Abstract
IMPORTANCE
Posttraumatic stress disorder (PTSD) is a common, debilitating mental disorder that has been associated with type 2 diabetes mellitus (T2D) and its risk factors, including obesity, in cross-sectional studies. If PTSD increases risk of incident T2D, enhanced surveillance in high-risk populations may be warranted.
OBJECTIVE
To conduct one of the first longitudinal studies of PTSD and incidence of T2D in a civilian sample of women.
DESIGN, SETTING, AND PARTICIPANTS
The Nurses’ Health Study II, a US longitudinal cohort of women (N = 49 739). We examined the association between PTSD symptoms and T2D incidence over a 22-year follow-up period.
MAIN OUTCOMES AND MEASURES
Type 2 diabetes, self-reported and confirmed with self-report of diagnostic test results, symptoms, and medications, a method previously validated by physician medical record review. Posttraumatic stress disorder was assessed by the Short Screening Scale for DSM-IV PTSD. We examined longitudinal assessments of body mass index, smoking, alcohol intake, diet quality, physical activity, and antidepressant use as mediators of possible increased risk of T2D for women with PTSD. The study hypothesis was formulated prior to PTSD ascertainment.
RESULTS
Symptoms of PTSD were associated in a dose-response fashion with T2D incidence (1-3 symptoms: hazard ratio, 1.4 [95% CI, 1.2-1.6]; 4 or 5 symptoms; hazard ratio, 1.5 [95% CI, 1.3-1.7]; 6 or 7 symptoms: hazard ratio, 1.8 [95% CI, 1.5-2.1]). Antidepressant use and a higher body mass index associated with PTSD accounted for nearly half of the increased risk of T2D for women with PTSD. Smoking, diet quality, alcohol intake, and physical activity did not further account for increased risk of T2D for women with PTSD.
CONCLUSIONS AND RELEVANCE
Women with the highest number of PTSD symptoms had a nearly 2-fold increased risk of T2D over follow-up than women with no trauma exposure. Health professionals treating women with PTSD should be aware that these patients are at risk of increased body mass index and T2D. Comprehensive PTSD treatment should be expanded to address the health behaviors that contribute to obesity and chronic disease in affected populations.
Posttraumatic stress disorder (PTSD) occurs following exposure to a potentially traumatic life event and is defined in DSM-V by 4 symptom clusters: intrusion, avoidance, negative alterations in cognition and mood, and alterations in arousal and reactivity. Posttraumatic stress disorder is a common and debilitating disorder with an estimated lifetime prevalence of 10.4% among women in the United States.1 Posttraumatic stress disorder has been associated with inflammation,2 neuroendocrine dysfunction,3 poor diet, and low physical activity,4 all risk factors for type 2 diabetes mellitus (T2D). Research has shown an association of PTSD with T2D,5-10 raising important questions about whether women with PTSD are at increased risk of T2D and whether the treatment of PTSD would prevent T2D.
However, most studies5,11 examining the association of PTSD with T2D have been limited in terms of causal inference by use of cross-sectional data. It is unclear from these studies, therefore, whether PTSD increases risk of T2D, whether T2D increases risk of PTSD, or whether the 2 conditions are associated owing to shared risk factors, such as childhood abuse or depression. To our knowledge, only 3 longitudinal studies have examined PTSD in relation to T2D onset. The first study,7 using a military sample of more than 44 000 persons, found that service members with PTSD at baseline were twice as likely to develop T2D over the 3 years of follow-up than those without PTSD. A study12 of male Vietnam-era veteran twins (N = 4340) also found PTSD associated with incident T2D. The applicability of these studies7,12 to the general population is uncertain, however, owing to their use of primarily or exclusively male participants from military samples. An additional study13 of survivors of the World Trade Center disaster found an increased risk of T2D over 9 years of follow-up among persons with PTSD compared with persons without PTSD at baseline (N = 36 899). However, the study13 relied exclusively on participant report to ascertain T2D incidence. In addition, to our knowledge, no studies have examined pathways by which PTSD may increase risk for T2D, such as increased body mass index (BMI), health behaviors, or antidepressant use.
Thus, it remains largely unknown whether (1) T2D and PTSD are associated because PTSD increases risk of T2D or, instead, because T2D increases risk of PTSD, or owing to shared risk factors; (2) PTSD increases risk for T2D in the civilian population; and (3) the extent to which increased BMI, health behaviors, or antidepressant use among persons with PTSD might account for possible increased risk of T2D. In the present study, we examine the association between PTSD symptoms and T2D incidence, validated in a substudy with medical record review, in a 22-year longitudinal study of women (the Nurses’ Health Study II). We further examine BMI, smoking, diet, alcohol intake, and physical activity as mediators of possible increased risk of T2D among women with PTSD.
Methods
Setting and Participants
The Nurses’ Health Study II is a cohort of 116 430 US female nurses, 24 to 42 years of age at enrollment in 1989 and followed biennially. In 2008, 60 804 women were mailed a supplementary questionnaire that assessed trauma exposure and PTSD symptoms (to retain participation in the main longitudinal study, only women who had returned the most recent biennial questionnaire were sent this supplemental questionnaire)14; 54 282 participants returned the questionnaire (for a response rate of 89%). This study was approved by the institutional review board of Brigham and Women's Hospital in Boston, Massachusetts. Return of the questionnaire via US mail constitutes implied consent.
Measures
Trauma and PTSD
Lifetime exposure to any of 16 traumatic events was queried with a modified version of the Brief Trauma Interview15 (Paula P. Schnurr, PhD, Melaine J. Vielhauer, PhD, and Frank W. Weathers, PhD, unpublished data, 1995; eTable in the Supplement). Participants were asked to indicate the event that they considered the worst and to report their age at occurrence. Women also reported the age at which they experienced their first traumatic event. Symptoms of PTSD were determined by querying participants with respect to the worst traumatic event using the 7-item Brief Screening Scale for DSM-IV PTSD16 (eg, “Since the event, have there ever been times when you became jumpy or got easily startled by ordinary noises”).
Trauma and PTSD were coded as time varying. For each year of the study, participants were categorized into 1 of 5 groups based on their report of the year of their first traumatic event and the year of their worst traumatic event: (1) no exposure to trauma, (2) exposure to trauma and no PTSD (endorsed at least 1 traumatic event and reported no PTSD symptoms), (3) exposure to trauma and reported 1 to 3 PTSD symptoms, (4) exposure to trauma and reported 4 or 5 PTSD symptoms, or (5) exposure to trauma and reported 6 or 7 PTSD symptoms. Prior to the year of their first trauma, or if they did not experience a trauma, women were categorized as having no trauma exposure. After the year they reported experiencing their first trauma, women were considered exposed to trauma, but with no PTSD symptoms. Following the year in which they reported their worst trauma as having occurred, women were assigned to a trauma/PTSD category according to the number of symptoms they reported. If a woman reported only 1 event, the year of her first trauma and her worst trauma would be the same.
Type 2 Diabetes
In each biennial questionnaire, women were asked whether they had received a diagnosis of T2D in the past 2 years. Respondents reporting a diagnosis were then mailed a questionnaire asking for the results of diagnostic tests and for information about symptoms and medications. Type 2 diabetes was diagnosed following the National Diabetes Data Group criteria.17,18 For T2D cases identified after 1998, the American Diabetes Association criteria were applied. This approach to T2D diagnosis (namely, self-report by medical professionals plus a follow-up questionnaire) has been validated by medical record review for 62 women in the Nurses’ Health Study I (98% confirmation of T2D)19 and for 59 men in the Health Professionals Follow-up Study (97% confirmation of T2D).18 In a validation study to determine undiagnosed T2D, only 1 of a random sample of 200 participants who had never reported T2D had a fasting plasma glucose or plasma fructosamine level in the diabetic range.20 Women who reported T2D but who did not return the follow-up questionnaire are not considered T2D cases and are censored at the year of the reported T2D (195 of the 3543 women who self-reported T2D [5.5%]).
Body Mass Index
Body mass index (calculated as weight in kilograms divided by height in meters squared) was calculated from biennially self-reported height and weight. Self-reported weight was validated with in-person weighing in a subgroup (n = 184; Spearman ρ = 0.96).
Health Behaviors
Cigarette smoking was queried biennially and was categorized as never, former, or current smoker of 1 to 14, 15 to 24, or 25 or more cigarettes per day. Diet was assessed in 1991, 1995, 1999, 2003, and 2005 with the Food Frequency Questionnaire,21 which queried consumption in the past 12 months of a wide range of foods. Diet quality was quantified using the Alternative Healthy Eating Index, which has been associated with T2D, cardiovascular disease, coronary heart disease, and stroke.22 We divided the index into quintiles, with the highest quintile representing the healthiest diet. Physical activity was queried in 1991, 1997, 2001, and 2005 and was categorized as less than 3.0, 3.0 to 8.9, 9.0 to 17.9, 18.0 to 26.9, or 27.0 or more metabolic equivalent task hours per week. Alcohol intake from wine, beer, and liquor was assessed in 1989, 1991, 1995, 1999, 2003, and 2007 and was measured in grams per day.
Antidepressant Use
Lifetime history of antidepressant use (any or none) was assessed in 1993. Regular antidepressant use in the past 2 years was assessed in 6 waves, from 1997 to 2009. We created a single time-varying recent antidepressant use variable. Women who endorsed lifetime antidepressant use in 1993 were coded as having used antidepressants for the years 1989-1993, and their use was updated as available.
Childhood Abuse
Childhood abuse was assessed in 2001. Physical and emotional abuse before the age of 12 years was assessed with 5 questions from the Physical and Emotional Abuse Subscale of the Childhood Trauma Questionnaire, coded continuously.23 Sexual abuse before the age of 18 years was assessed with 4 questions about unwanted, forced, or coerced sexual contact by an adult or older child and was coded as none, mild, moderate, or severe.24,25
Comorbid Mental Illness
Depressive symptoms were measured by the 5-item Mental Health Scale of the 36-Item Short Form Health Survey (SF-36) in 1993 and updated in 1997 and 2001. The score was dichotomized as recommended to indicate high or low depressive symptoms.26 Phobic anxiety, measured with the Crown-Crisp index,27 and past-year binge drinking (any or none) were assessed in 2005. Other mental disorders were not assessed in the Nurses’ Health Study II.
Women were not included in our analyses if they did not receive the PTSD questionnaire (62 148 of 116 430 women [53.4%]), did not return the questionnaire (6612 women [5.8%]), ever had type 1 diabetes (22 women [0.02%]), had T2D at baseline in 1989 or before (357 women [0.03%)], were exposed to trauma but did not report their age at the time of their worst trauma (2826 women [2.4%]), or were missing PTSD symptoms (1187 women [1.0%]). The women who were not included in our study were similar to those who were (N = 49 739) in terms of cumulative incidence of T2D from 1989 to 2011 (5.5% vs 6.2%; P = .13) and childhood somatotype (participants selected 1 of 9 pictograms of body size at 5 years of age28,29; highest somatotype, 7.1% vs 6.8%; P = .22). Parental T2D was slightly lower (maternal T2D, 12.2% vs 12.8% [P = .02]; paternal T2D, 14.7% vs 15.9% [P = .001]), and mean BMI at baseline was slightly higher (24.3 vs 23.9; P = .006), for women not included in our study than for those included.
Statistical Analysis
To determine whether women with PTSD symptoms were at greater risk for T2D, we calculated hazard ratios (HRs) for T2D incidence with PTSD as the independent variable. Participants contributed person-time from baseline in 1989 until their last returned questionnaire, T2D diagnosis, or the end of follow-up in 2011. As women entered the study at 24 to 44 years of age, we used left-truncated Cox proportional hazards models with age as the time scale to calculate HRs.
To investigate the extent to which higher BMI in women with vs without PTSD accounted for possible increased risk of T2D for women with PTSD, we added BMI, updated biennially, as a time-varying covariate to the model. Using the SAS MEDIATE Macro,30,31 we estimated the extent to which increased BMI in women with PTSD symptoms accounted for possible increased T2D incidence in this group compared with women who had not experienced a traumatic event. Next, to estimate the extent to which worse health behaviors among women with vs without PTSD accounted for possible increased risk of T2D for women with PTSD over and above the effects of BMI, we added diet, smoking, alcohol intake, and physical activity, updated every 2, 4, or 6 years as available, as time-varying covariates to the model. Finally, we added time-varying recent antidepressant use to the model to assess the possible effects of psychiatric mediations on risk of T2D for women with PTSD.
We conducted 4 additional analyses to further explore our results. First, because childhood abuse is associated with PTSD and has been associated with T2D in this cohort32 and therefore may have accounted for an association between PTSD and incidence of T2D, we repeated our analyses, further adjusted for childhood abuse. Second, because comorbid mental illnesses rather than PTSD itself may have accounted for an association between PTSD and incidence of T2D, we included depressive symptoms as a time-varying covariate, binge drinking, and phobic anxiety in an additional model. Because depression, binge drinking, and phobic anxiety may have been sequelae of PTSD, the estimate of the HR associated with PTSD may be biased toward the null in this model. Third, we examined the reverse of our hypothesis: namely, that T2D increased risk for PTSD. We calculated HRs for PTSD symptoms with T2D as the independent variable. Fourth, because our main analyses relied on retrospective recall of the timing of traumatic events, we conducted a prospective analysis examining T2D incidence after the assessment of trauma and PTSD (ie, 605 women had T2D in 2009-2011; women with T2D in 2008 or earlier were excluded).
All models were adjusted for respondent's self-reported race/ethnicity (self-reported and coded as white, African American, Asian, Hispanic, or other/missing), somatotype at 5 years of age (assessed in 9 levels ranging from very thin to extremely obese), the maximum of parents’ education, and maternal and paternal T2D.
Results
Just over a quarter of the sample (14 914 of 54 282 women [27.5%]) did not report experiencing a traumatic event at or before baseline in 1989 (Table 1); these women served as the reference group for the analyses. Fifty-one percent of this cohort reported experiencing a trauma and reported no history of PTSD symptoms. Four percent of this cohort reported the highest number of PTSD symptoms (6 or 7 symptoms). Compared with women who were unexposed to trauma, women with the highest number of PTSD symptoms were slightly more likely to have had the highest somatotype at 5 years of age (8.4% vs 6.1%), be white (94.7% vs 93.5%), and have had a mother with T2D (13.5% vs 11.9%) (Table 1).
Table 1.
Demographic and Family Factors, Stratified by Number of Symptoms of Posttraumatic Stress Disorder at Baseline (Nurses' Health Study II, 1989-2011)
| Women, No. (%) |
||||||
|---|---|---|---|---|---|---|
| Factor | No Trauma (n = 14 914) | Trauma, No Symptoms (n = 25 308) | Trauma, 1-3 Symptoms (n = 4752) | Trauma, 4 or 5 Symptoms (n = 2868) | Trauma, 6 or 7 Symptoms (n = 1897) | P Value |
| Parents' education at birth, ≥college | 3530 (23.7) | 5657 (22.4) | 1091 (23.0) | 633 (22.1) | 473 (24.9) | <.05 |
| Maternal T2D | 1767 (11.9) | 3283 (13.0) | 622 (13.1) | 403 (14.1) | 256 (13.5) | <.01 |
| Paternal T2D | 2317 (15.5) | 4093 (16.2) | 779 (16.4) | 428 (14.9) | 307 (16.2) | |
| Highest somatotype at 5 years of age | 916 (6.1) | 1784 (7.1) | 315 (6.6) | 221 (7.7) | 159 (8.4) | <.01 |
| Race | ||||||
| African American | 108 (0.7) | 261 (1.0) | 52 (1.1) | 24 (0.8) | 14 (0.7) | <.05 |
| Hispanic | 139 (0.9) | 343 (1.4) | 70 (1.5) | 37 (1.3) | 23 (1.2) | |
| Asian | 235 (1.6) | 295 (1.2) | 44 (0.9) | 18 (0.6) | 19 (1.0) | |
| White | 13 939 (93.5) | 23 750 (93.8) | 4472 (94.1) | 2716 (94.7) | 1796 (94.7) | |
| Other | 493 (3.3) | 659 (2.6) | 114 (2.4) | 73 (2.6) | 45 (2.4) | |
| Smoking | ||||||
| Never | 10 785 (72.3) | 16 633 (65.7) | 2957 (62.2) | 1744 (60.8) | 1053 (55.5) | <.001 |
| Past | 2699 (18.1) | 5672 (22.4) | 1182 (24.9) | 773 (27.0) | 524 (27.6) | |
| Currently 1-14 cigarettes/d | 612 (4.1) | 1173 (4.6) | 234 (4.9) | 125 (4.4) | 122 (6.4) | |
| Currently 15-24 cigarettes/d | 535 (3.6) | 1205 (4.8) | 237 (5.0) | 159 (5.5) | 115 (6.1) | |
| Currently ≥25 cigarettes/d | 237 (1.6) | 547 (2.2) | 123 (2.6) | 53 (1.9) | 76 (4.0) | |
| Missing data | 46 (0.3) | 78 (0.3) | 19 (0.4) | 14 (0.5) | 7 (0.4) | |
| Alcohol intake, g/d | ||||||
| 0 | 5349 (35.9) | 9164 (36.2) | 1720 (36.2) | 1090 (38.0) | 765 (40.3) | <.001 |
| 1 to <5 | 6435 (43.2) | 10 822 (42.8) | 1977 (41.6) | 1203 (42.0) | 716 (37.7) | |
| 5 to <10 | 1665 (11.2) | 2808 (11.1) | 528 (11.1) | 286 (10.0) | 203 (10.7) | |
| 10 to <20 | 1154 (7.7) | 1914 (7.6) | 409 (8.6) | 221 (7.7) | 156 (8.2) | |
| ≥20 | 197 (1.3) | 405 (1.6) | 90 (1.9) | 48 (1.7) | 39 (2.1) | |
| Missing data | 114 (0.8) | 195 (0.8) | 28 (0.6) | 20 (0.7) | 18 (1.0) | |
| Physical activity, MET h/wk | ||||||
| <3.0 | 2177 (14.6) | 3516 (13.9) | 721 (15.2) | 391 (13.6) | 289 (15.2) | <.05 |
| 3.0-8.9 | 3482 (23.4) | 5858 (23.2) | 1121 (23.6) | 655 (22.8) | 418 (22.0) | |
| 9.0-17.9 | 3139 (21.1) | 5454 (21.6) | 1008 (21.2) | 616 (21.5) | 364 (19.2) | |
| 18.0-26.9 | 1977 (13.3) | 3352 (13.2) | 659 (13.9) | 406 (14.2) | 272 (14.3) | |
| ≥27.0 | 4139 (27.8) | 7128 (28.2) | 1243 (26.2) | 800 (27.9) | 554 (29.2) | |
| Alternative Health Eating Index | ||||||
| 1st Quintile (worst) | 3056 (20.5) | 4576 (18.1) | 801 (16.9) | 480 (16.7) | 297 (15.7) | <.001 |
| 2nd Quintile | 2833 (19.0) | 4661 (18.4) | 888 (18.7) | 509 (17.8) | 302 (15.9) | |
| 3rd Quintile | 3874 (26.0) | 6535 (25.8) | 1207 (25.4) | 713 (24.9) | 483 (25.5) | |
| 4th Quintile | 17.4 (2591) | 4793 (18.9) | 856 (18.0) | 540 (18.8) | 361 (19.0) | |
| 5th Quintile (best) | 2498 (16.8) | 4629 (18.3) | 981 (20.6) | 616 (21.5) | 441 (23.3) | |
| Missing data | 62 (0.4) | 114 (0.5) | 19 (0.4) | 10 (0.4) | 13 (0.7) | |
Abbreviations: MET, metabolic equivalent task; T2D, type 2 diabetes mellitus.
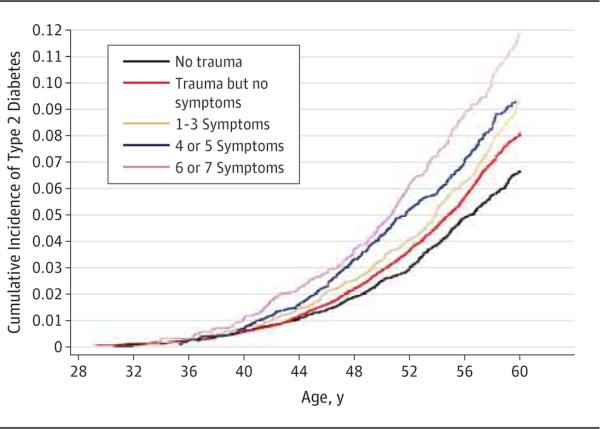
In the 22 years of follow-up, 3091 of 49 739 women developed T2D (6.2%). Women with PTSD symptoms had a higher incidence of T2D than women unexposed to a traumatic event in a dose-response fashion. There were 4.6 cases per 1000 person-years (236 cases per 50 835 person-years) among women with 6 or 7 PTSD symptoms; 3.9 cases per 1000 person-years (313 cases per 80 058 person-years) among women with 4 or 5 symptoms; 3.7 cases per 1000 person-years (472 cases per 128 344 person-years) among women with 1 to 3 symptoms; 2.8 cases per 1000 person-years (1467 cases per 516 784 person-years) among trauma-exposed women with no PTSD symptoms; and 2.1 cases per 1000 person-years (540 cases per 252 308 person-years) in the reference group of women unexposed to trauma (Figure).
Figure.
Cumulative Incidence of Type 2 Diabetes, Stratified by Number of Posttraumatic Stress Disorder Symptoms (Nurses’ Health Study II, 1989-2011)
We also observed a dose-response relationship between PTSD symptoms and the incidence rate of T2D in survival analyses adjusted for race, childhood somatotype, and parental factors (model 1 in Table 2). Women with 6 or 7 PTSD symptoms had the highest risk of T2D onset (HR, 1.8 [95% CI, 1.5-2.1]), followed by women with 4 or 5 symptoms and women with 1 to 3 symptoms, compared with women unexposed to trauma.
Table 2.
Risk of Type 2 Diabetes Onset by Symptoms of Posttraumatic Stress Disorder (Nurses' Health Study II, 1989-2011)a
| Status | Hazard Ratio (95% CI) | No. of Cases/Person-Years | |||
|---|---|---|---|---|---|
| Model 1 | Model 2b | Model 3c | Model 4d | ||
| No trauma | 1.0 [Reference] | 1.0 [Reference] | 1.0 [Reference] | 1.0 [Reference] | 540/252 308 |
| Trauma, no symptoms | 1.23 (1.11-1.35) | 1.21 (1.09-1.34) | 1.22 (1.10-1.35) | 1.20 (1.08-1.33) | 1467/516 784 |
| 1-3 Symptoms | 1.41 (1.24-1.60) | 1.36 (1.20-1.55) | 1.36 (1.20-1.55) | 1.30 (1.15-1.48) | 472/128 344 |
| 4 or 5 Symptoms | 1.52 (1.32-1.74) | 1.42 (1.23-1.64) | 1.41 (1.22-1.62) | 1.30 (1.12-1.50) | 313/80 058 |
| 6 or 7 Symptoms | 1.78 (1.52-2.07) | 1.50 (1.28-1.76) | 1.48 (1.26-1.73) | 1.33 (1.13-1.56) | 236/50 835 |
Adjusted for race, somatotype at 5 years of age, parental education, and maternal and paternal type 2 diabetes mellitus (N = 49 739).
Further adjusted for time-varying body mass index.
Further adjusted for time-varying smoking, physical activity, diet, and alcohol intake.
Further adjusted for time-varying recent antidepressant use.
After adjusting for BMI, the relationship between PTSD and the incidence of T2D was attenuated (model 2 in Table 2). Further adjustment for smoking, physical activity, and diet did not alter the association between PTSD and T2D (model 3 in Table 2). Additional adjustment for antidepressant use further attenuated the association between PTSD and T2D, although it remained statistically significant (model 4 in Table 2). In this final model, BMI accounted for 14.3%, smoking, physical activity, and diet accounted for 2.6%, and antidepressant use accounted for 33.8% of the association between PTSD and incidence of T2D.
In analyses adjusted for childhood abuse, the relationship between PTSD status and T2D incidence was somewhat attenuated (6 or 7 symptoms: HR, 1.5 [95% CI, 1.2-1.7]; 4 or 5 symptoms: HR, 1.4 [95% CI, 1.2-1.6]; 1-3 symptoms: HR, 1.2 [95% CI, 1.1-1.5]; P < .001 for all). In analyses adjusted for depressive symptoms, binge drinking, and phobic anxiety, the relationship between PTSD and T2D was somewhat attenuated but remained statistically significant (6 or 7 symptoms: HR, 1.3 [95% CI, 1.1-1.7]; 4 or 5 symptoms: HR, 1.3 [95% CI, 1.2-1.7]; 1-3 symptoms: HR, 1.3 [95% CI, 1.1-1.5]). Results were similar in analyses adjusted for both mental health indicators and childhood abuse.
In models examining the reverse of our hypothesis, we found no evidence that T2D increased risk for PTSD. There was no association between T2D onset before 2000 and risk of PTSD between 2000 and 2007 (730 women with T2D and 46 319 women without T2D; HR, 1.0 [95% CI, 0.3-3.1]).
In the prospective analysis estimating the association of PTSD with risk of T2D from 2009 to 2011, we found a statistically significant increased risk of T2D among women with PTSD symptoms (trauma/no PTSD symptoms: HR, 1.19 [95% CI, 0.96-1.47] [P = .11]; 1-3 PTSD symptoms: HR, 1.69 [95% CI, 1.32-2.15] [P < .001]; 4 or 5 PTSD symptoms: HR, 1.40 [95% CI, 1.05-1.85] [P = .02]; 6 or 7 PTSD symptoms: HR, 1.72 [95% CI, 1.26-2.34] [P = .006]).
Because women with PTSD symptoms may be more heavily involved with the health care system than women without PTSD systems, and therefore more likely to receive a diagnosis of T2D if they have T2D, we examined the association of PTSD with incident T2D, excluding women whose T2D was detected from screening only (eg, women who did not have T2D symptoms when they received their diagnosis). The association of PTSD with T2D incidence was the same for these women. In addition, because women with PTSD symptoms may be more likely than women without PTSD to receive fasting glucose tests owing to increased involvement in the health care system, and therefore more likely to have existing T2D detected, we adjusted for self-reported time-varying fasting glucose tests, beginning in 1999 and updated biennially; the PTSD-T2D relationship was only slightly attenuated in these analyses.
Discussion
We found that over 22 years of follow-up, PTSD symptoms were associated in a dose-response fashion with the onset of T2D. Women with the highest number of PTSD symptoms had a nearly 2-fold increased risk of T2D compared with women without exposure to trauma. Our results provide the strongest evidence to date that there may be a causal relationship between PTSD symptoms and T2D incidence. In addition, we identified antidepressant use and increased BMI as likely principal mechanisms for the increased risk of T2D among women with PTSD symptoms. However, even after adjustment for BMI, smoking, diet, alcohol consumption, physical activity, and antidepressant use, PTSD remained a significant predictor of T2D incidence, suggesting that other pathways may link PTSD with T2D risk. In addition, we found no support for the reverse of our hypothesis: that T2D increased risk for PTSD.
Our findings are consistent with the 3 prior longitudinal studies that have examined PTSD and risk of T2D onset.7,12,13 Our study builds on this work by examining T2D onset, assessed longitudinally, in a large population-based sample of women followed for 22 years. In addition, a T2D diagnosis was ascertained on the basis of T2D symptoms and treatment, a method previously validated against medical record review. Thus, our estimates of T2D risk are likely to be more generalizable to the general civilian population and unlikely to represent reverse causation (namely, the effects of T2D on mental health) compared with prior cross-sectional studies.2,5,9,10
Posttraumatic stress disorder may increase the risk of T2D through several pathways aside from BMI, antidepressant use, and the health risk behaviors that we examined. Posttraumatic stress disorder is associated with elevated inflammatory markers,33 and the molecules involved in the inflammatory process (eg, IL-6 and tumor necrosis factor) may increase insulin resistance.2 Posttraumatic stress disorder has been linked to an increased blood concentration of C-reactive protein.34 C-reactive protein has been found to predict T2D onset in a dose-response fashion in prospective studies. Posttraumatic stress disorder is also associated with sleep disturbance,35-37 which has been associated with risk of T2D.38-40 Posttraumatic stress disorder has further been associated with alterations to the hypothalamic-pituitary-adrenal axis.41 Dysfunction of the hypothalamic-pituitary-adrenal axis has been linked to visceral obesity, insulin resistance, and T2D.3
The association of PTSD with T2D remained after adjustment for depressive symptoms, binge drinking, phobic anxiety, and childhood abuse. Because depressive symptoms, binge drinking, and phobic anxiety may have been subsequent to PTSD onset, and childhood abuse may have been the PTSD-triggering event, adjustment for these factors likely represents an overadjustment and biases estimates toward the null. Nonetheless, adjustment for childhood abuse may capture a host of negative childhood circumstances that could be associated with both PTSD and T2D, such as low socioeconomic status, poor parent-child relationship, and emotional dysregulation. Thus, these models likely capture potential prior common causes of PTSD and T2D not included in other studies. Future studies should also consider other anxiety disorders as potential confounders of the association between PTSD and T2D because generalized anxiety disorder, social phobia, and agoraphobia have been associated with T2D.42-44
Our study included predominantly white professional women; therefore, we cannot be certain that results apply to other demographic groups. Owing to the large size of the Nurses’ Health Study II cohort, we used a screening instrument to assess symptoms of PTSD. The use of screening instruments is consistent with a large body of epidemiologic research but does not constitute a clinical diagnosis. Our results may therefore underestimate the relationship between PTSD and risk of T2D onset, to the extent that women with PTSD symptoms based on the screening instrument did not actually have PTSD. Future research using a clinician-administered structured interview is needed. Such research should also consider whether subthreshold PTSD also increases risk, given the dose-response nature of our findings.
Data were collected using DSM-IV PTSD criteria. Although studies comparing PTSD diagnosis under DSM-IV and DSM-V criteria have found that the vast majority of cases diagnosed using DSM-V also meet criteria under DSM-IV, future research should use the DSM-V criteria.45 Our use of a retrospective assessment of trauma and PTSD symptoms may have reduced accuracy in both the assessment of PTSD and the timing of the triggering traumatic event, compared with contemporaneous assessment of trauma and PTSD.
Moreover, to reduce participant burden, we did not collect data on age of participant at every traumatic event, and we assessed PTSD symptoms only with respect to the participant's self-identified worst event. Because many women experienced multiple traumas, women may have had PTSD symptoms from an event prior to their worst event. This measurement error may have biased our estimates of the association between PTSD and T2D. If T2D onset affected recall of traumatic events or PTSD symptoms, this may also have biased our results.
In addition, differential dropout from the study of women with T2D and PTSD symptoms may have attenuated our results because PTSD was not assessed until 2008, the 19th year of the study. Body mass index was assessed by self-report, which, although found to have good validity in this cohort, may nonetheless have introduced measurement error and bias in our estimates of the extent to which BMI accounted for the increased risk of T2D among women with PTSD symptoms. Finally, duration of antidepressant use and dosage were not assessed. Had we assessed antidepressant use more precisely, it may have accounted for a larger portion of the association between PTSD and T2D.
Conclusions
Our findings have implications for research and practice. Further research must identify the biochemical and possible additional behavioral changes, such as sleep disturbance, that mediate the relationship between PTSD and onset of T2D. A better understanding of pathways will facilitate interventions to prevent this disabling disease. For example, if research found that PTSD increases risk of T2D primarily through inflammatory pathways, this would suggest that inflammatory biomarkers may be a useful tool for early detection of T2D risk in women with PTSD. At this time, however, the biological pathways through which PTSD affects risk for T2D have yet to be identified. Research is also needed to determine whether effective treatment of PTSD reduces risk of T2D. Such research could be conducted by including biomarkers of T2D in clinical trials for PTSD. For practice, clinicians should note the nearly 2-fold increased risk of T2D for women with the highest number of PTSD symptoms. Because our results suggest that a higher BMI substantially mediates this increased risk, attention to possible weight gain in women with PTSD is crucial. Health professionals treating women with PTSD should therefore be aware that these patients are at risk of increased BMI and T2D. Comprehensive PTSD treatment should be expanded to address the health behaviors that contribute to obesity and chronic disease in PTSD-affected populations.
Supplementary Material
Acknowledgments
Funding/Support: Dr Roberts is supported by National Institutes of Health (NIH) grants MH078928 and MH093612. Dr Agnew-Blais is supported by National Institute of Mental Health grant T32MH017119. Dr Galea is supported by NIH grants MH095718, MH082598, MH 082729, DA013336, and DA034244 and by US Department of Defense grant W81XWH-07-1-0409. Dr Koenen is supported by NIH grants MH078928 and MH093612.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Information: The Nurses’ Health Study II is funded in part by NIH grant CA50385.
Footnotes
Author Contributions: Dr Agnew-Blais had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Roberts, Agnew-Blais, Kubzansky, Galea, Koenen.
Acquisition, analysis, or interpretation of data: Roberts, Agnew-Blais, Spiegelman, Mason, Galea, Hu, Rich-Edwards, Koenen.
Drafting of the manuscript: Roberts, Agnew-Blais, Hu, Koenen.
Critical revision of the manuscript for important intellectual content: Roberts, Spiegelman, Kubzansky, Mason, Galea, Rich-Edwards, Koenen.
Statistical analysis: Roberts, Agnew-Blais, Spiegelman, Kubzansky, Rich-Edwards, Koenen.
Obtained funding: Galea, Koenen.
Administrative, technical, or material support: Mason, Galea.
Study supervision: Roberts, Koenen.
Conflict of Interest Disclosures: None reported.
Additional Contributions: We acknowledge the Channing Division of Network Medicine within the Department of Medicine at Brigham and Women's Hospital and Harvard Medical School for their management of the Nurses’ Health Study II and the Growing Up Today Study.
REFERENCES
- 1.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 2.Black PH. The inflammatory consequences of psychologic stress: relationship to insulin resistance, obesity, atherosclerosis and diabetes mellitus, type II. Med Hypotheses. 2006;67(4):879–891. doi: 10.1016/j.mehy.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Björntorp P, Holm G, Rosmond R. Hypothalamic arousal, insulin resistance and Type 2 diabetes mellitus. Diabet Med. 1999;16(5):373–383. doi: 10.1046/j.1464-5491.1999.00067.x. [DOI] [PubMed] [Google Scholar]
- 4.Buckley TC, Mozley SL, Bedard MA, Dewulf AC, Greif J. Preventive health behaviors, health-risk behaviors, physical morbidity, and health-related role functioning impairment in veterans with post-traumatic stress disorder. Mil Med. 2004;169(7):536–540. doi: 10.7205/milmed.169.7.536. [DOI] [PubMed] [Google Scholar]
- 5.Boscarino JA. Posttraumatic stress disorder and physical illness: results from clinical and epidemiologic studies. Ann N Y Acad Sci. 2004;1032:141–153. doi: 10.1196/annals.1314.011. [DOI] [PubMed] [Google Scholar]
- 6.Agyemang C, Goosen S, Anujuo K, Ogedegbe G. Relationship between post-traumatic stress disorder and diabetes among 105,180 asylum seekers in the Netherlands. Eur J Public Health. 2012;22(5):658–662. doi: 10.1093/eurpub/ckr138. [DOI] [PubMed] [Google Scholar]
- 7.Boyko EJ, Jacobson IG, Smith B, et al. Millennium Cohort Study Team. Risk of diabetes in U.S. military service members in relation to combat deployment and mental health. Diabetes Care. 2010;33(8):1771–1777. doi: 10.2337/dc10-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.David D, Woodward C, Esquenazi J, Mellman TA. Comparison of comorbid physical illnesses among veterans with PTSD and veterans with alcohol dependence. Psychiatr Serv. 2004;55(1):82–85. doi: 10.1176/appi.ps.55.1.82. [DOI] [PubMed] [Google Scholar]
- 9.Goodwin RD, Davidson JR. Self-reported diabetes and posttraumatic stress disorder among adults in the community. Prev Med. 2005;40(5):570–574. doi: 10.1016/j.ypmed.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Weisberg RB, Bruce SE, Machan JT, Kessler RC, Culpepper L, Keller MB. Nonpsychiatric illness among primary care patients with trauma histories and posttraumatic stress disorder. Psychiatr Serv. 2002;53(7):848–854. doi: 10.1176/appi.ps.53.7.848. [DOI] [PubMed] [Google Scholar]
- 11.Sledjeski EM, Speisman B, Dierker LC. Does number of lifetime traumas explain the relationship between PTSD and chronic medical conditions? answers from the National Comorbidity Survey-Replication (NCS-R). J Behav Med. 2008;31(4):341–349. doi: 10.1007/s10865-008-9158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaccarino V, Goldberg J, Magruder KM, et al. Posttraumatic stress disorder and incidence of type-2 diabetes: a prospective twin study. J Psychiatr Res. 2014;56:158–164. doi: 10.1016/j.jpsychires.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller-Archie SA, Jordan HT, Ruff RR, et al. Posttraumatic stress disorder and new-onset diabetes among adult survivors of the World Trade Center disaster. Prev Med. 2014;66:34–38. doi: 10.1016/j.ypmed.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 14.Morgan CA III, Hazlett G, Wang S, Richardson EG, Jr, Schnurr P, Southwick SM. Symptoms of dissociation in humans experiencing acute, uncontrollable stress: a prospective investigation. Am J Psychiatry. 2001;158(8):1239–1247. doi: 10.1176/appi.ajp.158.8.1239. [DOI] [PubMed] [Google Scholar]
- 15.Schnurr PP, Spiro A III, Vielhauer MJ, Findler MN, Hamblen JL. Trauma in the lives of older men: findings from the Normative Aging Study. J Clin Geropsychology. 2002;8(3):175–187. doi:10.1023/A:1015992110544. [Google Scholar]
- 16.Breslau N, Peterson EL, Kessler RC, Schultz LR. Short screening scale for DSM-IV posttraumatic stress disorder. Am J Psychiatry. 1999;156(6):908–911. doi: 10.1176/ajp.156.6.908. [DOI] [PubMed] [Google Scholar]
- 17.National Diabetes Data Group Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28(12):1039–1057. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 18.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med. 2001;161(12):1542–1548. doi: 10.1001/archinte.161.12.1542. [DOI] [PubMed] [Google Scholar]
- 19.Manson JE, Rimm EB, Stampfer MJ, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991;338(8770):774–778. doi: 10.1016/0140-6736(91)90664-b. [DOI] [PubMed] [Google Scholar]
- 20.Field AE, Coakley EH, Must A, et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001;161(13):1581–1586. doi: 10.1001/archinte.161.13.1581. [DOI] [PubMed] [Google Scholar]
- 21.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 22.Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–1018. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernstein DP, Fink L, Handelsman L, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151(8):1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- 24.Moore D, Gallup G, Schussel R. Disciplining Children in America: A Gallup Poll Report. Gallup Organization; Princeton, NJ: 1995. [Google Scholar]
- 25.Roberts AL, Lyall K, Rich-Edwards JW, Ascherio A, Weisskopf MG. Association of maternal exposure to childhood abuse with elevated risk for autism in offspring. JAMA Psychiatry. 2013;70(5):508–515. doi: 10.1001/jamapsychiatry.2013.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II, psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Crown S, Crisp AH. The Middlesex Hospital Questionnaire (M.H.Q.). A short clinical diagnostic self-rating scale for psychoneurotic patients. Br J Psychiatry. 1966;112(490):917–923. doi: 10.1192/bjp.112.490.917. [DOI] [PubMed] [Google Scholar]
- 28.Must A, Willett WC, Dietz WH. Remote recall of childhood height, weight, and body build by elderly subjects. Am J Epidemiol. 1993;138(1):56–64. doi: 10.1093/oxfordjournals.aje.a116777. [DOI] [PubMed] [Google Scholar]
- 29.Magnusson C, Baron J, Persson I, et al. Body size in different periods of life and breast cancer risk in post-menopausal women. Int J Cancer. 1998;76(1):29–34. doi: 10.1002/(sici)1097-0215(19980330)76:1<29::aid-ijc6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 30.The SAS MEDIATE Macro [computer program] Brigham and Women's Hospital, Channing Laboratory; Boston, MA: 2009. [Google Scholar]
- 31.Lin DY, Fleming TR, De Gruttola V. Estimating the proportion of treatment effect explained by a surrogate marker. Stat Med. 1997;16(13):1515–1527. doi: 10.1002/(sici)1097-0258(19970715)16:13<1515::aid-sim572>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 32.Rich-Edwards JW, Spiegelman D, Lividoti Hibert EN, et al. Abuse in childhood and adolescence as a predictor of type 2 diabetes in adult women. Am J Prev Med. 2010;39(6):529–536. doi: 10.1016/j.amepre.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Känel R, Hepp U, Kraemer B, et al. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J Psychiatr Res. 2007;41(9):744–752. doi: 10.1016/j.jpsychires.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 34.Spitzer C, Barnow S, Völzke H, et al. Association of posttraumatic stress disorder with low-grade elevation of C-reactive protein: evidence from the general population. J Psychiatr Res. 2010;44(1):15–21. doi: 10.1016/j.jpsychires.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Harvey AG, Jones C, Schmidt DA. Sleep and posttraumatic stress disorder: a review. Clin Psychol Rev. 2003;23(3):377–407. doi: 10.1016/s0272-7358(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 36.Lamarche LJ, De Koninck J. Sleep disturbance in adults with posttraumatic stress disorder: a review. J Clin Psychiatry. 2007;68(8):1257–1270. doi: 10.4088/jcp.v68n0813. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi I, Boarts JM, Delahanty DL. Polysomnographically measured sleep abnormalities in PTSD: a meta-analytic review. Psychophysiology. 2007;44(4):660–669. doi: 10.1111/j.1469-8986.2007.537.x. [DOI] [PubMed] [Google Scholar]
- 38.Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med. 2006;166(16):1768–1774. doi: 10.1001/archinte.166.16.1768. [DOI] [PubMed] [Google Scholar]
- 39.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep. 2007;30(12):1667–1673. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):414–420. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morris MC, Compas BE, Garber J. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: a systematic review and meta-analysis. Clin Psychol Rev. 2012;32(4):301–315. doi: 10.1016/j.cpr.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith KJ, Béland M, Clyde M, et al. Association of diabetes with anxiety: a systematic review and meta-analysis. J Psychosom Res. 2013;74(2):89–99. doi: 10.1016/j.jpsychores.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 43.Lin EHB, Von Korff M, Alonso J, et al. Mental disorders among persons with diabetes—results from the World Mental Health Surveys. J Psychosom Res. 2008;65(6):571–580. doi: 10.1016/j.jpsychores.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grigsby AB, Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. Prevalence of anxiety in adults with diabetes: a systematic review. J Psychosom Res. 2002;53(6):1053–1060. doi: 10.1016/s0022-3999(02)00417-8. [DOI] [PubMed] [Google Scholar]
- 45.Stein DJ, McLaughlin KA, Koenen KC, et al. DSM-5 and ICD-11 definitions of posttraumatic stress disorder: investigating “narrow” and “broad” approaches. Depress Anxiety. 2014;31(6):494–505. doi: 10.1002/da.22279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.