In Brief
Glucosuria, the presence of glucose in the urine, has long been regarded as a consequence of uncontrolled diabetes. However, glucose excretion can be induced by blocking the activity of the renal sodium-glucose cotransporter 2 (SGLT-2). This mechanism corrects hyperglycemia independently of insulin. This article provides an overview of the paradigm shift that triggered the development of the SGLT-2 inhibitor class of agents and summarizes the available evidence from clinical studies to date.
Type 2 diabetes is a progressive, chronic metabolic disease characterized by hyperglycemia.1 Beyond being a diagnostic marker, elevated glucose is a key factor in the two abnormalities that are at the core of type 2 diabetes: pancreatic β-cell failure and insulin resistance. Chronic hyperglycemia can induce apoptosis of β-cells that is not countered by a compensatory increase in β-cell neogenesis and can lead to decreased insulin gene transcription. The detrimental effect of excessive glucose concentrations is referred to as “glucotoxicity.”2
Despite therapeutic advances, the incidence and prevalence of diabetes continue to surge. An estimated 25.8 million people in the United States have diabetes.3 The incidence could triple to one in three by 2050.4 Worldwide, the number of individuals with diabetes is projected to rise from 366 million in 2011 to 552 million by 2030, which is the equivalent of approximately three new cases being diagnosed every 10 seconds.5
Type 2 diabetes doubles the risk of cardiovascular disease,6 and macrovascular complications (myocardial infarction and stroke) are a common cause of death in patients with type 2 diabetes.3 The U.K. Prospective Diabetes Study showed that every 1% absolute decline in mean A1C was associated with a 37% reduction in the risk of microvascular complications and a 21% reduction in the risk of any diabetes-related complication or death.7 Diabetes also exacts a tremendous economic burden; in the United States, direct and indirect costs totaled $174 billion in 2007.3
Meeting treatment goals is elusive for many people with diabetes.8–10 Data from the National Health and Nutrition Examination Survey from 2003 to 2006 showed that only 57.1% of adults with diagnosed diabetes achieved an A1C < 7%, 45.5% had a blood pressure level < 130/80 mmHg, and 46.5% had an LDL cholesterol level < 100 mg/dl.10 Only 12.2% of people with diabetes reached all three goals.10
There are multiple barriers to achieving optimal glycemic control. The pathophysiology of diabetes is complex and involves multiple defects: β-cell failure (decreased insulin secretion); insulin resistance in muscle, brain, and liver; increased glucagon secretion in α-cells; increased lipolysis in adipose tissue; incretin deficiency and resistance in the gastrointestinal (GI) tract; and increased glucose reabsorption in the kidney.11 Other obstacles include clinical inertia, or the failure to start or intensify therapy when clinically indicated.12 There is some evidence that patients with type 2 diabetes who have lower medication adherence are less likely to undergo treatment intensification.13 Reaching glycemic targets may also be hampered by aversion to adding insulin or implementing lifestyle changes. Barriers such as cost and formulary restrictions also present challenges. Current medications for type 2 diabetes have potential adverse effects; sulfonylureas and insulin, for example, can cause hypoglycemia and weight gain.14 Thus, the search continues for novel therapeutic agents that can help patients avoid these limiting side effects while providing glycemic control.
Although the concept of the kidney playing a significant role in glucose balance is not new, only recently has this organ been considered a potential therapeutic target. Sodium-glucose cotransporters (SGLTs), namely SGLT-1 and SGLT-2, facilitate reabsorption of glucose back into the plasma. Inhibiting this process promotes glucosuria and thus reduces blood glucose. This review describes the mechanism of action of this new class of treatment for type 2 diabetes, as well as published data on its efficacy and safety.
Role of the Kidney in Glucose Homeostasis
Despite wide fluctuations in the daily supply of glucose and the body’s demand for it, homeostatic mechanisms maintain plasma glucose levels within a narrow range, with average levels of ∼ 90–100 mg/dl in a 24-hour period.15,16 The kidney’s crucial role in maintaining glucose balance was first described as early as 1938.17 Along with the liver, the kidney supplies glucose during periods of fasting. The renal contribution to gluconeogenesis is ∼ 15–55 g/day, or 20–25% of the glucose released into the circulation after an overnight fast.17,18
The reabsorption of glucose filtered into the glomerular filtrate is the primary mechanism by which the kidney influences glucose homeostasis.18 Glucose excretion in urine is the net difference between the amount of glucose filtered by the kidney and the amount reabsorbed. In healthy individuals, the kidney contributes significantly to glucose homeostasis by reabsorbing essentially all of the ∼ 180 g of glucose that it filters per day.19 Individuals without diabetes thus have very little or no glucose present in the urine.
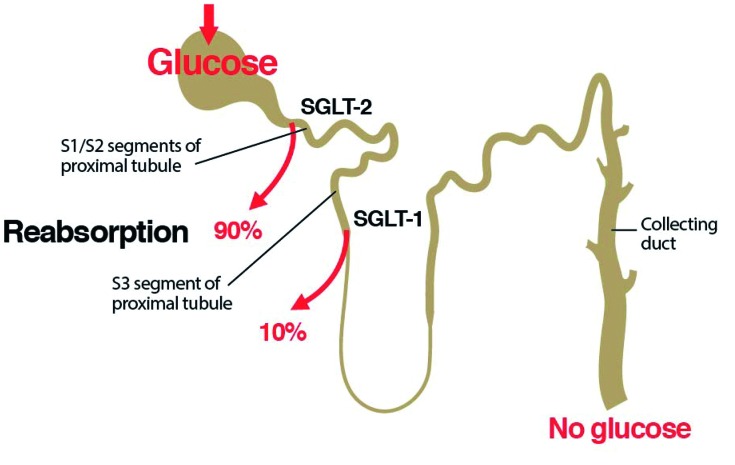
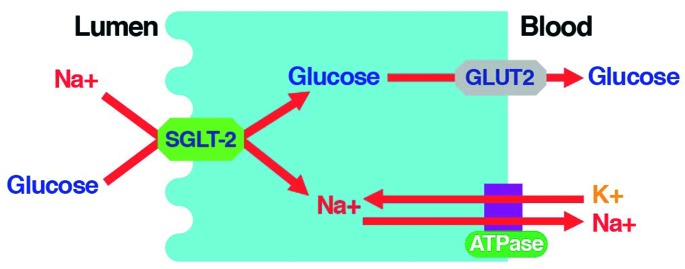
Reabsorption occurs in the proximal convoluted tubule (PCT) and is carried out by two isoforms of SGLT.20 SGLT-2 is located in the S1 and S2 segments of the PCT and has a high capacity but low affinity for glucose transport. In healthy individuals, it reabsorbs ∼ 90% of filtered glucose (Figure 1).20 SGLT-1 governs glucose transport in the S3 segment and is a low-capacity, high-affinity glucose transporter that reabsorbs the remaining 10% of the filtered glucose.20 The active transport of glucose is linked to downhill sodium transport, which is maintained by active extrusion of sodium across the basolateral surface into the intracellular fluid (Figure 2).20 Facilitated glucose transporters (GLUTs) carry glucose across the basolateral membrane by facilitated diffusion.20
Figure 1.
Renal glucose handling. In healthy individuals, the vast majority of the glucose filtered by the kidney is reabsorbed by SGLT-2 in the S1 and S2 segments of the proximal convoluted tubule, and the remaining glucose is reabsorbed by SGLT-1 in the S3 segment.20
Figure 2.
SGLT-2 mediates glucose reabsorption in the kidney. SGLT-2 catalyzes the active transport of glucose (against a concentration gradient) across the luminal membrane by coupling it with the downhill transport of Na+. The inward Na+ gradient across the luminal epithelium is maintained by active extrusion of Na+ across the basolateral surface into the intracellular fluid. Glucose diffuses out of the cell down a concentration gradient via the basolateral facilitative transporter GLUT2.20 Adapted from Ref. 20.
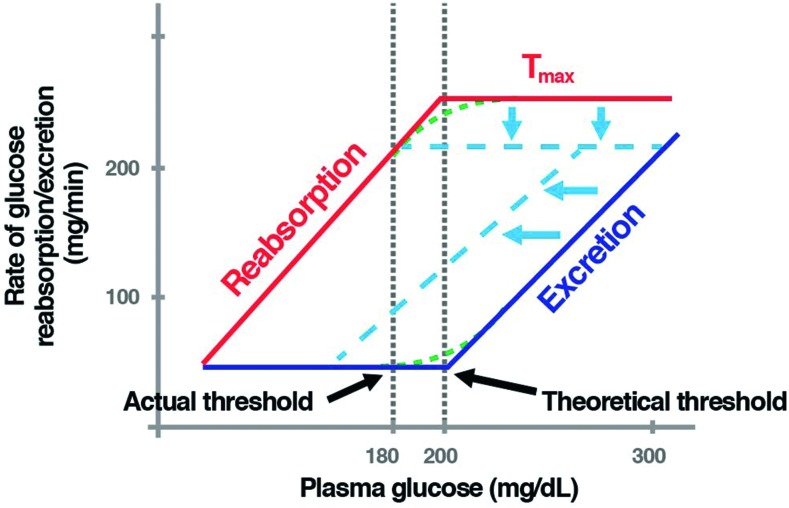
Glucose reabsorption in the PCT increases with rising plasma glucose levels until the transport maximum for glucose (Tmax) is reached. The Tmax is usually considered to occur at a glomerular filtration rate of 260–350 mg/min/1.73 m2. The renal glucose threshold (RTG) is the plasma glucose concentration above which the SGLT capacity becomes saturated and urinary glucose excretion (UGE) occurs. It is estimated to occur at a plasma glucose concentration of ∼ 200 mg/dl (Figure 3).15,16 The actual threshold is not abrupt and differs from the theoretical threshold for both the reabsorption and excretion curves (Figure 3). One reason for this splay, or difference in thresholds, is physiological variation among individual nephrons.
Figure 3.
Renal glucose handling before and following inhibition of SGLT-2. As the plasma glucose concentration increases, renal glucose reabsorption increases, following the line marked “Reabsorption” (in red). At plasma glucose concentrations greater than ∼ 200 mg/dl, all the filtered glucose is reabsorbed, and there is no excretion. When glucose reaches a threshold, at ∼ 200 mg/dl, the maximum capacity of the renal tubule to reabsorb glucose—or Tmax—is exceeded. Once past this threshold, glucose begins to be excreted via the urine (dark blue line, labeled “Excretion”). The actual thresholds for both reabsorption and excretion differ from the theoretical thresholds because of physiological variation among individual nephrons (i.e., slight differences in their glucose-handling abilities). This is known as “splay” (green dashed lines). The dashed light blue lines depict renal glucose handling after SGLT-2 inhibition. SGLT-2 inhibitors lower the renal glucose threshold, leading to urinary glucose excretion.16 Adapted from Ref. 16.
The rate of renal glucose reabsorption is elevated in people with type 2 diabetes; the Tmax is increased by 20–40% compared to healthy individuals.21 What was once an adaptive response to ensure sufficient caloric intake thus becomes the opposite: a maladaptive action that fuels further increases in plasma glucose. Both expression and function of SGLT-2 are upregulated in people with type 2 diabetes.22,23
Introduction to SGLT-2 Inhibitors
In the 2nd century, the Greek physician Areataeus postulated that diabetes was caused by a derangement in the kidney.24 Phlorizin, the first known SGLT inhibitor, was isolated from the root bark of apple trees.25 Interest in the compound was dormant until the 1970s, with discovery of the location of the transporters.
Phlorizin nonselectively blocks both SGLT-2 and SGLT-1, leading to UGE. When administered to diabetic rats, phlorizin normalized both fasting and postprandial plasma glucose concentrations and completely eliminated insulin resistance.26
The safety of chronic UGE is supported by a benign genetic condition termed familial renal glucosuria (FRG). This genetic disorder involves loss-of-function mutations in the gene coding for SGLT-2 that cause UGE ranging from 20 to 200 g/day.27,28 Individuals with FRG do not experience hypoglycemia, are asymptomatic, do not exhibit evidence of renal tubular dysfunction or renal insufficiency, and have a normal life expectancy.27,28
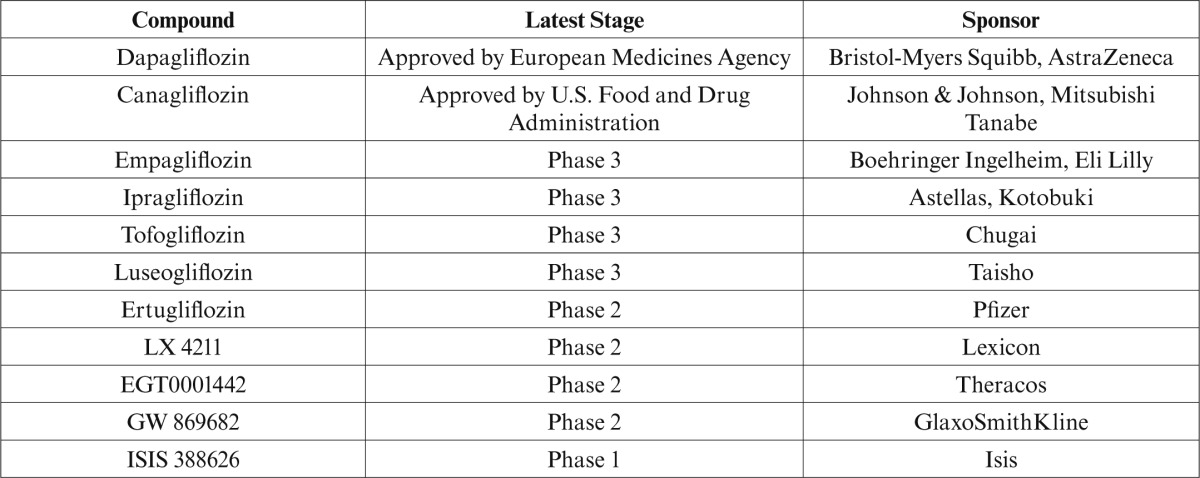
Phlorizin was not developed for use in humans because of its low bioavailability (∼ 15%) and its action on SGLT-1, which can result in GI side effects, including diarrhea. Other SGLT-2 inhibitors, such as sergliflozin, did not reach advanced stages of clinical development for reasons related to their pharmacokinetic profiles (i.e., their susceptibility to hydrolysis by GI tract enzymes resulted in relatively short half-lives and poor bioavailability).29–31 Canagliflozin has been approved in the United States, and dapagliflozin has been approved in Europe. Several other SGLT-2 inhibitors are in development (Table 1).
Table 1.
SGLT-2 Inhibitors and Phase of Development
Mechanism of Action and Potential Advantages
By lowering the renal threshold for glucose excretion, SGLT-2 inhibitors suppress renal glucose reabsorption and thereby increase UGE.19 Hyperglycemia is thus ameliorated. However, SGLT-2 inhibitors inhibit reabsorption of only ∼ 30–50% of the glucose filtered by the kidney.32 The reasons for this are unclear. One hypothesis is that SGLT-2 inhibitors may be actively secreted into the PCT such that the amount of SGLT-2 inhibitor in the PCT is limited by saturation of renal secretion of the inhibitor at high doses, and, depending on the site of secretion, the inhibitors may be unable to act on upstream SGLT-2.32 Another hypothesis is that SGLTs other than SGLT-2 may play a greater role in glucose reabsorption than is currently believed.32
SGLT-2 inhibition offers several putative advantages. Acting independently of insulin, these agents should not confer a risk of hypoglycemia and could be employed as monotherapy or in combination with other agents. Given their mechanism of action, these agents should be effective in patients with any degree of insulin resistance or β-cell function. They should also be associated with weight loss resulting from the loss of glucose (calories) in urine and glucose-induced osmotic diuresis.33 Their mild osmotic diuretic effect could potentially also reduce blood pressure.33 Taken together, these effects may have a beneficial impact on cardiovascular outcomes.34
Inducing UGE initially appears to be a counterintuitive strategy for treatment of patients with type 2 diabetes because it employs what was once thought of only as a signal of uncontrolled diabetes. This conceptual leap from symptom to tool for studying physiology to potential treatment will be further examined.
Clinical Studies
SGLT-2 inhibitors have improved glycemic control as monotherapy in patients with type 2 diabetes in phase 2 and 3 clinical trials. Placebo-adjusted reductions in A1C of up to 1.2% have been reported in studies ranging from 4 weeks to 90 weeks in duration,35–46 in addition to decreased fasting plasma glucose35–37,40–45 and postprandial glucose.35, 41–43
Phase 2 and 3 clinical trials of SGLT-2 inhibitors used as add-on therapy demonstrated improved glycemic control with low rates of hypoglycemia. An SGLT-2 inhibitor added to metformin,38,44,47–54 or to metformin plus a sulfonylurea resulted in absolute reductions in A1C of up to ∼ 1% from a baseline of ∼ 8%.55,56 A1C declined by ∼ 2% in one study of dapagliflozin added to metformin, in which patients had elevated baseline A1C levels (∼ 9%).40 Reductions in A1C have also been reported when SGLT-2 inhibitors were added to pioglitazone (∼ 1%),57 glimepiride (up to 0.8%),58 insulin (up to 1%),59,60 or insulin plus oral antidiabetic agents (up to 0.7%).61
Dapagliflozin has been shown to be effective in patients with early type 2 diabetes (i.e., treatment-naive patients), as well as in patients dependent on insulin plus insulin sensitizers.62 A pooled analysis of data from five phase 3 studies of dapagliflozin demonstrated that higher baseline A1C levels were associated with greater reductions in A1C. For example, at 24 weeks, dapagliflozin lowered A1C by 0.44% (placebo-adjusted) in patients with a baseline A1C < 8.0%, by 0.54% in patients with baseline A1C ≥ 8.0 to < 9.0%, and by 1.01% in patients with baseline A1C ≥ 9%.63
Because the glomerular filtration rate (GFR) is a factor in determining the extent to which SGLT-2 inhibitors can produce glucosuria, their efficacy would be expected to be reduced in patients with impaired renal function.16 Attenuated glucosuria associated with ipragliflozin was observed in patients with type 2 diabetes and moderate or severe renal impairment (estimated GFR [eGFR] 15–59 ml/min/1.73 m2) compared to those with mild renal impairment or normal renal function.64 UGE decreased by 42–90% in patients with type 2 diabetes and renal impairment (eGFR 30–89 ml/min/1.73 m2) receiving dapagliflozin compared to patients with type 2 diabetes and normal renal function.65
Clinical trials of SGLT-2 inhibitors in patients with type 2 diabetes and renal impairment have shown mixed results in their ability to reduce A1C. In a study involving patients with type 2 diabetes and moderate renal impairment (eGFR 30–60 ml/min/1.73 m2), reductions in A1C were observed with dapagliflozin, 5 and 10 mg, but were not significantly different from placebo.65 However, in a phase 3 study of canagliflozin in patients with type 2 diabetes and moderate renal impairment (eGFR 30–50 ml/min/1.73 m2), A1C was significantly lower with canagliflozin, 100 and 300 mg, compared to placebo at week 26.66 Further data are required to establish the efficacy of SGLT-2 inhibitors in patients with type 2 diabetes and renal impairment.
SGLT-2 inhibitors have produced weight reductions of up to 4.7 kg in phase 2 and 3 clinical trials when administered as monotherapy or as add-on therapy to metformin, a sulfonylurea, or insulin over study periods ranging from 4 to 104 weeks.35–45,47–56,58–61,66 Dapagliflozin also attenuates the weight gain associated with pioglitazone.57 Weight loss is accompanied by loss of body fat. A body composition study found that two-thirds of the 2.1 kg (placebo-adjusted) weight loss achieved with 10 mg dapagliflozin added to metformin for 24 weeks in patients with type 2 diabetes resulted from a reduction in body fat, both visceral and subcutaneous.54
A lower systolic blood pressure of ∼ 2–10 mmHg was observed in studies of SGLT-2 inhibitors in patients with type 2 diabetes.35,37–39,41–43,47,53,55–60,66 Declines in diastolic blood pressure were smaller and less consistent across clinical trials.33
Patients with type 2 diabetes receiving SGLT-2 inhibitors have decreased serum uric acid levels,67 a potentially beneficial effect given evidence that hyperuricemia is an independent risk factor for hypertension, renal disease, and cardiovascular disease.68 This effect may be mediated by GLUT9 (SLC2A9b) on the apical membrane of the PCT, which exchanges glucose for urate. The high concentration of glucose in the tubule would favor the exchange of glucose for urate, resulting in increased excretion of urate in the urine.69
Safety and Tolerability
Given the insulin-independent mechanism of action of SGLT-2 inhibitors, hypoglycemia would not be expected, and indeed, very low rates of hypoglycemia have been observed in clinical trials.35–62,70
Glucose in the urine supplies an environment that may encourage bacteria in the urinary tract to flourish and can result in infection. Some studies have shown an increased incidence of events suggestive of urinary tract infections (UTIs) in patients given SGLT-2 inhibitors.37,39,40,42–44,48,49,51,54,60,66,71 However, in many of these studies, the infections were not culture-verified, and some studies of SGLT-2 inhibitors have demonstrated a rate of UTIs similar to that with placebo.35,36,38,47,50,53,55–58,61,72 There was no increase in asymptomatic bacteriuria in a phase 2 study with canagliflozin in which midstream urine was collected and cultured.72 In general, events suggestive of UTI are reported more often in female patients receiving SGLT-2 inhibitors than in male patients.51,54,58,60,72
Some studies of SGLT-2 inhibitors have found that vulvovaginitis and balanitis approximately doubled,43,61,73 but this observation has not been consistent across all studies. Furthermore, the genital infections reported in studies of SGLT-2 inhibitors have not always been confirmed by culture. As with UTIs, events consistent with genital infection are generally reported more often in female patients than in male patients receiving SGLT-2 inhibitors.49,51,53–56,58,60 In a study of canagliflozin in patients with type 2 diabetes, 12% of the female patients had a positive culture for Candida at baseline; after 12 weeks of treatment with canagliflozin, 31% of women with a negative culture for Candida at baseline had a positive culture, compared to 14% of those receiving placebo.73 In most cases, genital infections seen in these studies responded to routine management, usually with azoles, and did not lead to drug discontinuation.
Small increases in hematocrit of 1–2% have been observed in some studies of SGLT-2 inhibitors, consistent with mild volume contraction, although urine volume rises only slightly; electrolytes, including sodium and potassium, are not significantly lost.74 There is no evidence of deleterious effects such as decreased hematocrit that would lead to orthostatic hypotension or renal impairment.16
From 11 phase 3 clinical trials of dapagliflozin, 9 cases of bladder cancer out of 5,478 patients administered dapagliflozin (0.16%) and 9 cases of breast cancer out of 2,223 female patients (0.4%) were detected, compared to the placebo groups, in which 1 of 3,156 subjects had bladder cancer (0.03%) and 1 of 1,053 female patients had breast cancer (0.09%).65 The number of cases was too small to establish causality. Half of the bladder cancer cases were found within 6–12 months of entering the trial and were in more advanced stages of this cancer. Six of the nine patients with bladder cancer demonstrated hematuria at the beginning of the trial. The increased incidence of UTIs in the dapagliflozin groups may have produced a detection bias for bladder cancer. Animal studies with doses up to 100 times the clinical dosage of dapagliflozin did not yield observations of carcinogenesis or mutagenesis.
There was one case of suspected drug-induced liver injury in the dapagliflozin arms. At 50 weeks, there was no change in bone mineral density, markers of bone formation, or bone resorption compared to placebo in 165 patients with type 2 diabetes inadequately controlled on metformin who were treated with dapagliflozin.75 No fractures or sex differences were noted in postmenopausal females or males.
Dapagliflozin had a largely neutral effect on blood lipids.65,76 Small increases in LDL cholesterol47,76 and HDL cholesterol47,57,76 and a small reduction in triglycerides47,76 have been observed in some placebo-controlled studies.
Outlook for SGLT-2 Inhibitors
On 19 January 2012, the U.S. Food and Drug Administration (FDA) informed Bristol-Myers Squibb and AstraZeneca that it would not approve dapagliflozin. This decision followed the agency’s Endocrinologic and Metabolic Drugs Advisory Committee recommendation against approval of dapagliflozin by a vote of nine to six. Further data from ongoing clinical trials and possibly data from new studies will be forthcoming. The Committee for Medicinal Products for Human Use of the European Medicines Agency granted marketing authorization for dapagliflozin on 12 November 2012 for use in adults with type 2 diabetes as monotherapy and as combination therapy with other glucose-lowering medicinal products including insulin, together with diet and exercise.77 On 29 March 2013, the FDA approved canagliflozin to improve glycemic control in adults with type 2 diabetes as an adjunct to diet and exercise.78
Several clinical trials of SGLT-2 inhibitors used as monotherapy or in combination with a range of other treatments are ongoing. The largest clinical trial program in progress is for empagliflozin, involving > 14,000 patients. Very large trials of dapagliflozin, canagliflozin, and empagliflozin are ongoing to assess effects on cardiovascular outcomes. In addition, basic science and mechanistic studies are underway to provide a greater understanding of the mechanism of action of SGLT-2 inhibitors and its implications for the pathophysiological processes involved in the progression of type 2 diabetes.
Conclusion
The concept of inhibition of SGLT-2 marks a departure in how diabetes is viewed and approached for treatment. SGLT-2 inhibitors have a novel mechanism of action that is independent of insulin secretion and action. These agents block glucose reabsorption, leading to urinary glucose excretion. The advantages of this approach are reduced hyperglycemia without hypoglycemia, along with weight loss and blood pressure reduction. Data from multiple phase 3 studies of > 5,000 subjects demonstrate these findings. However, increases in UTIs and genitourinary infections have been observed in some studies. SGLT-2 inhibitors could be used as monotherapy or in combination with other medications in patients with type 2 diabetes and potentially earlier in the continuum in those with prediabetes.
Taken together, the clinical evidence to date suggests that SGLT-2 inhibitors hold promise as an important addition to the toolbox of treatment options for type 2 diabetes.
ACKNOWLEDGMENTS
This review was supported by the Veterans Administration San Diego Healthcare System and the University of California, San Diego, School of Medicine. The author was fully responsible for all content and editorial decisions, was involved at all stages of manuscript development, and has approved the final version of this review that reflects the author’s interpretation and conclusions. Medical writing assistance, supported financially by Boehringer Ingelheim, was provided by Isobel Lever, PhD, and Wendy Morris, MSc, of Fleishman-Hillard Group Limited, London, U.K. Boehringer Ingelheim was given the opportunity to check the data used in the review for factual accuracy only.
REFERENCES
- 1.American Diabetes Association : Standards of medical care in diabetes—2012. Diabetes Care 35 (Suppl. 1):S11–S63, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaiser N, Leibowitz G, Nesher R: Glucotoxicity and beta-cell failure in type 2 diabetes mellitus. J Pediatr Endocrinol Metab 16:5–22, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention : National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, Ga., U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011 Available from http://www.cdc.gov/diabetes/pubs/factsheet11.htm. Accessed 13 August 2013 [Google Scholar]
- 4.Centers for Disease Control and Prevention : Press release: Number of Americans with diabetes projected to double or triple by 2050. Older, more diverse population and longer lifespans contribute to increase. 22 October 2010. Available from http://www.cdc.gov/media/pressrel/2010/r101022.html. Accessed 13 August 2013 [Google Scholar]
- 5.International Diabetes Federation : IDF Diabetes Atlas. 5th ed. Brussels, Belgium, International Diabetes Federation, 2011 Available from http://www.idforg/diabetesatlas. Accessed 13 August 2013 [Google Scholar]
- 6.Emerging Risk Factors Collaboration : Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 375:2215–2222, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR: Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321:405–412, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esposito K, Chiodini P, Bellstella G, Maiorino MI, Guigliano D: Proportion of patients at HbA1c target <7% with 8 classes of antidiabetic drugs in type 2 diabetes: systematic review of 218 randomized controlled trials with 78 945 patients. Diabetes Obes Metab 14:228–233, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Resnick HE, Foster GL, Bardsley J, Ratner RE: Achievement of American Diabetes Association clinical practice recommendations among U.S. adults with diabetes, 1999–2002: the National Health and Nutrition Examination Survey. Diabetes Care 29:531–537, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Cheung BM, Ong KL, Cherny SS, Sham PC, Tso AW, Lam KS: Diabetes prevalence and therapeutic target achievement in the United States, 1999 to 2006. Am J Med 122:443–453, 2009 [DOI] [PubMed] [Google Scholar]
- 11.DeFronzo RA: Banting lecture: From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58:773–795, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips LS, Branch WT, Cook CB, Doyle JP, El-Kebbi IM, Gallina DL, Miller CD, Ziemer DC, Barnes CS: Clinical inertia. Ann Intern Med 135:825–834, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Grant R, Adams AS, Trinacty CM, Zhang F, Kleinman K, Soumerai SB, Meigs JB, Ross-Degna D: Relationship between patient medication adherence and subsequent clinical inertia in type 2 diabetes glycemic management. Diabetes Care 30:807–812, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Inzucchi SE: Oral antihyperglycemic therapy for type 2 diabetes. JAMA 287:360–372, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Gerich JE: Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med 27:136–142, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeFronzo RA, Davidson JA, del Prato S: The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab 14:5–14, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Bergman H, Drury DR: The relationship of kidney function to the glucose utilization of the extra abdominal tissues. Am J Physiol 124:279–284, 1938 [Google Scholar]
- 18.Gerich JE, Meyer C, Woerle HJ, Stumvoll M: Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care 24:382–391, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Mather A, Pollock C: Glucose handling by the kidney. Kidney Int Suppl 120:S1–S6, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Wright EM, Loo DD, Hirayama BA: Biology of human sodium glucose transporters. Physiol Rev 91:733–794, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Farber SJ, Berger EY, Earle DP: Effect of diabetes and insulin of the maximum capacity of the renal tubules to reabsorb glucose. J Clin Invest 30:125–129, 1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mogensen CE: Maximum tubular reabsorption capacity for glucose and renal hemodynamics during rapid hypertonic glucose infusion in normal and diabetic subjects. Scand J Clin Lab Invest 28:101–109, 1971 [DOI] [PubMed] [Google Scholar]
- 23.Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J: Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes 54:3427–3434, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Krall LP, Levine R, Barnett D: The history of diabetes. In Joslin’s Diabetes Mellitus. 13th ed. Kahn CR, Weir GC, Eds. Philadelphia, Pa, Lea and Febiger, 1994, p. 2 [Google Scholar]
- 25.Ehrenkranz JR, Lewis NG, Kahn CR, Roth J: Phlorizin: a review. Diabetes Metab Res Rev 21:31–38, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Rossetti L, Smith D, Shulman GI, Papachristou D, DeFronzo RA: Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest 79:1510–1515, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calado J, Santer R, Rueff J: Effect of kidney disease on glucose handling (including genetic defects). Kidney Int Suppl 120:S7–S13, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Santer R, Calado J: Familial renal glucosuria and SGLT2: from a Mendelian trait to a therapeutic target. Clin J Am Soc Nephrol 5:133–141, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Hussey EK, Clark RV, Amin DM, Kipnes MS, O’Connor-Semmes RL, O’Driscoll EC, Leong J, Murray SC, Dobbins RL, Layko D, Nunez DJ: Single-dose pharmacokinetics and pharmacodynamics of sergliflozin etabonate, a novel inhibitor of glucose reabsorption, in healthy volunteers and patients with type 2 diabetes mellitus. J Clin Pharmacol 50:623–635, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Hussey EK, Dobbins RL, Stoltz RR, Stockman NL, O’Connor-Semmes RL, Kapur A, Murray SC, Layko D, Nunez DJ: Multiple-dose pharmacokinetics and pharmacodynamics of sergliflozin etabonate, a novel inhibitor of glucose reabsorption, in healthy overweight and obese subjects: a randomized double-blind study. J Clin Pharmacol 50:636–646, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Dobbins RL, O’Connor-Semmes R, Kapur A, Kapitza C, Golor G, Mikoshiba I, Tao W, Hussey EK: Remogliflozin etabonate, a selective inhibitor of the sodium-dependent transporter 2 reduces serum glucose in type 2 diabetes mellitus patients. Diabetes Obes Metab 14:15–22, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Liu JJ, Lee T, DeFronzo RA: Why do SGLT2 inhibitors inhibit only 30–50% of renal glucose reabsorption in humans? Diabetes 61:2199–2204, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.List JF, Whaley JM: Glucose dynamics and mechanistic implications of SGLT-2 inhibitors in animals and humans. Kidney Int Suppl 120:S20–S27, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Foote C, Perkovic V, Neal B: Effects of SGLT-2 inhibitors on cardiovascular outcomes. Diab Vasc Dis Res 9:117–123, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Bailey CJ, Igbal N, T’joen C, List JF: Dapagliflozin monotherapy in drug-naive patients: a randomised controlled trial of low-dose range. Diabetes Obes Metab 14:951–959, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Ferrannini E, Seman L, Seewaldt-Becker E, Hantel S, Pinnetti S, Woerle HJ: A phase IIb, randomized, placebo-controlled study of the SGLT2 inhibitor empagliflozin in patients with type 2 diabetes. Diabetes Obes Metab 15:721–728, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF: Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care 33:2217–2224, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kadowaki T, Ikeda S, Takano Y, Cynshi O, Christ AD, Boerlin V, Beyer U, Beck A: Tofogliflozin, a novel and selective SGLT2 inhibitor improves glycemic control and lowers body weight in patients with type 2 diabetes mellitus inadequately controlled on stable metformin or diet and exercise alone [Abstract]. Diabetes 61 (Suppl. 1):A22 (80-OR), 2012 [Google Scholar]
- 39.Kawano H, Kashiwagi A, Kazuta K, Yoshida S, Ueyama E, Utsuno A: Long-term safety, tolerability and efficacy of ipragliflozin in Japanese patients with type 2 diabetes mellitus: IGNITE [Abstract]. Diabetes 61 (Suppl. 1):A611 (2422-PO), 2012 [Google Scholar]
- 40.Henry RR, Murray AV, Marmolejo MH, Hennicken D, Ptaszynska A, List JF: Dapagliflozin, metformin XR, or both: initial pharmacotherapy for type 2 diabetes, a randomised controlled trial. Int J Clin Pract 66:446–456, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Seino Y, Sasaki T, Fukatsu A, Samukawa Y, Sakai S, Watanabe T: Luseogliflozin (TS-071), a selective SGLT2 inhibitor, improves glycemic control and lowers body weight in Japanese patients with type 2 diabetes mellitus [Abstract]. Diabetes 61 (Suppl. 1):A266–A267 (1039-P), 2012 [Google Scholar]
- 42.Stenlöf K, Cefalu WT, Kim KA, Alba M, Usiskin K, Tong C, Canovatchel W, Meininger G: Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab 15:372–382, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.List J, Woo V, Morales E, Tang W, Fiedorek FT: Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care 32:650–657, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woerle H-J, Ferrannini E, Berk A, Manun’Ebo M, Pinnetti S, Broedl UC: Safety and efficacy of empagliflozin as monotherapy or add-on to metformin in a 78-week open-label extension study in patients with type 2 diabetes [Abstract]. Diabetes 61 (Suppl. 1A):LB13 (49-LB), 2012 [Google Scholar]
- 45.Schwartz SL, Akinlade B, Klasen S, Kowalski D, Zhang W, Wilpshaar W: Safety, pharmacokinetic, and pharmacodynamic profiles of ipragliflozin (ASP1941), a novel and selective inhibitor of sodium-dependent glucose co-transporter in patients with type 2 diabetes mellitus. Diabetes Technol Ther 13:1219–1227, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Polidori D, Zhao Y, Alba M, Ferrannini E: Treatment with canagliflozin (CANA), a sodium glucose co-transporter 2 (SGLT2) inhibitor, for 26 weeks improves indices of beta-cell function (BCF) [Abstract]. Diabetes 61 (Suppl. 1):A265 (1032-P), 2012 [Google Scholar]
- 47.Bailey CJ, Gross JL, Pieters A, Bastien A, List JF: Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet 375:2223–2233, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Bailey CJ, Gross JL, Hennicken D, Iqbal N, Mansfield TA, List JF: Dapagliflozin add-on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled 102-week trial. BMC Med 11:43, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cefalu WT, Leiter LA, Niskanen L, Xie J, Millington D, Canovatchel W, Meininger G: Efficacy and safety of canagliflozin, a sodium glucose co-transporter 2 inhibitor, compared with glimepiride in patients with type 2 diabetes on background metformin [Abstract]. Diabetes 61 (Suppl. 1A ):LB10 (38-LB), 2012 [Google Scholar]
- 50.Goto K, Kashiwagi A, Kazuta K, Yoshida S, Ueyama E, Utsuno A: Ipragliflozin reduces A1c and body weight in type 2 diabetes patients who have inadequate glycemic control on metformin alone: ILLUMINATE study. Diabetes 61 (Suppl. 1):A269, 2012 [Google Scholar]
- 51.Nauck MA, del Prato S, Meier JJ, Duran-Garcia S, Rohwedder K, Elze M, Parikh SJ: Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin. Diabetes Care 34:2015–2022, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenstock J, Seman LJ, Jelaska A, Hantel S, Pinnetti S, Hach T, Woerle HJ: Efficacy and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, as add-on to metformin in type 2 diabetes with mild hyperglycaemia. Diabetes Obes Metab. Electronically published ahead of print on 1 August 2103 (doi: 10.1111/dom.12185) [DOI] [PubMed]
- 53.Rosenstock J, Aggarwal N, Polidori D, Zhao Y, Arbit D, Usiskin K, Capuano G, Canovatchel W, Canagliflozin DIA. 2001 Study Group: Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care 35:1232–1238, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bolinder J, Ljunggren Ö KullbergJ, Johansson L, Wilding J, Langkilde AM, Sugg J, Parikh S: Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 97:1020–1031, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Schernthaner G, Gross JL, Rosenstock J, Guarisco M, Fu M, Yee J, Kawaguchi M, Canovatchel W, Meininger G: Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care. Electronically published ahead of print on 5 April 2013 (doi: 10.2337/dc12–2491) [DOI] [PMC free article] [PubMed]
- 56.Wilding JP, Mathieu C, Vercruysse F, Usiskin K, Deng L, Canovatchel W: Canagliflozin (CANA), a sodium glucose co-transporter 2 inhibitor, improves glycemic control and reduces body weight in subjects with type 2 diabetes (T2D) inadequately controlled with metformin (MET) and sulfonylurea (SU) [Abstract]. Diabetes 61 (Suppl. 1):A262 (1022-P), 2012 [Google Scholar]
- 57.Rosenstock J, Vico M, Wei L, Salsali A, List JF: Effects of dapagliflozin, an SGLT2 inhibitor, on HbA1c, body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care 35:1473–1478, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strojek K, Yoon KH, Hruba V, Elze M, Langkilde AM, Parikh S: Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab 13:928–938, 2011 [DOI] [PubMed] [Google Scholar]
- 59.Devineni D, Morrow L, Hompesch M, Skee D, Vandebosch A, Murphy J, Ways K, Schwartz S: Canagliflozin improves glycaemic control over 28 days in subjects with type 2 diabetes not optimally controlled on insulin. Diabetes Obes Metab 14:539–545, 2012 [DOI] [PubMed] [Google Scholar]
- 60.Wilding JP, Woo V, Soler NG, Pahor A, Sugg J, Rohwedder K, Parikh S: Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med 156:405–415, 2012 [DOI] [PubMed] [Google Scholar]
- 61.Wilding JPH, Norwood P, T’joen C, Bastien A, List JF, Fiedorek FT: A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers. Diabetes Care 32:1656–1662, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang L, Feng Y, List J, Kasichayanula S, Pfister M: Dapagliflozin treatment in patients with different stages of type 2 diabetes mellitus: effects on glycaemic control and body weight. Diabetes Obes Metab 12:510–516, 2010 [DOI] [PubMed] [Google Scholar]
- 63.Hardy E, Salsali A, Hruba V, Mansfield T, Rohwedder K, Wessman C, Sugg JE, Wei L, Ptaszynska A, Parikh SJ: Efficacy increases with increasing baseline HbA1c category with dapagliflozin therapy. Diabetes 61 (Suppl. 1):A23, 2012 [Google Scholar]
- 64.Veltkamp SA, Van Dijk J, Krauwinkel WJJ, Smulders RA: The effect of renal impairment on the pharmacokinetics and urinary glucose excretion of the SGLT2 inhibitor ASP1941 in type 2 diabetes mellitus patients (Abstract). Diabetes 60 (Suppl. 1):A309 (1127-P), 2011 [Google Scholar]
- 65.U.S. Food and Drug Administration : FDA briefing document: NDA 202293. Dapagliflozin tablets, 5 and 10 mg, 2011. Available from http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/ucm262994.pdf. Accessed 21 September 2012 [Google Scholar]
- 66.Yale J, Bakris G, Cariou B, Yue D, David-Neto E, Xi L, Figueroa K, Wajs E, Usiskin K, Meininger G: Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab 15:463–473, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Musso G, Gambino R, Cassader M, Pagano G: A novel approach to control hyperglycemia in type 2 diabetes: sodium glucose co-transport (SGLT) inhibitors: systematic review and meta-analysis of randomized trials. Ann Med 44:375–393, 2012 [DOI] [PubMed] [Google Scholar]
- 68.Feig DI, Kang DH, Johnson RJ: Uric acid and cardiovascular risk. N Engl J Med 359:1811–1821, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheeseman C: Solute carrier family 2, member 9 and uric acid homeostasis. Curr Opin Nephrol Hypertens 18:428–432, 2009 [DOI] [PubMed] [Google Scholar]
- 70.Veltkamp SA, van Dijk J, Collins C, van Bruijnsvoort M, Kadokura T, Smulders RA: Combination treatment with ipragliflozin and metformin: a randomized, double-blind, placebo-controlled study in patients with type 2 diabetes mellitus. Clin Ther 34:1761–1771, 2012 [DOI] [PubMed] [Google Scholar]
- 71.Parikh S, Johnsson K, Ptaszynska A, Schmitz B, Sugg J, List JF: Characterization of urinary tract infection in the setting of pharmacologically induced glucosuria [Abstract]. Diabetologia 54 (Suppl.):A841, 2011 [Google Scholar]
- 72.Nicolle LE, Capuano G, Ways K, Usisikin K: Effect of canagliflozin, a sodium glucose co-transporter 2 (SGLT2) inhibitor, on bacteriuria and urinary tract infection in subjects with type 2 diabetes enrolled in a 12-week, phase 2 study. Curr Med Res Opin 28:1167–1171, 2012 [DOI] [PubMed] [Google Scholar]
- 73.Nyirjesy P, Zhao Y, Ways K, Usiskin K: Evaluation of vulvovaginal symptoms and Candida colonization in women with type 2 diabetes mellitus treated with canagliflozin, a sodium glucose co-transporter 2 inhibitor. Curr Med Res Opin 28:1173–1178, 2012 [DOI] [PubMed] [Google Scholar]
- 74.Ferrannini E: Sodium-glucose transporter-2 inhibition as an antidiabetic therapy. Nephrol Dial Transplant 25:2041–2043, 2010 [DOI] [PubMed] [Google Scholar]
- 75.Ljunggren O, Bolinder J, Johansson L, Wilding J, Langkilde AM, Sjöström CD, Sugg J, Parikh S: Dapagliflozin has no effect on markers of bone formation and resorption or bone mineral density in patients with inadequately controlled type 2 diabetes mellitus on metformin. Diabetes Obes Metab 14:990–999, 2012 [DOI] [PubMed] [Google Scholar]
- 76.Bristol-Myers Squibb and AstraZeneca : Background document, dapagliflozin, BMS-512148, NDA 202293, 2011. Available from http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM262996.pdf. Accessed 9 October 2012 [Google Scholar]
- 77.European Medicines Agency : Forxiga (dapagliflozin) authorisation details. Available from http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002322/human_med_001546.jsp&mid=WC0b01ac058001d124. Accessed 17 April 2013
- 78.U.SFood and Drug Administration : News release: FDA approves Invokana to treat type 2 diabetes, 2013. Available from http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm345848.htm. Accessed 17 April 2013