Abstract
Fungal fimbriae are surface appendages that were first described on the haploid cells of the smut fungus, Microbotryum violaceum. They are long (1-20 microm), narrow (7 nm) flexuous structures that have been implicated in cellular functions such as mating and pathogenesis. Since the initial description, numerous fungi from all five phyla have been shown to produce fimbriae on their extracellular surfaces. The present study analyses the protein component of M.violaceum fimbriae. The N-terminus and three internal amino acid sequences were determined. All four show a strong similarity to sequences which are characteristic of the collagen gene family. Enzymatic digests and immunochemical analyses support this finding. Based on these results, it is suggested that the proteinaceous subunits of fimbriae should be termed fungal collagens. Previously, collagen has been found only among members of the kingdom Animalia where it is the principal component of the animal extracellular matrix and is the most abundant animal protein. The unexpected finding of collagen in the members of the Mycota suggests that it may have evolved from a common ancestor that existed before the divergence of fungi and animals. Further, native fungal fimbriae can function as a mammalian extracellular matrix component. They can act as a substratum which permits animal cells to adhere, spread, and proliferate in a manner similar to animal collagens. The implications of this finding to both phylogeny and pathology are discussed.
Full text
PDF

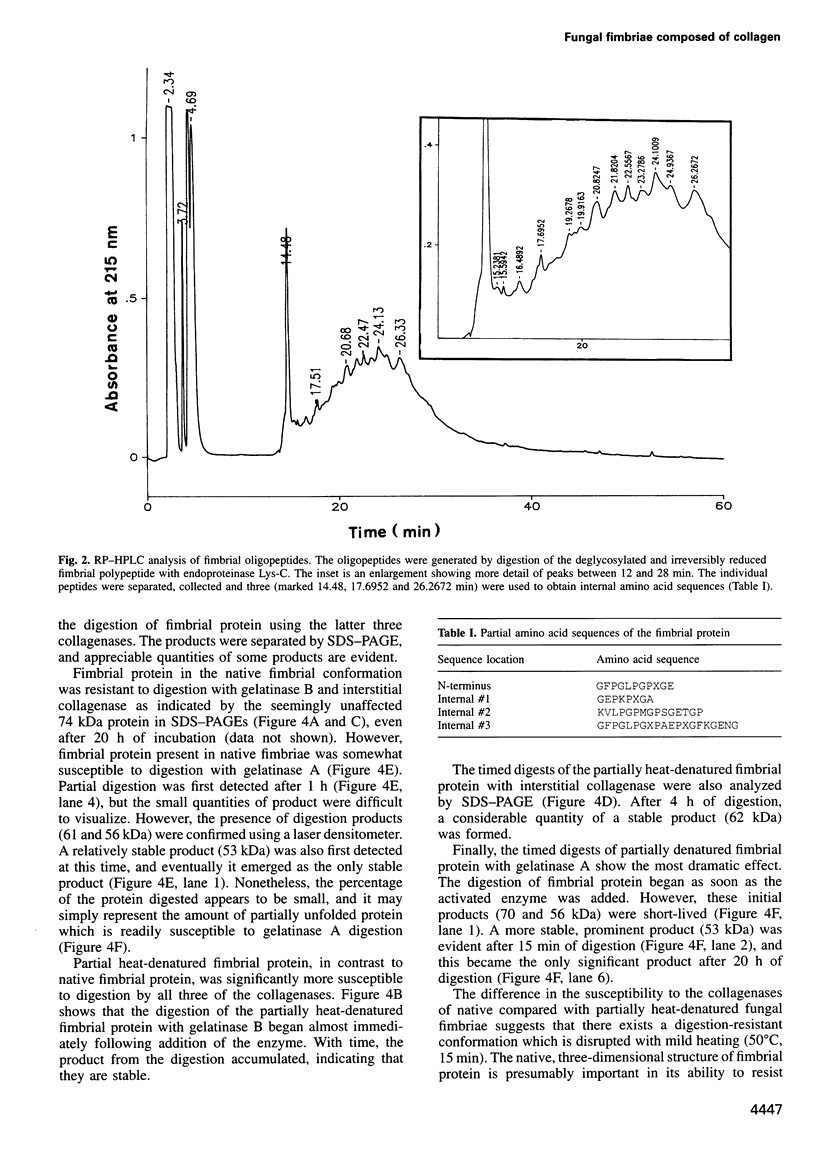
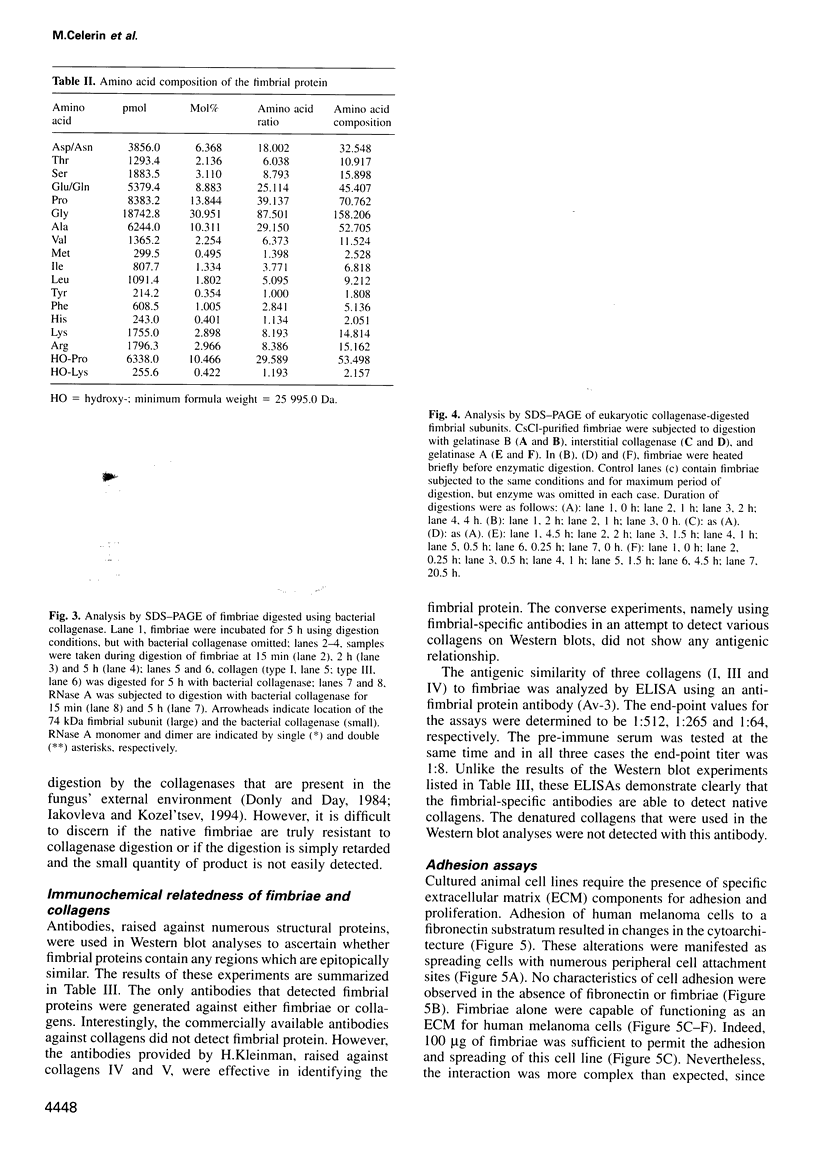

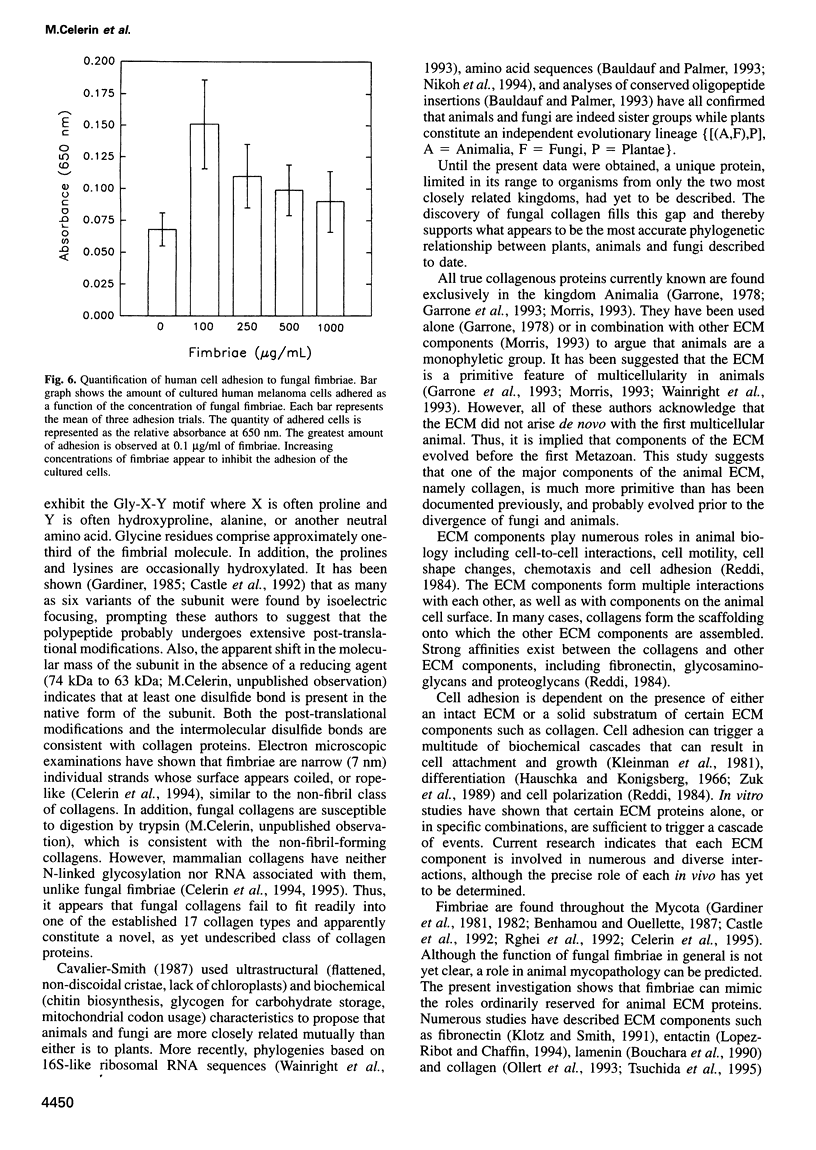



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldauf S. L., Palmer J. D. Animals and fungi are each other's closest relatives: congruent evidence from multiple proteins. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11558–11562. doi: 10.1073/pnas.90.24.11558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchara J. P., Tronchin G., Annaix V., Robert R., Senet J. M. Laminin receptors on Candida albicans germ tubes. Infect Immun. 1990 Jan;58(1):48–54. doi: 10.1128/iai.58.1.48-54.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celerin M., Laudenbach D. E., Bancroft J. B., Day A. W. Evidence that fimbriae of the smut fungus Microbotryum violaceum contain RNA. Microbiology. 1994 Oct;140(Pt 10):2699–2704. doi: 10.1099/00221287-140-10-2699. [DOI] [PubMed] [Google Scholar]
- Garrone R., Exposito J. Y., Franc J. M., Franc S., Humbert-David N., Qin L., Tillet E. La phylogenèse de la matrice extracellulaire. C R Seances Soc Biol Fil. 1993;187(2):114–123. [PubMed] [Google Scholar]
- Hauschka S. D., Konigsberg I. R. The influence of collagen on the development of muscle clones. Proc Natl Acad Sci U S A. 1966 Jan;55(1):119–126. doi: 10.1073/pnas.55.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., Klebe R. J., Martin G. R. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981 Mar;88(3):473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz S. A. Adherence of Candida albicans to components of the subendothelial extracellular matrix. FEMS Microbiol Lett. 1990 Mar 15;56(3):249–254. doi: 10.1016/s0378-1097(05)80049-7. [DOI] [PubMed] [Google Scholar]
- Klotz S. A., Smith R. L. A fibronectin receptor on Candida albicans mediates adherence of the fungus to extracellular matrix. J Infect Dis. 1991 Mar;163(3):604–610. doi: 10.1093/infdis/163.3.604. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li K., Tamai K., Tan E. M., Uitto J. Cloning of type XVII collagen. Complementary and genomic DNA sequences of mouse 180-kilodalton bullous pemphigoid antigen (BPAG2) predict an interrupted collagenous domain, a transmembrane segment, and unusual features in the 5'-end of the gene and the 3'-untranslated region of the mRNA. J Biol Chem. 1993 Apr 25;268(12):8825–8834. [PubMed] [Google Scholar]
- López-Ribot J. L., Chaffin W. L. Binding of the extracellular matrix component entactin to Candida albicans. Infect Immun. 1994 Oct;62(10):4564–4571. doi: 10.1128/iai.62.10.4564-4571.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. J., Gay S. The collagens: an overview and update. Methods Enzymol. 1987;144:3–41. doi: 10.1016/0076-6879(87)44170-0. [DOI] [PubMed] [Google Scholar]
- Neuhoff V., Arold N., Taube D., Ehrhardt W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis. 1988 Jun;9(6):255–262. doi: 10.1002/elps.1150090603. [DOI] [PubMed] [Google Scholar]
- Nikoh N., Hayase N., Iwabe N., Kuma K., Miyata T. Phylogenetic relationship of the kingdoms Animalia, Plantae, and Fungi, inferred from 23 different protein species. Mol Biol Evol. 1994 Sep;11(5):762–768. doi: 10.1093/oxfordjournals.molbev.a040156. [DOI] [PubMed] [Google Scholar]
- Ollert M. W., Söhnchen R., Korting H. C., Ollert U., Bräutigam S., Bräutigam W. Mechanisms of adherence of Candida albicans to cultured human epidermal keratinocytes. Infect Immun. 1993 Nov;61(11):4560–4568. doi: 10.1128/iai.61.11.4560-4568.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon H., Day A. W. 'Fimbriae' in the fungus Ustilago violacea. Nature. 1974 Aug 23;250(5468):648–649. doi: 10.1038/250648a0. [DOI] [PubMed] [Google Scholar]
- Poon N. H., Day A. W. Fungal fimbriae. I. Structure, origin, and synthesis. Can J Microbiol. 1975 Apr;21(4):537–546. doi: 10.1139/m75-076. [DOI] [PubMed] [Google Scholar]
- Sakiyama F., Masaki T. Lysyl endopeptidase of Achromobacter lyticus. Methods Enzymol. 1994;244:126–137. doi: 10.1016/0076-6879(94)44011-5. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Krutzsch H. C., Wacher M. P., Margulies I. M., Liotta L. A. The activation of human type IV collagenase proenzyme. Sequence identification of the major conversion product following organomercurial activation. J Biol Chem. 1989 Jan 25;264(3):1353–1356. [PubMed] [Google Scholar]
- Todaro G. J., Fryling C., De Larco J. E. Transforming growth factors produced by certain human tumor cells: polypeptides that interact with epidermal growth factor receptors. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5258–5262. doi: 10.1073/pnas.77.9.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronchin G., Bouchara J. P., Annaix V., Robert R., Senet J. M. Fungal cell adhesion molecules in Candida albicans. Eur J Epidemiol. 1991 Jan;7(1):23–33. doi: 10.1007/BF00221338. [DOI] [PubMed] [Google Scholar]
- Tsuchida K., Aoyagi Y., Odani S., Mita T., Isemura M. Isolation of a novel collagen-binding protein from the mushroom, Hypsizigus marmoreus, which inhibits the Lewis lung carcinoma cell adhesion to type IV collagen. J Biol Chem. 1995 Jan 27;270(4):1481–1484. doi: 10.1074/jbc.270.4.1481. [DOI] [PubMed] [Google Scholar]
- Vuorio E., de Crombrugghe B. The family of collagen genes. Annu Rev Biochem. 1990;59:837–872. doi: 10.1146/annurev.bi.59.070190.004201. [DOI] [PubMed] [Google Scholar]
- Vázquez-Juárez R., Ascencio F., Andlid T., Gustafsson L., Wadström T. The expression of potential colonization factors of yeasts isolated from fish during different growth conditions. Can J Microbiol. 1993 Dec;39(12):1135–1141. doi: 10.1139/m93-171. [DOI] [PubMed] [Google Scholar]
- Wainright P. O., Hinkle G., Sogin M. L., Stickel S. K. Monophyletic origins of the metazoa: an evolutionary link with fungi. Science. 1993 Apr 16;260(5106):340–342. doi: 10.1126/science.8469985. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Avvedimento V. E., Mudryj M., Ohkubo H., Vogeli G., Irani M., Pastan I., de Crombrugghe B. The collagen gene: evidence for its evolutinary assembly by amplification of a DNA segment containing an exon of 54 bp. Cell. 1980 Dec;22(3):887–892. doi: 10.1016/0092-8674(80)90565-6. [DOI] [PubMed] [Google Scholar]
- Yu L., Lee K. K., Ens K., Doig P. C., Carpenter M. R., Staddon W., Hodges R. S., Paranchych W., Irvin R. T. Partial characterization of a Candida albicans fimbrial adhesin. Infect Immun. 1994 Jul;62(7):2834–2842. doi: 10.1128/iai.62.7.2834-2842.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Lee K. K., Sheth H. B., Lane-Bell P., Srivastava G., Hindsgaul O., Paranchych W., Hodges R. S., Irvin R. T. Fimbria-mediated adherence of Candida albicans to glycosphingolipid receptors on human buccal epithelial cells. Infect Immun. 1994 Jul;62(7):2843–2848. doi: 10.1128/iai.62.7.2843-2848.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk A., Matlin K. S., Hay E. D. Type I collagen gel induces Madin-Darby canine kidney cells to become fusiform in shape and lose apical-basal polarity. J Cell Biol. 1989 Mar;108(3):903–919. doi: 10.1083/jcb.108.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]