pnas.88.20.9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palfi Z., Xu G. L., Bindereif A. Spliced leader-associated RNA of trypanosomes. Sequence conservation and association with protein components common to trans-spliceosomal ribonucleoproteins. J Biol Chem. 1994 Dec 2;269(48):30620–30625. [PubMed] [Google Scholar]
- Patzelt E., Perry K. L., Agabian N. Mapping of branch sites in trans-spliced pre-mRNAs of Trypanosoma brucei. Mol Cell Biol. 1989 Oct;9(10):4291–4297. doi: 10.1128/mcb.9.10.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry K. L., Watkins K. P., Agabian N. Trypanosome mRNAs have unusual "cap 4" structures acquired by addition of a spliced leader. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8190–8194. doi: 10.1073/pnas.84.23.8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shambaugh J. D., Hannon G. E., Nilsen T. W. The spliceosomal U small nuclear RNAs of Ascaris lumbricoides. Mol Biochem Parasitol. 1994 Apr;64(2):349–352. doi: 10.1016/0166-6851(94)00040-9. [DOI] [PubMed] [Google Scholar]
- Singh R., Reddy R. Gamma-monomethyl phosphate: a cap structure in spliceosomal U6 small nuclear RNA. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8280–8283. doi: 10.1073/pnas.86.21.8280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer E. J., Steitz J. A. The U5 and U6 small nuclear RNAs as active site components of the spliceosome. Science. 1993 Dec 24;262(5142):1989–1996. doi: 10.1126/science.8266094. [DOI] [PubMed] [Google Scholar]
- Steitz J. A. Splicing takes a holliday. Science. 1992 Aug 14;257(5072):888–889. doi: 10.1126/science.1386941. [DOI] [PubMed] [Google Scholar]
- Szkukalek A., Myslinski E., Mougin A., Luhrmann R., Branlant C. Phylogenetic conservation of modified nucleotides in the terminal loop 1 of the spliceosomal U5 snRNA. Biochimie. 1995;77(1-2):16–21. doi: 10.1016/0300-9084(96)88099-0. [DOI] [PubMed] [Google Scholar]
- Ségault V., Will C. L., Sproat B. S., Lührmann R. In vitro reconstitution of mammalian U2 and U5 snRNPs active in splicing: Sm proteins are functionally interchangeable and are essential for the formation of functional U2 and U5 snRNPs. EMBO J. 1995 Aug 15;14(16):4010–4021. doi: 10.1002/j.1460-2075.1995.tb00072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarn W. Y., Steitz J. A. SR proteins can compensate for the loss of U1 snRNP functions in vitro. Genes Dev. 1994 Nov 15;8(22):2704–2717. doi: 10.1101/gad.8.22.2704. [DOI] [PubMed] [Google Scholar]
- Terns M. P., Grimm C., Lund E., Dahlberg J. E. A common maturation pathway for small nucleolar RNAs. EMBO J. 1995 Oct 2;14(19):4860–4871. doi: 10.1002/j.1460-2075.1995.tb00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J., Lea K., Zucker-Aprison E., Blumenthal T. The spliceosomal snRNAs of Caenorhabditis elegans. Nucleic Acids Res. 1990 May 11;18(9):2633–2642. doi: 10.1093/nar/18.9.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. F., Hearst J. E. Structure of E. coli 16S RNA elucidated by psoralen crosslinking. Cell. 1983 Apr;32(4):1355–1365. doi: 10.1016/0092-8674(83)90316-1. [DOI] [PubMed] [Google Scholar]
- Tschudi C., Krainer A. R., Ullu E. The U6 small nuclear RNA from Trypanosoma brucei. Nucleic Acids Res. 1988 Dec 9;16(23):11375–11375. doi: 10.1093/nar/16.23.11375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschudi C., Richards F. F., Ullu E. The U2 RNA analogue of Trypanosoma brucei gambiense: implications for a splicing mechanism in trypanosomes. Nucleic Acids Res. 1986 Nov 25;14(22):8893–8903. doi: 10.1093/nar/14.22.8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschudi C., Ullu E. Destruction of U2, U4, or U6 small nuclear RNA blocks trans splicing in trypanosome cells. Cell. 1990 May 4;61(3):459–466. doi: 10.1016/0092-8674(90)90527-l. [DOI] [PubMed] [Google Scholar]
- Ullu E., Tschudi C. Permeable trypanosome cells as a model system for transcription and trans-splicing. Nucleic Acids Res. 1990 Jun 11;18(11):3319–3326. doi: 10.1093/nar/18.11.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umen J. G., Guthrie C. The second catalytic step of pre-mRNA splicing. RNA. 1995 Nov;1(9):869–885. [PMC free article] [PubMed] [Google Scholar]
- Wassarman D. A., Steitz J. A. Interactions of small nuclear RNA's with precursor messenger RNA during in vitro splicing. Science. 1992 Sep 25;257(5078):1918–1925. doi: 10.1126/science.1411506. [DOI] [PubMed] [Google Scholar]
- Watkins K. P., Agabian N. In vivo UV cross-linking of U snRNAs that participate in trypanosome trans-splicing. Genes Dev. 1991 Oct;5(10):1859–1869. doi: 10.1101/gad.5.10.1859. [DOI] [PubMed] [Google Scholar]
- Watkins K. P., Dungan J. M., Agabian N. Identification of a small RNA that interacts with the 5' splice site of the Trypanosoma brucei spliced leader RNA in vivo. Cell. 1994 Jan 14;76(1):171–182. doi: 10.1016/0092-8674(94)90181-3. [DOI] [PubMed] [Google Scholar]
- Will C. L., Behrens S. E., Lührmann R. Protein composition of mammalian spliceosomal snRNPs. Mol Biol Rep. 1993 Aug;18(2):121–126. doi: 10.1007/BF00986766. [DOI] [PubMed] [Google Scholar]
- Wyatt J. R., Sontheimer E. J., Steitz J. A. Site-specific cross-linking of mammalian U5 snRNP to the 5' splice site before the first step of pre-mRNA splicing. Genes Dev. 1992 Dec;6(12B):2542–2553. doi: 10.1101/gad.6.12b.2542. [DOI] [PubMed] [Google Scholar]
- Zuker M., Jaeger J. A., Turner D. H. A comparison of optimal and suboptimal RNA secondary structures predicted by free energy minimization with structures determined by phylogenetic comparison. Nucleic Acids Res. 1991 May 25;19(10):2707–2714. doi: 10.1093/nar/19.10.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]