Abstract
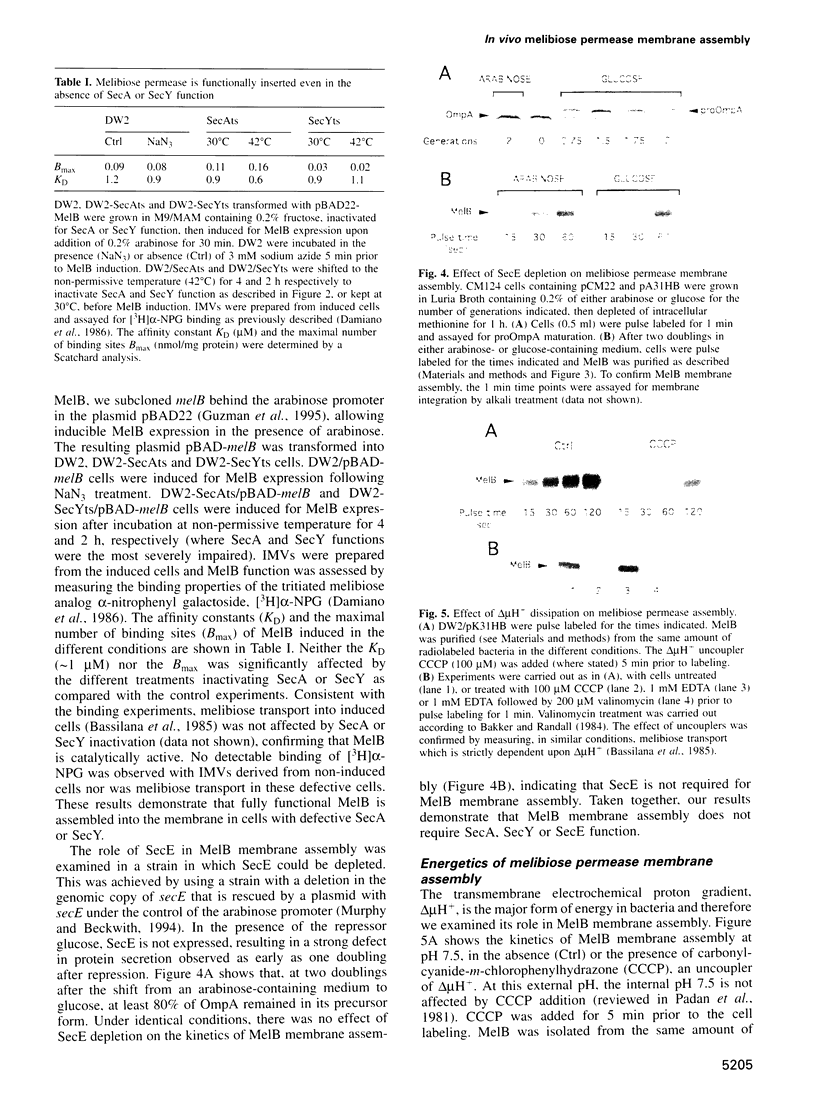
To investigate the mechanism of polytopic membrane protein insertion in Escherichia coli, we have examined the protein and energy requirements for in vivo membrane assembly of the prototypic 12 transmembrane domain sugar co-transporter, melibiose permease (MelB). MelB membrane assembly was analyzed both kinetically, by pulse labeling experiments, and functionally by measuring the activity of the inserted permease. Strikingly, the rate of MelB membrane assembly is decreased approximately 4-fold upon dissipation of the transmembrane electrochemical proton gradient, delta(mu)H+, indicative of a strong requirement for delta(mu)H+. Interestingly, selective dissipation of either the electrical (delta(psi)) or the chemical (delta(pH)) component of delta(mu)H+ demonstrates that either form of energy is required for MelB membrane assembly. In contrast, MelB membrane assembly does not require SecA, SecY or SecE, all three proteins which are strictly required for protein translocation. Neither the rate of MelB membrane assembly nor the amount of functional permease is affected by inactivation or depletion of these Sec proteins. These results strongly suggest that polytopic membrane proteins such as MelB insert into the cytoplasmic membrane by a mechanism fundamentally different from protein translocation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahrem B., Hoffschulte H. K., Müller M. In vitro membrane assembly of a polytopic, transmembrane protein results in an enzymatically active conformation. J Cell Biol. 1989 May;108(5):1637–1646. doi: 10.1083/jcb.108.5.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama Y., Ito K. Export of Escherichia coli alkaline phosphatase attached to an integral membrane protein, SecY. J Biol Chem. 1989 Jan 5;264(1):437–442. [PubMed] [Google Scholar]
- Andersson H., von Heijne G. Membrane protein topology: effects of delta mu H+ on the translocation of charged residues explain the 'positive inside' rule. EMBO J. 1994 May 15;13(10):2267–2272. doi: 10.1002/j.1460-2075.1994.tb06508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkowitz R. A., Bassilana M. Protein translocation in Escherichia coli. Biochim Biophys Acta. 1994 Dec 9;1197(3):311–343. doi: 10.1016/0304-4157(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Bakker E. P., Mangerich W. E. The effects of weak acids on potassium uptake by Escherichia coli K-12 inhibition by low cytoplasmic pH. Biochim Biophys Acta. 1983 May 5;730(2):379–386. doi: 10.1016/0005-2736(83)90355-3. [DOI] [PubMed] [Google Scholar]
- Bakker E. P., Randall L. L. The requirement for energy during export of beta-lactamase in Escherichia coli is fulfilled by the total protonmotive force. EMBO J. 1984 Apr;3(4):895–900. doi: 10.1002/j.1460-2075.1984.tb01902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassilana M., Damiano-Forano E., Leblanc G. Effect of membrane potential on the kinetic parameters of the Na+ or H+ melibiose symport in Escherichia coli membrane vesicles. Biochem Biophys Res Commun. 1985 Jun 28;129(3):626–631. doi: 10.1016/0006-291x(85)91937-0. [DOI] [PubMed] [Google Scholar]
- Botfield M. C., Naguchi K., Tsuchiya T., Wilson T. H. Membrane topology of the melibiose carrier of Escherichia coli. J Biol Chem. 1992 Jan 25;267(3):1818–1822. [PubMed] [Google Scholar]
- Botfield M. C., Wilson T. H. Mutations that simultaneously alter both sugar and cation specificity in the melibiose carrier of Escherichia coli. J Biol Chem. 1988 Sep 15;263(26):12909–12915. [PubMed] [Google Scholar]
- Botfield M. C., Wilson T. H. Peptide-specific antibody for the melibiose carrier of Escherichia coli localizes the carboxyl terminus to the cytoplasmic face of the membrane. J Biol Chem. 1989 Jul 15;264(20):11649–11652. [PubMed] [Google Scholar]
- Brandolin G., Doussiere J., Gulik A., Gulik-Krzywicki T., Lauquin G. J., Vignais P. V. Kinetic, binding and ultrastructural properties of the beef heart adenine nucleotide carrier protein after incorporation into phospholipid vesicles. Biochim Biophys Acta. 1980 Oct 3;592(3):592–614. doi: 10.1016/0005-2728(80)90103-6. [DOI] [PubMed] [Google Scholar]
- Cao G., Cheng S., Whitley P., von Heijne G., Kuhn A., Dalbey R. E. Synergistic insertion of two hydrophobic regions drives Sec-independent membrane protein assembly. J Biol Chem. 1994 Oct 28;269(43):26898–26903. [PubMed] [Google Scholar]
- Cao G., Kuhn A., Dalbey R. E. The translocation of negatively charged residues across the membrane is driven by the electrochemical potential: evidence for an electrophoresis-like membrane transfer mechanism. EMBO J. 1995 Mar 1;14(5):866–875. doi: 10.1002/j.1460-2075.1995.tb07068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiano-Forano E., Bassilana M., Leblanc G. Sugar binding properties of the melibiose permease in Escherichia coli membrane vesicles. Effects of Na+ and H+ concentrations. J Biol Chem. 1986 May 25;261(15):6893–6899. [PubMed] [Google Scholar]
- Date T., Goodman J. M., Wickner W. T. Procoat, the precursor of M13 coat protein, requires an electrochemical potential for membrane insertion. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4669–4673. doi: 10.1073/pnas.77.8.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A. The spontaneous insertion of proteins into and across membranes: the helical hairpin hypothesis. Cell. 1981 Feb;23(2):411–422. doi: 10.1016/0092-8674(81)90136-7. [DOI] [PubMed] [Google Scholar]
- Guzman L. M., Belin D., Carson M. J., Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995 Jul;177(14):4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Akiyama Y. In vivo analysis of integration of membrane proteins in Escherichia coli. Mol Microbiol. 1991 Sep;5(9):2243–2253. doi: 10.1111/j.1365-2958.1991.tb02154.x. [DOI] [PubMed] [Google Scholar]
- Klionsky D. J., Brusilow W. S., Simoni R. D. In vivo evidence for the role of the epsilon subunit as an inhibitor of the proton-translocating ATPase of Escherichia coli. J Bacteriol. 1984 Dec;160(3):1055–1060. doi: 10.1128/jb.160.3.1055-1060.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn A., Kreil G., Wickner W. Both hydrophobic domains of M13 procoat are required to initiate membrane insertion. EMBO J. 1986 Dec 20;5(13):3681–3685. doi: 10.1002/j.1460-2075.1986.tb04699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn A., Wickner W., Kreil G. The cytoplasmic carboxy terminus of M13 procoat is required for the membrane insertion of its central domain. Nature. 1986 Jul 24;322(6077):335–339. doi: 10.1038/322335a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Macfarlane J., Müller M. The functional integration of a polytopic membrane protein of Escherichia coli is dependent on the bacterial signal-recognition particle. Eur J Biochem. 1995 Nov 1;233(3):766–771. doi: 10.1111/j.1432-1033.1995.766_3.x. [DOI] [PubMed] [Google Scholar]
- McGovern K., Beckwith J. Membrane insertion of the Escherichia coli MalF protein in cells with impaired secretion machinery. J Biol Chem. 1991 Nov 5;266(31):20870–20876. [PubMed] [Google Scholar]
- Murphy C. K., Beckwith J. Residues essential for the function of SecE, a membrane component of the Escherichia coli secretion apparatus, are located in a conserved cytoplasmic region. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2557–2561. doi: 10.1073/pnas.91.7.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. E. coli mutant pleiotropically defective in the export of secreted proteins. Cell. 1981 Sep;25(3):765–772. doi: 10.1016/0092-8674(81)90184-7. [DOI] [PubMed] [Google Scholar]
- Oliver D. B., Cabelli R. J., Dolan K. M., Jarosik G. P. Azide-resistant mutants of Escherichia coli alter the SecA protein, an azide-sensitive component of the protein export machinery. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8227–8231. doi: 10.1073/pnas.87.21.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padan E., Arbel T., Rimon A., Shira A. B., Cohen A. Biosynthesis of the lactose permease in Escherichia coli minicells and effect of carrier amplification on cell physiology. J Biol Chem. 1983 May 10;258(9):5666–5673. [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Schuldiner S. pH homeostasis in bacteria. Biochim Biophys Acta. 1981 Dec;650(2-3):151–166. doi: 10.1016/0304-4157(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Phillips G. J., Silhavy T. J. The E. coli ffh gene is necessary for viability and efficient protein export. Nature. 1992 Oct 22;359(6397):744–746. doi: 10.1038/359744a0. [DOI] [PubMed] [Google Scholar]
- Pourcher T., Bibi E., Kaback H. R., Leblanc G. Membrane topology of the melibiose permease of Escherichia coli studied by melB-phoA fusion analysis. Biochemistry. 1996 Apr 2;35(13):4161–4168. doi: 10.1021/bi9527496. [DOI] [PubMed] [Google Scholar]
- Pourcher T., Leclercq S., Brandolin G., Leblanc G. Melibiose permease of Escherichia coli: large scale purification and evidence that H+, Na+, and Li+ sugar symport is catalyzed by a single polypeptide. Biochemistry. 1995 Apr 4;34(13):4412–4420. doi: 10.1021/bi00013a033. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993 Mar;57(1):50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Reizer A., Saier M. H., Jr A functional superfamily of sodium/solute symporters. Biochim Biophys Acta. 1994 Jun 29;1197(2):133–166. doi: 10.1016/0304-4157(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr, Werner P. K., Müller M. Insertion of proteins into bacterial membranes: mechanism, characteristics, and comparisons with the eucaryotic process. Microbiol Rev. 1989 Sep;53(3):333–366. doi: 10.1128/mr.53.3.333-366.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba K., Ito K., Yura T., Cerretti D. P. A defined mutation in the protein export gene within the spc ribosomal protein operon of Escherichia coli: isolation and characterization of a new temperature-sensitive secY mutant. EMBO J. 1984 Mar;3(3):631–635. doi: 10.1002/j.1460-2075.1984.tb01859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan M. M., Jacobson G. R. Membrane disposition of the Escherichia coli mannitol permease: identification of membrane-bound and cytoplasmic domains. Biochemistry. 1986 Dec 16;25(25):8230–8234. doi: 10.1021/bi00373a016. [DOI] [PubMed] [Google Scholar]
- Swidersky U. E., Rienhöfer-Schweer A., Werner P. K., Ernst F., Benson S. A., Hoffschulte H. K., Müller M. Biochemical analysis of the biogenesis and function of the Escherichia coli export factor SecY. Eur J Biochem. 1992 Jul 15;207(2):803–811. doi: 10.1111/j.1432-1033.1992.tb17111.x. [DOI] [PubMed] [Google Scholar]
- Taura T., Baba T., Akiyama Y., Ito K. Determinants of the quantity of the stable SecY complex in the Escherichia coli cell. J Bacteriol. 1993 Dec;175(24):7771–7775. doi: 10.1128/jb.175.24.7771-7775.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner P. K., Saier M. H., Jr, Müller M. Membrane insertion of the mannitol permease of Escherichia coli occurs under conditions of impaired SecA function. J Biol Chem. 1992 Dec 5;267(34):24523–24532. [PubMed] [Google Scholar]
- Wolfe P. B., Rice M., Wickner W. Effects of two sec genes on protein assembly into the plasma membrane of Escherichia coli. J Biol Chem. 1985 Feb 10;260(3):1836–1841. [PubMed] [Google Scholar]
- Wolfe P. B., Wickner W. Bacterial leader peptidase, a membrane protein without a leader peptide, uses the same export pathway as pre-secretory proteins. Cell. 1984 Apr;36(4):1067–1072. doi: 10.1016/0092-8674(84)90056-4. [DOI] [PubMed] [Google Scholar]
- Wolfe P. B., Wickner W., Goodman J. M. Sequence of the leader peptidase gene of Escherichia coli and the orientation of leader peptidase in the bacterial envelope. J Biol Chem. 1983 Oct 10;258(19):12073–12080. [PubMed] [Google Scholar]
- Yazyu H., Shiota-Niiya S., Shimamoto T., Kanazawa H., Futai M., Tsuchiya T. Nucleotide sequence of the melB gene and characteristics of deduced amino acid sequence of the melibiose carrier in Escherichia coli. J Biol Chem. 1984 Apr 10;259(7):4320–4326. [PubMed] [Google Scholar]
- von Heijne G. Control of topology and mode of assembly of a polytopic membrane protein by positively charged residues. Nature. 1989 Oct 5;341(6241):456–458. doi: 10.1038/341456a0. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Sec-independent protein insertion into the inner E. coli membrane. A phenomenon in search of an explanation. FEBS Lett. 1994 Jun 6;346(1):69–72. doi: 10.1016/0014-5793(94)00296-7. [DOI] [PubMed] [Google Scholar]







