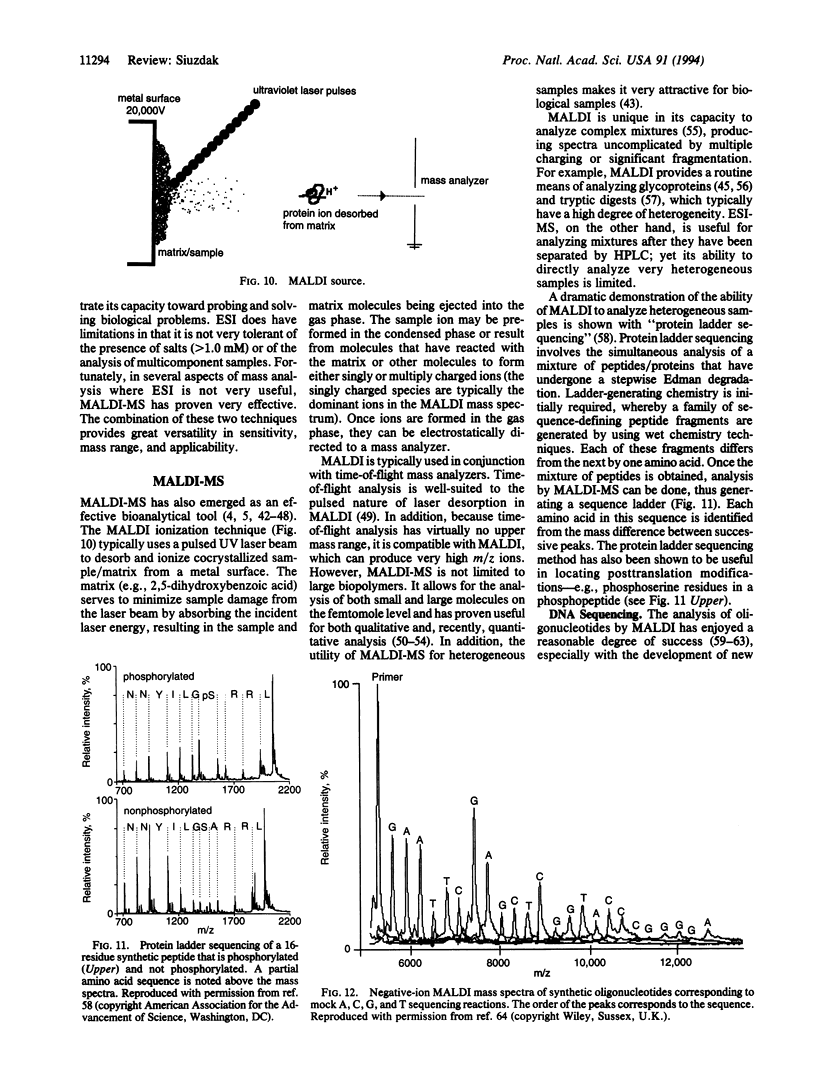
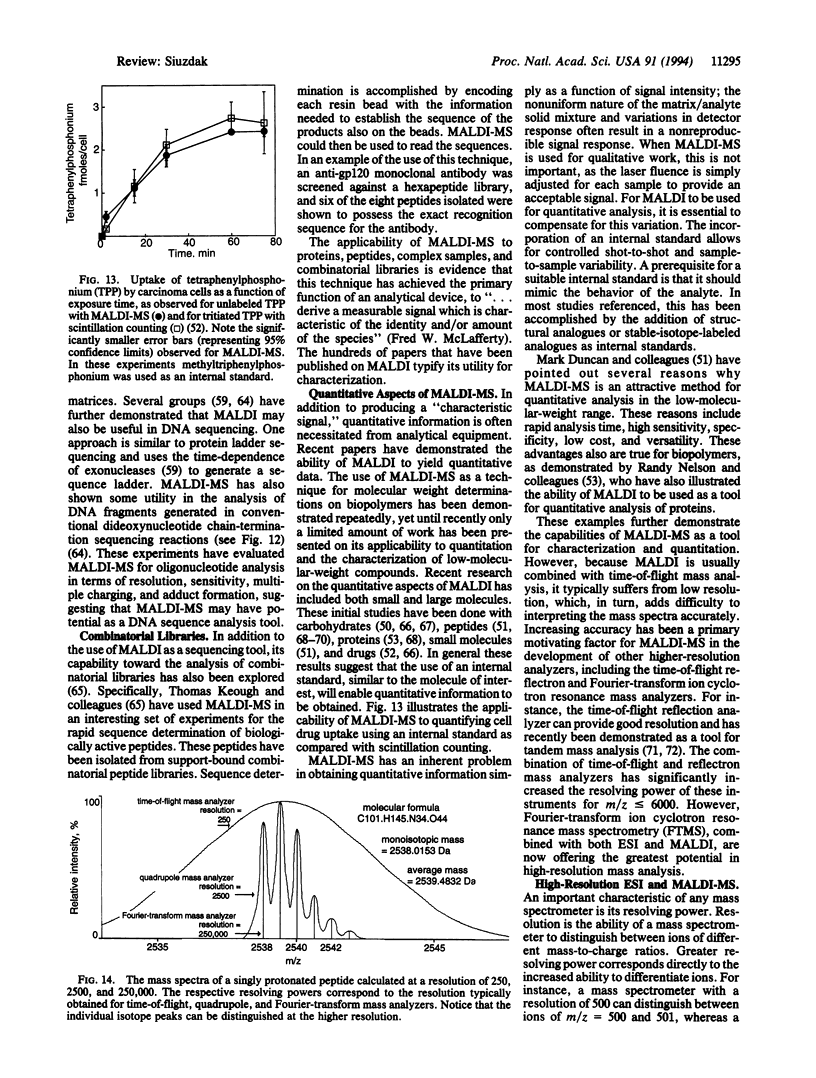
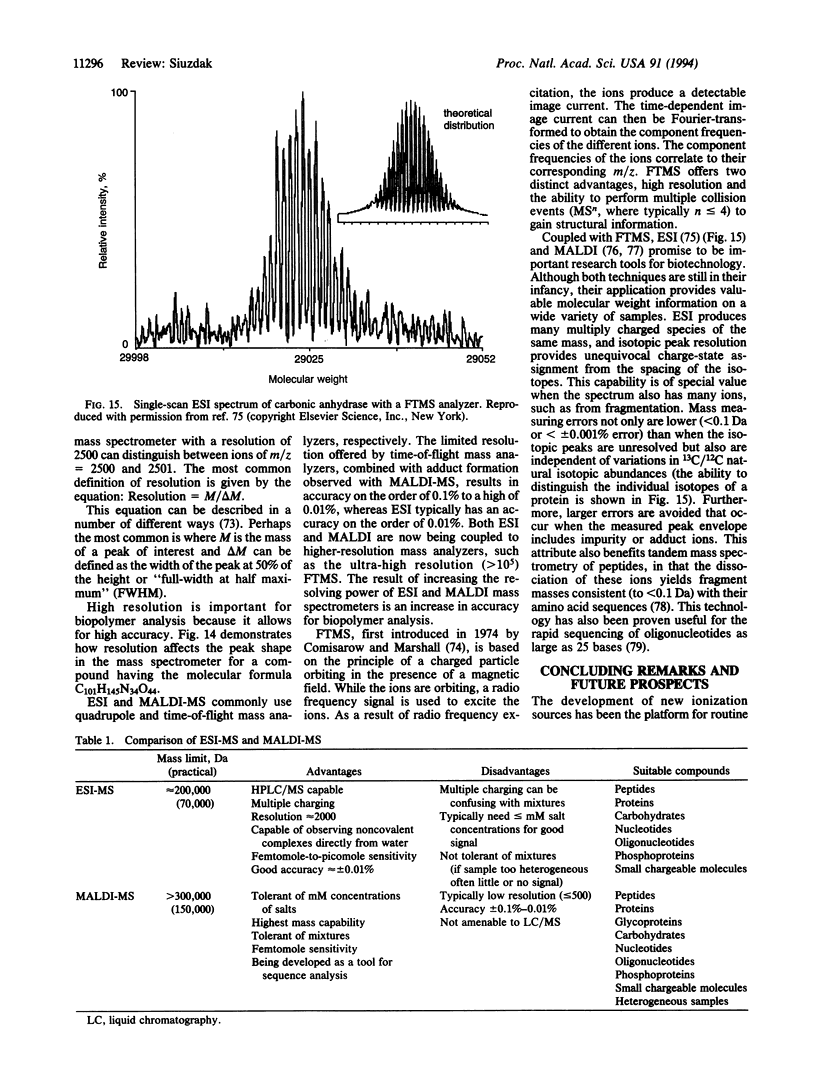
Abstract
The initial steps toward routinely applying mass spectrometry in the biochemical laboratory have been achieved. In the past, mass spectrometry was confined to the realm of small, relatively stable molecules; large or thermally labile molecules did not survive the desorption and ionization processes intact. Electrospray ionization (ESI) and matrix-assisted laser desorption/ionization (MALDI) mass spectrometry allow for the analysis of both small and large biomolecules through "mild" desorption and ionization methods. The use of ESI and MALDI mass spectrometry extends beyond simple characterization. Noncovalent interactions, protein and peptide sequencing, DNA sequencing, protein folding, in vitro drug analysis, and drug discovery are among the areas to which ESI and MALDI mass spectrometry have been applied. This review summarizes recent developments and major contributions in mass spectrometry, focusing on the applications of MALDI and ESI mass spectrometry.
Full text
PDF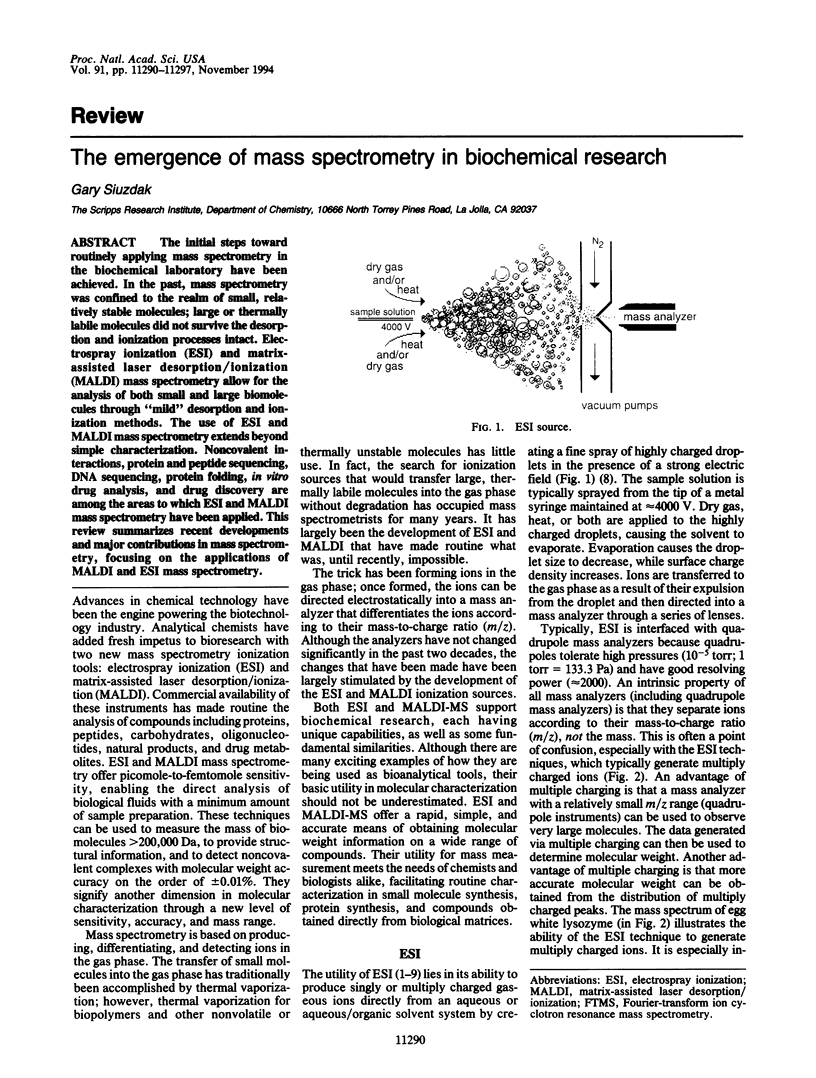
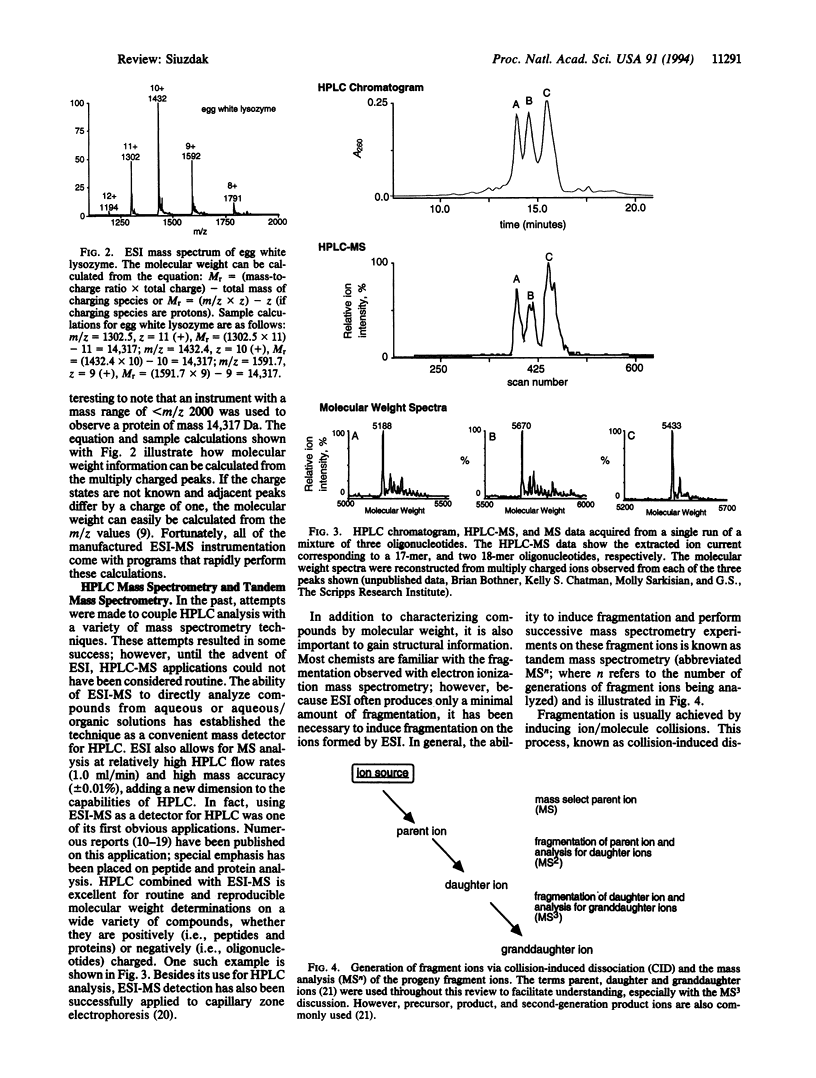

Images in this article
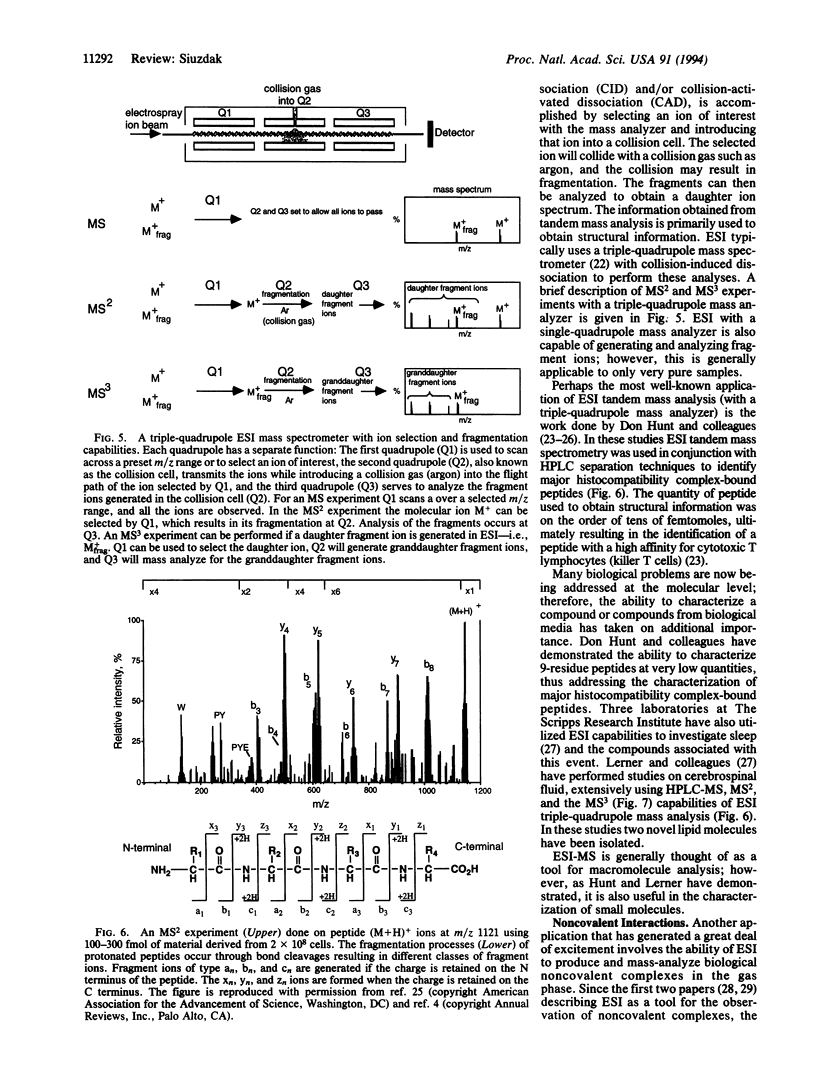
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
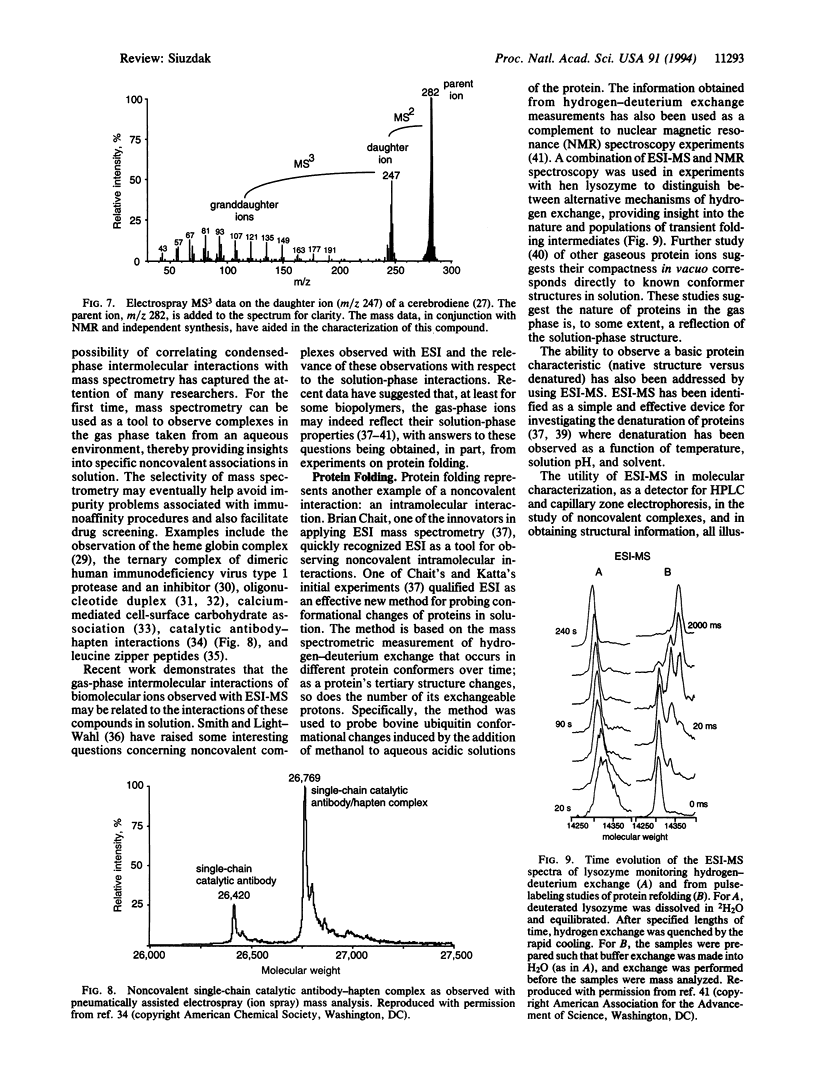
- Arnott D., Shabanowitz J., Hunt D. F. Mass spectrometry of proteins and peptides: sensitive and accurate mass measurement and sequence analysis. Clin Chem. 1993 Sep;39(9):2005–2010. [PubMed] [Google Scholar]
- Ashton D. S., Beddell C. R., Green B. N., Oliver R. W. Rapid validation of molecular structures of biological samples by electrospray-mass spectrometry. FEBS Lett. 1994 Mar 28;342(1):1–6. doi: 10.1016/0014-5793(94)80572-5. [DOI] [PubMed] [Google Scholar]
- Biemann K. Mass spectrometry of peptides and proteins. Annu Rev Biochem. 1992;61:977–1010. doi: 10.1146/annurev.bi.61.070192.004553. [DOI] [PubMed] [Google Scholar]
- Billeci T. M., Stults J. T. Tryptic mapping of recombinant proteins by matrix-assisted laser desorption/ionization mass spectrometry. Anal Chem. 1993 Jul 1;65(13):1709–1716. doi: 10.1021/ac00061a013. [DOI] [PubMed] [Google Scholar]
- Burlingame A. L., Boyd R. K., Gaskell S. J. Mass spectrometry. Anal Chem. 1994 Jun 15;66(12):634R–683R. doi: 10.1021/ac00084a024. [DOI] [PubMed] [Google Scholar]
- Carr S. A., Hemling M. E., Bean M. F., Roberts G. D. Integration of mass spectrometry in analytical biotechnology. Anal Chem. 1991 Dec 15;63(24):2802–2824. doi: 10.1021/ac00024a003. [DOI] [PubMed] [Google Scholar]
- Castoro J. A., Wilkins C. L. Ultrahigh resolution matrix-assisted laser desorption/ionization of small proteins by Fourier transform mass spectrometry. Anal Chem. 1993 Oct 1;65(19):2621–2627. doi: 10.1021/ac00067a013. [DOI] [PubMed] [Google Scholar]
- Chait B. T., Kent S. B. Weighing naked proteins: practical, high-accuracy mass measurement of peptides and proteins. Science. 1992 Sep 25;257(5078):1885–1894. doi: 10.1126/science.1411504. [DOI] [PubMed] [Google Scholar]
- Chait B. T., Wang R., Beavis R. C., Kent S. B. Protein ladder sequencing. Science. 1993 Oct 1;262(5130):89–92. doi: 10.1126/science.8211132. [DOI] [PubMed] [Google Scholar]
- Cox A. L., Skipper J., Chen Y., Henderson R. A., Darrow T. L., Shabanowitz J., Engelhard V. H., Hunt D. F., Slingluff C. L., Jr Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science. 1994 Apr 29;264(5159):716–719. doi: 10.1126/science.7513441. [DOI] [PubMed] [Google Scholar]
- Duncan M. W., Matanovic G., Cerpa-Poljak A. Quantitative analysis of low molecular weight compounds of biological interest by matrix-assisted laser desorption ionization. Rapid Commun Mass Spectrom. 1993 Dec;7(12):1090–1094. doi: 10.1002/rcm.1290071207. [DOI] [PubMed] [Google Scholar]
- Eshraghi J., Chowdhury S. K. Factors affecting electrospray ionization of effluents containing trifluoroacetic acid for high-performance liquid chromatography/mass spectrometry. Anal Chem. 1993 Dec 1;65(23):3528–3533. doi: 10.1021/ac00071a035. [DOI] [PubMed] [Google Scholar]
- Harvey D. J. Quantitative aspects of the matrix-assisted laser desorption mass spectrometry of complex oligosaccharides. Rapid Commun Mass Spectrom. 1993 Jul;7(7):614–619. doi: 10.1002/rcm.1290070712. [DOI] [PubMed] [Google Scholar]
- Heath T. G., Giordani A. B. Reversed-phase capillary high-performance liquid chromatography with on-line UV, fluorescence and electrospray ionization mass spectrometric detection in the analysis of peptides and proteins. J Chromatogr. 1993 May 21;638(1):9–19. doi: 10.1016/0021-9673(93)85002-o. [DOI] [PubMed] [Google Scholar]
- Hemling M. E., Roberts G. D., Johnson W., Carr S. A., Covey T. R. Analysis of proteins and glycoproteins at the picomole level by on-line coupling of microbore high-performance liquid chromatography with flow fast atom bombardment and electrospray mass spectrometry: a comparative evaluation. Biomed Environ Mass Spectrom. 1990 Nov;19(11):677–691. doi: 10.1002/bms.1200191107. [DOI] [PubMed] [Google Scholar]
- Henderson R. A., Michel H., Sakaguchi K., Shabanowitz J., Appella E., Hunt D. F., Engelhard V. H. HLA-A2.1-associated peptides from a mutant cell line: a second pathway of antigen presentation. Science. 1992 Mar 6;255(5049):1264–1266. doi: 10.1126/science.1546329. [DOI] [PubMed] [Google Scholar]
- Hess D., Covey T. C., Winz R., Brownsey R. W., Aebersold R. Analytical and micropreparative peptide mapping by high performance liquid chromatography/electrospray mass spectrometry of proteins purified by gel electrophoresis. Protein Sci. 1993 Aug;2(8):1342–1351. doi: 10.1002/pro.5560020817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberty M. C., Vath J. E., Yu W., Martin S. A. Site-specific carbohydrate identification in recombinant proteins using MALD-TOF MS. Anal Chem. 1993 Oct 15;65(20):2791–2800. doi: 10.1021/ac00068a015. [DOI] [PubMed] [Google Scholar]
- Hunt D. F., Henderson R. A., Shabanowitz J., Sakaguchi K., Michel H., Sevilir N., Cox A. L., Appella E., Engelhard V. H. Characterization of peptides bound to the class I MHC molecule HLA-A2.1 by mass spectrometry. Science. 1992 Mar 6;255(5049):1261–1263. doi: 10.1126/science.1546328. [DOI] [PubMed] [Google Scholar]
- Hunt D. F., Michel H., Dickinson T. A., Shabanowitz J., Cox A. L., Sakaguchi K., Appella E., Grey H. M., Sette A. Peptides presented to the immune system by the murine class II major histocompatibility complex molecule I-Ad. Science. 1992 Jun 26;256(5065):1817–1820. doi: 10.1126/science.1319610. [DOI] [PubMed] [Google Scholar]
- Katta V., Chait B. T. Conformational changes in proteins probed by hydrogen-exchange electrospray-ionization mass spectrometry. Rapid Commun Mass Spectrom. 1991 Apr;5(4):214–217. doi: 10.1002/rcm.1290050415. [DOI] [PubMed] [Google Scholar]
- Lawrence J. F., Lau B. P., Cleroux C., Lewis D. Comparison of UV absorption and electrospray mass spectrometry for the high-performance liquid chromatographic determination of domoic acid in shellfish and biological samples. J Chromatogr A. 1994 Jan 21;659(1):119–126. doi: 10.1016/0021-9673(94)85013-5. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Siuzdak G., Prospero-Garcia O., Henriksen S. J., Boger D. L., Cravatt B. F. Cerebrodiene: a brain lipid isolated from sleep-deprived cats. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9505–9508. doi: 10.1073/pnas.91.20.9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling V., Guzzetta A. W., Canova-Davis E., Stults J. T., Hancock W. S., Covey T. R., Shushan B. I. Characterization of the tryptic map of recombinant DNA derived tissue plasminogen activator by high-performance liquid chromatography-electrospray ionization mass spectrometry. Anal Chem. 1991 Dec 15;63(24):2909–2915. doi: 10.1021/ac00024a020. [DOI] [PubMed] [Google Scholar]
- Loo J. A., Quinn J. P., Ryu S. I., Henry K. D., Senko M. W., McLafferty F. W. High-resolution tandem mass spectrometry of large biomolecules. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):286–289. doi: 10.1073/pnas.89.1.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood W. A., Watson C. J., Ogden A. R., Hawkins R. V. Use of image analysis in determining masticatory efficiency in patients presenting for immediate dentures. Int J Prosthodont. 1992 Jul-Aug;5(4):359–366. [PubMed] [Google Scholar]
- McIver R. T., Jr, Li Y., Hunter R. L. High-resolution laser desorption mass spectrometry of peptides and small proteins. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4801–4805. doi: 10.1073/pnas.91.11.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranker A., Robinson C. V., Radford S. E., Aplin R. T., Dobson C. M. Detection of transient protein folding populations by mass spectrometry. Science. 1993 Nov 5;262(5135):896–900. doi: 10.1126/science.8235611. [DOI] [PubMed] [Google Scholar]
- Mirza U. A., Cohen S. L., Chait B. T. Heat-induced conformational changes in proteins studied by electrospray ionization mass spectrometry. Anal Chem. 1993 Jan 1;65(1):1–6. doi: 10.1021/ac00049a003. [DOI] [PubMed] [Google Scholar]
- Pieles U., Zürcher W., Schär M., Moser H. E. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry: a powerful tool for the mass and sequence analysis of natural and modified oligonucleotides. Nucleic Acids Res. 1993 Jul 11;21(14):3191–3196. doi: 10.1093/nar/21.14.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston L. M., Murray K. K., Russell D. H. Reproducibility and quantitation of matrix-assisted laser desorption ionization mass spectrometry: effects of nitrocellulose on peptide ion yields. Biol Mass Spectrom. 1993 Sep;22(9):544–550. doi: 10.1002/bms.1200220908. [DOI] [PubMed] [Google Scholar]
- Rideout D., Bustamante A., Siuzdak G. Cationic drug analysis using matrix-assisted laser desorption/ionization mass spectrometry: application to influx kinetics, multidrug resistance, and intracellular chemical change. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10226–10229. doi: 10.1073/pnas.90.21.10226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R. P., Ericson J. F., Lynch M. J., Fouda H. G. Confirmation of danofloxacin residues in chicken and cattle liver by microbore high-performance liquid chromatography electrospray ionization tandem mass spectrometry. Biol Mass Spectrom. 1993 Oct;22(10):595–599. doi: 10.1002/bms.1200221007. [DOI] [PubMed] [Google Scholar]
- Schnölzer M., Jones A., Alewood P. F., Kent S. B. Ion-spray tandem mass spectrometry in peptide synthesis: structural characterization of minor by-products in the synthesis of ACP(65-74). Anal Biochem. 1992 Aug 1;204(2):335–343. doi: 10.1016/0003-2697(92)90249-7. [DOI] [PubMed] [Google Scholar]
- Siegel M. M., Hollander I. J., Hamann P. R., James J. P., Hinman L., Smith B. J., Farnsworth A. P., Phipps A., King D. J., Karas M. Matrix-assisted UV-laser desorption/ionization mass spectrometric analysis of monoclonal antibodies for the determination of carbohydrate, conjugated chelator, and conjugated drug content. Anal Chem. 1991 Nov 1;63(21):2470–2481. doi: 10.1021/ac00021a016. [DOI] [PubMed] [Google Scholar]
- Smith R. D., Loo J. A., Edmonds C. G., Barinaga C. J., Udseth H. R. New developments in biochemical mass spectrometry: electrospray ionization. Anal Chem. 1990 May 1;62(9):882–899. doi: 10.1021/ac00208a002. [DOI] [PubMed] [Google Scholar]
- Straub R. F., Voyksner R. D. Determination of penicillin G, ampicillin, amoxicillin, cloxacillin and cephapirin by high-performance liquid chromatography-electrospray mass spectrometry. J Chromatogr. 1993 Sep 10;647(1):167–181. doi: 10.1016/0021-9673(93)83336-q. [DOI] [PubMed] [Google Scholar]
- Suckau D., Shi Y., Beu S. C., Senko M. W., Quinn J. P., Wampler F. M., 3rd, McLafferty F. W. Coexisting stable conformations of gaseous protein ions. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):790–793. doi: 10.1073/pnas.90.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada Y., Gu J., Okamoto N., Inui K. Diagnosis of carbohydrate-deficient glycoprotein syndrome by matrix-assisted laser desorption time-of-flight mass spectrometry. Biol Mass Spectrom. 1994 Feb;23(2):108–109. doi: 10.1002/bms.1200230211. [DOI] [PubMed] [Google Scholar]
- Wang B. H., Biemann K. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry of chemically modified oligonucleotides. Anal Chem. 1994 Jun 1;66(11):1918–1924. doi: 10.1021/ac00083a023. [DOI] [PubMed] [Google Scholar]
- Wu K. J., Shaler T. A., Becker C. H. Time-of-flight mass spectrometry of underivatized single-stranded DNA oligomers by matrix-assisted laser desorption. Anal Chem. 1994 May 15;66(10):1637–1645. doi: 10.1021/ac00082a007. [DOI] [PubMed] [Google Scholar]
- Youngquist R. S., Fuentes G. R., Lacey M. P., Keough T. Matrix-assisted laser desorption ionization for rapid determination of the sequences of biologically active peptides isolated from support-bound combinatorial peptide libraries. Rapid Commun Mass Spectrom. 1994 Jan;8(1):77–81. doi: 10.1002/rcm.1290080115. [DOI] [PubMed] [Google Scholar]




