Abstract
The zebrafish (Danio rerio) has become a powerful model organism for studying developmental processes and genetic diseases. However, there remain several problems in previous rearing methods. In this study, we demonstrate a novel method for rearing zebrafish larvae by using a new first food, freshwater rotifers (Brachionus calyciflorus). Feeding experiments indicated that freshwater rotifers are suitable as the first food for newly hatched larval fish. In addition, we revisited and improved a feeding schedule from 5 to 40 days postfertilization. Our feeding method using freshwater rotifers accelerated larval growth. At 49 dpf, one pair out of 10 pairs successfully produced six fertilized eggs. At 56, 63, and 71 dpf, 6 out of the 10 pairs constantly produced normal embryos. Our method will improve the husbandry of the zebrafish.
Introduction
The zebrafish system offers several advantages to the investigation of early development and genetic diseases.1–3 For example, researchers have been able to isolate a number of mutants and create tissue-specific transgenic fish. The zebrafish model now provides targeted disruption of interest genes with TALENs and the CRISPR/Cas system.4–7 In addition to these genetic engineering techniques, a comparatively short generation time (∼3 months after fertilization) is also one of the advantages in zebrafish.2,8–10 However, the generation time seems to be extended because there remain several problems with the previous rearing methods.
A shorter generation time is preferable to conduct research with zebrafish effectively, particularly for genetic analyses. Generation time in zebrafish is impacted by diet, nutrients, and environment such as water quality.11 Saltwater rotifers (Brachionus plicatilis) were reported as a good source of nutrients for newly hatched larval fish.12 Moreover, fish have reached sexual maturity ∼60 days postfertilization (dpf)13 after consuming saltwater rotifers. Thus, provision of saltwater rotifers greatly improves zebrafish generation time and survival rate (∼90%),12,13 but several problems remain. The main issue concerns the saltwater rotifer itself. Saltwater rotifers require a high salt concentration in the water; therefore, larval fish must be able to tolerate the high salt condition.12 In addition, larval fish were reported to show restricted growth after they were moved to a freshwater aquarium.12 Possible, nonmutually exclusive reasons for this outcome may be that saltwater rotifers could not survive in freshwater and/or that larval fish were physiologically stressed from the environmental change they experienced. Regardless, this restricts the usability of saltwater rotifers as a feed to increase zebrafish generation time.
In this study, we used freshwater rotifers (B. calyciflorus) instead of saltwater rotifers to overcome the main problem described above. Moreover, we revisited and improved a feeding schedule from newly hatched larvae to 40 dpf fish with freshwater rotifers and/or brine shrimp (Artemia spp.). Our aim was to develop a new feeding method for growing larval fish that shortens zebrafish generation time and has a high survival rate. An improved feeding method will accelerate zebrafish research and strengthen the advantages of the zebrafish as a model organism.
Materials and Methods
Animal experiments
All animal experiments were performed under the ethical guidelines of the Kyoto University and approved by the Animal Experimentation Committee of the Kyoto University (No. Info24-6).
Zebrafish
AB strain zebrafish (Danio rerio) were obtained from the Zebrafish International Resource Center, University of Oregon (Eugene, OR). The fish were kept in a recirculating flow-through aquarium (Meito system, Nagoya, Japan) under a 14-h light–10-h dark cycle (14 L/10D). A male and a female AB fish were placed in a 1-L breeding tank with a spawn insert (Aqua Schwarz GmbH, Göttingen, Germany) on the evening before the embryos were required. Three sets of four AB fish pairs were set up in a 2-L hand-made breeding tank, which was previously described,13 to obtain enough embryos for the rearing experiments (see next section). The next morning, fertilized embryos were selected and kept in 10-cm Petri dishes (50 embryos/dish) filled with 1/3 Ringer solution (39 mM NaCl, 0.97 mM KCl, 1.8 mM CaCl2, and 1.7 mM HEPES at pH 7.2) at 28.5°C.
Culture of freshwater rotifers
Freshwater rotifers (B. calyciflorus) were obtained from Wamushiya, Shizuoka, Japan. The rotifers were precultured in 2 L of artificial freshwater (60 mg/L artificial sea salt) and aerated. Concentrated Chlorella spp. (Nikkai Center, Tokyo, Japan) was added twice daily as food for the rotifers. To determine rotifer density in the culture, three 1-L cultures were set up. Triplicate sampling of 1 mL from each culture was performed. We renewed the main 2-L culture every 5 days, starting with 10,000 rotifers from the previous culture. To obtain a large number of rotifers, the culture was increased to 10 L after 3 days, with 2-L culture as the base. Two 10-L cultures were maintained using concentrated Chlorella for 1 week; the concentration of rotifers in the 10-L culture was ∼20 rotifers/mL. We recorded images and videos of the rotifers under a SZX16 stereomicroscope (Olympus, Tokyo, Japan) using a MicroPublisher 5.0 RTV digital camera (QImaging, Surrey, Canada) for photographs or an Infinity 2-1C (Lumenera, Ottawa, Canada) for video. Fifty freshwater rotifers were randomly selected from the images, and the size of the rotifers was then measured using ImageJ software (ImageJ, U. S. National Institutes of Health, Bethesda, MD)
Rearing experiments
At 5 days postfertilization (dpf), one clutch of hatched larval fish was divided into six groups and placed in a 1.8-L plastic tank with 200 mL of artificial freshwater (0.006% red sea salt [Red Sea, Houston, TX]). Each tank contained 30 hatched larval fish. Three tanks were prepared for rotifer feeding (experimental diet) and the others prepared for processed food feeding (control diet). We used ∼15 mg (15.3±2.9 mg, mean±SD, n=9) of the processed diet (Kyowa N250, Kyowa Hakko Bio, Tokyo, Japan) per feeding. Each processed food pellet was less than 250 μm in diameter. To concentrate the rotifers for feeding, ∼4 L of each rotifer culture was filtered through a 30-μm mesh filter. Before feeding, the concentrated rotifers were washed with deionized water on the filter and suspended in deionized water. The density of freshwater rotifers was determined before feeding. Approximately 55,000 rotifers in 12–24 mL deionized water were added to each nursery tank containing 200 mL of artificial freshwater at each feed. As a control, the same amount of deionized water without rotifers was added to each nursery tank for processed diets.
The plastic tanks containing larval fish were placed on a shelf at 27.4°C under 14L/10D conditions. Up to 12 dpf, rotifers or the processed diet were provided once daily. We did not change water or clean the nursery tanks for either group during this period. From 9 to 12 dpf, ∼200 brine shrimp (Artemia spp.) were also provided to both groups twice daily, in addition to the freshwater rotifers or the processed food. On the evening of the 12th day after fertilization, the larvae from each group were divided into two 2-L holding tanks connected to the recirculating flow-through aquarium. One of the 2-L holding tanks held 10 larval fish. Another 2-L tank held the remaining larval fish. Because over half of the larval fish fed with processed diet died at 12 dpf in the preliminary experiment, ten of the larval fish from this control group were put in one tank and the remaining were put into another tank. The density of larval fish was 5/L in one tank and 10/L in another tank when all larval fish survived at 12 dpf. The 10 fish in one tank were subjected to further experiments for growth to remove any effects of density. There was no obvious difference in size and the timing of first spawning between fish in the 5/L tank and in the 10/L tank (unpublished data). Rate of water change was adjusted to at least 4 L per hour until the larvae reached 30 dpf. After 13 dpf, only brine shrimp were provided to both treatments. From 12 to 15 dpf, ∼12,000 brine shrimp were provided twice daily. From 16 to 29 dpf, ∼24,000 brine shrimp were provided three times daily. After 30 dpf, ∼36,000 brine shrimp were provided three times daily until the end of the trial. Three tanks for each feed condition were set up to perform triplicated sampling. Three independent sets of the rearing trials were carried out side by side.
Determination of rotifer densities
We measured the density of living freshwater rotifers in the static nursery tanks following previously described methods used for saltwater rotifers, with the following modifications.12 After stirring the water, we removed 10 mL of water from each tank. Two hundred microliters of water was then placed on a Petri dish and the rotifers were counted under a SZX15 stereomicroscope (Olympus, Tokyo, Japan). We continued adding 200 μL of water until the observed number of rotifers reached over 100. Density of the rotifers was calculated based on the final volume of water and recorded number of rotifers. Determination of rotifer and brine shrimp density for feeding was carried out with the same method. The mean±SEM of density counts per day were calculated and plotted.
Growth and survival measurements
At 5, 9, 12, 16, 23, 30, 35, and 40 dpf, we used a Lumix DMC-FZ200 digital camera (Panasonic, Osaka, Japan) to take photographs of both the experimental larval fish fed and the control larval fish. Fish from the holding tanks of all three independent sets of rearing experiments were included. Five larvae in the photographs were randomly selected for measuring the total length and the standard length. Definitions of total length and standard length are the same as described in previous publications.8,13 Measurement of the total and standard lengths of five larvae was performed using ImageJ software, and the means of both lengths were calculated. Results are depicted as means±SEM.
During the rearing experiments described above, we also counted the number of living larval fish in both groups. Until 12 dpf, the number of living larvae was recorded daily. We then counted the number of living fish at 16, 23, and 30 dpf. Survival rates were calculated by dividing with 270, the number of larvae at 5 dpf.
Water quality measurements
We measured water quality in all tanks, using methods described in previous research.12,13 We used the same characteristics indicating water quality as those studies. Temperature and salinity were measured with SaltTestr 11 (MK Scientific, Kanagawa, Japan). We measured pH with a pH meter (Horiba, Kyoto, Japan) and ammonia concentration with a Mi407 (Milwaukee, North Carolina, USA meter). Concentrations of nitrate and nitrite were determined with a digital pack test kit (Kyoritsu, Tokyo, Japan). Each characteristic was measured daily from 5 to 12 dpf, then measured weekly after larval fish were moved to our recirculating flow-through aquarium. The average concentrations of each compound from the daily then weekly measurements were calculated and plotted as means±SEM.
Breeding trials
Sexual maturity of larval fish was first determined based on visual characteristics (body shape and color), as well as the standard length of the fish. At 47-dpf, 10 pairs of male and female fish were randomly selected from individual rearing trials for the first breeding trial. As the first breeding, two 2-L breeding tanks were set up in the evening. Each 2-L tank held 5 pairs of 47-dpf male and female fish. We collected produced embryos next morning and observed the embryos at 24 hpf. The 49-, 56-, 63-, and 71-dpf, 10 pairs of male and female fish were also randomly selected from the individual rearing trials for breeding. A male and female fish were placed together in a 1-L breeding tank on the evening of the day before we collected the embryos. During the next day, the fish were naturally crossed for 4 h after the room lights were turned on. The number of pairs that produced fertilized embryos was determined and the success rate was plotted. After collecting the embryos, we recorded the number of embryos in one clutch. The average number of embryos in one clutch was calculated and plotted (mean±SEM). Viability of the embryos at 1 dpf was determined based on morphology.
Statistical analyses
Unpaired two-sided t-tests were used to compare the means of total length from the two conditions (rotifer-fed vs. control-fed). For the comparison of survival rates between larvae raised on freshwater rotifers versus processed feed, a Gehan–Breslow–Wilcoxon test was conducted. A linear regression was applied to estimate the timing of sexual maturity. All statistical analyses were carried out in GraphPad Prism software (version 5.04 for Windows, GraphPad Software, La Jolla, CA).
Results
Freshwater rotifers, B. calyciflorus
We first observed the freshwater rotifers to examine whether they were suitable as a food for newly hatched larval fish. The size of the freshwater rotifers was ∼260 μm (266.2±32.4 μm, mean±SD; n=50) (Fig. 1A). The size of freshwater rotifers is essentially the same as the size of saltwater rotifers previously reported (221.5–283.3 μm).14 In addition to the size, the freshwater rotifers swam freely as essentially same as the saltwater rotifers (Supplementary Movie S1; Supplementary Data are available online at www.liebertpub.com/zeb).15 Due to the rotifers' smaller size, we actually consider them to be more suitable than brine shrimp for newly hatched larvae (Fig. 1B). The growth curve of freshwater rotifers in a 1-L culture is shown in Supplementary Figure. S1. The estimated doubling time was ∼19 h.
FIG. 1.
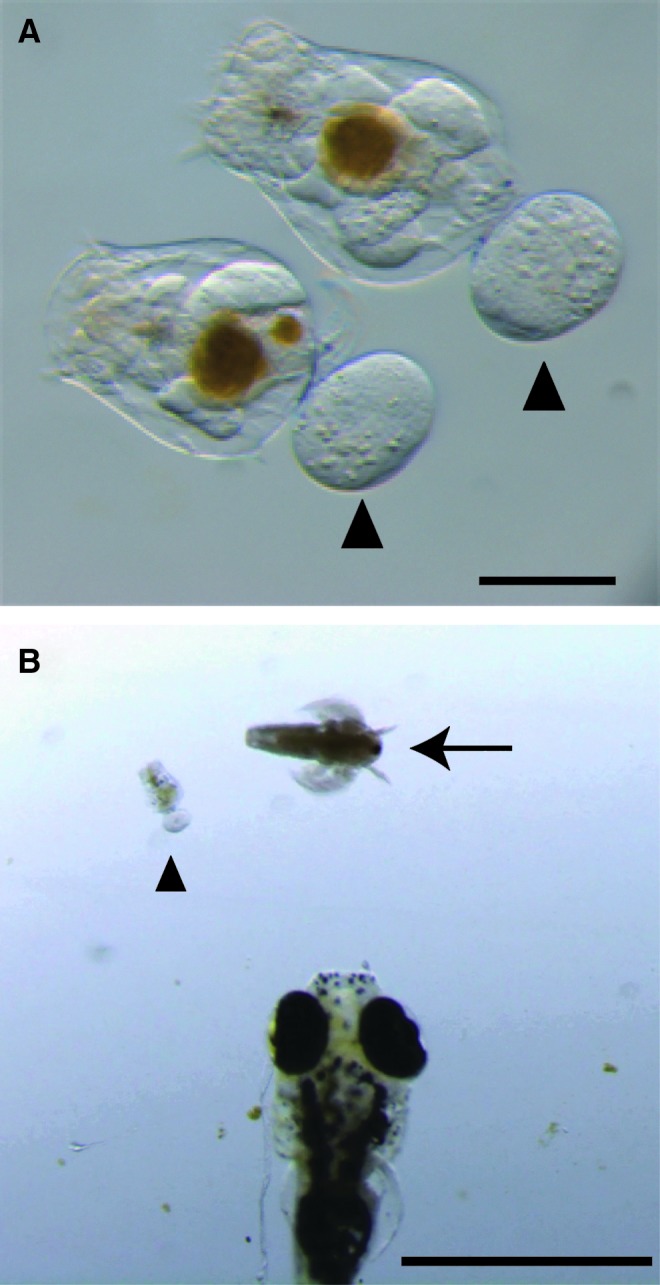
Freshwater rotifers (Brachionus calyciflorus). (A) Freshwater rotifers used in the present study. The arrowheads indicate an egg in each rotifer. The bar represents 100 μm. (B) Comparison of a freshwater rotifer (arrowhead) and a brine shrimp (Artemia spp.; arrow) as food for larval zebrafish (Danio rerio). A 7-dpf (days postfertilization) larval zebrafish is shown at the bottom of the panel. The bar represents 1 mm. Color images available online at www.liebertpub.com/zeb
To examine whether newly hatched larvae can eat the freshwater rotifers, we fed rotifers to 5-dpf larval fish (experimental diet). An artificial processed diet (Kyowa N250, particle size less than 250 μm) was used as a control. The larval fish fed with the processed food showed no visible materials in their intestine (Fig. 2A). In contrast, we were able to observe swallowed rotifers in the intestine of larval fish fed with the experimental diet (Fig. 2B), indicating that the larval fish are indeed able to eat the freshwater rotifers at 5 dpf.
FIG. 2.
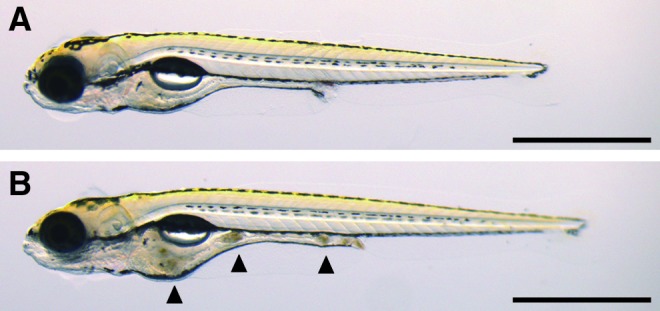
Digested freshwater rotifers (B. calyciflorus). The arrowheads in the panel (B) indicate swallowed and digested rotifers by a 5-dpf (days postfertilization) zebrafish (Danio rerio) larva; the images were taken immediately after feeding. The upper panel (A) shows a 5-dpf fish fed with the processed diet, and the lower panel (B) shows a 5-dpf fish fed with freshwater rotifers. The bar represents 1 mm. Color images available online at www.liebertpub.com/zeb
A new feeding schedule
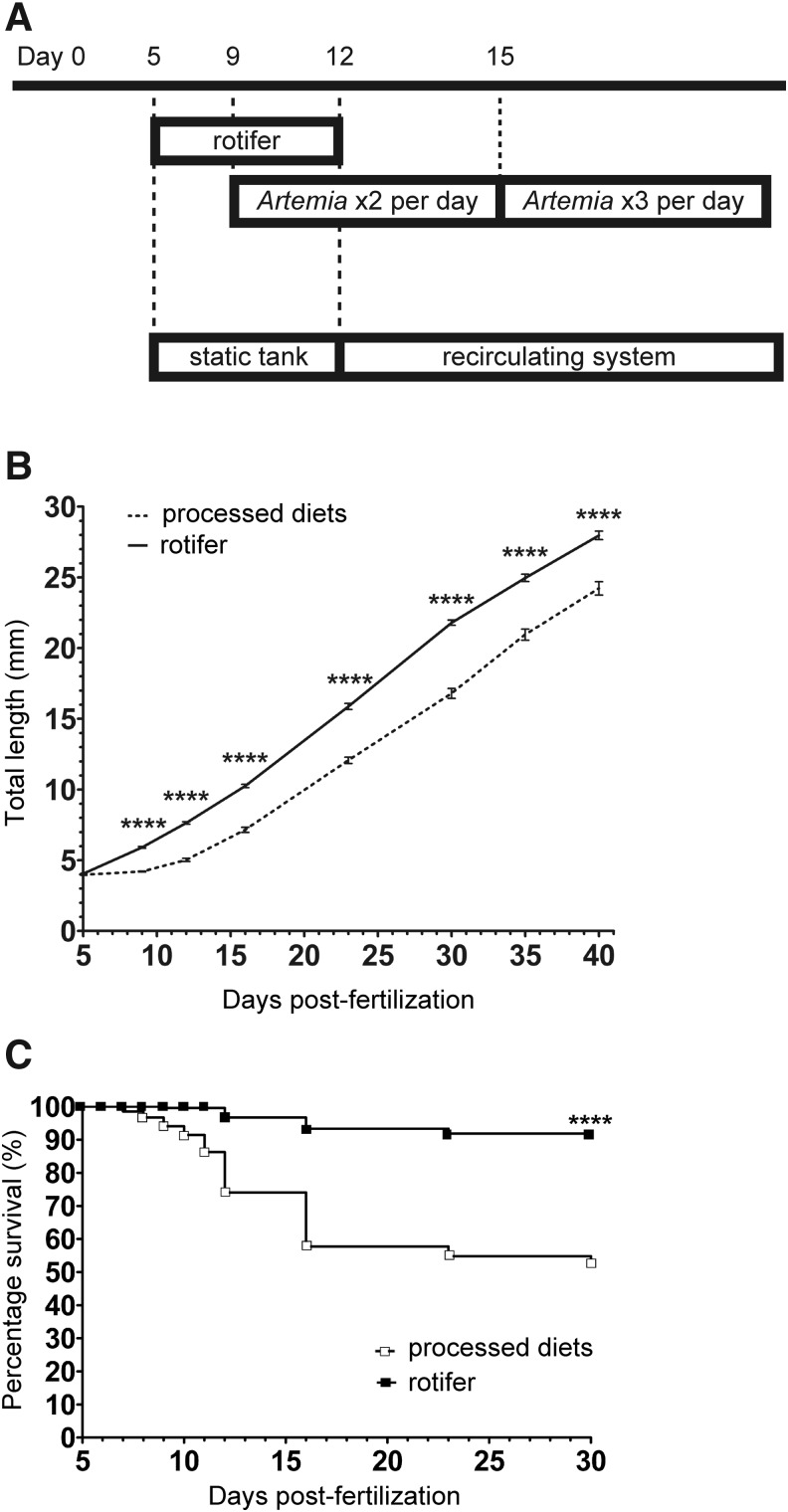
Based on our observations, we next revisited and designed a new feeding schedule using the freshwater rotifers and/or brine shrimp (Fig. 3A). At 5 dpf, 30 larval fish were moved to a nursery tank and fed freshwater rotifers. From 9 to 12 dpf, in addition to the rotifers, brine shrimp were provided twice daily. At 12 dpf, the larval fish were placed into a recirculating flow-through aquarium.
FIG. 3.
Feeding freshwater rotifers (B. calyciflorus) to zebrafish (Danio rerio) larvae results in higher growth and survival rates. **** indicates p<0.0001. (A) Schematic representation of the feeding schedule. (B) Growth curves of larval fish fed with the processed diet (dotted line) and with the freshwater rotifers (solid line). The data represent the mean±standard error of mean (SEM) (n=45 for both diets). (C) Kaplan–Meier survival curves of larval fish fed with the processed diet (open box, n=270) and with the freshwater rotifers (filled box, n=270).
Growth and survival
To examine any effects of the freshwater rotifers on larval growth, we conducted rearing experiments. One group of larvae was fed freshwater rotifers, whereas the other group was fed the processed control diet.16 The total body lengths of the larval fish in both groups were measured. Figure 3B clearly illustrates that from 9 to 40 dpf, rotifer-fed larval fish were significantly longer than the control-fed fish (freshwater rotifer diet vs. control diet: 9 dpf, 6.00±0.07 vs. 4.33±0.04 mm; 16 dpf, 10.40±0.12 vs. 7.36±0.18 mm; 30 dpf, 21.99±0.19 vs. 17.10±0.35 mm; and 40 dpf, 28.25±0.31 vs. 24.58±0.48 mm). From 5 to 12 dpf, the larvae fed with freshwater rotifers exhibited continuous larval growth in contrast to those fed with saltwater rotifers in previous studies (Fig. 3B and Supplementary Fig. S2).12 Our results indicate that freshwater rotifers are suitable as a first food for larval fish, and that first feeding is crucial for subsequent larval and juvenile growth in zebrafish. In addition to faster growth, the survival rate of the rotifer-fed fish (91.1%) was significantly higher than that of the control-fed fish (52.2%) (Fig. 3C).
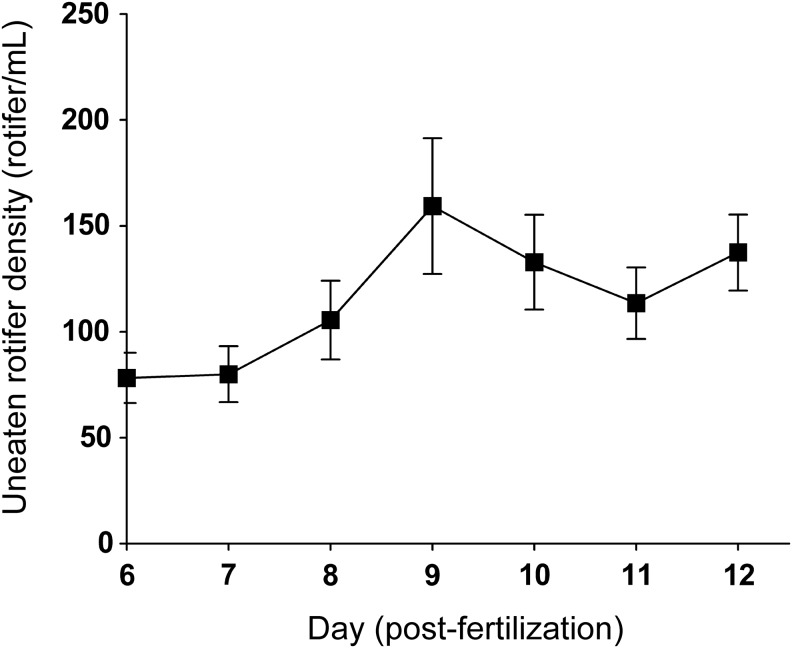
Rotifer densities
To examine if uneaten freshwater rotifers can survive to the following day after being placed in the nursery tanks, we determined the density of living freshwater rotifers in each static nursery tank before feeding rotifers during the rearing experiments described above. The starting density of freshwater rotifers was ∼275 rotifers/mL (i.e., we fed 5-dpf larvae housed in 200 mL of water ∼55,000 freshwater rotifers). On the next day, the average rotifer density in one tank dropped to 78.2±11.9/mL (mean±SEM; n=9; Fig. 4). Until larval fish were moved to the recirculating flow-through aquarium, average density of freshwater rotifers (per mL) on day 7, 8, 9, 10, 11, and 12 was 80.0±13.2, 105.6±18.6, 159.3±32.0, 132.9±22.4, 113.6±16.9, and 137.4±17.9 (mean±SEM), respectively. The results suggest that uneaten freshwater rotifers are able to survive until the next day, and that the amount of freshwater rotifers we used should be enough for 30 larval fish. We conclude, therefore, that freshwater rotifers are a beneficial and practical species to use as a first food for larval zebrafish in terms of growth and survival.
FIG. 4.
The density of uneaten freshwater rotifers (B. calyciflorus) in the static nursery tanks. The density of the rotifers was measured each day and plotted. The data represent the mean±SEM (n=9).
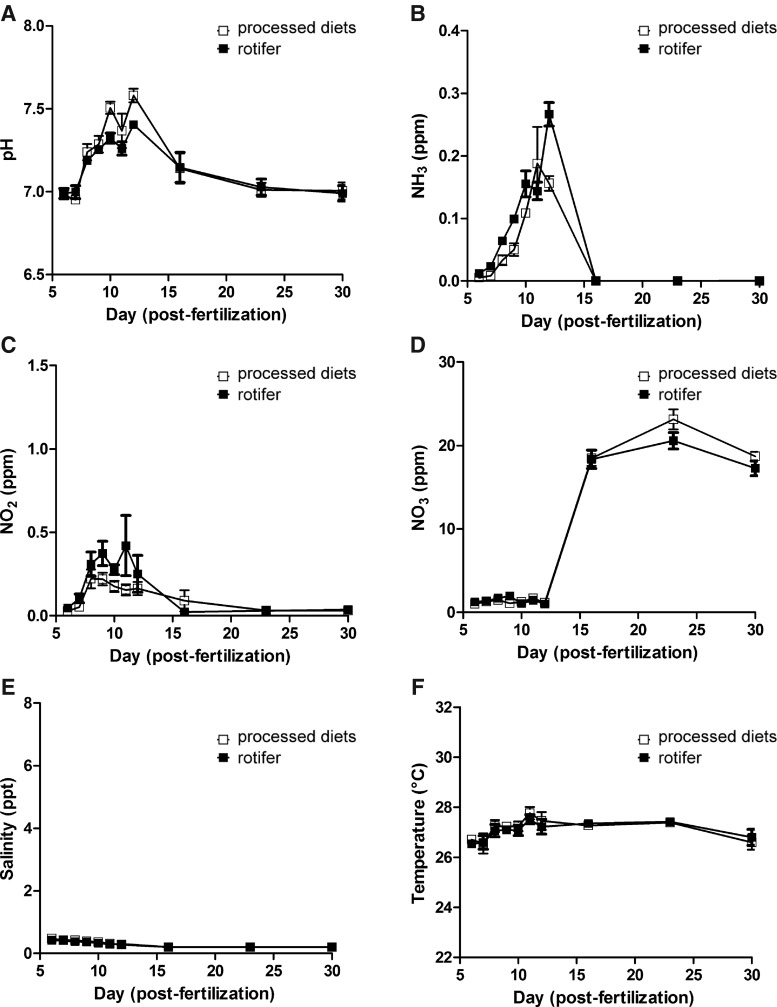
Water quality
Water quality is an important factor affecting the growth and survival of aquatic organisms. To better understand how water quality affects zebrafish larval growth and survival, we examined water quality in both the rotifer-fed tanks and the control-fed tanks during rearing experiments. We measured six characteristics of water based on previous studies with saltwater rotifers.12,13 Water pH increased from 7.0 to 7.6 in the nursery phase (from day 5 to 12) for both groups. In addition, the concentration of un-ionized ammonia increased up to 0.28 ppm during the same period (Fig. 5A, B). After transferring the fish to the recirculating flow-through aquarium, both parameters dropped to lower levels (pH to ∼7.0 and NH3 to 0 ppm) and stabilized. Unexpectedly, from day 6 to 9, the concentration of un-ionized ammonia in the rotifer-fed tank was significantly higher than that in the control-fed tank. After day 8, the concentrations of un-ionized ammonia in the tank fed with freshwater rotifers exceeded 0.05 ppm and reached 0.28±0.022 pm at day 12, a concentration that is generally believed to be toxic in cultured fish.12 However, none of the larval fish fed with freshwater rotifers died under these concentrations (Fig. 3C). In contrast, fish fed with the processed diets were found dead after day 8.
FIG. 5.
Characteristics of water quality during the rearing experiments: (A) pH, (B) NH3, (C) NO2, (D) NO3, (E) salinity, and (F) temperature. Open boxes indicate the results from tanks holding larval fish fed with processed food. Filled boxes indicate the results from tanks holding larval fish fed with freshwater rotifers (B. calyciflorus). Each water quality marker was measured at 6, 7, 8, 9, 10, 11, 12, 16, 23, and 30 days postfertilization. The data in the panels represent the mean±SEM (n=9 for both diets).
These results indicate that the cause of death observed in the processed diet condition may be starvation, not poor water quality. Nonetheless, the increasing concentration of un-ionized ammonia we observed implied that un-ionized ammonia was being produced from the unconsumed food in the tank. Given that we found uneaten, live, freshwater rotifers remaining in the nursery tank, it may be possible to prevent the increase of un-ionized ammonia by reducing the amount of freshwater rotifers given in one feeding, without any effect on larval fish growth and survival.
In addition to the increase of un-ionized ammonia, we also observed that nitrite concentrations increased during the nursery phase (Fig. 5C). However, in contrast to the concentrations of un-ionized ammonia and nitrite, nitrate concentrations did not drastically change during the same period (Fig. 5D). These findings suggested that there were few nitrifying bacteria present during this time, when the water in the tank was static. After being connected to the recirculating flow-through aquarium, nitrite and nitrate concentrations were constant and in the range considered normal.10
Water salinity was 0.2–0.6 ppt in both groups. Compared to the salinity from saltwater rotifers (∼5 ppt),12 water salinity in the present study was unsurprisingly lower and was within a range more consistent with that of a freshwater species.17 Temperature ranged from 25.2°C to 28.7°C during the rearing experiments.
Breeding trials
Sexual maturation in zebrafish correlates with body size (i.e., standard length [SL]), rather than age.18 Eaton and Farley reported that they observed the first spawning when the SL of male and female fish reached 22.0–24.0 mm and 24.0–26.0 mm, respectively.19 To ascertain when the rotifer-fed fish can begin to reproduce, the SL of the larval fish was measured (Fig. 6A). Regression analysis indicated that most male and female fish reached 24.9 mm SL at 42 dpf, suggesting that the fish should be ready to spawn fertilized eggs at that point in time. Consistent with this estimate, both male and female fish at 46 dpf exhibited sexually dimorphic, adult characteristics, such as differences in body color and body shape (Fig. 6B). We then investigated whether 47dpf zebrafish are indeed able to produce normal fertilized eggs by crossing male and female fish. We found that the 47-dpf fish succeeded in producing fertilized embryos that exhibited normal development (Fig. 6C). The results indicated that first spawning could happen as early as 47 dpf in the AB strain.
FIG. 6.
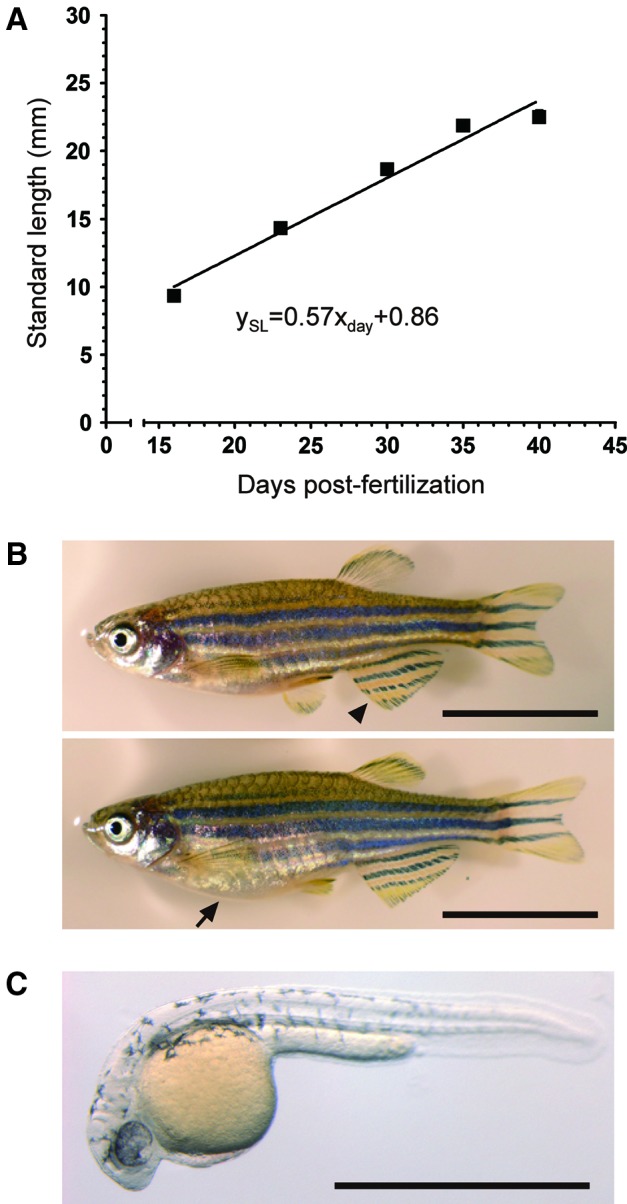
The onset of the first spawning is 47 days postfertilization (dpf). (A) Estimation of the sexual maturation of larval fish. The regression equation is displayed on the figure (R2=0.92). (B) A 46-dpf male (upper photograph) and female (lower photograph) zebrafish exhibiting sexually mature characteristics. The major secondary sex characteristic of a mature male fish is the body color, especially in the anal fin. The arrowhead in the upper photograph indicates the yellowish anal fin of the male compared with that of the female fish. The major sex characteristic of the female is a whiter body than that of the male fish. In addition, the larger belly (indicated by the arrow in the lower photograph) is also a typical female characteristic. The bar represents 10 mm. (C) One of the typical embryos from incross of 47-dpf zebrafish. The embryo exhibits the typical morphology at 1 dpf. The bar represents 1 mm. Color images available online at www.liebertpub.com/zeb
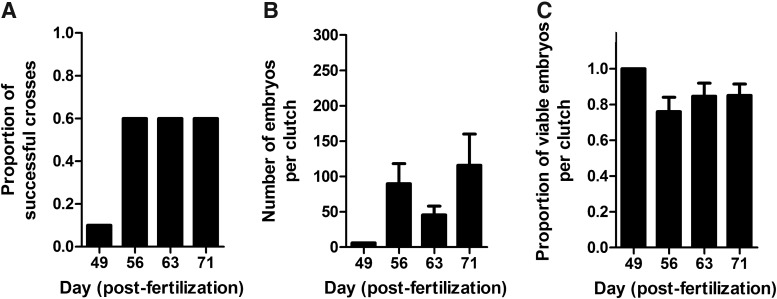
To determine when the fish can produce fertilized eggs routinely, we performed breeding trials at 49, 56, 63, and 71 dpf (Fig. 7). At 49 dpf, one pair out of 10 pairs produced six fertilized eggs (Fig. 7A, B). The six embryos developed normally at 1 dpf (Fig. 7C). At 56 dpf, 6 out of 10 pairs successfully produced normally developed fertilized eggs (Fig. 7A). In addition, the number of collected embryos (89.8±28.4, mean±SEM) increased (Fig. 7B). The survival rate of these embryos was 81.1% (Fig. 7C). At 63 and 71 dpf, success rate was constant at 60% (Fig. 7A). In addition, clutch size per day was 45.7±12.5 at 63 dpf or 115.7±44.3 at 71 dpf, respectively (Fig. 7B). The survival rate of these embryos was 84.7% at 63 dpf or 85.1% at 71 dpf, respectively (Fig. 7C). Based on the results, we believe that 56-dpf fish can successfully reproduce, and that zebrafish reared with our methods reach sexual maturity within 60 days across the board.
FIG. 7.
Sexual maturation of zebrafish (Danio rerio) within 60 days postfertilization (dpf). (A) The proportion of successful crosses in 10 pairs. (B) The number of collected embryos per clutch. (C) The proportion of viable embryos per clutch. At 49 dpf, one pair out of 10 pairs successfully produced six normally developed embryos. The data from the crosses at 56, 63, and 71 dpf represent the mean±SEM (n=6).
Discussion
In this study, we demonstrate that freshwater rotifers are suitable as feed for zebrafish and introduce a novel feeding strategy for rearing larval fish using freshwater rotifers and brine shrimp. Our choice of freshwater over saltwater rotifers offers an advantage. Larval fish are never exposed to high salt condition. With saltwater rotifers, larval fish must tolerate osmotic stress because saltwater rotifers cannot survive in freshwater. Thus, our method should be less stressful for zebrafish larvae.
We demonstrated our new feeding strategy with the freshwater rotifers. We combine freshwater rotifers and brine shrimp for 9–12 dpf larval fish. Fish at this stage occasionally vary in size, and the slightly smaller fish cannot physically ingest brine shrimp. Thus, our revised strategy allows smaller-than-average larval fish to have a better rate of survival when rearing zebrafish in the laboratory.
However, our proposed feeding strategy is not without challenges, particularly when we apply our method to larger fish facilities. Currently, we have two 10-L cultures of freshwater rotifers set up, which can feed at least 100 larval fish. Maintenance for cultures of this size is not difficult, requiring only the addition of concentrated Chlorella daily (∼10 min of labor). However, the density of freshwater rotifers in our cultures must be kept at ∼20 rotifers/mL, because a higher density sometimes causes sudden death in the rotifers.20 In addition, the density at which freshwater rotifers can be safely kept is very low compared to that of saltwater rotifers (>1000/mL).15 Thus, rotifer density imposes a clear limit on the number of larval fish we are able to raise at any one time. To overcome this limitation, we are designing a method that will allow us to keep highly concentrated cultures of freshwater rotifers. In preliminary tests, we succeeded in scaling up the rotifer culture to a volume of 80 L and collected one million rotifers every day (unpublished data). We are able to feed up to 600 larval fish per day with this amount. We should also note that because some of the freshwater rotifers survive in the nursery tank to the following day (Fig. 4), this suggests that we require far fewer rotifers than we have fed to obtain the same growth and survival of larvae. If that is indeed the case, one million rotifers might be enough for over 1000 larval fish. Further study will be needed to determine the most efficient amount of freshwater rotifers that will maintain high growth and survival in zebrafish larvae.
Our new method for rearing larvae facilitates larval fish to grow faster and survive better, shortening the generation time of zebrafish. Lawrence et al. discussed the generation time of zebrafish based on first egg production.13 In their report, fish reared with saltwater rotifers successfully laid fertilized eggs at 57 dpf, and thus zebrafish generation time was ∼60 days. Similar to their results, first eggs production we observed occurred at 47 dpf, and most of the 56-dpf fish seemed to be mature enough for regular usage for breeding in laboratories. We believe our method will advance genetic studies using zebrafish and improve the use of the zebrafish as a model organism.
Supplementary Material
Acknowledgments
The authors thank Mr. Satoshi Ansai for helpful suggestions and valuable discussions and Ms. Akari Inazuki for the maintenance of their fish facility. The authors also thank the members of their lab for critiques and comments. This work was supported by the Grant-in-Aid for Scientific Research (KAKENHI) for Young Scientists (A).
Disclosure Statement
No competing financial interests exist.
References
- 1.Driever W, Stemple D, Schier A, Solnicakrezel L. Zebrafish—genetic tools for studying vertebrate development. Trends Genet 1994;10:152–159 [DOI] [PubMed] [Google Scholar]
- 2.Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet 2007;8:353–367 [DOI] [PubMed] [Google Scholar]
- 3.Pickart MA, Klee EW. Zebrafish approaches enhance the translational research tackle box. Transl Res 2014;163:65–78 [DOI] [PubMed] [Google Scholar]
- 4.Huang P, Xiao A, Zhou MG, Zhu ZY, Lin S, Zhang B. Heritable gene targeting in zebrafish using customized TALENs. Nat Biotechnol 2011;29:699–700 [DOI] [PubMed] [Google Scholar]
- 5.Sander JD, Cade L, Khayter C, Reyon D, Peterson RT, Joung JK, et al. . Targeted gene disruption in somatic zebrafish cells using engineered TALENs. Nat Biotechnol 2011;29:697–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bedell VM, Wang Y, Campbell JM, Poshusta TL, Starker CG, Krug RG, et al. . In vivo genome editing using a high-efficiency TALEN system. Nature 2012;491:114–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hwang WY, Fu YF, Reyon D, Maeder ML, Tsai SQ, Sander JD, et al. . Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol 2013;31:227–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schilling TF: The morphology of larval and adult zebrafish. In: Zebrafish. Nüsslein-Volhard C. and Dahm R, (eds), pp. 59–94. Oxford University Press, New York, NY, 2002 [Google Scholar]
- 9.Sprague J, Bayraktaroglu L, Clements D, Conlin T, Fashena D, Frazer K, et al. . The Zebrafish Information Network: the zebrafish model organism database. Nucleic Acids Res 2006;34:D581–D585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harper C, Lawrence C: Husbandry. In: The Laboratory Zebrafish. Harper C. and Lawrence C, (eds), pp. 27–84, CRC Press, Boca Raton, FL, 2010 [Google Scholar]
- 11.Danielsen ET, Moeller ME, Rewitz KF. Nutrient signaling and developmental timing of maturation. Curr Top Dev Biol 2013;105:37–67 [DOI] [PubMed] [Google Scholar]
- 12.Best J, Adatto I, Cockington J, James A, Lawrence C. A novel method for rearing first-feeding larval zebrafish: polyculture with Type L saltwater rotifers (Brachionus plicatilis). Zebrafish 2010;7:289–295 [DOI] [PubMed] [Google Scholar]
- 13.Lawrence C, Adatto I, Best J, James A, Maloney K. Generation time of zebrafish (Danio rerio) and medakas (Oryzias latipes) housed in the same aquaculture facility. Lab Animal 2012;41:158–165 [DOI] [PubMed] [Google Scholar]
- 14.Hagiwara A, Suga K, Akazawa A, Kotani T, Sakakura Y. Development of rotifer strains with useful traits for rearing fish larvae. Aquaculture 2007;268:44–52 [Google Scholar]
- 15.Lawrence C, Sanders E, Henry E. Methods for culturing saltwater rotifers (Brachionus plicatilis) for rearing larval zebrafish. Zebrafish 2012;9:140–146 [DOI] [PubMed] [Google Scholar]
- 16.Carvalho AP, Araujo L, Santos MM. Rearing zebrafish (Danio rerio) larvae without live food: evaluation of a commercial, a practical and a purified starter diet on larval performance. Aquac Res 2006;37:1107–1111 [Google Scholar]
- 17.Lawrence C. The husbandry of zebrafish (Danio rerio): a review. Aquqculture 2007;269:1–20 [Google Scholar]
- 18.Spence R, Gerlach G, Lawrence C, Smith C. The behaviour and ecology of the zebrafish, Danio rerio. Biol Rev 2008;83:13–34 [DOI] [PubMed] [Google Scholar]
- 19.Eaton RC, Farley RD. Growth and reduction of depensation of the zebrafish Brachydanio rerio, reared in the laboratory. Copeia 1974;1974:204–209 [Google Scholar]
- 20.Arimoro FO. Culture of the freshwater rotifer, Brachionus calyciflorus, and its application in fish larviculture technology. Afr J Biotechnol 2006;5:536–541 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.