Abstract
Expression patterns of four candidate AREB/ABF genes and four DREB/CBF genes were evaluated in leaf and root tissues of five grape varieties (‘Qalati’, ‘Kaj Angoor’, ‘Sabz Angoor’, ‘Siahe Zarghan’, ‘Bidane Safid’) with differential response to drought stress. Among the AREB/ABF genes, AREB1 and ABF2 showed up-regulation in response to drought stress in leaf and root tissues of all varieties while AREB2 and ABF1 showed down-regulation in both leaf and root tissues of the sensitive variety ‘Bidane Sefid’ in response to drought and salt stress. Among the DREB/CBF genes, CBF4 was the most responsive to drought stress in both leaf and root tissues. CBF2 and CBF3 showed up-regulation in all varieties in response to drought stress in leaf except in ‘Bidane Sefid’. Under salinity stress, AREB2 and ABF2 showed up-regulation in response to the increasing level of salinity in the leaf tissues but in the root tissues ABF2 was up-regulated in response to increasing NaCl concentration while AREB2 was down-regulated. Therefore, it seems AREB2 has tissue-specific response to salinity stress. All CBF genes were up-regulated in response to salinity stress in the leaf and root tissues. Expression data suggested that CBF2 is more responsive to NaCl stress. Among all four promising and stress tolerant varieties ‘Siah Zarghan’ and ‘Kaj Angoor’ were more tolerant than ‘Qalati’ and ‘Sabz Angoor’ to drought and salinity.
Introduction
Plants respond and adapt to stress conditions through different mechanisms at the molecular, cellular, physiological, and biochemical levels. An important step in understanding how a genome functions in a different environmental cue is to determine the pattern as how the expression of the genes is regulated. Various adverse environmental stresses change the expression pattern of a variety of genes in many plant species [1]. Among the stress related genes, transcription factors (TFs) play an important role in regulating plant’s response to stress conditions. TFs act as master switches and trigger simultaneous expression of a large number of stress-response genes that contribute to the stress tolerance phenotype. With the exception of a few TFs that play a role in primary growth processes, most TFs are up-regulated under stress conditions [2][3]. Several families of plant TFs play significant roles in regulation of gene expression under abiotic stresses [4]. Genes are often linked by several binding sites for distinct transcription factors, and different transcription factors coordinately regulate the transcription of these genes.
One of the most important adjustments under stress condition in plant is the change in the level of its phytohormones, such as abscisic acid (ABA). ABA, in addition to playing important roles in many physiological processes such as seed dormancy and development, embryo morphogenesis, leaf senescence and promotion of stomata closure, is considered as a plant stress hormone that is accumulated under osmotic imbalance caused by abiotic stresses. ABA is known to play a key role in stress responses and tolerance in plants. Genes that are altered in their expression in response to ABA accumulation in plant during stress belong to ABA-dependent pathway, and other ones belong to ABA-independent pathway. Although these two pathways are distinct, crosstalk between the two pathways are documented in several plants [5][6].
ABA perceives stress uniquely and acts as an endogenous messenger in plant cells to induce a double negative regulatory pathway in which ABA is bound to the ABA receptors RCARs/PYR1/PYLs (Regulatory Components of ABA Receptor/Pyrabactin Resistance Protein1/PYR-Like proteins) to form the complex that provides an active site for the PP2Cs (type 2C protein phosphatases). This causes inhibition of the activity of PP2C as a negative regulator of the pathway which leads to induction of SnRK2 as a positive regulator of downstream signaling and subsequent phosphorylation of the target proteins [7][8]. Thus, in the presence of ABA, the PP2Cs are inactivated to repress SnRK2 phosphatase activity and then SnRK2 could initiate ABA-responsive regulation pathway and activate the most significant cis-element ABA-responsive element (ABRE) to regulate the expression of many genes under osmotic stress conditions. Various TFs regulate the expression of ABA-responsive genes through ABRE, which contains the core sequence PyACGTGGC that regulates dehydration- and high salinity-responsive gene expression in plants in the ABA-dependent pathway. ABRE-binding protein (AREB)/ABRE binding factors (ABFs), as a member of basic domain leucine zipper (bZIP) TF, are activated in the presence of ABA and regulate the expression of genes by binding to the ABRE in their promoter regions. Several studies showed that exogenous application of ABA induced a number of genes that respond to dehydration and cold stress in the ABA-dependent pathways [5][9]. On the other hand, several studies reported induction of AREB1, AREB2 and ABF3 under drought, high salinity and ABA treatments in plants [9–11]. Previous studies showed that of the nine members of AREB/ABFs in Arabidopsis, AREB1/ABF2, AREB2/ABF4, and ABF3 were induced by ABA and osmotic stress in vegetative tissues [12–16], which suggested that they are involved in ABA and/or stress signaling. In rice, OsABF2 was shown to be induced by drought, salinity, and ABA treatment [10]. Fujita et al. [15] reported that transgenic Arabidopsis plants overexpressing the active form of AREB1 showed ABA hypersensitivity and enhanced drought tolerance. Overexpression of ABF2 in Arabidopsis enhanced its tolerance to drought, high salt, heat and oxidative stresses [17]. Lettuce and Agrostis mongolica transgenic plants ectopically expressing ABF/AREB members were more tolerant than wild type to drought and cold/heat stresses [18][19]. Similarly, overexpression of the PtABF gene of Poncirus trifoliata enhanced dehydration and drought tolerance in tobacco [20]. These studies validated the significance of ABF/AREB family members in plant’s response to various environmental stresses [21]. Yeast two-hybrid screening in grape (Vitis vinifera) identified positive interactions between two VvABFs and VvSNRK2 [11]. The authors reported that VvABFs expression under various abiotic stresses was tissue-specific where VvABF1 was highly induced in roots and VvABF2 was up-regulated in the leaves under drought, salt and ABA treatments.
Some of the most important TFs in the ABA-independent pathway that play significant roles in responses to important abiotic stresses include dehydration-responsive element binding protein (DREB)/C-repeat binding factor (CBF), NAC (NAM, ATAF, and CUC) and MYB/MYC [22–24]. The core sequence for dehydration-responsive element (DRE) is A/GCCGAC, which is a Cis-acting element for regulation of cold and dehydration-responsive gene expression in plant [22–26]. DREB/CBFs were known as low temperature responsive genes but their expression pattern in some plants were changed in response to different stress conditions. For example, AtCBF4 was responsive to drought stress, OsDREB1A was induced in response to salt stress, and AtDREB1D, VvCBF1, VvCBF2 and VvCBF3 were responsive to ABA [22][27][28].
In grape genome, Xiao et al. [28] identified three CBF/DREB1 genes; the deduced proteins were 42–51% identical to AtCBF1 and contain CBF-specific amino acid motifs, and were responsive to low temperature. The expression of these genes also changed under different levels of drought stress and ABA treatment. In another study [29], the authors also reported that the expression of CBF4 genes in V. vinifera and V. riparia was regulated in response to low temperature, drought, and salinity. Over-expression of AtCBF3 increased stress tolerance in transgenic Arabidopsis, whereas overexpression of the tomato LeCBF1 did not have the same effect in transgenic tomato [30]. ABA-mediated induction of DREB/CBF genes has also been identified in some plants. The crosstalk among ABA-dependent and ABA-independent stress response pathways and TF families could be because of the commonality in plant’s responses to different stresses, such as salinity and cold stresses, which create osmotic stress that crosstalk with drought stress [22][27][28][31–34].
Grape is an economically important most widely cultivated woody fruit crop in the world. Viticulture is closely associated with Iran history as the center of origin of V. vinifera. Traditional rainfed cultivation of grape in different edaphoclimatic zones of Iran shows that some genotypes have evolved greater tolerance than others to stress conditions, especially drought and salinity [35][36]. Considering the fact that AREB/ABFs and DREB/CBFs are major transcription factors that respond to abiotic stresses by regulating downstream genes stress-responsive genes in ABA-dependent and independent pathways, the present study was undertaken to determine the expression pattern of these genes in four promising grape varieties of Iran in response to drought and salinity stress.
Materials and Methods
Plant material and stress treatments
Cuttings of four promising drought and salt tolerant varieties [‘Qalati (Q)’, ‘Kaj Angoor (KA)’, ‘Sabz Angoor (SA)’, ‘Siahe Zarghan’ (SZ)] and the white seedless drought and salt-sensitive cultivar ‘Bidane Sefid’ (BS) [37] of grape (Vitis vinifera) with winter-dormant buds were obtained in 2012 from the Grapevine Research Station of Takestan- Qazvin, Iran. All of these cuttings were from the middle part of the branch with same diameter (1.0–1.5 cm) with 3–4 dormant buds. Cuttings were rooted in pots filled with sand under greenhouse conditions first, and rooted shoots during spring were planted in 25 cm (height) x 19 cm (dia) pots containing a mixture of soil and composts (1:2) to grow for one year out door. One-year-old rooted plants were pruned to three winter buds and roots of 8 to 10 cm in length in the following winter, and planted in 20 L pots containing approximately 15 L mixture of sand and Garden soil (2:1). Plants were grown out door and irrigated every 3 to 4 d with tap water mixed with one fourth concentration of Hoagland nutrient solution. In 2013 August, which is the warmest month of the year in most parts of Iran with a mean temperature of ~35°C and ~0.64 mm rainfall, seven-month-old uniform plants were selected for stress experiments. For drought stress treatments (S1a Fig), four levels of water stress (well-watered control, -0.3 MPa; medium stress, -0.7 MPa; high stress, -1 MPa; and severe stress: -1.5 MPa) were applied based on curve soil moisture. For salinity stress, two levels (100 mM and 200 mM) of NaCl were applied, and to reduce the negative effects of NaCl on calcium absorption, CaCl2 (one tenth of NaCl concentration) was added to the salt solution. Salinity stress was imposed (S1b Fig) with daily watering of salinized plants with 6 L of salt solution throughout the experiment. Salt concentration of the irrigation solution started with 10 mM NaCl in the first day and NaCl concentration increased 20 mM each two days until day 9 (for 100 mM stress level) and day 19 (for 200 mM stress level). Primary experiment showed that 6 L water irrigation each day creates -0.3 MPa suction in the soil, which is same as the control used in the study. Three different plants (biological replicates) were used for each treatment. The leaf and root tissues were harvested individually from each plant in liquid nitrogen at different stress levels for drought and 9 d and 19 d for salt stress treatment and stored in a -80°C freezer until RNA extraction.
RNA purification and cDNA synthesis
Total RNA was extracted according to Reid et al. [38] using an extraction buffer that contained 300 mM Tris HCL (pH 8.0), 25 mM EDTA, 2 M NaCl, 2% CTAB, 2% PVPP, 0.05% spermidine trihydrochloride, and 2% β-mercaptoethanol. The RNA was further purified using an RNeasy kit (Qiagen, Valencia, CA). First strand cDNA was synthesized from 2 μg of total RNA using the iScript cDNA synthesis kit (Bio-rad, Hercules, CA) according to the manufacturer's instructions.
Gene Identification and PCR primer design
In order to identify the V. vinifera AREB/ABF genes, protein sequences of A. thaliana AREB/ABF genes were used for a BLASTP search against the V. vinifera genome. The bZIP domain was checked in all the grape candidates. All known AREB/ABF gene sequences in the NCBI (http://ncbi.nlm.nih.gov) database along with A. thaliana genes and grape candidates were clustered (S2 Fig), and based on their classification in the cluster, four grape AREB/ABF candidate genes were selected. DREB/CBF genes were identified from V. vinifera genome based on their annotation against the NCBI database. Primers were designed for each of the genes using Primer 3 (http://bioinfo.ut.ee/primer3) and NCBI primer blast (http://www.ncbi.nlm.nih.gov/tools/primer-blast). For family gene distinction, one of the primer pairs was designed from the bZIP domain and the other one was designed from the conserved sequences of the particular gene. Details of the primers are provided in Table 1. Quantitative real time PCR (qRT-PCR) reactions were performed using SYBR Green kit (Bio-rad, Hercules, CA). Reactions were performed in a 20 μl volume containing 3 μl of diluted cDNA (5 times diluted from the first strand cDNA stock) and 10 μl 2× iQ SYBR Green Master Mix Supermix as described earlier [39]. Elongation factor (VvEF1α; accession no. LOC100261589) was used as the internal reference gene (S3 Fig).
Table 1. Primer sequences of the genes used for expression analysis in grape.
| Primer | Sequence (5’– 3’) |
|---|---|
| VvAREB1-F | CTTCCATATACTCCTTGACC |
| VvAREB1-R | AGGCAATGTCAAAGAACCC |
| VvAREB2-F | TAACCACATTAGCAACTCCC |
| VvAREB2-R | CATTATGAACGCTGTCCTGC |
| VvABF1-F | TGATAAACACATGGCTGACC |
| VvABF1-R | TCTTCCAAAGTCATCTCCCC |
| VvABF2-F | GCAGGTGTGATTAGTTTAGGG |
| VvABF2-R | CTTGAGTCCAACACTCCTGG |
| VvCBF1-F | AGAGAAGGTTGGAGATGGTTCA |
| VvCBF1-R | CAGGTGGAGTAAGGAGCAAAC |
| VvCBF2-F | CTGCTTCTTCCGACTCTC |
| VvCBF2-R | GCACTTCACTCACCCATTTGTT |
| VvCBF3-F | AAGTGCGGGATCCCAAAACC |
| VvCBF3-R | GGAGTCGGGGAAATTGAGC |
| VvCBF4-F | ACCCTCACCCGCTCGTATG |
| VvCBF4-R | CCGCGTCTCCCGAAACTT |
| VvEF1a-F | CGGGCAAGAGATACCTCAAT |
| VvEF1a-R | AGAGCCTCTCCCTCAAAAGG |
Results
Eight genes, four each belonging to AREB/ABF and DREB/CBF, in five grape varieties showed time- and tissue-dependent variation in their expression patterns in the leaf and root tissues under drought (Figs 1 and 2; S1 and S2 Tables) and salt (Figs 3 and 4; S3 and S4 Tables) stress treatments.
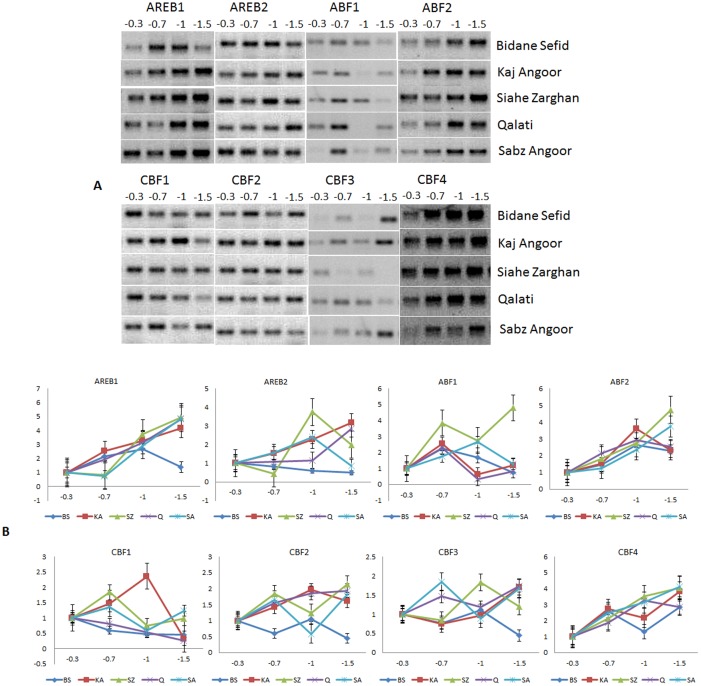
Fig 1. Semiquantitative (A) and Quantitative (B) expression analysis of AREB/ABF and DREB/CBF genes in the leaf tissues of five different varieties of grape under drought stress.
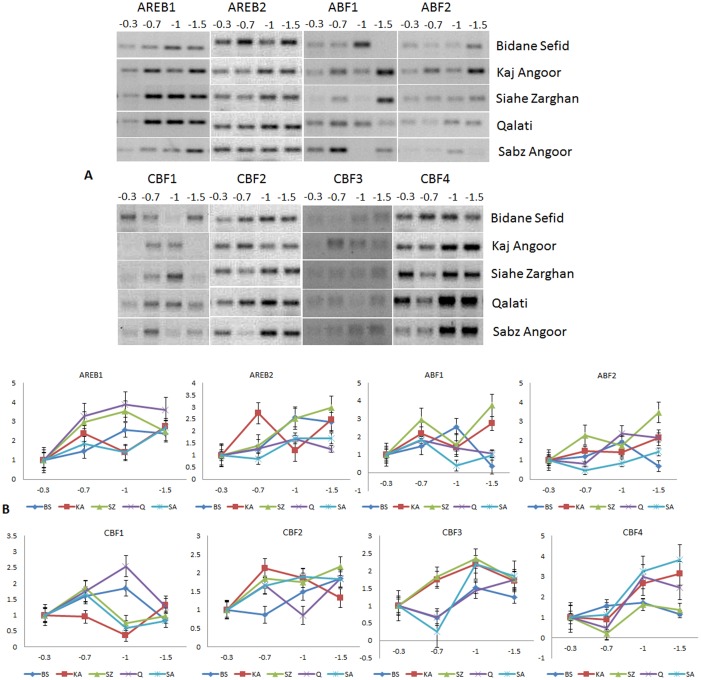
Fig 2. Semiquantitative (A) and Quantitative (B) expression analysis of AREB/ABF and DREB/CBF genes in the root tissues of five different varieties of grape under drought stress.
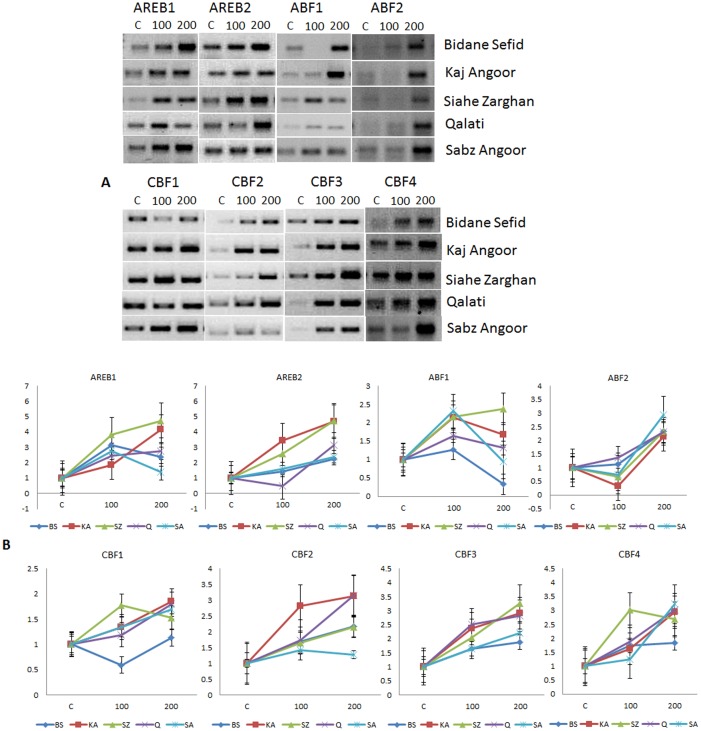
Fig 3. Semiquantitative (A) and Quantitative (B) expression analysis of AREB/ABF and DREB/CBF genes in the leaf tissues of five different varieties of grape under salt stress.
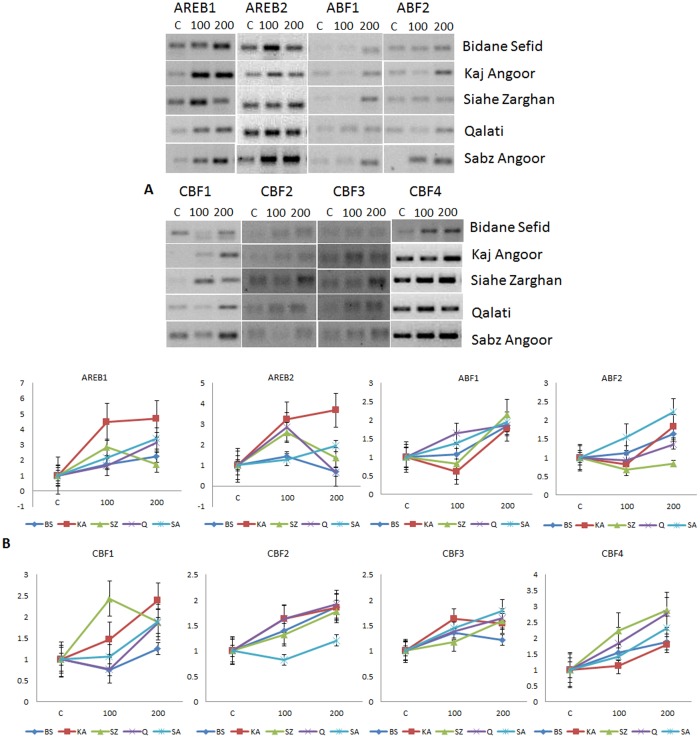
Fig 4. Semiquantitative (A) and Quantitative (B) expression analysis of AREB/ABF and DREB/CBF genes in the root tissues of five different varieties of grape under salt stress.
Expression of AREB/ABF genes under drought
Under drought stress, VvAREB1 gene in grape, a homolog of AREB1/ABF2, was significantly up-regulated in the leaf of all genotypes at high (-1 MPa) and severe (-1.5 MPa) stress levels, although its expression in BS under severe stress was lower than that under moderate and high drought (Fig 1; S1 Table). The gene showed an incremental up-regulation along with the severity of stress level in KA and Q. In SZ and SA, the gene showed down-regulation under medium stress level following which its expression was upregulated. In the root tissue (Fig 2; S2 Table), the gene showed up-regulation under drought stress in all five varieties. The expression of AREB1 was similar in the leaf and root tissues in BS where the level of induction across different stress levels was, in general, lower than the four resistant varieties.
VvAREB2 was closer to AREB1/ABF2 in the cluster (S2 Fig); its transcript accumulation, like AREB1, showed an increasing trend of up-regulation with the severity of drought stress in the leaf of KA and Q. In SZ and SA, the transcript accumulation reached its peak at the high level drought stress. However, it was down-regulated in SZ immediately after imposition of low level of stress, whereas it was down-regulated in SA at the severe drought condition. VvAREB2 showed down-regulation under drought in the leaf of the sensitive variety BS, but the level of its expression was higher compared to the control condition of other varieties. However, in the root, it was up-regulated in BS, Q, and SA under drought stress, although with a slight reduction in BS and Q at severe stress and in SA at the low level stress. On the other hand, SZ showed increased accumulation of VvAREB2 mRNA with increasing stress level while KA showed an up-regulation compared to the control but the level was highest under low followed by severe level drought stress, and the level dropped at high level stress.
VvABF1, the grape homolog of ABF3 gene, showed a different expression pattern than other AREB/ABF genes. In the leaf tissue, all five varieties had increased mRNA abundance under low level drought stress, after which the pattern was variable in the varieties. SZ showed highest up-regulation of VvABF1 at severe stress level with a slight reduction at high level stress. In SA, up-regulation was observed at all stress levels, although the level of accumulation at severe stress was slightly less than moderate and high level stress. In the leaf of KA and Q, the level of ABF1 expression, after an up-regulation at moderate level stress, was down-regulated at high level drought and then slightly increased, but the level was still lower than the control. The sensitive variety BS showed a declining trend after 2-fold up-regulation under moderate stress until the level was lower than the control under severe stress. In the root tissue (Fig 2), ABF1 in BS was down-regulated under severe stress while there was an increasing up-regulation under moderate to high drought stress. SZ and KA showed similar pattern of expression with up-regulation under all levels of stress and a slight decline (still higher than the control) under high level drought stress. The variety Q showed up-regulation of ABF1 at -0.7 MPa and then the level declined during subsequent levels of stress with down-regulation under severe stress compared to the control. However, VvABF1 had an up-regulation in its transcript accumulation under moderate stress following which it was down-regulated.
VvABF2, the homolog of AtABF3 gene, was up-regulated in the leaf under stress in all varieties. SZ and SA showed an up-regulation of ABF2 with the highest mRNA accumulation under severe drought stress, whereas the peak for other varieties was observed at high level drought. The expression level of ABF2 in the leaf of SZ was significantly higher than other varieties under control condition. However, expression pattern of ABF2 in the root varied with genotypes. In the drought sensitive variety BS, it was slightly up-regulated under moderate stress followed by a 2-fold increase under high stress level and then significantly down-regulated to an undetectable level under severe drought. It was up-regulated in SZ and SA with highest expression under severe stress, but in SA, it was down-regulated under moderate and high level drought. On the other hand, it had the highest expression under high level of drought stress in Q, with slight down-regulation under moderate stress. The expression pattern of ABF2 in the leaf and root of BS was same at all stress levels and also in the SA and Q except the medium level in the other stress level has same trend in both leaf and root.
Expression of DREB/CBF genes under drought stress
In addition to AREB/ABF genes, four grape genes, showing similarity with DREB/CBF genes, were also evaluated for their expression under different levels of drought stress. VvCBF1 showed down-regulation in its transcript accumulation in the leaf of BS in response to increasing stress level. Same trend was observed in the leaf of variety Q. The gene also showed similar pattern in the leaf of SA and SZ where after an up-regulation at the moderate drought stress, its expression was down-regulated under high level stress and then slightly up-regulated under severe drought. KA showed a different expression pattern; it showed an up-regulation under both moderate and high level stress, but under severe stress level, its transcript accumulation was significantly down-regulated. In the root tissue, SZ and SA showed similar pattern like in the leaf where the gene showed up-regulation under moderate stress level and then down-regulation under high and severe stress conditions. The expression pattern of VvCB1 was same in the root of Q and BS where it was increasingly up-regulated under moderate and high stress levels and then declined in its transcript accumulation under severe drought stress. On the other hand, in KA it was down-regulated in response to moderate and high levels of drought but was up-regulated under severe stress condition.
VvCBF2 expression pattern in the leaf of KA and Q was similar where the mRNA content showed up-regulation at all levels of stress but with a slight decline in KA under severe stress. SA and SZ also displayed same trend; up-regulation under moderate and severe stress, and down-regulation under high level of drought. The gene showed significant down-regulation in the sensitive variety BS compared to the control, especially under moderate and severe drought conditions. On the contrary, the expression level of VvCBF2 in the root of BS was higher than the control under high and severe drought. In the root of KA, Q and SZ, the gene showed varying level of up-regulation under different levels of drought. For example, SA showed increasing CBF2 mRNA accumulation with increasing stress level; KA had the highest accumulation at moderate drought, SZ and Q under severe drought condition, although CBF2 was down-regulated under high drought in Q.
In the leaf tissue of BS and SZ, VvCBF3 showed similar pattern of expression where it showed down-regulation under moderate drought followed by increased transcript accumulation under high and severe stress, but the mRNA content was still lower than the control in BS. Similarly, Q and SA showed similar pattern in the expression of CBF3. The gene was up-regulated under moderate stress and, after a brief decline, was up-regulated to its maximum peak. The three varieties Q, SA and BS showed similar trend in the root tissue where the gene showed up-regulation after being down-regulated at moderate stress and the highest up-regulation at high (SA and BS) and severe drought (Q). The expression of CBF3 in the root of KA and SZ showed up-regulation under all levels of stress, although with a slight decline under severe drought stress.
VvCBF4 showed up-regulation in its transcript accumulation in response to increasing drought stress in the leaf tissue of all varieties, although at varying level in comparison to the control. Interestingly, the CBF4 expression level in the leaf was very high under non-stressed control in SZ and KA. All varieties had a higher basal mRNA content of CBF4 than the sensitive variety BS. Variety Q had its highest peak of CBF4 under high drought condition, whereas all others had its highest expression under severe drought stress. KA and BS showed a reduction of CBF4 expression under high stress but the level was still higher compared to the control. Increasing trend of CBF4 accumulation was observed with increasing severity of drought stress in SA and SZ. In the root of KA and SA, the gene showed up-regulation under high and severe stress, and a slight down-regulation under moderate stress in Q. In the sensitive variety BS, after an up-regulation under moderate and high level drought, the expression of CBF4 returned to the basal level under severe drought. Expression level of CBF4 in the root of SZ and SA was higher than other varieties under non-stressed control condition. At the same time, SA and SZ displayed down-regulation of CBF4 at the primary stage (moderate level) of drought stress.
Expression of AREB/ABF genes under salt stress
Expression profiles of the AREB/ABF genes in all the five varieties were also evaluated under two salinity levels, 100 mM and 200 mM NaCl. Like drought stress, the genes showed temporal and spatial variation in their expression pattern under salinity (Figs 3 and 4). AREB1 was up-regulated in the leaf tissues of all the varieties under both levels (100 mM and 200 mM NaCl) of salinity stress as compared to the unstressed control (Fig 3). However, at 200 mM salinity level, its expression in KA, SZ and Q was higher in comparison to that under 100 mM NaCl, whereas its expression level declined in BS and SA at 200 mM compared to 100 mM. In the root tissue (Fig 4), AREB1 was up-regulated in all the varieties under both levels of salinity except SZ, where the level was higher at 200 mM NaCl in comparison to 100 mM NaCl. AREB1 expression was highest in SZ (4.7-fold) in the leaf under 200 mM NaCl, but KA maintained its high expression of 4.2-fold and 4.7-fold in leaf and root tissues at 200 mM salinity level, respectively, in comparison to other varieties.
AREB2 expression in the leaf tissue of all the varieties was up-regulated with increasing salt stress level, although in the variety Q, down-regulation was observed at 100 mM salinity level. In the root tissue, AREB2 was up-regulated in all the varieties under 100 mM NaCl. But, under 200 mM NaCl, KA and SZ maintained AREB2 abundance while Q and BS showed down-regulation, and SZ showed decreased level of up-regulation in comparison to 100 mM NaCl. The varieties KA and SZ behaved similarly for both AREB1 and AREB2.
ABF1 was up-regulated under 100 mM NaCl in the leaf of all the varieties, but under 200 mM NaCl, BS showed 0.3-fold down-regulation while SA was slightly down-regulated (0.9-fold) as compared to the control. SZ showed an increased accumulation of ABF1 transcript, whereas KA and Q showed its up-regulation at a lower level than 100 mM NaCl. In the root tissues, ABF1 was down-regulated in KA and SZ at 100 mM salinity level, but remained unchanged in BS, whereas in SA and Q, it was up-regulated. With increasing salinity level (200 mM NaCl), all the varieties showed up-regulation of ABF1.
ABF2 was down-regulated in the leaf tissues of KA, SZ and SA at 100 mM NaCl. On the other hand, it remained almost unchanged in BS, while it was up-regulated in Q. Under 200 mM NaCl, its mRNA content was up-regulated in the leaf tissues of all varieties with the level being highest in SA (2.9-fold). In the root tissues, ABF2 was up-regulated under 200 mM NaCl in all varieties except SZ, which showed down-regulation under both 100 and 200 mM NaCl. At 100 mM salinity level, except SA and BS, which showed ABF2 up-regulation, other varieties showed down-regulation as compared to the control.
Expression of DREB/CBF genes under salt stress
Under both levels of salinity, CBF1 mRNA accumulation was up-regulated in the leaf tissues of all varieties except BS, which showed its down-regulation at 100 mM salinity. SZ showed a slightly reduced level of CBF1 mRNA under 200 mM NaCl stress as compared to 100 mM NaCl. CBF1 expression pattern in root was similar to leaf, where BS and Q showed CBF1 down-regulation at 100 mM salinity level and the expression level did not change in SA. The transcript accumulation of CBF2 was up-regulated under both 100 mM and 200 mM NaCl in the leaf and root tissues of all varieties, except in SA where it was down-regulated in root tissue at 100 mM salinity as compared to the control, and showed a slight decline in CBF1 mRNA at 200 mM compared to 100 mM NaCl. Variety Q had the highest abundance of CBF2 mRNA under 200 mM salinity in both the leaf and root tissues (S3 and S4 Tables).
CFB3 was up-regulated in all the varieties in the leaf and root tissues under both 100 mM and 200 mM NaCl stress. At 100 mM salinity level, up-regulation of CBF3 in the leaf tissue was the highest in Q (2.5-fold), whereas under 200 mM NaCl stress condition it was highest in SZ (3.3-fold) in comparison to the control. In BS and KA, CBF3 mRNA content was slightly lower in 200 mM compared to 100 mM salinity. Similarly, CBF4 transcript was up-regulated in the leaf and root tissues of all the varieties under both levels of salinity. The mRNA level of all varieties increased with increasing salinity level in both leaf and root tissues except SZ, where its expression was reduced in the leaf at 200 mM NaCl than at 100 mM NaCl. Fold-change expression of the genes under drought and salinity for all genes are provided as S1, S2, S3 and S4 Tables,and heatmaps as S4 and S5 Figs.
Discussion
ABA accumulates under osmotic stress and other water stresses. It mediates stress responses in vegetative tissues in plant at both genetic and biochemical levels, although not all stress responses are ABA-dependent [40–43]. Expression pattern of ABA-responsive genes is regulated mainly by two different family of bZIP TFs: one that is active in seeds (ABIs) and the other in vegetative tissues (AREB/ABFs) [6][44–47]. ABFs are generally highly conserved although the conserved regions do not have easily recognizable motifs. AREB/ABF genes are active in two different ways: they produce functional proteins to stabilize the plant cells and interact with other regulatory proteins under stress conditions; and they play a central role by modulating expression of downstream genes directly or indirectly through ABA [12]. ABFs may also be involved in nuclear translocation and transcriptional activation [48]. Each ABF gene may function in different ABA-dependent stress signaling pathways. Each AREB/ABF protein may play a specific role in addition to the shared roles with other AREB/ABFs. Although all AREB/ABFs are ABA-inducible and can bind to same ABREs, they are induced by various stress treatments including ABA, and their expression patterns are different from each other in a tissue-dependent manner [48]. The difference in their expression level in response to different stress conditions may be related to the roles of each AREB/ABF and to the list of their target genes [15][49].
In the present study AREB1 expression was up-regulated under both drought and high salt stress, but AREB2 was more responsive to salt stress than drought stress. Both ABF1 and ABF2 were overexpressed under both drought and salinity stresses, but ABF2 expression was enhanced mostly under drought stress condition. Boneh et al. [11] and Choi et al. [48] showed that the expression of AREB1/ABF2 and ABF3 was induced by high salt, and AREB2/ABF4 was induced by cold, high salt and drought in Arabidopsis, which indicated that AREB1/ABF2 and ABF3 are involved in high salt signal transduction, whereas AREB2/ABF4 participates in multiple stress responses [48]. Yoshida et al. [12] also showed that AREB1, AREB2, and ABF3 were up-regulated by water stress and ABA. Also, all these three AREB/ABF TFs required ABA for full activation in both Arabidopsis and rice. These studies and the present study clearly suggested that AREB/ABF genes have largely overlapping functions. Arabidopsis mutants for each of these genes showed reduced drought tolerance, whereas a triple mutant was more resistant to ABA and sensitive to drought stress. Several other studies reported that AREB/ABF-overexpressing plants showed ABA hypersensitivity and enhanced tolerance to abiotic stresses, such as freezing, drought and salt stress [15][49–51]. The present results showed that AREB/ABFs had different expression levels in different tissues in grape in response to drought and salinity stress. Boneh et al. [11] reported that the stress and ABA-regulated expression of the ABF genes were organ-specific in grape. In grape, two putative ABFs were reported to interact with SnRK2, which is upstream of the phosphorylation cascade of ABA double-negative regulatory mechanism, and thus phosphorylated ABF to its functionally active form [11].
The CBF/DREB proteins play a critical role in abiotic stress-mediated gene expression and represent one of the most attractive regulons for breeding programs. Most CBF genes are known to be induced/up-regulated in response to cold but not drought stress [29][31]. However, several studies showed that DREB/CBF genes are induced by various stresses, and that ABA is capable of activating some DREB/CBF genes [29][31] [52][53]. Thirty eight VvDREB members, organized into A1 through A6 subgroups of Arabidopsis DREBs, were identified from the entire grapevine genome and its expression sequence tag assembly [54]. In the present study, CBF1 and CBF3 were expressed more under salinity, whereas CBF2 was responsive to both drought and high salinity stress. Up-regulation of CBF4 was observed under both drought and salinity stresses in the leaf and root tissues of grape. In Arabidopsis, AtCBF4 was induced by drought but not cold [27][31][32]. CBF4 is known to be more responsive to osmotic stress in plants and was induced by exogenous ABA application in Arabidopsis and grape [27][29]. Expression of CBF4 in response to cold stress could be because of osmotic stress [29]. In Medicago, it was expressed in response to cold, drought and high salt stresses, and ABA treatment [55]. CBF1-3 transcript level also increased in response to elevated ABA levels, which was partly due to the increased activity of CBF promoters in response to ABA [27][52]. In the present study, CBF genes were most responsive to drought stress, although they were expressed at high level under salt stress. Overexpression of CBF genes enhanced drought and salt tolerance in Arabidopsis, rice and Medicago [55][56]. Evaluation of the expression patterns of three nucleus-localized DREB/CBF (CBF1, CBF2 and CBF3) genes in grape showed that their transcripts were more in young tissues than in old tissues [28]. Varying amounts of CBF1 transcript observed in grape could be explained by the plant condition that influenced its stress response to stress or could be because of plant cells memory to the previously encountered stress [52][57]. Further, DREB was shown to interact with AREB in ABA-induced slow gene expression in vegetative tissues under dehydration stress conditions [58]. In the present study, varietal difference in the amounts of ABF and CBF transcripts was observed even under unstressed control conditions, although no significant difference was observed in the expression of the CBF genes between V. vinifera and V. riparia [28]. High levels of these TFs were observed in stress tolerant varieties in comparison to the sensitive variety. Based on the phenotype data (S1 Fig) supported by the gene expression results, varieties SZ, KA and Q were considered resistant to drought and salinity stresses; KA appeared to be the most tolerant variety to salt stress. In all of these tolerant varieties a high level of expression of AREB/ABF and DREB/CBF genes was observed. High level of expression of these TFs under the control condition in the resistant varieties is an indicative of their anticipatory preparedness to respond better to a particular stress condition compared to the susceptible variety. DREB proteins can function with AREB in the expression of dehydration responsive rd29A gene under high-salinity conditions. Therefore, further detail studies are needed especially in resistant varieties of grape to unravel interaction between the AREB/ABRE and DREB/DRE regulatory systems that modulates expression of downstream stress responsive genes in response to ABA accumulated under drought and high-salinity conditions. Further validation of the expression of these TFs in a diverse collection of grape varieties will facilitate their use as candidate expression markers in breeding programs to screen drought and/or salinity resistant/susceptible genotypes.
Supporting Information
Q = Qalati, SA = Sabz Angoor, SZ = Sabz angoor, KA = Kaj angoor, B = Bidaneh sefid; 1 = control, 2 = - 1.5 Mpa drought stress. Picture was taken three weeks and two weeks after drought (a) and salinity (b) stress treatment, respectively.
(PPTX)
(PPTX)
(PPTX)
T1, -0.3 Mpa; T2, -0.7 Mpa; T3, -1.0 Mpa; T4, -1,5 Mpa.
(PPTX)
T1, control; T2, 100 mM NaCl; T3, 200 mM NaCl.
(PPTX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors thank Dr. Mohammad Ali Nejatian of Takistan Grape Research Station for providing the grape cuttings. The manuscript has been approved for publication by the Louisiana Agricultural Experimental Station as MS# 2015-306-22459.
Data Availability
All relevant data are available in the paper and its Supporting Information files.
Funding Statement
This is a self-funded research program by HZ.
References
- 1. Bartels D, Sunkar R. Drought and salt tolerance in plants. Critical Reviews in Plant Sciences. 2005; 24: 23–58. [Google Scholar]
- 2. Bartels D, Souer E. Molecular responses of higher plants to dehydration In: Hirt H, and Shinozaki K, editors. Plant Responses to Abiotic Stress. Berlin: Springer-Verlag; 2004. pp. 9–38. [Google Scholar]
- 3. Bhargava S, Sawant K. Drought stress adaptation: metabolic adjustment and regulation of gene expression. Plant Breeding. 2013; 132: 21–32. [Google Scholar]
- 4. Lindemose S, O’ Shea C, Jensen MK, Skriver K. Structure, function and networks of transcription factors involved in abiotic stress responses. International Journal of Molecular Sciences. 2013; 14: 5842–78. 10.3390/ijms14035842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu JK. Salt and drought stress signal transduction in plants. Annual Review of Plant Biology. 2002; 53: 247–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fujita Y, Fujita M, Shinozaki K, Yamaguchi-Shinozaki K. ABA-mediated transcriptional regulation in response to osmotic stress in plants. Journal of Plant Research 2011; 124: 509–525. 10.1007/s10265-011-0412-3 [DOI] [PubMed] [Google Scholar]
- 7. Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, et al. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science. 2009; 324: 1064–1068. 10.1126/science.1172408 [DOI] [PubMed] [Google Scholar]
- 8. Park S, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science. 2009; 324: 1068–1071. 10.1126/science.1173041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shinozaki K, Yamaguchi-Shinozaki K, Seki M. Regulatory network of gene expression in the drought and cold stress responses. Current Opinion in Plant Biology 2003; 6: 410–417. [DOI] [PubMed] [Google Scholar]
- 10. Hossain MA, Cho JI, Han M, Ahn CH, Jeon JS, An G, et al. The ABRE binding bZIP transcription factor OsABF2 is a positive regulator of abiotic stress and ABA signaling in rice. Journal of Plant Physiology. 2010; 167: 1512–20. 10.1016/j.jplph.2010.05.008 [DOI] [PubMed] [Google Scholar]
- 11. Boneh U, Biton I, Schwartz A, Ben-Ari G. Characterization of the ABA signal transduction pathway in Vitis vinifera . Plant Science. 2012; 187: 89–96. 10.1016/j.plantsci.2012.01.015 [DOI] [PubMed] [Google Scholar]
- 12. Yoshida T, Fujita Y, Sayama H, Kidokoro S, Maruyama K, Mizoi J, et al. AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA for full activation. Plant Journal. 2010; 61: 672–685. 10.1111/j.1365-313X.2009.04092.x [DOI] [PubMed] [Google Scholar]
- 13. Choi H, Hong J, Ha J, Kang J, Kim SY. ABFs, a family of ABA-responsive element binding factors. Journal of Biological Chemistry. 2000; 275: 1723–1730. [DOI] [PubMed] [Google Scholar]
- 14. Uno Y, Furihata T, Abe H, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K. Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proceedings of the National Academy of Sciences, USA. 2000; 97: 11632–11637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fujita Y, Fujita M, Satoh R, Maruyama K, Parvez MM, Seki M, et al. AREB1 is a transcription activator of novel ABRE-dependent ABA signaling that enhances drought stress tolerance in Arabidopsis . Plant Cell. 2005; 17: 3470–3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fujita Y, Fujita M, Shinozaki K, Yamaguchi-Shinozaki K. ABA-mediated transcriptional regulation in response to osmotic stress in plants. Journal of Plant Research. 2011; 124: 509–525. 10.1007/s10265-011-0412-3 [DOI] [PubMed] [Google Scholar]
- 17. Li XY, Liu X, Yao Y, Li YH, Liu S, He CY, et al. Overexpression of Arachis hypogaea AREB1 gene enhances drought tolerance by modulating ROS scavenging and maintaining endogenous ABA content. International Journal of Molecular Sciences. 2013; 14: 12827–12842. 10.3390/ijms140612827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vanjildorj E, Bae TW, Riu KZ, Kim SY, Lee HY. Overexpression of Arabidopsis ABF3 gene enhances tolerance to drought and cold in transgenic lettuce (Lactuca sativa). Plant Cell Tissue and Organ Culture. 2005; 83: 41–50. [Google Scholar]
- 19. Vanjildorj E, Bae TW, Riu KZ, Yun PY, Park SY, Lee CH, et al. Transgenic Agrostis mongolica Roshev. with enhanced tolerance to drought and heat stresses obtained from Agrobacterium-mediated transformation. Plant Cell Tissue and Organ Culture 2006; 87: 109–120. [Google Scholar]
- 20. Huang XS, Liu JH, Chen XJ. Overexpression of PtrABF gene, a bZIP transcription factor isolated from Poncirus trifoliata, enhances dehydration and drought tolerance in tobacco via scavenging ROS and modulating expression of stress-responsive genes. BMC Plant Biology. 2010; 10: 230–248. 10.1186/1471-2229-10-230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Umezawa T, Fujita M, Fujita Y, Yamaguchi-Shinozaki K, Shinozaki K. Engineering drought tolerance in plants: discovering and tailoring genes to unlock the future. Current Opinion in Biotechnology. 2006; 17: 113–122. [DOI] [PubMed] [Google Scholar]
- 22. Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, et al. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis . Plant Cell. 1998; 10: 1391–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nature Biotechnology. 1999; 17: 287–291. [DOI] [PubMed] [Google Scholar]
- 24. Zhu JK. Salt and drought stress signal transduction in plants. Annual Review of Plant Biology. 2002; 53: 247–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yamaguchi-Shinozaki K, Shinozaki K. A novel cis-acting element in an Arabidopsis gene is in volved in responsiveness to drought, low temperature, or high-salts tress. Plant Cell. 1994; 6: 251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shinozaki K, Yamaguchi-Shinozaki K. Molecular responses to dehydration and low temperature: differences and cross-talk between two stress signaling pathways. Current Opinion in Plant Biology. 2000; 3: 217–223. [PubMed] [Google Scholar]
- 27. Haake V, Cook D, Riechmann JL, Pineda O, Thomashow MF, Zhang JZ. Transcription factor CBF4 is a regulator of drought adaptation in Arabidopsis . Plant Physiology. 2002; 130: 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xiao H, Siddiqua M, Braybrook S. Three grape CBF/DREB1 genes respond to low temperature, drought and abscisic acid. Plant, Cell and Environment. 2006; 29: 1410–1421. [DOI] [PubMed] [Google Scholar]
- 29. Xiao H, Tattersall E, Siddiqua M, Cramer GR, Nassuth A. CBF4 is a unique member of the CBF transcription factor family of Vitis vinifera and Vitis riparia . Plant Cell and Environment. 2008; 31: 1–10. [DOI] [PubMed] [Google Scholar]
- 30. Zhang X, Fowler SG, Cheng H, Lou Y, Rhee SY, Stockinger EJ, et al. Freezing-sensitive tomato has a functional CBF cold-response pathway, but a CBF regulon that differs from that of freezing-tolerant Arabidopsis . Plant Journal. 2004; 39: 905–919. [DOI] [PubMed] [Google Scholar]
- 31. Gilmour SJ, Zarka DG, Stockinger EJ, Salazar MP, Houghton JM, Thomashow MF. Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in c old-induced COR gene expression. Plant Journal. 1998; 16: 433–442. [DOI] [PubMed] [Google Scholar]
- 32. Medina J, Bargues M, Terol J, Perez-Alonso M, Salinas J. The Arabidopsis CBF gene family is composed of three genes encoding AP2 domain-containing proteins whose expression i s regulated by low temperature but not by abscisic acid or dehydration. Plant Physiology. 1999; 119: 463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hong JP, Kim WT. Isolation and functional characterization of the CaDREBLP1 gene encoding a dehydration responsive element binding-factor-like protein 1 in hot pepper (Capsicum annuum L. cv. Pukang). Planta. 2005; 220: 875–888. [DOI] [PubMed] [Google Scholar]
- 34. Skinner JS, Zitzewitz J, Szucs P, Marquez-Cedillo L, Filichkin T, Amundsen K, et al. Structural, functional, and phylogenetic characterization of a large CBF gene family in barley. Plant Molecular Biology. 2005; 59: 533–551. [DOI] [PubMed] [Google Scholar]
- 35. McGovern PE. Ancient wine: The search for the origins of viniculture. 2007. Princeton, NJ: Princeton University Press. [Google Scholar]
- 36. Ghaderi N, Talaie A, Ebadi A, Lessani H. The physiological response of three Iranian grape cultivars to progressive drought stress. Journal of Agricultural Science and Technology. 2011; 13: 601–610. [Google Scholar]
- 37. Haddadinejad M, Ebadi A, Fattahi MR, Nejatian MA. Primary morphological screening of 698 grapevine genotypes to select drought tolerant rootstocks. Iranian Journal of Horticultural Science. 2012; 44: 193–207. [Google Scholar]
- 38. Reid KE, Niclas O, James S, Fred P, Steven TL. An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biology. 2006; 6: 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Baisakh N, Subudhi P, Varadwaj P. Primary responses to salt stress in a halophyte Spartina alterniflora (Loisel). Functional & Integrative Genomics. 2008; 8: 287–300. [DOI] [PubMed] [Google Scholar]
- 40. Leung J, Giraudat J. Abscisic acid signal transduction. Annual Review of Plant Physiology and Plant Molecular Biology. 1998; 49: 199–222. [DOI] [PubMed] [Google Scholar]
- 41. Shinozaki K, Yamaguchi-Shinozaki K. Molecular responses to drought and cold stress. Current Opinion in Biotechnology. 1996; 7: 161–167. [DOI] [PubMed] [Google Scholar]
- 42. Thomashow MF. Role of Cold-Responsive Genes in Plant Freezing Tolerance. Plant Physiology. 1998; 118: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ingram J, Bartels D. The molecular basis of dehydration tolerance in plants. Annual Review of Plant Physiology and Plant Molecular Biology 1996; 47: 377–403. [DOI] [PubMed] [Google Scholar]
- 44. Finkelstein RR, Gampala SS, Rock CD. Abscisic acid signaling in seeds and seedlings. Plant Cell. 2002; 14: S15–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yamaguchi-Shinozaki K, Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annual Review of Plant Biology. 2006; 57: 781–803. [DOI] [PubMed] [Google Scholar]
- 46. Nakashima K, Ito Y, Yamaguchi-Shinozaki K. Transcriptional regulatory networks in response to abiotic stresses in Arabidopsis and grasses. Plant Physiology. 2009; 149: 88–95. 10.1104/pp.108.129791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nakashima K, Yamagushi-shinozaki K, Shinozaki K. The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold and heat. Frontiers in Plant Science. 2014; 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Choi H, Hong J, Ha J, Kang J, Kim SY. ABFs, a family of ABA-responsive element binding factors. Journal of Biological Chemistry. 2000; 275: 1723–1730. [DOI] [PubMed] [Google Scholar]
- 49. Kim S, Kang J, Cho D, Park J, Kim S. ABF2, an ABRE-binding bZIP factor, is an essential component of glucose signaling and its overexpression affects multiple stress tolerance. Plant Journal 2004; 40: 75–87. [DOI] [PubMed] [Google Scholar]
- 50. Furihata T, Maruyama K, Fujita Y, Umezawa T, Yoshida R, Shinozaki K, et al. Abscisic acid-dependent multisite phosphorylation regulates the activity of a transcription activator AREB1. Proceedings of the National Academy of Sciences, USA. 2006; 103: 1988–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kang JY, Choi HI, Im MY, Kim SY. Arabidopsis basic leucine zipper proteins that mediate stress-responsive abscisic acid signaling. Plant Cell. 2002; 14: 343–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Knight H, Zarka DG, Okamoto H, Thomashow MF, Knight R. Abscisic acid induces CBF gene transcription and subsequent induction of cold-regulated genes via the CRT promoter element. Plant Physiology. 2004; 135: 1710–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kidokoro S, Watanabe K, Ohori T, Moriwaki T, Maruyama K, Mizoi J, et al. Soybean DREB1/CBF-type transcription factors function in heat and drought as well as cold stress-responsive gene expression. Plant Journal. 2015; 81: 505–518. 10.1111/tpj.12746 [DOI] [PubMed] [Google Scholar]
- 54. Zhao T, Xia H, Liu J, Ma F. The gene family of dehydration responsive element-binding transcription factors in grape (Vitis vinifera): genome-wide identification and analysis, expression profiles, and involvement in abiotic stress resistance. Molecular Biology Reports. 2014; 41: 1577–1590. 10.1007/s11033-013-3004-6 [DOI] [PubMed] [Google Scholar]
- 55. Li D, Zhang Y, Hu X, Shen X, Ma L, Zhen, et al. Transcriptional profiling of Medicago truncatula under salt stress identified a novel CBF transcription factor MtCBF4 that plays an important role in abiotic stress responses. BMC Plant Biology. 2011; 11: 109–128. 10.1186/1471-2229-11-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ito Y, Katasura K, Maruyama K, Taji T, Kobayashi M, Seki M, et al. Functional analysis of rice DREB1/CBF-type transcription factors involved in cold-responsive gene expression in transgenic rice. Plant and Cell Physiol. 2006; 47: 141–153. [DOI] [PubMed] [Google Scholar]
- 57. Knight H, Brandt S, Knight MR. A history of stress alters drought calcium signaling pathways in Arabidopsis . Plant Journal. 1998; 16: 681–687. [DOI] [PubMed] [Google Scholar]
- 58. Narusaka Y, Nakashima K, Shinwari Z, Sakuma Y, Furihata T, Abe H, et al. Interaction between two cis-acting elements, ABRE and DRE, in ABA-dependent expression of Arabidopsis rd29A gene in response to dehydration and high-salinity stresses. Plant Journal. 2003; 34: 137–148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Q = Qalati, SA = Sabz Angoor, SZ = Sabz angoor, KA = Kaj angoor, B = Bidaneh sefid; 1 = control, 2 = - 1.5 Mpa drought stress. Picture was taken three weeks and two weeks after drought (a) and salinity (b) stress treatment, respectively.
(PPTX)
(PPTX)
(PPTX)
T1, -0.3 Mpa; T2, -0.7 Mpa; T3, -1.0 Mpa; T4, -1,5 Mpa.
(PPTX)
T1, control; T2, 100 mM NaCl; T3, 200 mM NaCl.
(PPTX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are available in the paper and its Supporting Information files.