Abstract
We combine immunofluorescence and single-molecule fluorescence in situ hybridization (smFISH), followed by automated image analysis, to quantify the concentration of nuclear transcription factors, number of transcription factors bound, and number of nascent mRNAs synthesized at individual gene loci. A theoretical model is used to decipher how transcription-factor binding modulates the stochastic kinetics of mRNA production. We demonstrate this approach by examining the regulation of hunchback in the early Drosophila embryo.
Sequence-specific transcription factors bind to regulatory DNA elements and interact with the cell’s transcriptional machinery to regulate mRNA production. However, a quantitative mapping between transcription-factor binding and the activity of the regulated gene is still lacking 1. To obtain this mapping, transcriptional regulation needs to be characterized at the level of the individual gene copy, capturing simultaneously the stochastic events of transcription-factor binding and mRNA production 2. Here we present an approach to pursue this goal.
To demonstrate our method, we examine the regulation of the zygotic gene hunchback (hb) by the maternal transcription factor Bicoid (Bcd) in the early embryo of Drosophila melanogaster. As a morphogen, nuclear Bcd concentration naturally varies by >50 fold within a single embryo 3, allowing us to examine its concentration-dependent activity without needing to externally perturb the system. To simultaneously quantify hb mRNA production and Bcd protein concentration, we combined single-molecule fluorescence in situ hybridization (smFISH) 4, 5 with antibody staining (immunofluorescence) (Online Methods). Wild type (OreR) embryos at nuclear cleavage cycles 11-14 were collected, labeled, and imaged using laser scanning confocal microscopy (Fig. 1a, Online Methods). The images were analyzed as described below (Supplementary Note).
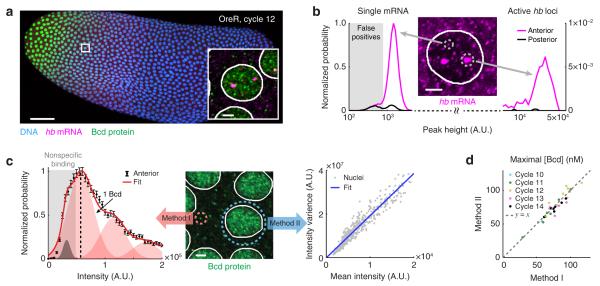
Figure 1. Simultaneous quantification of Bcd protein and hb mRNA in a single embryo.
(a) Confocal image of a wild-type Drosophila embryo labeled for Bcd protein, hb mRNA and DNA. Scale bar, 50 μm. Inset: A magnified view of a single anterior nucleus. Scale bar, 2 μm.
(b) The intensity histograms of smFISH spots in the anterior and posterior parts of a single embryo (>40,000 spots). The histograms were used to discard false-positive spots and to discriminate individual mRNAs from sites of active transcription. Middle: smFISH Image of a single nucleus in the anterior region. Scale bar, 2 μm.
(c) Two methods for converting the immunofluorescence signal to absolute Bcd concentration. Middle: Immunofluorescence signal in the anterior part of the embryo. Scale bar, 2 μm. Left: The intensity histogram of cytoplasmic Bcd spots at the anterior (>10,000 spots). Error bars represent S.E.M. By fitting the histogram to the sum of Gaussian functions, the intensity corresponding to a single Bcd protein was identified. Right: The relation between the mean and variance of Bcd fluorescence intensity of individual nuclei (>500 nuclei). The data was fitted to a straight line and the slope was used to calculate the intensity of a single labeled Bcd protein.
(d) The average Bcd concentration in the brightest 1% nuclei of individual embryos, calculated using the two methods (see Panel c). Data from 31 embryos, nuclear cycles 10-14.
In the hb mRNA channel, we observed two distinct groups of foci (spots), distinguishable by their intensity and size (Fig. 1b). We identified the smaller, weaker spots as individual mature hb mRNAs, while the larger, brighter ones were mapped to the accumulation of multiple nascent mRNAs at the sites of active hb transcription 6. The measured lifetime of both species following inhibition of transcription initiation supported this interpretation (Supplementary Fig. 1, Online Methods), and the measured numbers of active hb sites were consistent with previous reports 7-9 (Supplementary Fig. 2). We performed automated recognition of the individual fluorescent foci and extracted the intensity value corresponding to a single hb mRNA (Supplementary Fig. 3, Supplementary Note). We then used this value to convert the intensity of the smFISH signal at each transcription site to the total amount of hb mRNA (in units of mRNA molecules) at that gene locus 4. The maximal measured level of hb transcription was in excellent agreement with a previous report 6 (Supplementary Fig. 3). Using a number of methods, we estimated our mRNA detection efficiency to be >80% and the resulting error in measuring nascent mRNA at <10% (Supplementary Fig. 4).
In the Bcd protein channel, we verified that the antibody signal was proportional to Bcd concentration by measuring both the auto-fluorescence and immunofluorescence signal in a transgenic fly strain where Bcd is fused to EGFP 10 (Supplementary Fig. 5). To convert the immunofluorescence signal to absolute Bcd concentration, we used two methods (Supplementary Note). In the first method, we automatically detected individual, diffraction-limited immunofluorescent spots in the cytoplasmic regions of the embryo (Fig. 1c). After discarding false positive spots (likely due to nonspecific labeling by primary antibodies), we used the extracted single-protein intensity to convert nuclear fluorescence to Bcd concentration in each nucleus of a given embryo. In the second method, we made use of the spatial fluctuations of the fluorescence signal inside each nucleus (Fig. 1c). We used the slope of the variance-versus-mean curve in each embryo (Fig. 1c) to obtain the single-Bcd fluorescence value and convert nuclear fluorescence to Bcd concentration. The two methods showed good agreement in estimating the maximal Bcd concentration in a given embryo (ratio of 1.01 with a standard deviation of ~10%, 31 embryos, Fig. 1d). Measuring the maximal concentration of EGFP-Bcd in a transgenic fly strain yielded values that lie between the estimates of two previous works 3, 11 (Supplementary Fig. 5).
To analyze hb regulation, we first plotted the number of nascent hb mRNAs versus nuclear Bcd concentration for all hb loci (within the range 0.25-0.7 embryo length (EL)) in each embryo (Fig. 2a). Binning the data along the Bcd axis yielded the gene regulation function 12 (Fig. 2a, Supplementary Fig. 6, Supplementary Note). We fitted the gene regulation function of each embryo to a Hill function 3 (Fig. 2a, Supplementary Fig. 6). The Hill coefficient h was approximately constant during cycles 11-13, h=6.6±0.4 (designates mean ± S.E.M. throughout, Fig. 2b). A possible interpretation is that hb transcription is activated through the cooperative binding of 6-7 Bcd proteins at the hb regulatory region 3, 13. To test this idea, we performed the same analysis for a fly strain in which a lacZ reporter gene is driven by three high-affinity Bcd binding sites fused to a minimal promoter (bcd3-lacZ) 14 (Online Methods). The analysis yielded h=3.1±0.2 (Fig. 2b, Supplementary Fig. 7), i.e. equal to the known number of Bcd binding sites in this construct. These results suggest that the measured Hill coefficient in wild-type embryos estimates the number of Bcd proteins that bind cooperatively to regulate hb expression.
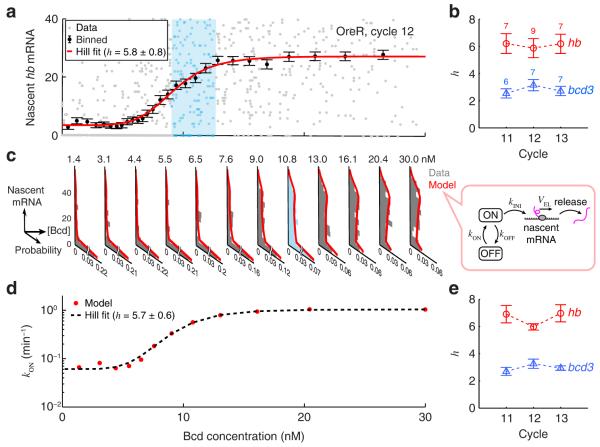
Figure 2. The regulatory relation between Bcd concentration and hb transcription.
(a) The number of nascent mRNAs at individual hb loci plotted against nuclear Bcd concentration (single embryo, >600 nuclei, 0.25-0.7 EL). The single-locus data was binned along the Bcd axis (mean ± S.E.M.) and fitted to a Hill function. One window of Bcd concentration (corresponding to one histogram in Panel c) is highlighted in cyan.
(b) The Hill coefficient of the gene regulation function. Error bars represent S.E.M. from the indicated number of embryos.
(c) Nascent hb mRNA histograms at different Bcd concentration windows, covering the entire data set of Panel a. The histogram corresponding to the highlighted window in Panel a is marked in cyan. Each histogram was fitted to a two-state model of hb transcription (right).
(d) The rate of stochastic gene activation, kON, estimated using the procedure above, is plotted against nuclear Bcd concentration, and fitted to a Hill function.
(f) The Hill coefficient of kON activation, calculated for the same embryos as in Panel b. Error bars represent S.E.M.
Our next goal was to use the measured variability in number of nascent hb mRNAs (Supplementary Fig. 8, Supplementary Note) to extract information about the way Bcd modulates the stochastic kinetics of promoter activity. This was done by comparing the observed mRNA copy-number statistics to the prediction of a theoretical model describing hb kinetics. In the model, the gene stochastically switches between an inactive (“OFF”) and an active (“ON”) state, and transcription initiation is only possible in the active state. ON/OFF switching and transcription initiation are characterized by Poissonian rates kON, kOFF, and kINI respectively 2. Transcription initiation is followed by mRNA elongation and release, both modeled as deterministic processes (Fig. 2c). We solved the model to obtain the steady-state distribution of nascent hb mRNAs per gene locus as a function of the kinetic parameters (Supplementary Note).
To compare model predictions with the observed hb statistics, we binned the entire single-locus data set from each embryo into multiple Bcd-concentration windows (Fig. 2c). We then applied maximum-likelihood parameter estimation to fit the experimental nascent mRNA distribution in each window (Supplementary Fig. 9, Supplementary Note). We found that good agreement between theory and experiment was achieved by assuming that only kON is affected by Bcd concentration, while kOFF and kINI remain constant (Fig. 2d, Supplementary Fig. 9, 10). In support of the fitting procedure, the extracted value for the maximal transcription rate, kTX=kINI∙kONmax/(kONmax+kOFF)≈18.9±0.4 min−1, was consistent with previous estimates 9, 15. As another test, we used our calculated parameters to simulate nascent mRNA accumulation at a single hb locus and found good agreement with the results of a recent live-embryo study 7 (Supplementary Fig. 11, Supplementary Note).
Our analysis indicated that the dependence of kON on Bcd concentration during cycles 11-13 can be approximated by a Hill function (Fig. 2d, Supplementary Fig. 12), with a Hill coefficient h=6.5±0.3 (Fig. 2e), very close to what we found for the gene regulation function above (Fig. 2b). This observation suggests a direct relation between cooperative Bcd binding and the switching of hb to an active transcriptional state (Supplementary Fig. 13). Performing the same analysis for bcd3-lacZ yielded a Hill coefficient of 3.0 ± 0.1 (Fig. 2e, Supplementary Fig. 12), i.e. equal to the number of Bcd binding sites, thus directly supporting the connection between Bcd binding and kON modulation. We note that even at the highest Bcd concentration, the hb gene is active only kON/(kON+kOFF)=53±1% of the time, consistent with the higher-than-Poisson noise measured at the anterior part of the embryo (Supplementary Fig. 8 and 6).
We next set out to directly measure Bcd binding at the hb gene locus. We used the presence of nascent mRNA (smFISH signal) to identify the spatial positions of hb gene copies in the nucleus (Fig. 3a). We then defined Bcd enrichment at the hb locus as the difference between the Bcd immunofluorescence signal in the vicinity of the gene and the signal elsewhere in the nucleus 16. In a single nucleus, the measured enrichment is predicted to have too high an uncertainty to be significant, due to the Poissonian statistics of molecule positions in space (Supplementary Note). However, binning the data over ≳100 gene loci should allow the signal to emerge above the noise, and this is indeed what we observed (Supplementary Fig. 14). To convert the enrichment signal to the number of bound Bcd molecules, we normalized by the corresponding value measured for the bcd3-lacZ construct, where the number of bound Bcd proteins at saturating concentration was assumed to be 3 (Supplementary Fig. 14, Supplementary Note).
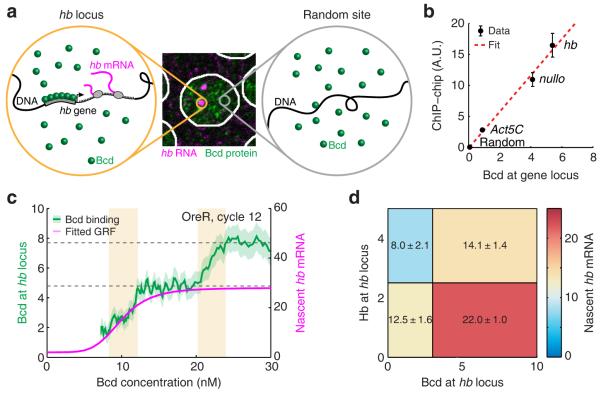
Figure 3. Transcription-factor binding at the hb locus.
(a) To detect Bcd binding at the hb locus, the Bcd signal from a random part of the nucleus (right, gray circle) was subtracted from the signal in the vicinity of the gene (left, orange circle).
(b) Comparison of our measured Bcd binding values with published ChIP-chip data (Berkeley Drosophila Transcription Network Project: http://bdtnp.lbl.gov/Fly-Net/). Error bars represent S.E.M.
(c) Bcd binding at the hb locus (shading indicates S.E.M.) and nascent hb mRNA (Hill fit of the gene regulation function, GRF) as a function of nuclear Bcd concentration (data from 9 embryos). Dashed gray lines highlight discrete binding plateaus. The binding curve exhibits two sharp increases (yellow). The left one is accompanied by the activation of hb, while the right one is not.
(d) hb transcription as a function of Bcd and Hb binding. The numbers of nascent hb mRNAs and bound Bcd and Hb were measured for >7600 loci in 18 embryos (nuclear cycles 11-14, 0.25-0.7 EL). The data was binned by nuclear position and then by Bcd and Hb binding levels. The mean number of nascent hb mRNAs in each bin is color coded, as well as written (mean ± S.E.M.).
Applying the procedure above, we measured an average binding of 5.36 ± 0.10 Bcd proteins at the hb locus (calculated from >21,000 loci in 31 embryos, Supplementary Fig. 14), close to the numbers we estimated above from the gene regulation function and the stochastic analysis of hb transcription. In contrast, measuring Bcd binding at a random position in the same nuclei yielded a value of 0.04 ± 0.09. We compared our estimation of Bcd binding at three gene loci (hb, Act5C and nullo) to published ChIP-chip data 17 (Supplementary Note), and found good agreement between the two data sets (Fig. 3b).
Next, we examined how Bcd binding at hb varied with nuclear Bcd concentration. The measured curves (Fig. 3c, Supplementary Fig. 15) exhibited plateaus suggestive of distinct binding states, with sharp transitions between these plateaus, consistent with the highly cooperative binding by Bcd at hb 18. When comparing the Bcd binding curve to the hb gene regulation function (Fig. 3c, Supplementary Fig. 15), we observed that hb activation coincides with a sharp increase in bound Bcd, to ~5 molecules. This value is again roughly consistent with our previous conclusion that Bcd activates hb transcription through the cooperative binding of 6-7 molecules. At higher Bcd concentrations, the transition to other binding states (Fig. 3c, Supplementary Fig. 15) is not accompanied by a noticeable change in hb transcription. We speculate that these binding states correspond to the occupation of additional Bcd binding sites, possibly at other hb enhancers 19.
Our analysis above of hb activity and Bcd binding showed distinct differences between nuclear cleavage cycles 11-13 and cycle 14 (Supplementary Fig. 2, 6, 7, 15), consistent with previous reports that hb expression in cycle 14 is subject to additional regulation 19. To further probe hb regulation during cycle 14, we focused on the role of its own gene product, the Hb transcription factor. Hb is believed to regulate hb expression, but it is debated whether Hb serves as an activator 20 or a repressor 19, and how it interacts with other transcription factors such as Bcd 13. We simultaneously measured the binding of both Bcd and Hb at each active hb locus (Supplementary Fig. 16, 17, Supplementary Note). Analyzing the full data set (Bcd binding, Hb binding, nascent hb mRNA) revealed that higher Bcd binding is accompanied by higher hb expression, while higher Hb binding is accompanied by lower hb expression (Fig. 3d, Supplementary Fig. 18). Thus, the simultaneous binding data confirmed the positive regulatory effect of Bcd, while demonstrating a repressive effect of Hb binding.
In closing, the simultaneous quantification of transcription factors and nascent mRNA in individual nuclei and individual gene loci, followed by the three layers of analysis—average transcriptional response, mRNA statistics, and transcription-factor binding—provides a powerful approach for investigating the stochastic process of gene regulation. Even in the absence of a well-defined gradient (as found for morphogens and the transcription factors they regulate 21), the natural cell-to-cell fluctuations in transcription-factor levels are often significant 22, and span—ipso facto—the physiologically relevant range of regulatory concentrations. When needed, the regulating transcription factor can be artificially expressed (or depleted) in order to scan a broader range of concentrations 23. Especially promising is the ability to delineate the combinatorial activity of multiple transcription factors acting on the same gene, as this remains a major challenge to our ability to decipher the genetic networks that drive cell behavior.
ONLINE METHODS
Fly strains
Oregon-R (OreR) strain was used as the wild type. egfp-bcd strain 10 was obtained from the Bloomington Drosophila Stock Center (#29018).
Construction of P[bcd3-lacZ] and bcd3-lacZ strain
The plasmid P[bcd3-lacZ] was constructed as follows. The oligonucleotide sequence containing 3 Bcd binding sites (AGGTTCTAATCCCGGTCTAATCCCTCGAGTCTAATCCCATGAGTCGACG, 14) was synthesized as an insertion in pIDTSMART-KAN, between the EcoRI and BamHI sites (performed by Integrated DNA Technologies), and subcloned into pCaSpeR-hs43-lacZ (24, plasmid obtained from the Drosophila Genomics Resource Center) upstream of the hsp70 minimal promoter. P element transformation (25, performed by BestGene) was used to generate bcd3-lacZ transgenic fly lines. A stock with the transgene inserted on chromosome 2 was used in this study.
α-Amanitin injection
For RNA lifetime measurement, α-Amanitin injection was performed following the method of 26. Briefly, embryos were collected at about 2 hours after deposition, dechorinated in 50% bleach, and staged by eye under the stereoscope. α-Amanitin was dissolved in nuclease-free water (Ambion AM9937) at a concentration of 1 mg/ml, and injected to stage 4-5 embryos (~50 pl per embryo) using an Eppendorf FemtoJet Express microinjector. Embryos were then incubated at room temperature for a delay time period (0, 2, 4, 6, 8, 30 minutes) before being fixed. hb mRNA in the embryo was labeled and imaged as described below.
Embryo collection, fixation and storage
All embryos were collected at 25°C (following the protocol of 27). EGFP-Bcd expressing embryos were fixed in 8% (v/v) paraformaldehyde/PBS : heptane (4 ml : 4ml) for 30 minutes, hand devitellinized, and stored in 1 ml 1× PBS (with 0.1% (w/v) BSA and 0.1% (v/v) Triton X-100) at 4°C. All other embryos were fixed in 4% (v/v) paraformaldehyde/PBS : heptane (4 ml : 4ml) for 15 minutes, vortexed in methanol : heptane (4 ml : 4ml) for 30 seconds for devitellinization, and stored in 1 ml methanol at −20°C.
Simultaneous labeling using smFISH and immunofluorescence
Combining smFISH and immunofluorescence protocols for simultaneous detection of mRNA and protein is challenging, as reaction conditions for the two procedures are conflicting in the following ways: (1) The typical smFISH hybridization solution is based on saline-sodium citrate (SSC) buffer 5, while immunofluorescence hybridization solution is usually based on Phosphate buffered saline (PBS) 28. (2) smFISH hybridization requires higher reaction temperature and harsher chemicals (≥ 30°C, usually with formamide, 5) than immunofluorescence (4-24°C, no harsh chemicals, 28). (3) smFISH is RNase sensitive, whereas many immunofluorescence reagents, including blocking reagents and antibodies, are usually not RNase free.
To find out the best way to combine smFISH and immunofluorescence labeling, we tested three different protocol designs: (1) smFISH and immunofluorescence labeling in the same reaction 5, (2) immunofluorescence labeling followed by smFISH 29-31, (3) smFISH labeling followed by immunofluorescence 6, 16. For each protocol, we characterized the following quantities: (a) the average smFISH intensity of the hb gene at the anterior side of the embryo (cycles 11-13, 0.25-0.4 EL), (b) the exponential slope (decay length) of the Bcd immunofluorescence signal. We found that, compared to smFISH- and immunofluorescence-only labeling, both design #1 and design #2 resulted in a decrease of the hb smFISH signal as well as a decrease of the Bcd immunofluorescence decay length, indicating that both smFISH and immunofluorescence signals have been distorted. In contrast, design #3 preserved both the smFISH intensity and the immunofluorescence slope. We therefore developed a combined labeling protocol based on design #3.
In this protocol, the smFISH labeling procedure was adapted from previous protocols 5, 32. Sets of DNA oligonucleotides complementary to the target gene (48 probes for hb, 72 probes for lacZ, 48 probes for Act5C and 20 probes for nullo, Supplementary Table 1) were designed and ordered (Biosearch Technologies). hb probes were directly synthesized with 3’-TAMRA labeling; all other probes were synthesized with 3’-amine-modification and were conjugated to various fluorophores in the lab, as described in 32. Before hybridization, embryos were rehydrated 4 times (10 minutes each) in 1 ml PBTx (1× PBS, 0.1% (v/v) Triton X-100) and washed twice (5 minutes each) in 1 ml hybridization wash buffer (2× SSC, 20% (w/v) formamide, 0.1% (v/v) Triton X-100). Hybridization was then performed at 30°C for 16 hours by incubating embryos with 100 nM of probes (or roughly 2 nM per probe) in 50 μl hybridization buffer (2× SSC, 20% (w/v) formamide, 0.2 mg/ml BSA [RNase free, Ambion AM2616], 2 mM Ribonucleoside Vanadyl Complex [NEB S1402S], 1 mg/ml E. coli tRNA [Sigma 83854-100MG], 0.1 g/ml Dextran sulfate). A shaker was used to facilitate mixing. Hybridized embryos were washed 3 times (30 minutes each) in hybridization wash buffer at 30°C, and twice (10 minutes each) in 2× SSC (with 0.1% (v/v) Triton X-100) at room temperature. All washing steps were performed with nutation.
Immunofluorescence was performed after smFISH, using a protocol adapted from 28, 33. Briefly, embryos were washed 4 times (10 minutes each) in 1 ml PBTx, and blocked in 1 ml PBT-B (1× PBS, 20% (v/v) Western blocking reagent [Roche 11921673001], 2 mM Ribonucleoside Vanadyl Complex [NEB S1402S], 0.1% (v/v) Triton X-100) at room temperature for 1 hour. 500 μl Rabbit anti-Bcd primary antibody (Santa Cruz Biotechnology SC-66818, 1:50 (v/v) dilution in PBT-B) or guinea pig anti-Hb primary antibody 28 (gift of J. Reinitz, 1:200 (v/v) dilution in PBT-B) were pre-absorbed (incubated with stage 15-16 wild-type embryos at 4°C for 20 hours), and then hybridized with the embryos of interest at 4°C for 20 hours. This was followed by 4 additional washes (10 minutes each) in 1 ml PBTx and a 1-hour blocking in 1 ml PBT-B. Next, embryos were incubated with 500 μl of either goat anti-rabbit or goat anti-guinea pig IgG secondary antibodies conjugated with either Alexa 488 or Alexa 647 (Invitrogen, A11008, A21244, A11073, 1:500 (v/v) dilution in PBT-B) at room temperature for 1 hour, and washed 4 times (10 minutes each) in 1 ml PBTx. All steps were performed with nutation.
DNA staining and embryo mounting
After immunofluorescence labeling, embryos were stained with 1 ml Hoechst 33342 (Invitrogen P36236, 1:10000 (v/v) dilution in PBTx) for 10 minutes on a nutator, and washed 4 times (10 minutes each, with nutation) in 1 ml PBTx before mounting in Aqua-Poly/Mount (Polyscience 18606). Imaging was performed at least 36 hours after mounting to allow complete solidification of the sample.
Microscopy
16-bit, high-resolution (xy pixel size: 83 nm, z spacing: 350 nm) 3D image stacks were acquired on a Zeiss LSM 710 laser scanning confocal microscope using a 63× oil immersion objective (1.4 NA) and a pinhole size of 1 Airy Unit. Embryos at the mitotic interphase of nuclear cleavage cycles 11-14 were selected based on the number and shape of the nuclei (Hoechst signal), and the appearance of bright spots in the smFISH channel. Three adjacent image stacks, with typical size of ~2300 × 2700 × 23 voxels each, were acquired automatically and stitched together (using the “tiling” function of the LSM710 imaging software) to cover the cortex layer of each embryo. Before image acquisition, the microscope was warmed up for at least 3 hours to avoid possible drift of the focal plane.
Data and code availability
Image processing and data analysis were performed using custom MATLAB scripts (Supplementary Software), and are described in detail in the Supplementary Note. The raw images themselves are available online at http://1drv.ms/1ztXI1a.
Supplementary Material
ACKNOWLEDGMENTS
We thank J. Reinitz (University of Chicago) for the gift of anti-Hb antibody, and the following people for their generous advice: A. Boettiger, J. Elf, H. Garcia, D. Larson, S. Little, J. Ma, A. Raj, A. Sanchez, E. Segal, D. van Dijk and all members of the Golding and Sokac labs. Work in the Golding lab was supported by grants from the US National Institutes of Health (R01 GM082837), the National Science Foundation (PHY 1147498, PHY 1430124 and PHY 1427654), and Welch Foundation (Q-1759). H.X. is supported by the Burroughs Wellcome Fund Career Award at the Scientific Interface. A.M.S. and L.F. are supported by a Computational and Integrative Biomedical Research (CIBR) Center Seed Award and Curtis Hankamer Basic Research Fund Award of Baylor College of Medicine. We gratefully acknowledge the computing resources provided by the CIBR Center of Baylor College of Medicine.
Footnotes
AUTHOR CONTRIBUTIONS
H.X., L.A.S. and I.G. conceived the experimental and analysis methods. H.X. and L.A.S. developed image and data analysis algorithms. H.X. performed the experiments, developed algorithms and theoretical models, and analyzed the data. L.F. performed fly injection experiments. A.M.S. provided guidance on fly biology and microscopy. I.G. supervised the project. H.X., A.M.S. and I.G. wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
REFERENCES
- 1.Segal E, Widom J. Nat Rev Genet. 2009;10:443–456. doi: 10.1038/nrg2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanchez A, Golding I. Science. 2013;342:1188–1193. doi: 10.1126/science.1242975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gregor T, Tank DW, Wieschaus EF, Bialek W. Cell. 2007;130:153–164. doi: 10.1016/j.cell.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Femino AM, Fay FS, Fogarty K, Singer RH. Science. 1998;280:585–590. doi: 10.1126/science.280.5363.585. [DOI] [PubMed] [Google Scholar]
- 5.Raj A, van den Bogaard P, Rifkin SA, van Oudenaarden A, Tyagi S. Nat Methods. 2008;5:877–879. doi: 10.1038/nmeth.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Little SC, Tikhonov M, Gregor T. Cell. 2013;154:789–800. doi: 10.1016/j.cell.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lucas T, et al. Curr Biol. 2013;23:2135–2139. doi: 10.1016/j.cub.2013.08.053. [DOI] [PubMed] [Google Scholar]
- 8.Porcher A, et al. Development. 2010;137:2795–2804. doi: 10.1242/dev.051300. [DOI] [PubMed] [Google Scholar]
- 9.Garcia HG, Tikhonov M, Lin A, Gregor T. Curr Biol. 2013;23:2140–2145. doi: 10.1016/j.cub.2013.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gregor T, Wieschaus EF, McGregor AP, Bialek W, Tank DW. Cell. 2007;130:141–152. doi: 10.1016/j.cell.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abu-Arish A, Porcher A, Czerwonka A, Dostatni N, Fradin C. Biophys J. 2010;99:L33–35. doi: 10.1016/j.bpj.2010.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenfeld N, Young JW, Alon U, Swain PS, Elowitz MB. Science. 2005;307:1962–1965. doi: 10.1126/science.1106914. [DOI] [PubMed] [Google Scholar]
- 13.Lopes FJ, Spirov AV, Bisch PM. Dev Biol. 2012;370:165–172. doi: 10.1016/j.ydbio.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ronchi E, Treisman J, Dostatni N, Struhl G, Desplan C. Cell. 1993;74:347–355. doi: 10.1016/0092-8674(93)90425-p. [DOI] [PubMed] [Google Scholar]
- 15.Boettiger AN, Levine M. Cell Rep. 2013;3:8–15. doi: 10.1016/j.celrep.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He F, Ren J, Wang W, Ma J. PLoS One. 2011;6:e19122. doi: 10.1371/journal.pone.0019122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li XY, et al. PLoS Biol. 2008;6:e27. doi: 10.1371/journal.pbio.0060027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma X, Yuan D, Diepold K, Scarborough T, Ma J. Development. 1996;122:1195–1206. doi: 10.1242/dev.122.4.1195. [DOI] [PubMed] [Google Scholar]
- 19.Perry MW, Bothma JP, Luu RD, Levine M. Curr Biol. 2012;22:2247–2252. doi: 10.1016/j.cub.2012.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopes FJ, Vieira FM, Holloway DM, Bisch PM, Spirov AV. PLoS Comput Biol. 2008;4:e1000184. doi: 10.1371/journal.pcbi.1000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashe HL, Briscoe J. Development. 2006;133:385–394. doi: 10.1242/dev.02238. [DOI] [PubMed] [Google Scholar]
- 22.Eldar A, Elowitz MB. Nature. 2010;467:167–173. doi: 10.1038/nature09326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niwa H, Miyazaki J, Smith AG. Nat Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
ONLINE METHODS REFERENCES
- 24.Thummel C, Pirrotta V. Dros. Inf. Serv. 1992;71:150. [Google Scholar]
- 25.Spradling AC, Rubin GM. Science. 1982;218:341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- 26.Edgar BA, Weir MP, Schubiger G, Kornberg T. Cell. 1986;47:747–754. doi: 10.1016/0092-8674(86)90517-9. [DOI] [PubMed] [Google Scholar]
- 27.Figard L, Sokac AM. J Vis Exp. 2011 doi: 10.3791/2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kosman D, Small S, Reinitz J. Dev Genes Evol. 1998;208:290–294. doi: 10.1007/s004270050184. [DOI] [PubMed] [Google Scholar]
- 29.Toledano H, D’Alterio C, Loza-Coll M, Jones DL. Nat Protoc. 2012;7:1808–1817. doi: 10.1038/nprot.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zimmerman SG, Peters NC, Altaras AE, Berg CA. Nat Protoc. 2013;8:2158–2179. doi: 10.1038/nprot.2013.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Namekawa SH, Lee JT. Nat Protoc. 2011;6:270–284. doi: 10.1038/nprot.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skinner SO, Sepulveda LA, Xu H, Golding I. Nat Protoc. 2013;8:1100–1113. doi: 10.1038/nprot.2013.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaeger J, et al. Nature. 2004;430:368–371. doi: 10.1038/nature02678. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Image processing and data analysis were performed using custom MATLAB scripts (Supplementary Software), and are described in detail in the Supplementary Note. The raw images themselves are available online at http://1drv.ms/1ztXI1a.