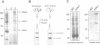Abstract
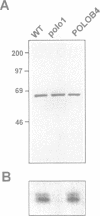
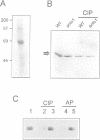
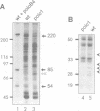
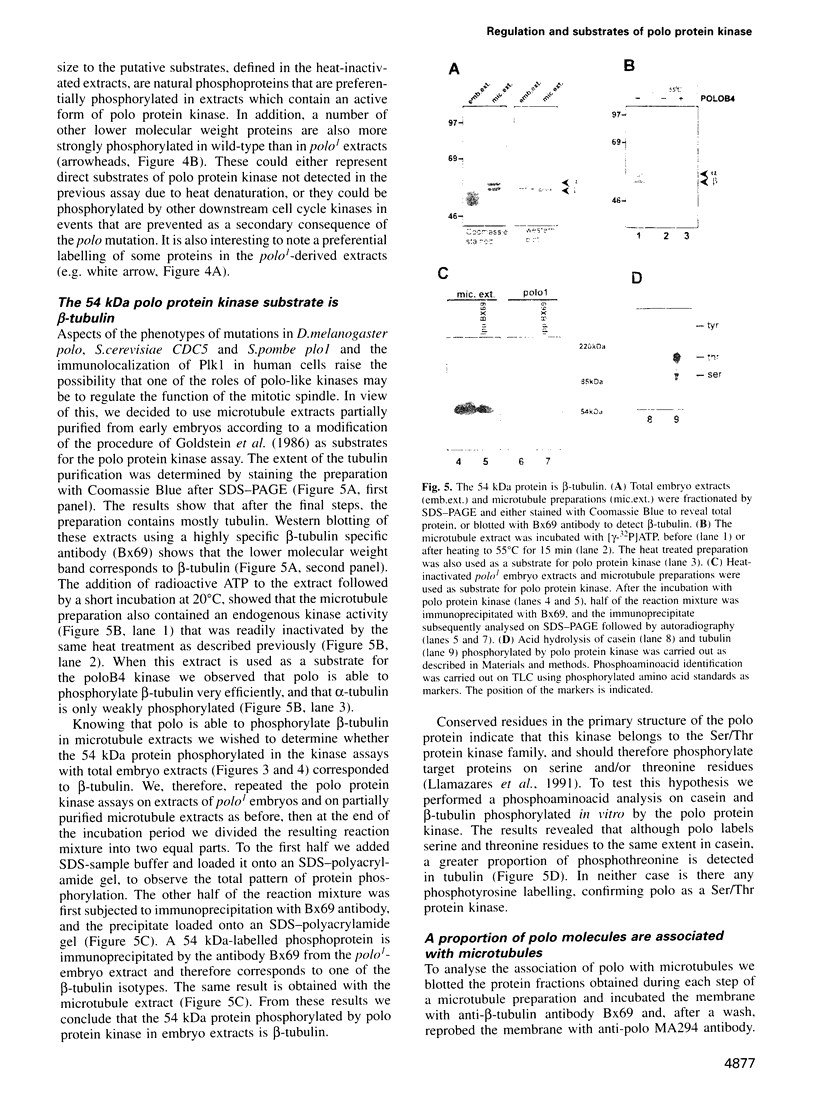
The Drosophila gene polo encodes a protein kinase required for progression through mitosis. Wild-type polo protein migrates as a tight doublet of 67 kDa which is converted to a single band by phosphatase treatment, which also inactivates the kinase. We have determined putative polo substrates in a cell-free system derived from mutant embryos. Exogenous polo protein kinase phosphorylates proteins of sizes 220 kDa, 85 kDa and 54 kDa, to a greater extent when added to extracts of polo(1)-derived embryos compared with extracts of wild-type embryos, which in both cases have been subject to mild heat treatment to inactivate endogenous kinases. Proteins of the same size are predominantly phosphorylated by the endogenous kinases present in wild-type extracts, and are either not phosphorylated or are poorly phosphorylated in extracts of polo(1)-derived embryos. We show that a specific monoclonal antibody to beta-tubulin precipitates the phosphorylated 54 kDa protein together with an associated 85 kDa protein also phosphorylated by polo protein kinase. Moreover polo binds to an 85 kDa protein which is enriched in microtubule preparations. We discuss the extent to which these in vitro phosphorylation results reflect the effects of mutations in polo on microtubule behaviour during the mitotic cycle.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byers B., Goetsch L. Duplication of spindle plaques and integration of the yeast cell cycle. Cold Spring Harb Symp Quant Biol. 1974;38:123–131. doi: 10.1101/sqb.1974.038.01.016. [DOI] [PubMed] [Google Scholar]
- Cambiazo V., González M., Maccioni R. B. DMAP-85: a tau-like protein from Drosophila melanogaster larvae. J Neurochem. 1995 Mar;64(3):1288–1297. doi: 10.1046/j.1471-4159.1995.64031288.x. [DOI] [PubMed] [Google Scholar]
- Clay F. J., McEwen S. J., Bertoncello I., Wilks A. F., Dunn A. R. Identification and cloning of a protein kinase-encoding mouse gene, Plk, related to the polo gene of Drosophila. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):4882–4886. doi: 10.1073/pnas.90.11.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue P. J., Alberts G. F., Guo Y., Winkles J. A. Identification by targeted differential display of an immediate early gene encoding a putative serine/threonine kinase. J Biol Chem. 1995 Apr 28;270(17):10351–10357. doi: 10.1074/jbc.270.17.10351. [DOI] [PubMed] [Google Scholar]
- Draetta G., Piwnica-Worms H., Morrison D., Druker B., Roberts T., Beach D. Human cdc2 protein kinase is a major cell-cycle regulated tyrosine kinase substrate. Nature. 1988 Dec 22;336(6201):738–744. doi: 10.1038/336738a0. [DOI] [PubMed] [Google Scholar]
- Eipper B. A. Rat brain microtubule protein: purification and determination of covalently bound phosphate and carbohydrate. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2283–2287. doi: 10.1073/pnas.69.8.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipper B. A. Rat brain tubulin and protein kinase activity. J Biol Chem. 1974 Mar 10;249(5):1398–1406. [PubMed] [Google Scholar]
- Fenton B., Glover D. M. A conserved mitotic kinase active at late anaphase-telophase in syncytial Drosophila embryos. Nature. 1993 Jun 17;363(6430):637–640. doi: 10.1038/363637a0. [DOI] [PubMed] [Google Scholar]
- Gard D. L., Kirschner M. W. A microtubule-associated protein from Xenopus eggs that specifically promotes assembly at the plus-end. J Cell Biol. 1987 Nov;105(5):2203–2215. doi: 10.1083/jcb.105.5.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard D. L., Kirschner M. W. A polymer-dependent increase in phosphorylation of beta-tubulin accompanies differentiation of a mouse neuroblastoma cell line. J Cell Biol. 1985 Mar;100(3):764–774. doi: 10.1083/jcb.100.3.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier J., Solomon M. J., Booher R. N., Bazan J. F., Kirschner M. W. cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991 Oct 4;67(1):197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- Goldstein L. S., Laymon R. A., McIntosh J. R. A microtubule-associated protein in Drosophila melanogaster: identification, characterization, and isolation of coding sequences. J Cell Biol. 1986 Jun;102(6):2076–2087. doi: 10.1083/jcb.102.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golsteyn R. M., Mundt K. E., Fry A. M., Nigg E. A. Cell cycle regulation of the activity and subcellular localization of Plk1, a human protein kinase implicated in mitotic spindle function. J Cell Biol. 1995 Jun;129(6):1617–1628. doi: 10.1083/jcb.129.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golsteyn R. M., Schultz S. J., Bartek J., Ziemiecki A., Ried T., Nigg E. A. Cell cycle analysis and chromosomal localization of human Plk1, a putative homologue of the mitotic kinases Drosophila polo and Saccharomyces cerevisiae Cdc5. J Cell Sci. 1994 Jun;107(Pt 6):1509–1517. doi: 10.1242/jcs.107.6.1509. [DOI] [PubMed] [Google Scholar]
- Hamanaka R., Maloid S., Smith M. R., O'Connell C. D., Longo D. L., Ferris D. K. Cloning and characterization of human and murine homologues of the Drosophila polo serine-threonine kinase. Cell Growth Differ. 1994 Mar;5(3):249–257. [PubMed] [Google Scholar]
- Hanks S. K., Hunter T. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 1995 May;9(8):576–596. [PubMed] [Google Scholar]
- Hartwell L. H., Mortimer R. K., Culotti J., Culotti M. Genetic Control of the Cell Division Cycle in Yeast: V. Genetic Analysis of cdc Mutants. Genetics. 1973 Jun;74(2):267–286. doi: 10.1093/genetics/74.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtrich U., Wolf G., Bräuninger A., Karn T., Böhme B., Rübsamen-Waigmann H., Strebhardt K. Induction and down-regulation of PLK, a human serine/threonine kinase expressed in proliferating cells and tumors. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1736–1740. doi: 10.1073/pnas.91.5.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez J., Alphey L., Nurse P., Glover D. M. Complementation of fission yeast cdc2ts and cdc25ts mutants identifies two cell cycle genes from Drosophila: a cdc2 homologue and string. EMBO J. 1990 Nov;9(11):3565–3571. doi: 10.1002/j.1460-2075.1990.tb07567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada K., Johnson A. L., Johnston L. H., Sugino A. A multicopy suppressor gene of the Saccharomyces cerevisiae G1 cell cycle mutant gene dbf4 encodes a protein kinase and is identified as CDC5. Mol Cell Biol. 1993 Jul;13(7):4445–4457. doi: 10.1128/mcb.13.7.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake R. J., Jelinek W. R. Cell cycle- and terminal differentiation-associated regulation of the mouse mRNA encoding a conserved mitotic protein kinase. Mol Cell Biol. 1993 Dec;13(12):7793–7801. doi: 10.1128/mcb.13.12.7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. G., Nurse P. Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature. 1987 May 7;327(6117):31–35. doi: 10.1038/327031a0. [DOI] [PubMed] [Google Scholar]
- Lee M. S., Ogg S., Xu M., Parker L. L., Donoghue D. J., Maller J. L., Piwnica-Worms H. cdc25+ encodes a protein phosphatase that dephosphorylates p34cdc2. Mol Biol Cell. 1992 Jan;3(1):73–84. doi: 10.1091/mbc.3.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llamazares S., Moreira A., Tavares A., Girdham C., Spruce B. A., Gonzalez C., Karess R. E., Glover D. M., Sunkel C. E. polo encodes a protein kinase homolog required for mitosis in Drosophila. Genes Dev. 1991 Dec;5(12A):2153–2165. doi: 10.1101/gad.5.12a.2153. [DOI] [PubMed] [Google Scholar]
- Mandelkow E., Mandelkow E. M. Microtubules and microtubule-associated proteins. Curr Opin Cell Biol. 1995 Feb;7(1):72–81. doi: 10.1016/0955-0674(95)80047-6. [DOI] [PubMed] [Google Scholar]
- Marshall C. J. Signal transduction. Hot lips and phosphorylation of protein kinases. Nature. 1994 Feb 24;367(6465):686–686. doi: 10.1038/367686a0. [DOI] [PubMed] [Google Scholar]
- Millar J. B., McGowan C. H., Lenaers G., Jones R., Russell P. p80cdc25 mitotic inducer is the tyrosine phosphatase that activates p34cdc2 kinase in fission yeast. EMBO J. 1991 Dec;10(13):4301–4309. doi: 10.1002/j.1460-2075.1991.tb05008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. O. Principles of CDK regulation. Nature. 1995 Mar 9;374(6518):131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- Nigg E. A. Targets of cyclin-dependent protein kinases. Curr Opin Cell Biol. 1993 Apr;5(2):187–193. doi: 10.1016/0955-0674(93)90101-u. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Ohkura H., Hagan I. M., Glover D. M. The conserved Schizosaccharomyces pombe kinase plo1, required to form a bipolar spindle, the actin ring, and septum, can drive septum formation in G1 and G2 cells. Genes Dev. 1995 May 1;9(9):1059–1073. doi: 10.1101/gad.9.9.1059. [DOI] [PubMed] [Google Scholar]
- Ookata K., Hisanaga S., Bulinski J. C., Murofushi H., Aizawa H., Itoh T. J., Hotani H., Okumura E., Tachibana K., Kishimoto T. Cyclin B interaction with microtubule-associated protein 4 (MAP4) targets p34cdc2 kinase to microtubules and is a potential regulator of M-phase microtubule dynamics. J Cell Biol. 1995 Mar;128(5):849–862. doi: 10.1083/jcb.128.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S. R., Drubin D. G., Kelly R. B. Identification of three coated vesicle components as alpha- and beta-tubulin linked to a phosphorylated 50,000-dalton polypeptide. J Cell Biol. 1983 Jul;97(1):40–47. doi: 10.1083/jcb.97.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport L., Leterrier J. F., Virion A., Nunez J., Osty J. Phosphorylation of microtubule-associated proteins. Eur J Biochem. 1976 Mar 1;62(3):539–549. doi: 10.1111/j.1432-1033.1976.tb10188.x. [DOI] [PubMed] [Google Scholar]
- Sandoval I. V., Cuatrecasas P. Protein kinase associated with tubulin: affinity chromatography and properties. Biochemistry. 1976 Aug 10;15(16):3424–3432. doi: 10.1021/bi00661a005. [DOI] [PubMed] [Google Scholar]
- Serrano L., Díaz-Nido J., Wandosell F., Avila J. Tubulin phosphorylation by casein kinase II is similar to that found in vivo. J Cell Biol. 1987 Oct;105(4):1731–1739. doi: 10.1083/jcb.105.4.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano L., Hernández M. A., Díaz-Nido J., Avila J. Association of casein kinase II with microtubules. Exp Cell Res. 1989 Mar;181(1):263–272. doi: 10.1016/0014-4827(89)90200-0. [DOI] [PubMed] [Google Scholar]
- Simmons D. L., Neel B. G., Stevens R., Evett G., Erikson R. L. Identification of an early-growth-response gene encoding a novel putative protein kinase. Mol Cell Biol. 1992 Sep;12(9):4164–4169. doi: 10.1128/mcb.12.9.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkel C. E., Glover D. M. polo, a mitotic mutant of Drosophila displaying abnormal spindle poles. J Cell Sci. 1988 Jan;89(Pt 1):25–38. doi: 10.1242/jcs.89.1.25. [DOI] [PubMed] [Google Scholar]
- Wandosell F., Serrano L., Hernández M. A., Avila J. Phosphorylation of tubulin by a calmodulin-dependent protein kinase. J Biol Chem. 1986 Aug 5;261(22):10332–10339. [PubMed] [Google Scholar]
- Wood J. S., Hartwell L. H. A dependent pathway of gene functions leading to chromosome segregation in Saccharomyces cerevisiae. J Cell Biol. 1982 Sep;94(3):718–726. doi: 10.1083/jcb.94.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R. P., Oskarsson M., Paules R. S., Schulz N., Cleveland D., Vande Woude G. F. Ability of the c-mos product to associate with and phosphorylate tubulin. Science. 1991 Feb 8;251(4994):671–675. doi: 10.1126/science.1825142. [DOI] [PubMed] [Google Scholar]