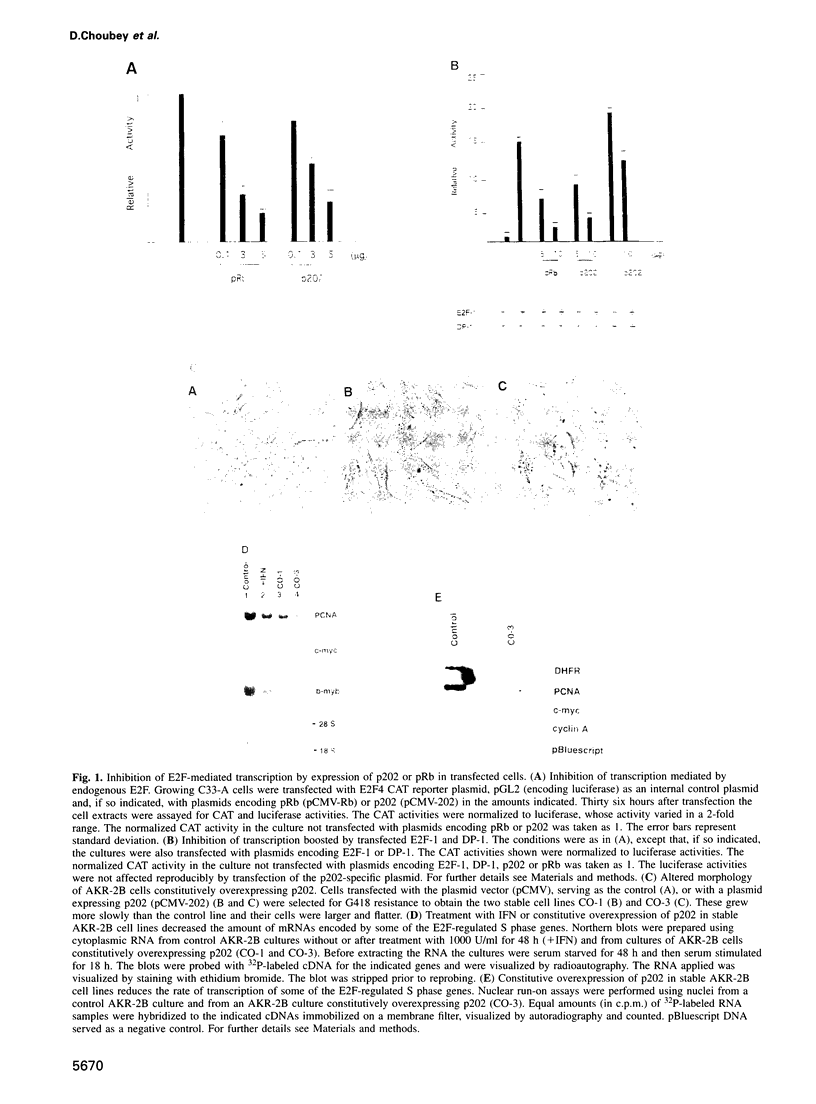
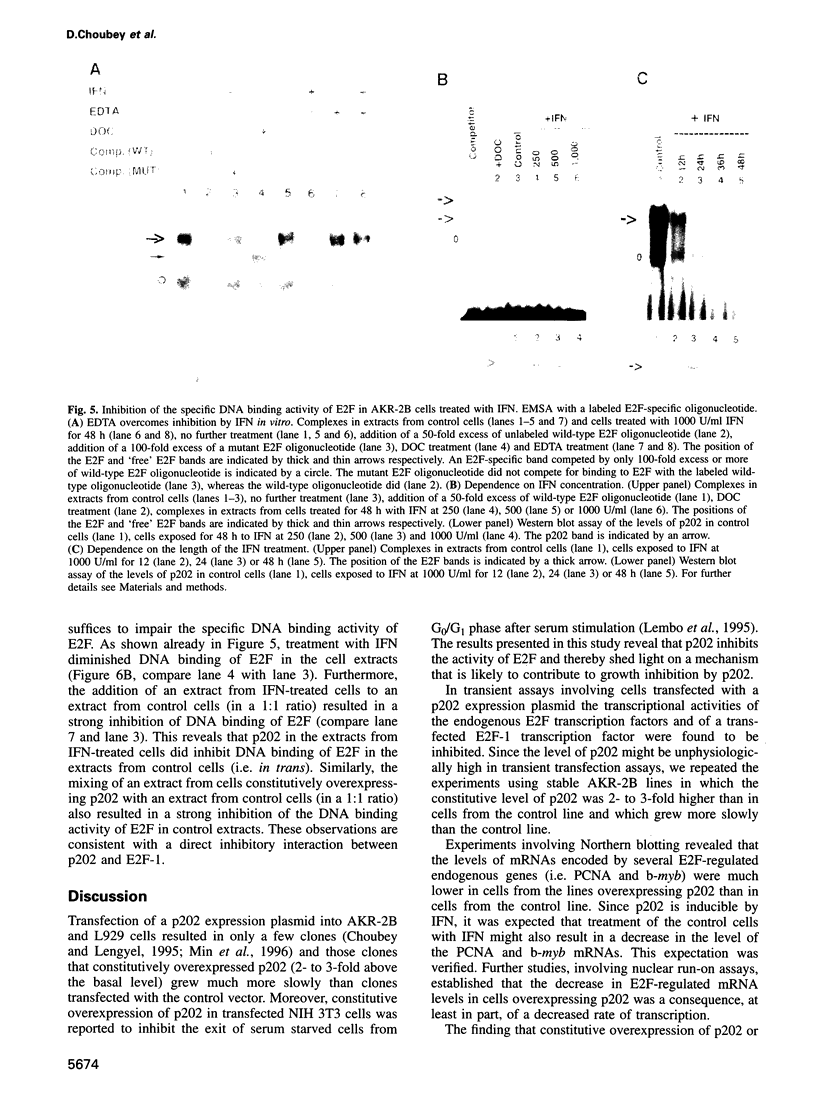
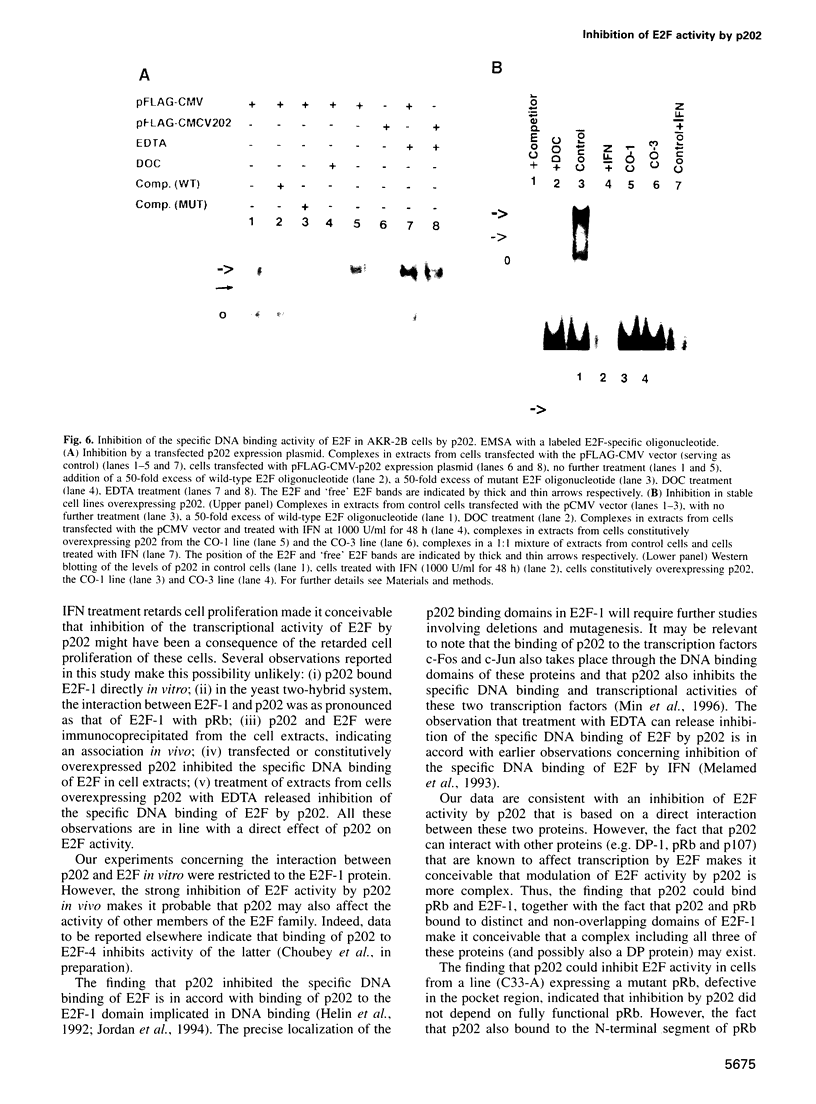
Abstract
Many of the antimicrobial, immunomodulatory and cell growth inhibitory activities of the interferons are mediated by interferon-inducible proteins. Earlier we characterized an interferon-inducible murine protein, p202, whose expression in transfected cells inhibits cell proliferation and which can form a complex with retinoblastoma protein (pRb). Here we report that in transfected cells expression of p202 inhibits E2F-stimulated transcription of a reporter gene and of endogenous genes. Inhibition of the transcriptional activity of E2F by p202 does not depend on fully functional pRb and is correlated with inhibition of the sequence-specific DNA binding of E2F. p202 interacts with the transcription factor E2F (E2F-1/DP-1) in vitro and in vivo. Inhibition of E2F activity by p202 may contribute to growth inhibition by the interferons.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. D., Kaelin W. G., Jr Transcriptional control by E2F. Semin Cancer Biol. 1995 Apr;6(2):99–108. doi: 10.1006/scbi.1995.0013. [DOI] [PubMed] [Google Scholar]
- Bagchi S., Raychaudhuri P., Nevins J. R. Adenovirus E1A proteins can dissociate heteromeric complexes involving the E2F transcription factor: a novel mechanism for E1A trans-activation. Cell. 1990 Aug 24;62(4):659–669. doi: 10.1016/0092-8674(90)90112-r. [DOI] [PubMed] [Google Scholar]
- Bagchi S., Weinmann R., Raychaudhuri P. The retinoblastoma protein copurifies with E2F-I, an E1A-regulated inhibitor of the transcription factor E2F. Cell. 1991 Jun 14;65(6):1063–1072. doi: 10.1016/0092-8674(91)90558-g. [DOI] [PubMed] [Google Scholar]
- Bandara L. R., Buck V. M., Zamanian M., Johnston L. H., La Thangue N. B. Functional synergy between DP-1 and E2F-1 in the cell cycle-regulating transcription factor DRTF1/E2F. EMBO J. 1993 Nov;12(11):4317–4324. doi: 10.1002/j.1460-2075.1993.tb06116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandara L. R., La Thangue N. B. Adenovirus E1a prevents the retinoblastoma gene product from complexing with a cellular transcription factor. Nature. 1991 Jun 6;351(6326):494–497. doi: 10.1038/351494a0. [DOI] [PubMed] [Google Scholar]
- Beijersbergen R. L., Carlée L., Kerkhoven R. M., Bernards R. Regulation of the retinoblastoma protein-related p107 by G1 cyclin complexes. Genes Dev. 1995 Jun 1;9(11):1340–1353. doi: 10.1101/gad.9.11.1340. [DOI] [PubMed] [Google Scholar]
- Beijersbergen R. L., Kerkhoven R. M., Zhu L., Carlée L., Voorhoeve P. M., Bernards R. E2F-4, a new member of the E2F gene family, has oncogenic activity and associates with p107 in vivo. Genes Dev. 1994 Nov 15;8(22):2680–2690. doi: 10.1101/gad.8.22.2680. [DOI] [PubMed] [Google Scholar]
- Blake M. C., Azizkhan J. C. Transcription factor E2F is required for efficient expression of the hamster dihydrofolate reductase gene in vitro and in vivo. Mol Cell Biol. 1989 Nov;9(11):4994–5002. doi: 10.1128/mcb.9.11.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappan S. P., Hiebert S., Mudryj M., Horowitz J. M., Nevins J. R. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991 Jun 14;65(6):1053–1061. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- Chittenden T., Livingston D. M., Kaelin W. G., Jr The T/E1A-binding domain of the retinoblastoma product can interact selectively with a sequence-specific DNA-binding protein. Cell. 1991 Jun 14;65(6):1073–1082. doi: 10.1016/0092-8674(91)90559-h. [DOI] [PubMed] [Google Scholar]
- Choubey D., Gutterman J. U. The interferon-inducible growth-inhibitory p202 protein: DNA binding properties and identification of a DNA binding domain. Biochem Biophys Res Commun. 1996 Apr 16;221(2):396–401. doi: 10.1006/bbrc.1996.0607. [DOI] [PubMed] [Google Scholar]
- Choubey D., Lengyel P. Binding of an interferon-inducible protein (p202) to the retinoblastoma protein. J Biol Chem. 1995 Mar 17;270(11):6134–6140. doi: 10.1074/jbc.270.11.6134. [DOI] [PubMed] [Google Scholar]
- Choubey D., Lengyel P. Interferon action: cytoplasmic and nuclear localization of the interferon-inducible 52-kD protein that is encoded by the Ifi 200 gene from the gene 200 cluster. J Interferon Res. 1993 Feb;13(1):43–52. doi: 10.1089/jir.1993.13.43. [DOI] [PubMed] [Google Scholar]
- Choubey D., Lengyel P. Interferon action: nucleolar and nucleoplasmic localization of the interferon-inducible 72-kD protein that is encoded by the Ifi 204 gene from the gene 200 cluster. J Cell Biol. 1992 Mar;116(6):1333–1341. doi: 10.1083/jcb.116.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choubey D., Snoddy J., Chaturvedi V., Toniato E., Opdenakker G., Thakur A., Samanta H., Engel D. A., Lengyel P. Interferons as gene activators. Indications for repeated gene duplication during the evolution of a cluster of interferon-activatable genes on murine chromosome 1. J Biol Chem. 1989 Oct 15;264(29):17182–17189. [PubMed] [Google Scholar]
- Cobrinik D., Whyte P., Peeper D. S., Jacks T., Weinberg R. A. Cell cycle-specific association of E2F with the p130 E1A-binding protein. Genes Dev. 1993 Dec;7(12A):2392–2404. doi: 10.1101/gad.7.12a.2392. [DOI] [PubMed] [Google Scholar]
- Dalton S. Cell cycle regulation of the human cdc2 gene. EMBO J. 1992 May;11(5):1797–1804. doi: 10.1002/j.1460-2075.1992.tb05231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGregori J., Kowalik T., Nevins J. R. Cellular targets for activation by the E2F1 transcription factor include DNA synthesis- and G1/S-regulatory genes. Mol Cell Biol. 1995 Aug;15(8):4215–4224. doi: 10.1128/mcb.15.8.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynlacht B. D., Flores O., Lees J. A., Harlow E. Differential regulation of E2F transactivation by cyclin/cdk2 complexes. Genes Dev. 1994 Aug 1;8(15):1772–1786. doi: 10.1101/gad.8.15.1772. [DOI] [PubMed] [Google Scholar]
- Field S. J., Tsai F. Y., Kuo F., Zubiaga A. M., Kaelin W. G., Jr, Livingston D. M., Orkin S. H., Greenberg M. E. E2F-1 functions in mice to promote apoptosis and suppress proliferation. Cell. 1996 May 17;85(4):549–561. doi: 10.1016/s0092-8674(00)81255-6. [DOI] [PubMed] [Google Scholar]
- Flemington E. K., Speck S. H., Kaelin W. G., Jr E2F-1-mediated transactivation is inhibited by complex formation with the retinoblastoma susceptibility gene product. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):6914–6918. doi: 10.1073/pnas.90.15.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg D., Vairo G., Chittenden T., Xiao Z. X., Xu G., Wydner K. L., DeCaprio J. A., Lawrence J. B., Livingston D. M. E2F-4, a new member of the E2F transcription factor family, interacts with p107. Genes Dev. 1994 Nov 15;8(22):2665–2679. doi: 10.1101/gad.8.22.2665. [DOI] [PubMed] [Google Scholar]
- Gutterman J. U. Cytokine therapeutics: lessons from interferon alpha. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1198–1205. doi: 10.1073/pnas.91.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel P. A., Gill R. M., Phillips R. A., Gallie B. L. Transcriptional repression of the E2-containing promoters EIIaE, c-myc, and RB1 by the product of the RB1 gene. Mol Cell Biol. 1992 Aug;12(8):3431–3438. doi: 10.1128/mcb.12.8.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helin K., Lees J. A., Vidal M., Dyson N., Harlow E., Fattaey A. A cDNA encoding a pRB-binding protein with properties of the transcription factor E2F. Cell. 1992 Jul 24;70(2):337–350. doi: 10.1016/0092-8674(92)90107-n. [DOI] [PubMed] [Google Scholar]
- Helin K., Wu C. L., Fattaey A. R., Lees J. A., Dynlacht B. D., Ngwu C., Harlow E. Heterodimerization of the transcription factors E2F-1 and DP-1 leads to cooperative trans-activation. Genes Dev. 1993 Oct;7(10):1850–1861. doi: 10.1101/gad.7.10.1850. [DOI] [PubMed] [Google Scholar]
- Hiebert S. W., Chellappan S. P., Horowitz J. M., Nevins J. R. The interaction of RB with E2F coincides with an inhibition of the transcriptional activity of E2F. Genes Dev. 1992 Feb;6(2):177–185. doi: 10.1101/gad.6.2.177. [DOI] [PubMed] [Google Scholar]
- Hinds P. W., Mittnacht S., Dulic V., Arnold A., Reed S. I., Weinberg R. A. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992 Sep 18;70(6):993–1006. doi: 10.1016/0092-8674(92)90249-c. [DOI] [PubMed] [Google Scholar]
- Hollenberg S. M., Sternglanz R., Cheng P. F., Weintraub H. Identification of a new family of tissue-specific basic helix-loop-helix proteins with a two-hybrid system. Mol Cell Biol. 1995 Jul;15(7):3813–3822. doi: 10.1128/mcb.15.7.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivey-Hoyle M., Conroy R., Huber H. E., Goodhart P. J., Oliff A., Heimbrook D. C. Cloning and characterization of E2F-2, a novel protein with the biochemical properties of transcription factor E2F. Mol Cell Biol. 1993 Dec;13(12):7802–7812. doi: 10.1128/mcb.13.12.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. G., Ohtani K., Nevins J. R. Autoregulatory control of E2F1 expression in response to positive and negative regulators of cell cycle progression. Genes Dev. 1994 Jul 1;8(13):1514–1525. doi: 10.1101/gad.8.13.1514. [DOI] [PubMed] [Google Scholar]
- Johnson D. G., Schwarz J. K., Cress W. D., Nevins J. R. Expression of transcription factor E2F1 induces quiescent cells to enter S phase. Nature. 1993 Sep 23;365(6444):349–352. doi: 10.1038/365349a0. [DOI] [PubMed] [Google Scholar]
- Jooss K., Lam E. W., Bybee A., Girling R., Müller R., La Thangue N. B. Proto-oncogenic properties of the DP family of proteins. Oncogene. 1995 Apr 20;10(8):1529–1536. [PubMed] [Google Scholar]
- Jordan K. L., Haas A. R., Logan T. J., Hall D. J. Detailed analysis of the basic domain of the E2F1 transcription factor indicates that it is unique among bHLH proteins. Oncogene. 1994 Apr;9(4):1177–1185. [PubMed] [Google Scholar]
- Kaelin W. G., Jr, Krek W., Sellers W. R., DeCaprio J. A., Ajchenbaum F., Fuchs C. S., Chittenden T., Li Y., Farnham P. J., Blanar M. A. Expression cloning of a cDNA encoding a retinoblastoma-binding protein with E2F-like properties. Cell. 1992 Jul 24;70(2):351–364. doi: 10.1016/0092-8674(92)90108-o. [DOI] [PubMed] [Google Scholar]
- Kato J., Matsushime H., Hiebert S. W., Ewen M. E., Sherr C. J. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993 Mar;7(3):331–342. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- Kovesdi I., Reichel R., Nevins J. R. Identification of a cellular transcription factor involved in E1A trans-activation. Cell. 1986 Apr 25;45(2):219–228. doi: 10.1016/0092-8674(86)90386-7. [DOI] [PubMed] [Google Scholar]
- Krek W., Ewen M. E., Shirodkar S., Arany Z., Kaelin W. G., Jr, Livingston D. M. Negative regulation of the growth-promoting transcription factor E2F-1 by a stably bound cyclin A-dependent protein kinase. Cell. 1994 Jul 15;78(1):161–172. doi: 10.1016/0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- Krek W., Livingston D. M., Shirodkar S. Binding to DNA and the retinoblastoma gene product promoted by complex formation of different E2F family members. Science. 1993 Dec 3;262(5139):1557–1560. doi: 10.1126/science.8248803. [DOI] [PubMed] [Google Scholar]
- La Thangue N. B. DRTF1/E2F: an expanding family of heterodimeric transcription factors implicated in cell-cycle control. Trends Biochem Sci. 1994 Mar;19(3):108–114. doi: 10.1016/0968-0004(94)90202-x. [DOI] [PubMed] [Google Scholar]
- Lees J. A., Saito M., Vidal M., Valentine M., Look T., Harlow E., Dyson N., Helin K. The retinoblastoma protein binds to a family of E2F transcription factors. Mol Cell Biol. 1993 Dec;13(12):7813–7825. doi: 10.1128/mcb.13.12.7813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembo D., Angeretti A., Benefazio S., Hertel L., Gariglio M., Novelli F., Landolfo S. Constitutive expression of the interferon-inducible protein p202 in NIH 3T3 cells affects cell cycle progression. J Biol Regul Homeost Agents. 1995 Apr-Jun;9(2):42–46. [PubMed] [Google Scholar]
- Martin K., Trouche D., Hagemeier C., Sørensen T. S., La Thangue N. B., Kouzarides T. Stimulation of E2F1/DP1 transcriptional activity by MDM2 oncoprotein. Nature. 1995 Jun 22;375(6533):691–694. doi: 10.1038/375691a0. [DOI] [PubMed] [Google Scholar]
- Melamed D., Tiefenbrun N., Yarden A., Kimchi A. Interferons and interleukin-6 suppress the DNA-binding activity of E2F in growth-sensitive hematopoietic cells. Mol Cell Biol. 1993 Sep;13(9):5255–5265. doi: 10.1128/mcb.13.9.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min W., Ghosh S., Lengyel P. The interferon-inducible p202 protein as a modulator of transcription: inhibition of NF-kappa B, c-Fos, and c-Jun activities. Mol Cell Biol. 1996 Jan;16(1):359–368. doi: 10.1128/mcb.16.1.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenarh M., Quaiser A. Two different protein-protein interactions in oligomeric complexes of SV40 large T antigen with the cellular oncoprotein p53. Oncogene. 1989 Mar;4(3):379–382. [PubMed] [Google Scholar]
- Nevins J. R. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992 Oct 16;258(5081):424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- O'Connor D. J., Lam E. W., Griffin S., Zhong S., Leighton L. C., Burbidge S. A., Lu X. Physical and functional interactions between p53 and cell cycle co-operating transcription factors, E2F1 and DP1. EMBO J. 1995 Dec 15;14(24):6184–6192. doi: 10.1002/j.1460-2075.1995.tb00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormondroyd E., de la Luna S., La Thangue N. B. A new member of the DP family, DP-3, with distinct protein products suggests a regulatory role for alternative splicing in the cell cycle transcription factor DRTF1/E2F. Oncogene. 1995 Oct 19;11(8):1437–1446. [PubMed] [Google Scholar]
- Sellers W. R., Rodgers J. W., Kaelin W. G., Jr A potent transrepression domain in the retinoblastoma protein induces a cell cycle arrest when bound to E2F sites. Proc Natl Acad Sci U S A. 1995 Dec 5;92(25):11544–11548. doi: 10.1073/pnas.92.25.11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen G. C., Lengyel P. The interferon system. A bird's eye view of its biochemistry. J Biol Chem. 1992 Mar 15;267(8):5017–5020. [PubMed] [Google Scholar]
- Shan B., Zhu X., Chen P. L., Durfee T., Yang Y., Sharp D., Lee W. H. Molecular cloning of cellular genes encoding retinoblastoma-associated proteins: identification of a gene with properties of the transcription factor E2F. Mol Cell Biol. 1992 Dec;12(12):5620–5631. doi: 10.1128/mcb.12.12.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin E. K., Tevosian S. G., Yee A. S. The N-terminal region of E2F-1 is required for transcriptional activation of a new class of target promoter. J Biol Chem. 1996 May 24;271(21):12261–12268. doi: 10.1074/jbc.271.21.12261. [DOI] [PubMed] [Google Scholar]
- Singh P., Wong S. H., Hong W. Overexpression of E2F-1 in rat embryo fibroblasts leads to neoplastic transformation. EMBO J. 1994 Jul 15;13(14):3329–3338. doi: 10.1002/j.1460-2075.1994.tb06635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. J., Nevins J. R. The Rb-related p107 protein can suppress E2F function independently of binding to cyclin A/cdk2. Mol Cell Biol. 1995 Jan;15(1):338–344. doi: 10.1128/mcb.15.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalmeier K., Synovzik H., Mertz R., Winnacker E. L., Lipp M. Nuclear factor E2F mediates basic transcription and trans-activation by E1a of the human MYC promoter. Genes Dev. 1989 Apr;3(4):527–536. doi: 10.1101/gad.3.4.527. [DOI] [PubMed] [Google Scholar]
- Vojtek A. B., Hollenberg S. M., Cooper J. A. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993 Jul 16;74(1):205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- Weber H., Valenzuela D., Lujber G., Gubler M., Weissmann C. Single amino acid changes that render human IFN-alpha 2 biologically active on mouse cells. EMBO J. 1987 Mar;6(3):591–598. doi: 10.1002/j.1460-2075.1987.tb04795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A. E2F and cell proliferation: a world turned upside down. Cell. 1996 May 17;85(4):457–459. doi: 10.1016/s0092-8674(00)81244-1. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. The retinoblastoma protein and cell cycle control. Cell. 1995 May 5;81(3):323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- Weintraub S. J., Prater C. A., Dean D. C. Retinoblastoma protein switches the E2F site from positive to negative element. Nature. 1992 Jul 16;358(6383):259–261. doi: 10.1038/358259a0. [DOI] [PubMed] [Google Scholar]
- Xu G., Livingston D. M., Krek W. Multiple members of the E2F transcription factor family are the products of oncogenes. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1357–1361. doi: 10.1073/pnas.92.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Sheppard K. A., Peng C. Y., Yee A. S., Piwnica-Worms H. Cyclin A/CDK2 binds directly to E2F-1 and inhibits the DNA-binding activity of E2F-1/DP-1 by phosphorylation. Mol Cell Biol. 1994 Dec;14(12):8420–8431. doi: 10.1128/mcb.14.12.8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki L., Jacks T., Bronson R., Goillot E., Harlow E., Dyson N. J. Tumor induction and tissue atrophy in mice lacking E2F-1. Cell. 1996 May 17;85(4):537–548. doi: 10.1016/s0092-8674(00)81254-4. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]