Abstract
Proteins of the tolloid/bone morphogenetic protein (BMP)-1 family play important roles in the differentiation of cell fates. Among those proteins are BMP-1, which plays a role in cartilage and bone formation in mammals, the TOLLOID protein, which is required for the establishment of the dorsoventral axis of Drosophila embryos and BP10/SpAN, which are thought to act in the morphogenesis of sea urchins. These proteins have some properties in common. First, they contain the astacin metalloprotease domain, the CUB domain and the epidermal growth factor-like domain. Second, they are expressed in embryos at stages expected for their role in cell differentiation. Third, at least BMP-1 and TOLLOID are thought to interact with proteins of the transforming growth factor-beta family. We report that the hch-1 gene of the nematode Caenorhabditis elegans encodes a tolloid/BMP-1 family protein. The protein has the characteristic domains common to the tolloid/ BMP-1 family. Like other members of the family, it is expressed in embryos. However, the phenotype of hch-1 mutants shows that it is required for normal hatching and normal migration of a post-embryonic neuroblast. Furthermore, in spite of its expression in embryogenesis, it is not required for the viability of embryos. These results show new functions of the tolloid/BMP-1 family proteins and give insight into their evolution.
Full text
PDF

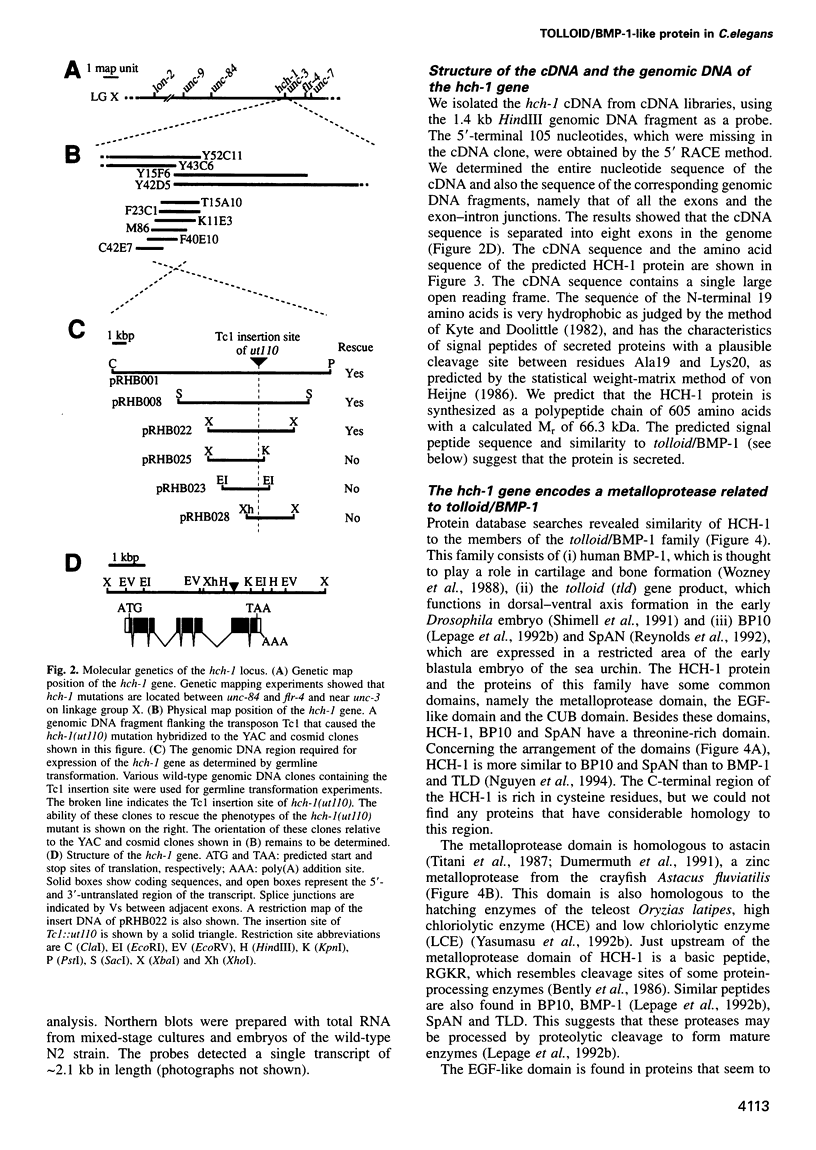


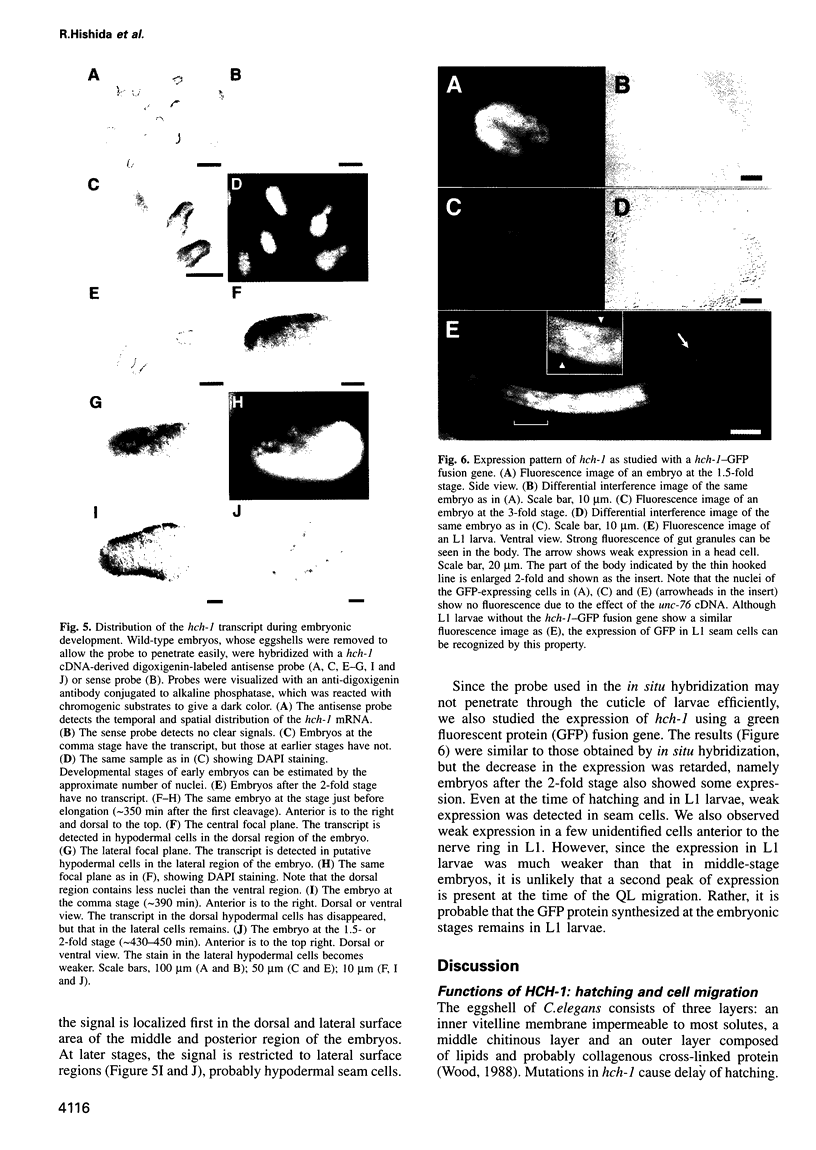






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appella E., Weber I. T., Blasi F. Structure and function of epidermal growth factor-like regions in proteins. FEBS Lett. 1988 Apr 11;231(1):1–4. doi: 10.1016/0014-5793(88)80690-2. [DOI] [PubMed] [Google Scholar]
- Bentley A. K., Rees D. J., Rizza C., Brownlee G. G. Defective propeptide processing of blood clotting factor IX caused by mutation of arginine to glutamine at position -4. Cell. 1986 May 9;45(3):343–348. doi: 10.1016/0092-8674(86)90319-3. [DOI] [PubMed] [Google Scholar]
- Bork P., Beckmann G. The CUB domain. A widespread module in developmentally regulated proteins. J Mol Biol. 1993 May 20;231(2):539–545. doi: 10.1006/jmbi.1993.1305. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974 May;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M., Tu Y., Euskirchen G., Ward W. W., Prasher D. C. Green fluorescent protein as a marker for gene expression. Science. 1994 Feb 11;263(5148):802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- Childs S. R., O'Connor M. B. Two domains of the tolloid protein contribute to its unusual genetic interaction with decapentaplegic. Dev Biol. 1994 Mar;162(1):209–220. doi: 10.1006/dbio.1994.1079. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clark S. G., Lu X., Horvitz H. R. The Caenorhabditis elegans locus lin-15, a negative regulator of a tyrosine kinase signaling pathway, encodes two different proteins. Genetics. 1994 Aug;137(4):987–997. doi: 10.1093/genetics/137.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson A., Kozono Y., Lutterbach B., Shownkeen R., Sulston J., Waterston R. YACs and the C. elegans genome. Bioessays. 1991 Aug;13(8):413–417. doi: 10.1002/bies.950130809. [DOI] [PubMed] [Google Scholar]
- Coulson A., Sulston J., Brenner S., Karn J. Toward a physical map of the genome of the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7821–7825. doi: 10.1073/pnas.83.20.7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson A., Waterston R., Kiff J., Sulston J., Kohara Y. Genome linking with yeast artificial chromosomes. Nature. 1988 Sep 8;335(6186):184–186. doi: 10.1038/335184a0. [DOI] [PubMed] [Google Scholar]
- Dumermuth E., Sterchi E. E., Jiang W. P., Wolz R. L., Bond J. S., Flannery A. V., Beynon R. J. The astacin family of metalloendopeptidases. J Biol Chem. 1991 Nov 15;266(32):21381–21385. [PubMed] [Google Scholar]
- Estevez M., Attisano L., Wrana J. L., Albert P. S., Massagué J., Riddle D. L. The daf-4 gene encodes a bone morphogenetic protein receptor controlling C. elegans dauer larva development. Nature. 1993 Oct 14;365(6447):644–649. doi: 10.1038/365644a0. [DOI] [PubMed] [Google Scholar]
- Ferguson E. L., Anderson K. V. Localized enhancement and repression of the activity of the TGF-beta family member, decapentaplegic, is necessary for dorsal-ventral pattern formation in the Drosophila embryo. Development. 1992 Mar;114(3):583–597. doi: 10.1242/dev.114.3.583. [DOI] [PubMed] [Google Scholar]
- Fire A. Integrative transformation of Caenorhabditis elegans. EMBO J. 1986 Oct;5(10):2673–2680. doi: 10.1002/j.1460-2075.1986.tb04550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukagawa M., Suzuki N., Hogan B. L., Jones C. M. Embryonic expression of mouse bone morphogenetic protein-1 (BMP-1), which is related to the Drosophila dorsoventral gene tolloid and encodes a putative astacin metalloendopeptidase. Dev Biol. 1994 May;163(1):175–183. doi: 10.1006/dbio.1994.1133. [DOI] [PubMed] [Google Scholar]
- Georgi L. L., Albert P. S., Riddle D. L. daf-1, a C. elegans gene controlling dauer larva development, encodes a novel receptor protein kinase. Cell. 1990 May 18;61(4):635–645. doi: 10.1016/0092-8674(90)90475-t. [DOI] [PubMed] [Google Scholar]
- Greenwald I. Structure/function studies of lin-12/Notch proteins. Curr Opin Genet Dev. 1994 Aug;4(4):556–562. doi: 10.1016/0959-437x(94)90072-b. [DOI] [PubMed] [Google Scholar]
- Greenwald I. lin-12, a nematode homeotic gene, is homologous to a set of mammalian proteins that includes epidermal growth factor. Cell. 1985 Dec;43(3 Pt 2):583–590. doi: 10.1016/0092-8674(85)90230-2. [DOI] [PubMed] [Google Scholar]
- Hamelin M., Scott I. M., Way J. C., Culotti J. G. The mec-7 beta-tubulin gene of Caenorhabditis elegans is expressed primarily in the touch receptor neurons. EMBO J. 1992 Aug;11(8):2885–2893. doi: 10.1002/j.1460-2075.1992.tb05357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgecock E. M., Culotti J. G., Hall D. H., Stern B. D. Genetics of cell and axon migrations in Caenorhabditis elegans. Development. 1987 Jul;100(3):365–382. doi: 10.1242/dev.100.3.365. [DOI] [PubMed] [Google Scholar]
- Heim R., Cubitt A. B., Tsien R. Y. Improved green fluorescence. Nature. 1995 Feb 23;373(6516):663–664. doi: 10.1038/373663b0. [DOI] [PubMed] [Google Scholar]
- Hwang S. P., Partin J. S., Lennarz W. J. Characterization of a homolog of human bone morphogenetic protein 1 in the embryo of the sea urchin, Strongylocentrotus purpuratus. Development. 1994 Mar;120(3):559–568. doi: 10.1242/dev.120.3.559. [DOI] [PubMed] [Google Scholar]
- Katsura I. In search of new mutants in cell-signaling systems of the nematode Caenorhabditis elegans. Review. Genetica. 1993;88(2-3):137–146. doi: 10.1007/BF02424470. [DOI] [PubMed] [Google Scholar]
- Kessler E., Takahara K., Biniaminov L., Brusel M., Greenspan D. S. Bone morphogenetic protein-1: the type I procollagen C-proteinase. Science. 1996 Jan 19;271(5247):360–362. doi: 10.1126/science.271.5247.360. [DOI] [PubMed] [Google Scholar]
- Kramer J. M., French R. P., Park E. C., Johnson J. J. The Caenorhabditis elegans rol-6 gene, which interacts with the sqt-1 collagen gene to determine organismal morphology, encodes a collagen. Mol Cell Biol. 1990 May;10(5):2081–2089. doi: 10.1128/mcb.10.5.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lepage T., Gache C. Early expression of a collagenase-like hatching enzyme gene in the sea urchin embryo. EMBO J. 1990 Sep;9(9):3003–3012. doi: 10.1002/j.1460-2075.1990.tb07493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage T., Gache C. Purification and characterization of the sea urchin embryo hatching enzyme. J Biol Chem. 1989 Mar 25;264(9):4787–4793. [PubMed] [Google Scholar]
- Lepage T., Ghiglione C., Gache C. Spatial and temporal expression pattern during sea urchin embryogenesis of a gene coding for a protease homologous to the human protein BMP-1 and to the product of the Drosophila dorsal-ventral patterning gene tolloid. Development. 1992 Jan;114(1):147–163. doi: 10.1242/dev.114.1.147. [DOI] [PubMed] [Google Scholar]
- Lepage T., Sardet C., Gache C. Spatial expression of the hatching enzyme gene in the sea urchin embryo. Dev Biol. 1992 Mar;150(1):23–32. doi: 10.1016/0012-1606(92)90004-z. [DOI] [PubMed] [Google Scholar]
- Leytus S. P., Kurachi K., Sakariassen K. S., Davie E. W. Nucleotide sequence of the cDNA coding for human complement C1r. Biochemistry. 1986 Aug 26;25(17):4855–4863. doi: 10.1021/bi00365a020. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- Mello C. C., Kramer J. M., Stinchcomb D., Ambros V. Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991 Dec;10(12):3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I., Moerman D. G., Waterston R. H. Analysis of a mutator activity necessary for germline transposition and excision of Tc1 transposable elements in Caenorhabditis elegans. Genetics. 1988 Oct;120(2):397–407. doi: 10.1093/genetics/120.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T., Jamal J., Shimell M. J., Arora K., O'Connor M. B. Characterization of tolloid-related-1: a BMP-1-like product that is required during larval and pupal stages of Drosophila development. Dev Biol. 1994 Dec;166(2):569–586. doi: 10.1006/dbio.1994.1338. [DOI] [PubMed] [Google Scholar]
- O'Connell B. C., Hagen F. K., Tabak L. A. The influence of flanking sequence on the O-glycosylation of threonine in vitro. J Biol Chem. 1992 Dec 15;267(35):25010–25018. [PubMed] [Google Scholar]
- Padgett R. W., St Johnston R. D., Gelbart W. M. A transcript from a Drosophila pattern gene predicts a protein homologous to the transforming growth factor-beta family. Nature. 1987 Jan 1;325(6099):81–84. doi: 10.1038/325081a0. [DOI] [PubMed] [Google Scholar]
- Reynolds S. D., Angerer L. M., Palis J., Nasir A., Angerer R. C. Early mRNAs, spatially restricted along the animal-vegetal axis of sea urchin embryos, include one encoding a protein related to tolloid and BMP-1. Development. 1992 Mar;114(3):769–786. doi: 10.1242/dev.114.3.769. [DOI] [PubMed] [Google Scholar]
- Rosenzweig B., Liao L. W., Hirsh D. Sequence of the C. elegans transposable element Tc1. Nucleic Acids Res. 1983 Jun 25;11(12):4201–4209. doi: 10.1093/nar/11.12.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage C., Das P., Finelli A. L., Townsend S. R., Sun C. Y., Baird S. E., Padgett R. W. Caenorhabditis elegans genes sma-2, sma-3, and sma-4 define a conserved family of transforming growth factor beta pathway components. Proc Natl Acad Sci U S A. 1996 Jan 23;93(2):790–794. doi: 10.1073/pnas.93.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydoux G., Fire A. Soma-germline asymmetry in the distributions of embryonic RNAs in Caenorhabditis elegans. Development. 1994 Oct;120(10):2823–2834. doi: 10.1242/dev.120.10.2823. [DOI] [PubMed] [Google Scholar]
- Shimell M. J., Ferguson E. L., Childs S. R., O'Connor M. B. The Drosophila dorsal-ventral patterning gene tolloid is related to human bone morphogenetic protein 1. Cell. 1991 Nov 1;67(3):469–481. doi: 10.1016/0092-8674(91)90522-z. [DOI] [PubMed] [Google Scholar]
- Stenzel P., Angerer L. M., Smith B. J., Angerer R. C., Vale W. W. The univin gene encodes a member of the transforming growth factor-beta superfamily with restricted expression in the sea urchin embryo. Dev Biol. 1994 Nov;166(1):149–158. doi: 10.1006/dbio.1994.1303. [DOI] [PubMed] [Google Scholar]
- Sulston J. E., Schierenberg E., White J. G., Thomson J. N. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983 Nov;100(1):64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Sulston J., Du Z., Thomas K., Wilson R., Hillier L., Staden R., Halloran N., Green P., Thierry-Mieg J., Qiu L. The C. elegans genome sequencing project: a beginning. Nature. 1992 Mar 5;356(6364):37–41. doi: 10.1038/356037a0. [DOI] [PubMed] [Google Scholar]
- Titani K., Torff H. J., Hormel S., Kumar S., Walsh K. A., Rödl J., Neurath H., Zwilling R. Amino acid sequence of a unique protease from the crayfish Astacus fluviatilis. Biochemistry. 1987 Jan 13;26(1):222–226. doi: 10.1021/bi00375a029. [DOI] [PubMed] [Google Scholar]
- Tosi M., Duponchel C., Meo T., Julier C. Complete cDNA sequence of human complement Cls and close physical linkage of the homologous genes Cls and Clr. Biochemistry. 1987 Dec 29;26(26):8516–8524. doi: 10.1021/bi00400a004. [DOI] [PubMed] [Google Scholar]
- Way J. C., Wang L., Run J. Q., Wang A. The mec-3 gene contains cis-acting elements mediating positive and negative regulation in cells produced by asymmetric cell division in Caenorhabditis elegans. Genes Dev. 1991 Dec;5(12A):2199–2211. doi: 10.1101/gad.5.12a.2199. [DOI] [PubMed] [Google Scholar]
- Wilson I. B., Gavel Y., von Heijne G. Amino acid distributions around O-linked glycosylation sites. Biochem J. 1991 Apr 15;275(Pt 2):529–534. doi: 10.1042/bj2750529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. B., Hecht R., Carr S., Vanderslice R., Wolf N., Hirsh D. Parental effects and phenotypic characterization of mutations that affect early development in Caenorhabditis elegans. Dev Biol. 1980 Feb;74(2):446–469. doi: 10.1016/0012-1606(80)90445-5. [DOI] [PubMed] [Google Scholar]
- Wozney J. M., Rosen V., Celeste A. J., Mitsock L. M., Whitters M. J., Kriz R. W., Hewick R. M., Wang E. A. Novel regulators of bone formation: molecular clones and activities. Science. 1988 Dec 16;242(4885):1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- Yan L., Pollock G. H., Nagase H., Sarras M. P., Jr A 25.7 x 10(3) M(r) hydra metalloproteinase (HMP1), a member of the astacin family, localizes to the extracellular matrix of Hydra vulgaris in a head-specific manner and has a developmental function. Development. 1995 Jun;121(6):1591–1602. doi: 10.1242/dev.121.6.1591. [DOI] [PubMed] [Google Scholar]
- Yasumasu S., Iuchi I., Yamagami K. Isolation and some properties of low choriolytic enzyme (LCE), a component of the hatching enzyme of the teleost, Oryzias latipes. J Biochem. 1989 Feb;105(2):212–218. doi: 10.1093/oxfordjournals.jbchem.a122641. [DOI] [PubMed] [Google Scholar]
- Yasumasu S., Iuchi I., Yamagami K. Purification and partial characterization of high choriolytic enzyme (HCE), a component of the hatching enzyme of the teleost, Oryzias latipes. J Biochem. 1989 Feb;105(2):204–211. doi: 10.1093/oxfordjournals.jbchem.a122640. [DOI] [PubMed] [Google Scholar]
- Yasumasu S., Katow S., Hamazaki T. S., Iuchi I., Yamagami K. Two constituent proteases of a teleostean hatching enzyme: concurrent syntheses and packaging in the same secretory granules in discrete arrangement. Dev Biol. 1992 Feb;149(2):349–356. doi: 10.1016/0012-1606(92)90290-w. [DOI] [PubMed] [Google Scholar]
- Yasumasu S., Yamada K., Akasaka K., Mitsunaga K., Iuchi I., Shimada H., Yamagami K. Isolation of cDNAs for LCE and HCE, two constituent proteases of the hatching enzyme of Oryzias latipes, and concurrent expression of their mRNAs during development. Dev Biol. 1992 Oct;153(2):250–258. doi: 10.1016/0012-1606(92)90110-3. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]