Abstract
Background and Purpose
Angiotensin AT1 receptor antagonists induce weight loss; however, the mechanism underlying this phenomenon is unknown. The Mas receptor agonist angiotensin-(1-7) is a metabolite of angiotensin I and of angiotensin II. As an agonist of Mas receptors, angiotensin-(1-7) has beneficial cardiovascular and metabolic effects.
Experimental Approach
We investigated the anti-obesity effects of transgenically overexpressed angiotensin-(1-7) in rats. We secondly examined whether weight loss due to telmisartan (8 mg·kg−1·d−1) in diet-induced obese Sprague Dawley (SD) rats can be blocked when the animals were co-treated with the Mas receptor antagonist A779 (24 or 72 μg·kg−1·d−1).
Key Results
In contrast to wild-type controls, transgenic rats overexpressing angiotensin-(1-7) had 1.) diminished body weight when they were regularly fed with chow; 2.) were protected from developing obesity although they were fed with cafeteria diet (CD); 3.) showed a reduced energy intake that was mainly related to a lower CD intake; 5.) remained responsive to leptin despite chronic CD feeding; 6.) had a higher, strain-dependent energy expenditure, and 7.) were protected from developing insulin resistance despite CD feeding. Telmisartan-induced weight loss in SD rats was partially antagonized after a high, but not a low dose of A779.
Conclusions and Implications
Angiotensin-(1-7) regulated food intake and body weight and contributed to the weight loss after AT1 receptor blockade. Angiotensin-(1-7)-like agonists may be drug candidates for treating obesity.
Tables of Links
| TARGETS |
|---|
| GPCRsa |
| Angiotensin AT1 receptor |
| Mas (MAS1) receptor |
| Enzymesb |
| ACE, angiotensin converting enzyme |
| ACE2, angiotensin converting enzyme 2 |
| LIGANDS |
|---|
| A779 |
| AgRP, Agouti related protein |
| CRH, corticotrophin-releasing hormone |
| Leptin |
| MCH, melanin concentrating hormone |
| NPY, neuropeptide Y |
| Telmisartan |
These tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,bAlexander et al., 2013a,b).
Introduction
Different antagonists of angiotensin AT1 receptors, such as telmisartan (Schupp et al., 2005; He et al., 2010; Miesel et al., 2012; Müller-Fielitz et al., 2012a; 2014; 2015,,), candesartan (Zorad et al., 2006; Müller-Fielitz et al., 2011; 2012a,) or olmesartan (Vazquez-Medina et al., 2013) reduce body weight in experimental studies. Weight loss was mostly found after high doses of AT1 receptor antagonists and was not related to a reduction in BP (Müller-Fielitz et al., 2011; 2014,). Weight loss was also seen in patients during irbesartan therapy (Kintscher et al., 2007).
A leptin-dependent mechanism is thought to be mechanistically involved as AT1 receptor antagonists failed to reduce body weight in animals in which leptin signalling was impaired (Müller-Fielitz et al., 2011). Furthermore, leptin sensitivity was restored by treatment with AT1 receptor antagonists (Müller-Fielitz et al., 2014; 2015,). A hypothalamic-pituitary-adrenal (HPA) axis-dependent mechanism seems conceivable since palatable food intake is increased to compensate for stress (Dallman et al., 2004) and treatment with AT1 receptor antagonists was shown to lessen HPA activity in parallel to a reduced intake of high-calorie and palatable food (Miesel et al., 2012; Müller-Fielitz et al., 2012a). In addition to these potential mechanisms, the AT1 receptor antagonist telmisartan prevented weight gain independently of food intake by activating the PPARδ-dependent pathways (He et al., 2010). A PPARγ-related mechanism accounting for weight reduction after AT1 receptor antagonists can definitely be excluded because typical PPARγ agonists such as the thiazolidinediones tended to increase body weight, food intake, fat quantity, and adipocyte size in rats and humans (de Souza et al., 2001; Larsen et al., 2003).
Nevertheless, the underlying mechanisms still remain a matter of debate due to the finding that angiotensin II (AngII) itself prevented weight gain (Müller-Fielitz et al., 2012b) or even to induce weight loss (Cabassi et al., 2005; Song et al., 2005; Zhang et al., 2009; Tabony et al., 2014). AngII-dependent weight loss has been attributed to (i) lipolysis and thermogenesis due to its stimulation of the sympathetic outflow to adipose tissue (Engeli et al., 2000; Cabassi et al., 2005; King et al., 2013), (ii) to decreased food intake (Brink et al., 1996; Yoshida et al., 2012) because it stimulated secretion of leptin from adipocytes (Skurk et al., 2005) and to suppression of the hypothalamic expression of neuropeptide Y (NPY) and orexin (Yoshida et al., 2012) and (iii) to muscle atrophy (Kadoguchi et al., 2015). The last effect has been related to multiple mechanisms, including activation of the ubiquitin proteasome pathway, inhibition of the insulin/IGF-1/Akt/mammalian target of rapamycin axis, activation of apoptosis and mitochondrial dysfunction (Song et al., 2005; Zhang et al., 2009; Tabony et al., 2014). The absence of AT1 receptors in skeletal muscle and the ability of AngII to increase cytokines provide strong evidence for an indirect mechanism for muscle wasting (Zhang et al., 2009).
The consensual effects of AngII and AT1 receptor antagonists raise the question of whether AT1-independent mechanisms contribute to the prevention of weight gain after these antagonists, especially considering that diet-induced obesity was not observed after telmisartan in AT1 receptor-deficient mice (Rong et al., 2010). We aimed in this study to investigate whether the endogenous AngII metabolite, angiotensin-(1–7) [Ang(1–7)] was involved in the prevention of weight gain following treatment with AT1 receptor antagonists. This hypothesis is also based on observations that not only AngII (Müller-Fielitz et al., 2012a; 2014,) but also Ang(1–7) is increased during AT1 receptor blockade (Ishiyama et al., 2004; Igase et al., 2005). It is widely accepted that Ang(1–7) (i) is cleaved by ACE2 from AngII and/or AngI by ACE and the neutral endopeptidase (Santos et al., 2012a); (ii) has cardiovascular functions, mostly showing effects opposing those of AngII (Bader, 2013; Santos, 2014); and (iii) acts via the GPCR Mas (Santos et al., 2012a). In addition to its cardiovascular effects, Ang(1–7) also beneficially affects metabolic parameters such as triglycerides and lipids (Santos et al., 2010; Silva et al., 2013), insulin sensitivity and glucose homeostasis (Santos et al., 2008; 2014,; Giani et al., 2009; 2012,; Liu et al., 2012). In the context of Ang(1–7) regulating weight, the abdominal fat mass was increased in Mas-deficient mice (Santos et al., 2008) and the captopril-induced prevention of weight gain was abolished by the Mas receptor antagonist A779 (Oh et al., 2012). Thus, we designed our study by particularly addressing the following hypotheses: (i) the development of diet-induced obesity can be prevented by Ang(1–7) and (ii) the prevention of weight gain by AT1 receptor antagonists can at least partly be attributed to an Ang(1–7)-dependent mechanism. To pursue our first hypothesis, we used the transgenic rat model TGR(A1-7)3292, which shows testicular-specific expression of a cytomegalovirus promoter-driven transgene, doubling the circulating levels of Ang(1–7), compared with non-transgenic control rats (Santos et al., 2004), and fed them with either standard chow or a cafeteria diet (CD). To investigate the second hypothesis, CD-fed Sprague-Dawley (SD) rats were treated with telmisartan plus A779, while controls only received telmisartan or vehicle.
Methods
Animals
All animal care and experimental procedures were conducted in accordance with the NIH guidelines for the care and use of laboratory animals and approved by the animal ethics committee of the local regulatory authority. The results of all studies involving animals are reported in accordance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath et al., 2010). A total of 112 animals were used in the experiments described here.
Male TGR(A1-7)3292 rats [referred to hereafter as transgenic (TG) rats, Max Delbrück Center for Molecular Medicine, Berlin, Germany] and SD rats (Saint Berthevin, Cedex, France) were used in the experiments. The animals were kept at room temperature with a 12 h/12 h dark (2:00 a.m.–2:00 p.m.)/light (2:00 p.m.–2:00 a.m.) cycle. The body weights of the rats and their food and water intakes were monitored by daily weighing at 2:00 p.m. at the beginning of the light cycle.
Feeding and treatment protocols
Protocol 1
At the age of 4 weeks, rats arrived in the laboratory and were kept in pairs. After a 2 week habituation period, TG and SD rats were randomized to one of the two groups per strain. One group of TG and SD rats (n = 11–14), respectively, was fed solely with standard chow (consisting of 6% disaccharides, 30% polysaccharides and 4% fat; Maintenance 1320, Altromin, Lage, Germany). This feeding regimen is designated as ‘control’ throughout the following. A second group of TG and SD rats (n = 11–14) had free access to standard chow plus six various commercial chocolate/ cookie bars, consisting of approximately 62% carbohydrates, 25% fat, 6% protein and 2% fibre, for the entire duration of the study (for details, see Supporting Information Table S1). The rats received only one kind of chocolate/cookie bar per day, these being switched daily in a regular manner (Miesel et al., 2010). This feeding regimen was designated as ‘CD’. Blood samples (non-fasting) were drawn from a tail nick before and at days 36 and 71, and again at day 72 (fasting for 18 h) to determine endocrine and metabolic parameters. Animals were phenotyped regarding BP (day 60), energy expenditure (days 64–66), insulin sensitivity [oral glucose tolerance test (OGTT) at day 72, insulin tolerance test (ITT) at day 74], leptin sensitivity (days 78–80) and fat mass (day 80). At day 80, abdominal girth and body length were determined without knowledge of the treatments. Body mass index (BMI) was calculated from body weight and body length (not including tail length). At day 82, rats were killed and organs removed.
Protocol 2
To further examine age dependency, 12-week-old TG and SD rats were fed with chow or CD as described above for 160 days (n = 6 each group). Animals were assessed only for body weight, food intake and insulin sensitivity (ITT at days 147 or 154).
Protocol 3
To address the question of whether the AT1 receptor antagonist prevented weight gain via an Ang(1–7)/Mas-dependent pathway, one group of SD rats on CD feeding (n = 12) was treated simultaneously with telmisartan (8 mg·kg−1·day−1, by gavage), whereas a second group (n = 12) received in addition to telmisartan, the Mas receptor antagonist A779 via s.c. implanted osmotic minipumps (2ML4, Alzet®, release rate 24 μg·kg−1·day−1; Müller-Fielitz et al., 2012b). Controls (n = 12) received vehicle instead of telmisartan. Rats that were treated with only telmisartan or vehicle-received saline instead of A779. All animals were monitored regarding gain in body weight, energy intake, glycaemic control (OGTT at day 24), BP (day 25), and energy expenditure (day 26–28). At day 29, rats were killed. Immediately after finishing protocol 3, a further group of SD rats was fed with CD and treated with 72 μg·kg−1·day−1 A779 in addition to telmisartan to investigate possible dose effects.
Test protocols
The systolic BP and heart rate were determined in conscious rats (Raasch et al., 2002). Randomized measurements were performed only between 2:00 p.m. and 5:00 p.m. The energy expenditure, the respiratory exchange rate (RER), the drinking and feeding behaviours, and locomotion of each rat were determined within 3 days by using the PhenoMaster System™ (TSE, Bad Homburg, Germany) but only the data from the third day were analysed (Müller-Fielitz et al., 2014).
OGTT, ITT and leptin resistance test (LRT) were performed as described previously (Miesel et al., 2012; Müller-Fielitz et al., 2012a; 2014,). For additional details, see Supporting Information Appendix S1. Fat distribution was determined in anaesthetized rats by employing the MRI technique (Miesel et al., 2012; Müller-Fielitz et al., 2012a; 2014,). After preparation, the length of the femur was measured using a vernier calliper.
Biochemical analysis
Plasma concentrations of insulin and leptin (all from Linco, St. Charles, MO, USA) or angiotensin II (IBL, Hamburg, Germany) were determined by radioimmunoassay using commercial kits (Müller-Fielitz et al., 2014). Blood glucose was determined using glucose sensors (Ascensia® ELITE XL, Bayer, Leverkusen, Germany). Triglycerides were quantified in plasma of fasting animals using a Roche/Hitachi Modular P Chemistry Analyzer (Mannheim, Germany; Müller-Fielitz et al., 2014). Formaldehyde-fixed, paraffin-embedded sections of the liver were stained with haematoxylin and eosin according to standard protocols. A pathologist, unaware of the treatment groups, evaluated the slides and scored each liver tissue specimen for hepatocytes with steatosis.
mRNA levels of (an-)orexigenic peptides and of AT1A/AT1B receptors, ACE2 and Mas receptors were determined in hypothalamus, liver, muscle and visceral adipose tissue by real-time quantitative PCR using SYBR Green reagent (Applied Biosystems, Weiterstadt, Germany) in an ABI Prism 7000 platform (Applied Biosystems) as previously described (Raasch et al., 2004; Miesel et al., 2010; Santos et al., 2010). For details, see Supporting Information Appendix S1.
Data analysis
Data are expressed as means ± SEM. As described above, rats were fed either with chow or with chow + chocolate/cookie bars. Because of the different calorie values of chow (11.7 kJ·g−1) and chocolate/cookie bars (20.8 kJ·g−1), we calculated the energy intake (in kJ) of each rat individually to correctly assess food intake on the basis of the amounts of chow and chocolate/cookie bars consumed.
The amount of fat was semi-automatically quantified in retroperitoneal fat pads and in subcutaneous fat on the basis of the transverse T1-weighted turbo spin-echo images by using the freeware MRIcro Version 1.4 build 1 (http://downloads.fyxm.net/MRIcro-117936.html) and the Vitom for Windows software (Essen, Deutschland). Only intensity signals of >80 grey scale were considered to ensure that fat was being analysed.
In order to quantify the total effect for changes in plasma concentrations of glucose in response to OGTT or ITT over the observation period, the AUCs were calculated for each individual animal on the basis of the changes from baseline values. Changes in insulin or glucose over time were similarly calculated. The rate of fall in glucose levels (as half-life of glucose) after insulin exposure was calculated after log-normal transformation of the glucose concentrations (between 6 and 42 min after insulin injections) and by determining the slopes of linear regression lines.
Correlation coefficients (two-tailed P values) were computed according to Pearson, assuming a Gaussian distribution, with GraphPad Prism, Version 4 (GraphPad Software, Inc., La Jolla, CA, USA). Statistical analysis was performed by one-way analysis of variance (anova), followed by appropriate post hoc tests (Bonferroni or Dunnett). Wilcoxon signed-rank test was used when Gaussian distribution differed between groups. A two-way anova, followed by Bonferroni’s post hoc test for multiple comparisons, was performed to examine the effects of two variables. Differences were considered to be statistically significant at P < 0.05.
Materials
Telmisartan: was supplied by Boehringer Ingelheim Pharmaceuticals, Inc. (Ingelheim, Germany) and A779: by Abcam plc (Cambridge, UK).
Results
Results in TG Ang(1–7)-overexpressing rats
Haemodynamics
CD feeding of SD rats induced mild hypertension. Such diet-related effects were not observed in TG rats. Heart rate and left ventricular weight were lower in TG rats without being influenced by diet (Supporting Information Fig. S1). AngII plasma concentrations were similar in SD and TG rats (Supporting Information Fig. S1). CD feeding tended to increase AngII in SD rats (P = 0.065).
Weight regulation and food behaviour
Gain in body weight was higher in young and old SD rats than in TG rats when they were fed with CD (Table 2013a, Supporting Information Fig. S2A/B). CD feeding selectively increased growth in the girth of SD rats since BMI and fat mass, but not body and femur length were increased (Figure 1A/B, Table 2013a). CD feeding increased the number of hepatocytes with steatosis in SD, but not in TG rats (Supporting Information Fig. S3). Energy intake was also higher in SD rats after CD than after control feeding, but less distinct in TG rats (Supporting Information Fig. S2C, Table 2013a). Ratio between chow and chocolate/cookie bars was changed in favor of chow intake in TG, compared with SD rats (Table 2013a; Supporting Information Fig. S2D). Water intake for the entire study duration was higher in SD than TG rats but lowered during CD feeding selectively in SD rats (Table 2013a). In SD but not TG rats, mRNA levels of the orexigenic peptide prepro-orexin (PPO) were higher after CD feeding while levels of the anorexigenic peptide, cocaine- and amphetamine-regulated transcript (CART), were diminished. No strain differences in (an-)orexigenic peptides were found except that levels of the Agouti related peptide (AgRP) were higher in TG rats (Supporting Information Table S2 and Appendix S1). Hypothalamic ACE2 or Mas receptor expression was not influenced either by strain or by diet (Supporting Information Fig. S4). However, ACE2 and Mas mRNA levels were increased only in the visceral fat of TG rats. This was not observed in other metabolic tissues. Mas expression decreased in SD but not TG rats, when fed with CD (Supporting Information Fig. S5).
Figure 1.
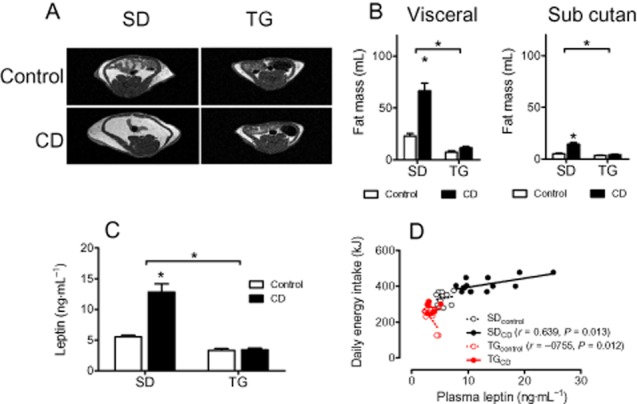
Fat mass and plasma leptin are enhanced by CD feeding in SD, but not in TG rats (protocol 1). (A) Typical MRI images obtained by transverse T1-weighted turbo spin-echo MRI. (B) The abundance of visceral and subcutaneous fat deposits was quantified by computer-assisted planimetry. (C) Plasma levels of leptin at the end of the study; (D) Correlation between plasma leptin and energy intake. Means ± SEM, n = 9–14, *P < 0.05, significantly different from control or as indicated.
Table 1.
Growth in SD and TG rats after long-term feeding with high-calorie cafeteria diet (CD)
| SDcontrol | SDCD | TGcontrol | TGCD | |
|---|---|---|---|---|
| Body weight gain (g) | 377 ± 12 | 429 ± 21* | 232 ± 21 | 267 ± 11† |
| BMI (kg·m−2) | 7.12 ± 0.13 | 7.76 ± 0.23* | 6.57 ± 0.14 | 6.34 ± 0.12† |
| Femur length (mm) | 40.8 ± 0.3 | 40.6 ± 0.3 | 38.7 ± 0.3 | 39.3 ± 0.3† |
| Girth (cm) | 21.1 ± 0.2 | 22.4 ± 0.5* | 18.6 ± 0.3 | 18.6 ± 0.3† |
| Liver (g) | 18.7 ± 0.9 | 18.0 ± 0.9 | 11.1 ± 0.4 | 11.2 ± 0.3† |
| Kidneys (g) | 1.77 ± 0.03 | 1.70 ± 0.5 | 1.11 ± 0.02 | 1.06 ± 0.03† |
| Adrenal glands (mg) | 27.5 ± 0.4 | 28.6 ± 0.4 | 30.7 ± 1.0 | 26.9 ± 0.8* |
| Total energy intake (MJ) | 32.6 ± 0.6 | 42.1 ± 1.0* | 23.9 ± 0.7 | 30.2 ± 0.6*,† |
| Total water intake (mL) | 3338 ± 7 | 2089 ± 132* | 3000 ± 49 | 2836 ± 94† |
| Ratio chow versus chocolate/cookie bars (%) | 100/0 | 24 ± 2/76 ± 2* | 100/0 | 40 ± 2/60 ± 2*,‡ |
| Triglycerides (mmol·L−1) | 1.13 ± 0.07 | 1.39 ± 0.09* | 0.74 ± 0.04 | 0.75 ± 0.02† |
Data shown are means ± SEM, n = 9–14.
P < 0.05, significantly different from corresponding control rats, fed standard chow.
P < 0.05, significant differences between SD and TG rats.
P < 0.05, significant differences between SDCD and TGCD.
Leptin sensitivity
Plasma leptin was selectively increased in SD rats when fed with CD (Figure 1C). The positive correlation between plasma leptin and food intake suggests that leptin resistance developed in CD-fed SD rats. In TG rats, however, energy intake was not positively associated with food intake after CD feeding or indeed negatively associated after control feeding, indicating leptin sensitivity (Figure 1D). The effects of Ang(1–7) on leptin sensitivity were further investigated by performing LRTs. Following leptin injections, energy intake was selectively enhanced in SD rats in response to CD, compared with control feeding; this change was not observed in TG rats (Figure 2A/B). Furthermore, body weight was reduced upon leptin injections in all groups except for CD-fed SD rats (Figure 2C/D), confirming that leptin sensitivity was preserved in TG rats, in spite of CD feeding.
Figure 2.
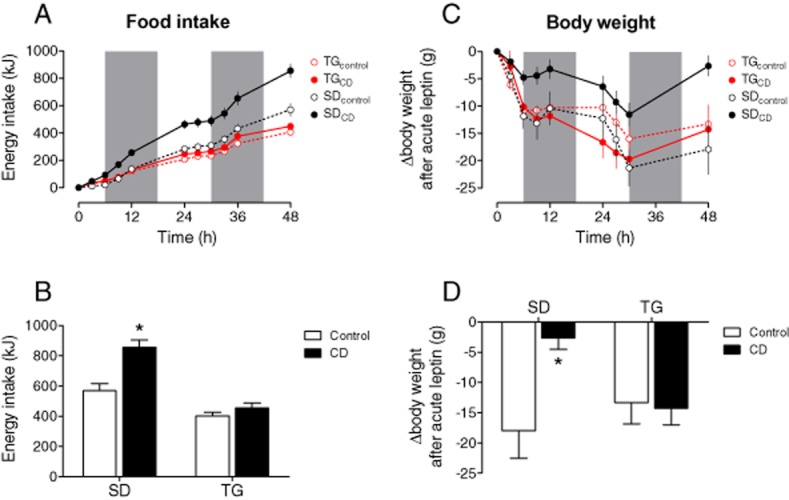
Leptin sensitivity is impaired in SD rats after CD feeding, but preserved in TG rats. CD feeding induces leptin resistance in SD rats, which is selectively prevented in TG rats. At the 1st day, leptin was injected at 8:00 a.m., 11:00 a.m., 2:00 p.m. and 5:00 p.m. (100 μg·kgbw−1 s.c. each time) and at 8:00 p.m. (200 μg·kgbw−1 s.c.). At the second day, rats were treated with leptin at 8:00 and 11:00 a.m. (100 μg·kgbw−1 s.c.) and at 2:00 p.m. (200 μg·kgbw−1 s.c.). The grey bars indicate the dark periods. (A/B) The energy intake after exogenous leptin is increased in CD- compared with chow-fed control SD, which indicates leptin resistance, but remained unaffected in TG rats. (C/D) The reduction in body weight after exogenous leptin is lessened in CD- compared with control-fed SD rats, but remained unaffected in TG rats. Means ± SEM, n = 11–14, *P < 0.05, significantly different from control.
Energy expenditure
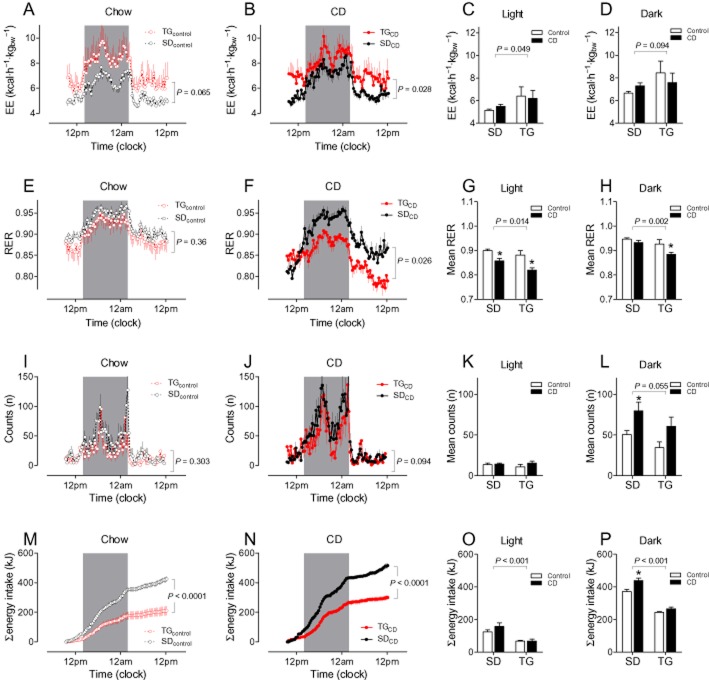
Energy expenditure of TG rats tended to be increased after control feeding (Figure 3A) or was increased after CD feeding (Figure 3B) compared with appropriate SD controls. Strain differences in energy expenditure were more pronounced during light than during dark periods and were only influenced to a minor degree by diet (Figure 3C/D). Respiratory index was lower in CD-fed TG than in CD-fed SD rats, which also was more evident during light than during dark periods (Figure 3E–H). No strain and diet differences were observed in locomotion except that locomotion was increased in SD rats after CD feeding during the dark period (Figure 3I–L). Energy intake (particularly during the dark phase) was also higher in SD rats than in TG rats in calorimetric experiments (Figure 3M–P).
Figure 3.
Energy expenditure (EE; A–D), respiratory ratio (RER, E–H), locomotion (I–L) and energy intake during indirect calorimetry measurements. Animals were housed for 3 days in calorimetry cages, but only the data from the third day are shown. The time-dependent comparison between SD and TG rats is shown in terms of feeding; control in A, E, I, M and CD in B, F, J, N. Mean values during light and dark periods were calculated for EE (C, D), RER (G, H), and locomotion (K, L), whereas total energy intake is shown separately for the light (O) and dark (P) periods. Means ± SEM, n = 11–14, *P < 0.05 significantly different from appropriate control-fed rats.
Glucose control
Fasting glucose and insulin levels were indeed lower in younger TG rats than in SD controls when only fed with chow (Figure 4A/D). However, we did not detect any strain differences regarding insulin sensitivity in lean animals by OGTT or ITT (Figures 4 and 5 and Supporting Information Fig. S6). CD feeding impaired glucose utilization selectively in SD rats as (i) baseline insulin (Figure 4D) was doubled, (ii) glucose (Figure 4B and C) and insulin responses (Figure 4E and F) in OGTT were clearly increased in SD but not in TG rats, and (iii) the glucose response was attenuated upon insulin challenge in ITT. Half-life of glucose decline and maximal glucose reduction were selectively lower in SD rats and the AUC was increased (Figure 5). This pattern of reaction could also be seen in the older animals (Supporting Information Fig. S6).
Figure 4.
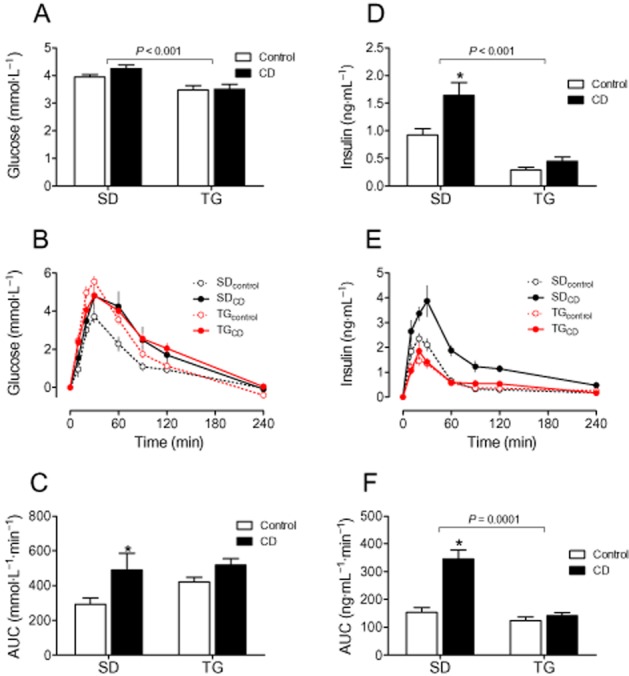
CD feeding impaired glucose utilization in SD rats, but not in TG rats. (A) baseline glucose levels, (B) plasma glucose levels in response to an oral glucose tolerance test (1 g glucose·kgbw−1), (C) AUC of plasma glucose time curves. (D) Baseline insulin levels; (E) plasma insulin levels in OGTT, (F) AUC of plasma insulin time curves. Means ± SEM, n = 11–14, *P < 0.05, significantly different from control.
Figure 5.
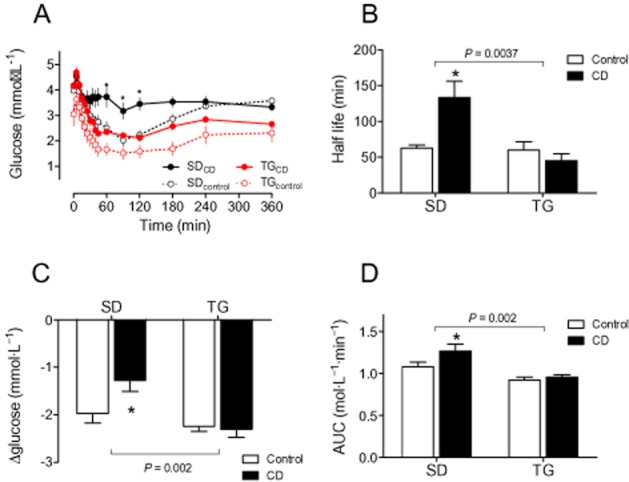
Insulin response is impaired by CD feeding in SD, but not in TG rats. (A) Glucose plasma concentrations after insulin injections (0.6 IU insulin·kgbw−1, s.c.). The maximal glucose decrease was lower (C) and both the half-life (B) and the AUC (D) were higher in SDCD than in SDcontrol, indicating impaired glucose utilization. Strain differences could be observed for all parameters. Means ± SEM, n = 11–14, *P < 0.05, significantly different from SDcontrol.
Results of treating SD rats with telmisartan + A779
Haemodynamics
Vehicle + saline-treated controls were hypertensive and BP was normalized by telmisartan but not further influenced by A779 (Supporting Information Fig. S7A). Heart rate did not differ between groups (Supporting Information Fig. S7B). Left ventricular weight was reduced with respect to BP reduction (Supporting Information Fig. S7C). AngII plasma levels markedly increased after telmisartan while A779 co-treatment had no further effect (Supporting Information Fig. S7D).
Weight regulation and food behaviour
Gain in body weight was lessened by 4 weeks of treatment with telmisartan. The anti-obesity effects of telmisartan were partly reduced when SD rats additionally received 24 μg·kg−1·day−1 (31%, ns) or 72 μg·kg−1·day−1 A779 (60%, P < 0.05; Figure 6A). Weights of livers and kidneys were not affected by A779 co-treatment. Energy expenditure, in terms of locomotion (Supporting Information Figure S8G–I), were slightly increased by telmisartan, compared with controls during the light period, but not during the dark period, while A779 co-treatment had no further effect on these parameters. RER was not altered by any treatment regimen (Supporting Information Fig. S8D–F). The energy intake of rats receiving 72 μg·kg−1·day−1 A779 was higher (especially during the dark period) than that of other groups (approximately 20%; Supporting Information Fig. S8K–M). The proportion of palatable CD intake on total energy intake of controls was 77 ± 2% and was not influenced by any treatment regimen. Total water intake of vehicle + saline-treated rats was 705 ± 27 mL and was not influenced by any treatment. Levels of the orexigenic peptides AgRP and NPY as well as the anorexigenic peptide CART were higher in telmisartan-treated rats than in controls (Supporting Information Table S3 and Appendix S1). When telmisartan-treated rats additionally received 72 μg·kg−1·day−1 A779, levels of mRNA for melanin concentrating hormone (MCH) and PPO were increased in the hypothalami of these rats, which corresponds to the higher energy intake found in these animals (Supporting Information Table S3). Hypothalamic mRNA levels of ACE2, Mas, and AT1A/AT1B receptors were not influenced either by telmisartan or by A779 co-treatment (Supporting Information Fig. S5). Moreover, neither telmisartan treatment nor co-treatment with A779 modified Mas mRNA levels in metabolic tissues (Supporting Information Fig. S6).
Figure 6.
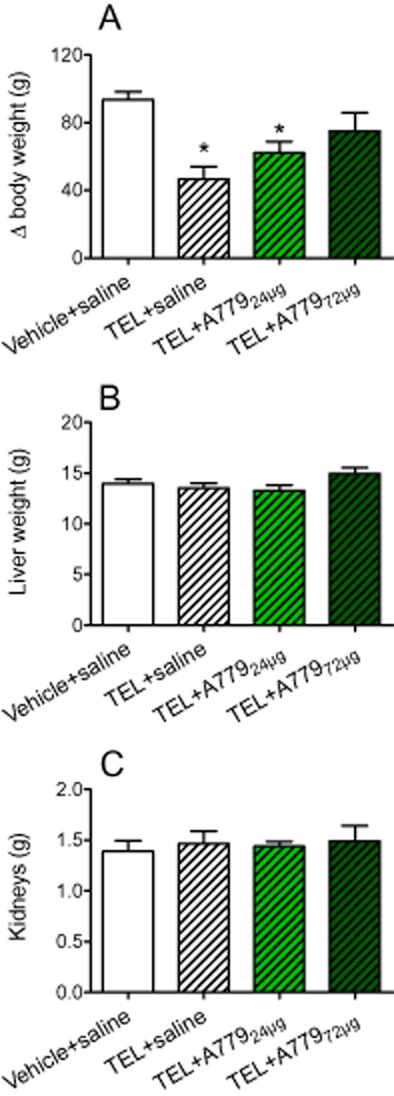
Regulation of growth in telmisartan (TEL) -treated animals in the presence or absence of the Mas receptor antagonist A779 (24 or 72 μg·kg−1·day−1); (B) liver weight; (C) kidney weight. Means + SEM, n = 11–12; P < 0.05, significantly different from vehicle + saline.
Glucose control
Baseline fasting glucose and glucose response in OGTT were similar between groups (Figure 7A–C). However, telmisartan improved glucose utilization as the insulin response to glucose challenge was decreased, compared with controls. A dose of 72 μg·kg−1·day−1 A779 increased the insulin response in OGTT (Figure 7D–F).
Figure 7.
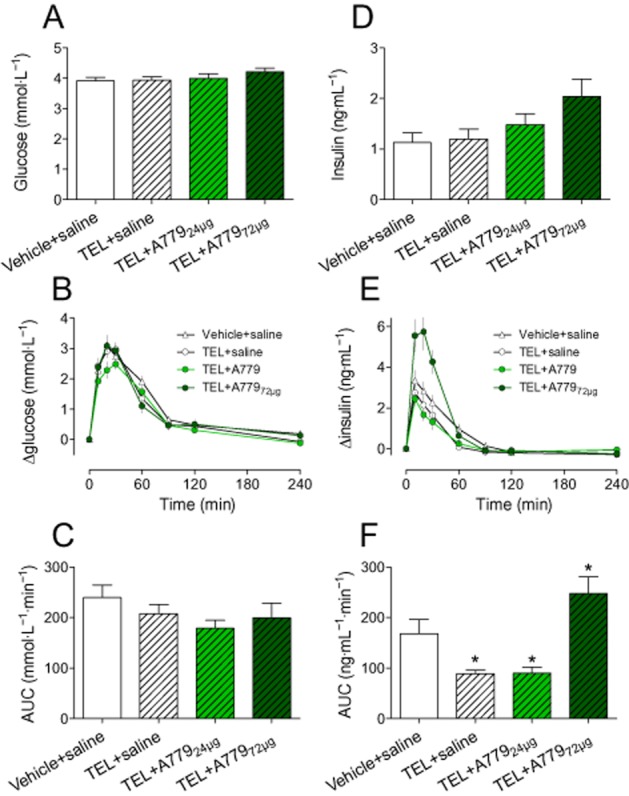
Effects of telmisartan (TEL) and telmisartan+A779 on glucose (A–C) and insulin levels (D–F) of CD-fed SD rats in response to an OGTT. (A/D) Baseline levels of fasting glucose and insulin, (B/E) plasma glucose and insulin levels in response to glucose challenge (1 g glucose·kgbw−1). (C/F) AUCs of glucose and insulin of concerning plasma concentration time curves; Means ± SEM, n = 12, *P < 0.05, significantly different from vehicle + saline.
Discussion
TG, but not SD rats, were protected from developing obesity
Body weight of control-fed TG rats was lower than that of SD rats, being associated with a lower energy intake but higher energy expenditure. SD rats became leptin and insulin resistant, hypophagic, obese and hypertensive when they were fed with CD (Müller-Fielitz et al., 2015) while none of these adverse effects was seen in TG rats although they were also fed with CD, confirming the anti-obesity effects of Ang(1–7) already seen previously (Santos et al., 2008; 2012b; 2013,,).
It seems rather unlikely that enhanced diuresis participates in the reduced weight gain since even water excretion was increased by Ang(1–7) (Handa et al., 1996) and urinary volume was decreased in Mas-deficient mice (Pinheiro et al., 2009). Here, we showed that the water intake of TG rats tended to decrease rather than increase, thus not supporting the involvement of diuresis, which nonetheless would induce only a short-lasting effect on body weight.
In contrast to the findings of others showing that Mas protein increased in obese rats (Rubio-Ruiz et al., 2014), we showed here that Mas mRNA levels were doubled in the adipose tissue of TG rats. It is possible that this increase might lower the fat mass of TG rats by increasing lipolysis as Ang(1–7) increased glycerol release from adipocytes in a Mas-dependent manner (Oh et al., 2012). Here, we observed that RER was lower in TG rats after CD feeding, which indicates a higher degree of fat burning and lipolysis. In addition to this direct effect, we could speculate that lipolysis may also be indirectly enhanced in response to Ang(1–7) by stimulating the sympathetic outflow as observed for AngII (King et al., 2013) since Ang(1–7) stimulates peripheral noradrenergic neurotransmission (Gironacci et al., 2013).
It is interesting that during CD feeding the energy intake actually increased in both strains but TG rats did not become obese as energy expenditure was coincidently higher. Regarding feeding behaviour, TG rats seemed to prefer standard chow to the CD, compared with SD rats, giving rise to speculation as to whether Ang(1–7) affects reward. Food preference for chow was also found in telmisartan-treated rats and, in the context of reward, to depend on diminished dopamine signalling (Müller-Fielitz et al., 2015). In contrast, Ang(1–7) increases dopamine release (Stragier et al., 2005), making this pathway rather unlikely to account for the food preference of TG rats. Reward was also shown to be associated with lower HPA axis activity (Corwin et al., 2011) and Ang(1–7) diminished HPA activity (Zhu et al., 2014), thus contributing to the observed food preference. Supporting this concept, intake of palatable food was increased by stress (Pecoraro et al., 2004) but reduced by telmisartan when stress reactions were also lessened (Miesel et al., 2012).
Within the last few decades, a firm link has been established between obesity and low-grade inflammation in peripheral tissues but also in the hypothalamus. Obesity-induced inflammation prompts the CNS to increase food intake and reduce energy expenditure, thus supplying more nutrients to an expanding adipose tissue (Pimentel et al., 2014). Ang(1–7) protects white adipose tissue from the proinflammatory state stimulated by a high-fat diet, thus contributing to the anti-obesity effects of this peptide (Santos et al., 2012b). Inflammation disrupts leptin signalling pathways (Pimentel et al., 2014). Here, we did not determine any cytokine levels in our groups of rats. Nevertheless, we did investigate whether leptin sensitivity was modified in TG rats as we have demonstrated here and elsewhere that triglycerides are increased in SD rats after CD feeding (Müller-Fielitz et al., 2015) while triglyceride levels of TG rats were per se lower and not affected by CD feeding. Accordingly, triglycerides were increased in Mas-deficient mice (Silva et al., 2013). High serum triglycerides impair the ability of the blood–brain barrier (BBB) to transport leptin (Banks et al., 2004). Chronic CD feeding induced leptin resistance (Müller-Fielitz et al., 2015), which also occurred in the SD rats in our study as (i) the total energy intake exceeded that of control-fed animals despite higher plasma leptin levels, (ii) plasma leptin positively correlated with energy intake and (iii) energy intake in response to exogenous leptin was higher. In contrast, diet did not affect baseline plasma leptin in TG rats, and TG rats also showed intact leptin function during CD feeding. We assume that leptin transport across the BBB is impaired in SD rats but still intact in TG rats as impaired transport of leptin across the BBB via a saturable leptin transporter is likely to represent a major component of the peripheral leptin resistance (Banks et al., 1999; Banks and Farrell, 2003).
TG, but not SD rats, were protected from developing insulin resistance
In agreement with others, we found better glucose control in TG than SD rats receiving the high-calorie diet (Santos et al., 2012b; 2013,), which may be related to the following mechanisms.
Ang(1–7) improved cellular glucose uptake in vitro (Silva et al., 2013; Santos et al., 2014), it also affected insulin signalling by stimulating the phosphorylation of JAK2 and insulin receptor substrate-1 (Giani et al., 2007) and ameliorated the inhibitory effect of AngII on glucose transport activity (Prasannarong et al., 2012).
Using different diabetic animal models, the beneficial effects of Ang(1–7) on glucose utilization were confirmed as (i) glycaemic control was better in diabetic db/db mice when they were infected with an adenovirus expressing human ACE2 (Bindom et al., 2010); (ii) pancreatic microcirculation and intra-islet microvessel density were improved (Yuan et al., 2013); (iii) short-term Ang(1–7) infusions improved insulin resistance in fructose-fed rats and attenuated diabetic nephropathy in Zucker diabetic fatty rats (Giani et al., 2009; 2012,); (iv) an oral Ang(1–7) formulation reversed hyperglycaemia in transgenic rats with inducible insulin resistance (Santos et al., 2014); and (v) Ang(1–7) improved the effect of insulin by recruiting muscle microvasculature (Fu et al., 2014). In line with these Ang(1–7) effects, Mas gene-deleted mice presented with glucose intolerance and reduced insulin sensitivity as well as a decrease in insulin-stimulated glucose uptake by adipocytes and decreased GLUT4 in adipose tissue (Santos et al., 2008).
Ang(1–7) improves glucose control in obesity. As expected, CD feeding of SD rats induced insulin resistance, which may be partly attributed to a hepatic mechanism because non-alcoholic steatohepatitis, a typical outcome of obesity, is strongly associated with hepatic insulin resistance and type 2 diabetes (Birkenfeld and Shulman, 2014) and, in our study, we found that SD rats fed solely with CD developed steatosis. None of these problems was observed in TG rats despite CD feeding. Accordingly, glucose control was improved, obesity was prevented and hepatic inflammation was reduced in rats receiving a high-fat diet and simultaneously being orally treated with Ang(1–7) (Feltenberger et al., 2013; Santos et al., 2013). In obesity, the intramyocellular triglyceride content contributes to aggravating insulin resistance (Turner et al., 2014). Higher triglyceride plasma levels in CD-fed SD rats but strain-dependent lower levels confirm this notion in our study. At least, inflammation has been proposed as a critical factor causing insulin resistance. In this regard, the anti-inflammatory potency of Ang(1–7) may also contribute to the improved glucose control (Santos et al., 2012b). Ang(1–7) may also improve glucose control by regulating energy intake and expenditure. It may be assumed that enhanced energy expenditure during the light period is functionally important for alterations in glucose metabolism in TG rats when the light period is interpreted as a sedentary state. It is well known that insulin sensitivity is impaired in sedentary conditions but can be restored as a result of physical activity (Duvivier et al., 2013). However, our study gave no evidence of increased physical activity in TG rats in terms of locomotion. Moreover, energy expenditure tended to be higher during the dark period and RER was lower in TG rats during the night, indicating better fat burning. To better investigate the relation between physical activity and energy balance, additional experiments should be performed by using a combined setting of treadmills and indirect calorimetry.
Telmisartan (partly) prevents weight gain via an Ang(1–7)/Mas-dependent mechanism
According to our previous findings in diet-induced rat obesity, telmisartan prevented weight gain (Miesel et al., 2012; Müller-Fielitz et al., 2012a; 2014,). A779 did not affect weight regulation when a dose of 24 μg·kg−1·day−1 was administered. However, treatment with a threefold higher A779 dose of A779 partly reversed the telmisartan-induced prevention of weight gain. This finding provides evidence that prevention of weight gain by AT1 receptor antagonists is partly mediated by an Ang(1–7)-dependent mechanism. Captopril also prevented weight gain in lean SD rats while Ang(1–7) was elevated and A779 (24 μg·kg−1·day−1) was demonstrated to antagonize captopril’s effect on weight regulation (Oh et al., 2012). In another report, captopril failed to reduce body weight but increased plasma and tissue Ang(1–7) (Benter et al., 2011). In fact, we previously observed a reduction in weight gain in lean rats after captopril, but not after ramipril, enalapril or fosinopril, raising the question of whether these effects of captopril on body weight were related to ACE inhibition (Raasch et al., 2002). Nonetheless, the anti-obesity effects of ACE inhibitors seem to be minor compared with those of AT1 receptor antagonists under equieffective antihypertensive dosing (Miesel et al., 2012).
Plasma Ang(1–7) is known to be increased in rats both after ACE inhibition (Benter et al., 2011; Oh et al., 2012) and after AT1 receptor blockade (Ishiyama et al., 2004; Igase et al., 2005) and was associated with a reduction in body weight. An increase in Ang(1–7) in the presence of AT1 receptor antagonists depends on more locally available AngII as renin is up-regulated in the circulation. The elevated levels of plasma Ang(1–7) observed following ACE inhibition are due to the fact that ACE is responsible for the breakdown of Ang(1–7) (Yamada et al., 1998; Ferrario et al., 2005). It is surprising that Ang(1–7) plasma levels normalize after captopril plus A779 (Oh et al., 2012) because (i) A779 blocks Ang(1–7) effects at Mas receptors and does not influence Ang(1–7) biosynthesis, and (ii) despite simultaneous Mas receptor blockade, captopril should block the degradation of AngII to Ang(1–7) via ACE. Circulating Ang(1–7) levels were not determined in our study as this peptide exerts its effects on the receptors at the local tissue level. There, ACE2 is expressed and metabolizes AngII to Ang(1–7), which is very short-lived and scarcely reaches the bloodstream before being degraded. An up-regulation of ACE2 and Mas receptors in the visceral fat of TG rats, as seen here may support the importance of local Ang(1–7) for its biological effects. Nevertheless, we cannot exclude the possibility that telmisartan exerts its effects via the Ang(1–7) receptor Mas or its downstream signalling pathways in this part of the study.
BP and glucose homeostasis were analysed as secondary parameters. BP was reduced by telmisartan but not further altered by administering A779 at the same time. In contrast, a captopril-induced BP reduction was related to Ang(1–7) (Benter et al., 2011) and seems to reflect the vasodilator effects of Ang(1–7) (Bader, 2013). However, the hydralazine-induced drop in BP was also antagonized by A779 although Ang(1–7) was normal, which might indicate a non-specificity due to 10-fold higher A779 dose (744 μg·kg−1·day−, i.p.) as used by Benter et al. (2011). Moreover, Ang(1–7)-induced vasodilatation need not necessarily be attributed to Mas receptors, as in younger rats (similar to those used in this study), the vasodepressor effects of Ang(1–7) were attributed to an AT2 receptor-dependent mechanism and only in older rats, were related to stimulation of Mas receptors (Bosnyak et al., 2012).
As has repeatedly been shown, insulin response to OGTT was lessened by telmisartan, indicating improved insulin sensitivity (Miesel et al., 2012; Müller-Fielitz et al., 2012a; 2014,). It is supposed that reduced insulin response in OGTT after telmisartan may be attributed to an Ang(1–7)-dependent mechanism because the insulin secretion after telmisartan+72 μg A779 exceeded control levels. This assumption is strengthened by observations showing that insulin was reduced in transgenic animals with high Ang(1–7) (Figure 4) and in fructose-fed rats after Ang(1–7) administration (Giani et al., 2009).
In summary, we clearly show here that Ang(1–7) lowers diet-induced obesity in rats and that the anti-obesity effects of telmisartan can partly be attributed to an Ang(1–7) mechanism.
Acknowledgments
This study was supported by the Excellent Funding of the Medical Section of the University of Lübeck and by funding from the German Center for Cardiovascular Research (Grant Number 81X2700101) and the Deutsche Forschungsgemeinschaft DFG (GRK 1957). Walter Raasch received telmisartan from Boehringer Ingelheim Pharmaceuticals, Inc. (Ingelheim, Germany).
Glossary
- Ang(1–7)
angiotensin-(1–7)
- AngI
angiotensin I
- AngII
angiotensin II
- BBB
blood–brain barrier
- BMI
body mass index
- CART
cocaine- and amphetamine-regulated transcript
- CD
cafeteria diet
- CRH
corticotropin-releasing hormone
- HPA axis
hypothalamic-pituitary-adrenal axis
- ITT
insulin tolerance test
- LRT
leptin resistance test
- MCH
melanin concentrating hormone
- NPY
neuropeptide Y
- OGTT
oral glucose tolerance test
- POMC
pro-opiomelanocortin
- PPO
prepro-orexin
- RER
respiratory exchange rate
- SD
Sprague-Dawley rat rats
- TG
transgenic
Author contributions
J. S., M. W., I. S., F. S., F. M. V., J. B., C. T., M. B. and W. R. performed the research; W. R., M. B. and R. A. S. designed the research study; W. R. and J. S. analysed the data; and W. R., J. S., R. A. S. and M. B. wrote the paper.
Conflict of interest
No conflict of interests.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Figure S1 Systolic blood pressure (SBP), heart rate (HR), left ventricular weight, and AngII plasma concentration in SD (open bars) or TG rats (closed bars) depending on chow or CD feeding. Means + SEM, n = 11–14. *P < 0.05.
Figure S2 Body weight and energy intake are enhanced by CD feeding in older SD but not in TG rats (protocol 2). (A) Time-dependent increase in body weight. (B) Gain in body weight within the 12-week feeding period. (C) Time-dependent energy intake. (D) Cumulative energy intake: open bars depict energy intake originating from chow intake whereas shaded bars represent energy intake from chocolate/cookie bars. Means ± SEM, n = 11–14. *P < 0.05. †Intake of chocolate/cookie bars: P < 0.05 versus SDCD. ‡Chow intake: P < 0.05 versus SDCD.
Figure S3 Histological findings of livers from control- or CD-fed SD and TG rats. Means ± SEM, n = 11–14. *P < 0.05 versus control. Tissue specimens were evaluated, without knowledge of treatments, based on and scored for hepatocytes with steatosis.
Figure S4 mRNA levels of components of the ACE2, Mas, and AT1A and AT1b receptors in hypothalami of rats of protocol 2 (control- or CD-fed SD and TG rats) and protocol 3, respectively [CD-fed SD rats that were treated with telmisartan (TEL; 8 mg·kg−1·day−1) or telmisartan plus A779 (24 or 72 μg·kg−1·day−1) while controls received vehicle + saline]. Means ± SEM.
Figure S5 mRNA levels of MAS and ACE2 in different metabolic tissues of rats of protocol 2 (control- or CD-fed SD and TG rats) and protocol 3, respectively [CD-fed SD rats that were treated with telmisartan (TEL; 8 mg·kg−1·day−1) or telmisartan plus A779 (24 or 72 μg·kg−1·day−1) while controls received vehicle + saline]. Means ± SEM.
Figure S6 Insulin response is impaired by CD feeding in SD but not in TG rats (protocol 2). (A) Fasting glucose levels; (B) glucose plasma concentrations after insulin injections (0.6 IU insulin·kgbw−1, s.c.). The AUC (C), the minimal glucose levels (D), the time points of minimal glucose levels (E) and the half-life of glucose decline (F) were higher in SDCD than in SDcontrol, indicating impaired glucose control. A strain difference could be observed for all parameters. Means ± SEM, n = 11–14, *P < 0.05.
Figure S7 Systolic blood pressure (SBP, A), heart rate (HR, B), left ventricular weight (C), and AngII plasma concentration (D) in CD-fed SD rats that were treated for 4 weeks with telmisartan (TEL; 8 mg·kg−1·day−1) or telmisartan plus A779 (14 or 72 μg·kg−1·day−1, by osmotic minipumps). Controls received vehicle and saline. Means ± SEM, n = 11–12. *P < 0.05 versus vehicle + saline.
Figure S8 Energy expenditure (EE; A–C) respiratory ratio (RER, D–F), locomotion (G–I) and energy intake (K–M) during indirect calorimetry measurements. Animals were housed for 3 days in calorimetry cages, but only the data of the 3rd day are depicted. Mean values during light and dark periods were calculated for EE (B, C), RER (E, F) and locomotion (H, I), whereas total energy intake was depicted specifically considering light (L) and dark periods (M). Means ± SEM, n = 12, *P < 0.05 versus vehicle + saline.
Table S1 Nutrition composition of chocolate and cookie bars.
Table S2 mRNA levels of orexigenic (PPO, NPY, MCH, AgRP) and anorexigenic peptides (POMC, CART, corticotropin-releasing hormone, CRH) in hypothalami of Sprague-Dawley (SD) rats or transgenic rats (TG) overexpressing Ang(1–7) that received control diet or cafeteria diet (CD).
Table S3 mRNA levels of orexigenic (PPO, NPY, MCH, AgRP) and anorexigenic peptides (POMC, CART, CRH) in hypothalami of rats of CD-fed SD rats that were treated with telmisartan (8 mg·kg−1·day−1) or telmisartan plus A779 (24 or 72 μg·kg−1·day−1) while controls received vehicle + saline.
Appendix S1 Supplementary methodical information to OGTT, ITT, LTT, RNA isolation, cDNA synthesis and quantification of mRNA as well as supplementary discussion on hypothalamic expression of (an-)orexigenic peptides.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013b;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader M. ACE2, angiotensin-(1–7), and Mas: the other side of the coin. Pflugers Arch. 2013;465:79–85. doi: 10.1007/s00424-012-1120-0. [DOI] [PubMed] [Google Scholar]
- Banks WA, Farrell CL. Impaired transport of leptin across the blood-brain barrier in obesity is acquired and reversible. Am J Physiol Endocrinol Metab. 2003;285:E10–E15. doi: 10.1152/ajpendo.00468.2002. [DOI] [PubMed] [Google Scholar]
- Banks WA, DiPalma CR, Farrell CL. Impaired transport of leptin across the blood-brain barrier in obesity. Peptides. 1999;20:1341–1345. doi: 10.1016/s0196-9781(99)00139-4. [DOI] [PubMed] [Google Scholar]
- Banks WA, Coon AB, Robinson SM, Moinuddin A, Shultz JM, Nakaoke R, et al. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes. 2004;53:1253–1260. doi: 10.2337/diabetes.53.5.1253. [DOI] [PubMed] [Google Scholar]
- Benter IF, Yousif MH, Al Saleh FM, Raghupathy R, Chappell MC, Diz DI. Angiotensin-(1–7) blockade attenuates captopril- or hydralazine-induced cardiovascular protection in spontaneously hypertensive rats treated with NG-nitro-L-arginine methyl ester. J Cardiovasc Pharmacol. 2011;57:559–567. doi: 10.1097/FJC.0b013e31821324b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindom SM, Hans CP, Xia H, Boulares AH, Lazartigues E. Angiotensin I-converting enzyme type 2 (ACE2) gene therapy improves glycemic control in diabetic mice. Diabetes. 2010;59:2540–2548. doi: 10.2337/db09-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenfeld AL, Shulman GI. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology. 2014;59:713–723. doi: 10.1002/hep.26672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosnyak S, Widdop RE, Denton KM, Jones ES. Differential mechanisms of ang (1–7)-mediated vasodepressor effect in adult and aged candesartan-treated rats. Int J Hypertens. 2012;2012:192567. doi: 10.1155/2012/192567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink M, Wellen J, Delafontaine P. Angiotensin II causes weight loss and decreases circulating insulin-like growth factor I in rats through a pressor-independent mechanism. J Clin Invest. 1996;97:2509–2516. doi: 10.1172/JCI118698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabassi A, Coghi P, Govoni P, Barouhiel E, Speroni E, Cavazzini S, et al. Sympathetic modulation by carvedilol and losartan reduces angiotensin II-mediated lipolysis in subcutaneous and visceral fat. J Clin Endocrinol Metab. 2005;90:2888–2897. doi: 10.1210/jc.2004-1995. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Avena NM, Boggiano MM. Feeding and reward: perspectives from three rat models of binge eating. Physiol Behav. 2011;104:87–97. doi: 10.1016/j.physbeh.2011.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, la Fleur SE, Pecoraro NC, Gomez F, Houshyar H, Akana SF. Minireview: glucocorticoids – food intake, abdominal obesity, and wealthy nations in 2004. Endocrinology. 2004;145:2633–2638. doi: 10.1210/en.2004-0037. [DOI] [PubMed] [Google Scholar]
- Duvivier BM, Schaper NC, Bremers MA, van Crombrugge G, Menheere PP, Kars M, et al. Minimal intensity physical activity (standing and walking) of longer duration improves insulin action and plasma lipids more than shorter periods of moderate to vigorous exercise (cycling) in sedentary subjects when energy expenditure is comparable. PLoS ONE. 2013;8:e55542. doi: 10.1371/journal.pone.0055542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeli S, Negrel R, Sharma AM. Physiology and pathophysiology of the adipose tissue renin-angiotensin system. Hypertension. 2000;35:1270–1277. doi: 10.1161/01.hyp.35.6.1270. [DOI] [PubMed] [Google Scholar]
- Feltenberger JD, Andrade JM, Paraiso A, Barros LO, Filho AB, Sinisterra RD, et al. Oral formulation of angiotensin-(1–7) improves lipid metabolism and prevents high-fat diet-induced hepatic steatosis and inflammation in mice. Hypertension. 2013;62:324–330. doi: 10.1161/HYPERTENSIONAHA.111.00919. [DOI] [PubMed] [Google Scholar]
- Ferrario CM, Jessup J, Gallagher PE, Averill DB, Brosnihan KB, Ann TE, et al. Effects of renin-angiotensin system blockade on renal angiotensin-(1–7) forming enzymes and receptors. Kidney Int. 2005;68:2189–2196. doi: 10.1111/j.1523-1755.2005.00675.x. [DOI] [PubMed] [Google Scholar]
- Fu Z, Zhao L, Aylor KW, Carey RM, Barrett EJ, Liu Z. Angiotensin-(1–7) recruits muscle microvasculature and enhances insulin’s metabolic action via mas receptor. Hypertension. 2014;63:1219–1227. doi: 10.1161/HYPERTENSIONAHA.113.03025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giani JF, Gironacci MM, Munoz MC, Pena C, Turyn D, Dominici FP. Angiotensin-(1–7) stimulates the phosphorylation of JAK2, IRS-1 and Akt in rat heart in vivo: role of the AT1 and Mas receptors. Am J Physiol Heart Circ Physiol. 2007;293:H1154–H1163. doi: 10.1152/ajpheart.01395.2006. [DOI] [PubMed] [Google Scholar]
- Giani JF, Mayer MA, Munoz MC, Silberman EA, Hocht C, Taira CA, et al. Chronic infusion of angiotensin-(1–7) improves insulin resistance and hypertension induced by a high-fructose diet in rats. Am J Physiol Endocrinol Metab. 2009;296:E262–E271. doi: 10.1152/ajpendo.90678.2008. [DOI] [PubMed] [Google Scholar]
- Giani JF, Burghi V, Veiras LC, Tomat A, Munoz MC, Cao G, et al. Angiotensin-(1–7) attenuates diabetic nephropathy in Zucker diabetic fatty rats. Am J Physiol Renal Physiol. 2012;302:F1606–F1615. doi: 10.1152/ajprenal.00063.2012. [DOI] [PubMed] [Google Scholar]
- Gironacci MM, Longo Carbajosa NA, Goldstein J, Cerrato BD. Neuromodulatory role of angiotensin-(1–7) in the central nervous system. Clin Sci (Lond) 2013;125:57–65. doi: 10.1042/CS20120652. [DOI] [PubMed] [Google Scholar]
- Handa RK, Ferrario CM, Strandhoy JW. Renal actions of angiotensin-(1–7): in vivo and in vitro studies. Am J Physiol. 1996;270:F141–F147. doi: 10.1152/ajprenal.1996.270.1.F141. [DOI] [PubMed] [Google Scholar]
- He H, Yang D, Ma L, Luo Z, Ma S, Feng X, et al. Telmisartan prevents weight gain and obesity through activation of peroxisome proliferator-activated receptor-{delta}-dependent pathways. Hypertension. 2010;55:869–879. doi: 10.1161/HYPERTENSIONAHA.109.143958. [DOI] [PubMed] [Google Scholar]
- Igase M, Strawn WB, Gallagher PE, Geary RL, Ferrario CM. Angiotensin II AT1 receptors regulate ACE2 and angiotensin-(1–7) expression in the aorta of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2005;289:H1013–H1019. doi: 10.1152/ajpheart.00068.2005. [DOI] [PubMed] [Google Scholar]
- Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43:970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- Kadoguchi T, Kinugawa S, Takada S, Fukushima A, Furihata T, Homma T, et al. Angiotensin II can directly induce mitochondrial dysfunction, decrease oxidative fibre number and induce atrophy in mouse hindlimb skeletal muscle. Exp Physiol. 2015;100:312–322. doi: 10.1113/expphysiol.2014.084095. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King VL, English VL, Bharadwaj K, Cassis LA. Angiotensin II stimulates sympathetic neurotransmission to adipose tissue. Physiol Rep. 2013;1:e00014. doi: 10.1002/phy2.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintscher U, Bramlage P, Paar WD, Thoenes M, Unger T. Irbesartan for the treatment of hypertension in patients with the metabolic syndrome: a sub analysis of the Treat to Target post authorization survey. Prospective observational, two armed study in 14,200 patients. Cardiovasc Diabetol. 2007;6:12. doi: 10.1186/1475-2840-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PJ, Jensen PB, Sorensen RV, Larsen LK, Vrang N, Wulff EM, et al. Differential influences of peroxisome proliferator-activated receptors gamma and -alpha on food intake and energy homeostasis. Diabetes. 2003;52:2249–2259. doi: 10.2337/diabetes.52.9.2249. [DOI] [PubMed] [Google Scholar]
- Liu C, Lv XH, Li HX, Cao X, Zhang F, Wang L, et al. Angiotensin-(1–7) suppresses oxidative stress and improves glucose uptake via Mas receptor in adipocytes. Acta Diabetol. 2012;49:291–299. doi: 10.1007/s00592-011-0348-z. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesel A, Müller H, Thermann M, Heidbreder M, Dominiak P, Raasch W. Overfeeding-induced obesity in spontaneously hypertensive rats: an animal model of the human metabolic syndrome. Ann Nutr Metab. 2010;56:127–142. doi: 10.1159/000278748. [DOI] [PubMed] [Google Scholar]
- Miesel A, Müller-Fielitz H, Jöhren O, Vogt FM, Raasch W. Double blockade of angiotensin II (AT(1))-receptors and ACE does not improve weight gain and glucose homeostasis better than single-drug treatments in obese rats. Br J Pharmacol. 2012;165:2721–2735. doi: 10.1111/j.1476-5381.2011.01726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Fielitz H, Markert A, Wittmershaus C, Pahlke F, Jöhren O, Raasch W. Weight loss and hypophagia after high-dose AT1-blockade is only observed after high dosing and depends on regular leptin signalling but not blood pressure. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:373–384. doi: 10.1007/s00210-011-0602-5. [DOI] [PubMed] [Google Scholar]
- Müller-Fielitz H, Landolt J, Heidbreder M, Werth S, Vogt FM, Jöhren O, et al. Improved insulin sensitivity after long-term treatment with AT1 blockers is not associated with PPARgamma target gene regulation. Endocrinology. 2012a;153:1103–1115. doi: 10.1210/en.2011-0183. [DOI] [PubMed] [Google Scholar]
- Müller-Fielitz H, Lau M, Jöhren O, Stellmacher F, Schwaninger M, Raasch W. Blood pressure response to angiotensin II is enhanced in obese zucker rats and is attributed to an aldosterone-dependent mechanism. Br J Pharmacol. 2012b;166:2417–2429. doi: 10.1111/j.1476-5381.2012.01953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Fielitz H, Hübel N, Mildner M, Vogt FM, Barkhausen J, Raasch W. Chronic blockade of angiotensin AT(1) receptors improves cardinal symptoms of metabolic syndrome in diet-induced obesity in rats. Br J Pharmacol. 2014;171:746–760. doi: 10.1111/bph.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Fielitz H, Lau M, Geissler C, Werner L, Piehl M, Raasch W. Preventing leptin resistance by blocking angiotensin II AT1 receptors in diet-induced obese rats. Br J Pharmacol. 2015;172:857–868. doi: 10.1111/bph.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh YB, Kim JH, Park BM, Park BH, Kim SH. Captopril intake decreases body weight gain via angiotensin-(1–7) Peptides. 2012;37:79–85. doi: 10.1016/j.peptides.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004;145:3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- Pimentel GD, Ganeshan K, Carvalheira JB. Hypothalamic inflammation and the central nervous system control of energy homeostasis. Mol Cell Endocrinol. 2014;397:15–22. doi: 10.1016/j.mce.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Pinheiro SV, Ferreira AJ, Kitten GT, da Silveira KD, da Silva DA, Santos SH, et al. Genetic deletion of the angiotensin-(1–7) receptor Mas leads to glomerular hyperfiltration and microalbuminuria. Kidney Int. 2009;75:1184–1193. doi: 10.1038/ki.2009.61. [DOI] [PubMed] [Google Scholar]
- Prasannarong M, Santos FR, Henriksen EJ. ANG-(1–7) reduces ANG II-induced insulin resistance by enhancing Akt phosphorylation via a Mas receptor-dependent mechanism in rat skeletal muscle. Biochem Biophys Res Commun. 2012;426:369–373. doi: 10.1016/j.bbrc.2012.08.093. [DOI] [PubMed] [Google Scholar]
- Raasch W, Bartels T, Schwartz C, Hauser W, Rutten H, Dominiak P. Regression of ventricular and vascular hypertrophy: are there differences between structurally different angiotensin-converting enzyme inhibitors? J Hypertens. 2002;20:2495–2504. doi: 10.1097/01.hjh.0000042885.24999.e9. [DOI] [PubMed] [Google Scholar]
- Raasch W, Jöhren O, Schwartz S, Gieselberg A, Dominiak P. Combined blockade of AT1-receptors and ACE synergistically potentiates antihypertensive effects in SHR. J Hypertens. 2004;22:611–618. doi: 10.1097/00004872-200403000-00025. [DOI] [PubMed] [Google Scholar]
- Rong X, Li Y, Ebihara K, Zhao M, Naowaboot J, Kusakabe T, et al. Angiotensin II type 1 receptor-independent beneficial effects of telmisartan on dietary-induced obesity, insulin resistance and fatty liver in mice. Diabetologia. 2010;53:1727–1731. doi: 10.1007/s00125-010-1744-6. [DOI] [PubMed] [Google Scholar]
- Rubio-Ruiz ME, Valle-Mondragon L, Castrejon-Tellez V, Carreon-Torres E, Diaz-Diaz E, Guarner-Lans V. Angiotensin II and 1–7 during aging in metabolic syndrome rats. Expression of AT1, AT2 and Mas receptors in abdominal white adipose tissue. Peptides. 2014;57:101–108. doi: 10.1016/j.peptides.2014.04.021. [DOI] [PubMed] [Google Scholar]
- Santos RA. Angiotensin-(1–7) Hypertension. 2014;63:1138–1147. doi: 10.1161/HYPERTENSIONAHA.113.01274. [DOI] [PubMed] [Google Scholar]
- Santos RA, Ferreira AJ, Nadu AP, Braga AN, de Almeida AP, Campagnole-Santos MJ, et al. Expression of an angiotensin-(1–7)-producing fusion protein produces cardioprotective effects in rats. Physiol Genomics. 2004;17:292–299. doi: 10.1152/physiolgenomics.00227.2003. [DOI] [PubMed] [Google Scholar]
- Santos RA, Ferreira AJ, Verano-Braga T, Bader M. Angiotensin-converting enzyme 2, Angiotensin-(1–7) and Mas: new players of the renin angiotensin system. J Endocrinol. 2012a;216:R1–R17. doi: 10.1530/JOE-12-0341. [DOI] [PubMed] [Google Scholar]
- Santos SH, Fernandes LR, Mario EG, Ferreira AV, Porto LC, Alvarez-Leite JI, et al. Mas deficiency in FVB/N mice produces marked changes in lipid and glycemic metabolism. Diabetes. 2008;57:340–347. doi: 10.2337/db07-0953. [DOI] [PubMed] [Google Scholar]
- Santos SH, Braga JF, Mario EG, Porto LC, Rodrigues-Machado MG, Murari A, et al. Improved lipid and glucose metabolism in transgenic rats with increased circulating angiotensin-(1–7) Arterioscler Thromb Vasc Biol. 2010;30:953–961. doi: 10.1161/ATVBAHA.109.200493. [DOI] [PubMed] [Google Scholar]
- Santos SH, Fernandes LR, Pereira CS, Guimaraes AL, de Paula AM, Campagnole-Santos MJ, et al. Increased circulating angiotensin-(1–7) protects white adipose tissue against development of a proinflammatory state stimulated by a high-fat diet. Regul Pept. 2012b;178:64–70. doi: 10.1016/j.regpep.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Santos SH, Andrade JM, Fernandes LR, Sinisterra RD, Sousa FB, Feltenberger JD, et al. Oral Angiotensin-(1–7) prevented obesity and hepatic inflammation by inhibition of resistin/TLR4/MAPK/NF-kappaB in rats fed with high-fat diet. Peptides. 2013;46:47–52. doi: 10.1016/j.peptides.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Santos SH, Giani JF, Burghi V, Miquet JG, Qadri F, Braga JF, et al. Oral administration of angiotensin-(1–7) ameliorates type 2 diabetes in rats. J Mol Med (Berl) 2014;92:255–265. doi: 10.1007/s00109-013-1087-0. [DOI] [PubMed] [Google Scholar]
- Schupp M, Clemenz M, Gineste R, Witt H, Janke J, Helleboid S, et al. Molecular characterization of new selective peroxisome proliferator-activated receptor gamma modulators with angiotensin receptor blocking activity. Diabetes. 2005;54:3442–3452. doi: 10.2337/diabetes.54.12.3442. [DOI] [PubMed] [Google Scholar]
- Silva AR, Aguilar EC, Alvarez-Leite JI, da Silva RF, Arantes RM, Bader M, et al. Mas receptor deficiency is associated with worsening of lipid profile and severe hepatic steatosis in ApoE-knockout mice. Am J Physiol Regul Integr Comp Physiol. 2013;305:R1323–R1330. doi: 10.1152/ajpregu.00249.2013. [DOI] [PubMed] [Google Scholar]
- Skurk T, van Harmelen V, Blum WF, Hauner H. Angiotensin II promotes leptin production in cultured human fat cells by an ERK1/2-dependent pathway. Obes Res. 2005;13:969–973. doi: 10.1038/oby.2005.113. [DOI] [PubMed] [Google Scholar]
- Song YH, Li Y, Du J, Mitch WE, Rosenthal N, Delafontaine P. Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J Clin Invest. 2005;115:451–458. doi: 10.1172/JCI22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza CJ, Eckhardt M, Gagen K, Dong M, Chen W, Laurent D, et al. Effects of pioglitazone on adipose tissue remodeling within the setting of obesity and insulin resistance. Diabetes. 2001;50:1863–1871. doi: 10.2337/diabetes.50.8.1863. [DOI] [PubMed] [Google Scholar]
- Stragier B, Hristova I, Sarre S, Ebinger G, Michotte Y. In vivo characterization of the angiotensin-(1–7)-induced dopamine and gamma-aminobutyric acid release in the striatum of the rat. Eur J Neurosci. 2005;22:658–664. doi: 10.1111/j.1460-9568.2005.04188.x. [DOI] [PubMed] [Google Scholar]
- Tabony AM, Yoshida T, Sukhanov S, Delafontaine P. Protein phosphatase 2C-alpha knockdown reduces angiotensin II-mediated skeletal muscle wasting via restoration of mitochondrial recycling and function. Skelet Muscle. 2014;4:20. doi: 10.1186/2044-5040-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner N, Cooney GJ, Kraegen EW, Bruce CR. Fatty acid metabolism, energy expenditure and insulin resistance in muscle. J Endocrinol. 2014;220:T61–T79. doi: 10.1530/JOE-13-0397. [DOI] [PubMed] [Google Scholar]
- Vazquez-Medina JP, Popovich I, Thorwald MA, Viscarra JA, Rodriguez R, Sonanez-Organis JG, et al. Angiotensin receptor-mediated oxidative stress is associated with impaired cardiac redox signaling and mitochondrial function in insulin-resistant rats. Am J Physiol Heart Circ Physiol. 2013;305:H599–H607. doi: 10.1152/ajpheart.00101.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Iyer SN, Chappell MC, Ganten D, Ferrario CM. Converting enzyme determines plasma clearance of angiotensin-(1–7) Hypertension. 1998;32:496–502. doi: 10.1161/01.hyp.32.3.496. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Semprun-Prieto L, Wainford RD, Sukhanov S, Kapusta DR, Delafontaine P. Angiotensin II reduces food intake by altering orexigenic neuropeptide expression in the mouse hypothalamus. Endocrinology. 2012;153:1411–1420. doi: 10.1210/en.2011-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Li Y, Li G, Song Y, Gong X. Ang(1–7) treatment attenuates beta-cell dysfunction by improving pancreatic microcirculation in a rat model of type 2 diabetes. J Endocrinol Invest. 2013;36:931–937. doi: 10.3275/8951. [DOI] [PubMed] [Google Scholar]
- Zhang L, Du J, Hu Z, Han G, Delafontaine P, Garcia G, et al. IL-6 and serum amyloid A synergy mediates angiotensin II-induced muscle wasting. J Am Soc Nephrol. 2009;20:604–612. doi: 10.1681/ASN.2008060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Tong Q, Liu W, Tian M, Xie W, Ji L, et al. Angiotensin (1–7) protects against stress-induced gastric lesions in rats. Biochem Pharmacol. 2014;87:467–476. doi: 10.1016/j.bcp.2013.10.026. [DOI] [PubMed] [Google Scholar]
- Zorad S, Dou JT, Benicky J, Hutanu D, Tybitanclova K, Zhou J, et al. Long-term angiotensin II AT1 receptor inhibition produces adipose tissue hypotrophy accompanied by increased expression of adiponectin and PPARgamma. Eur J Pharmacol. 2006;552:112–122. doi: 10.1016/j.ejphar.2006.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Systolic blood pressure (SBP), heart rate (HR), left ventricular weight, and AngII plasma concentration in SD (open bars) or TG rats (closed bars) depending on chow or CD feeding. Means + SEM, n = 11–14. *P < 0.05.
Figure S2 Body weight and energy intake are enhanced by CD feeding in older SD but not in TG rats (protocol 2). (A) Time-dependent increase in body weight. (B) Gain in body weight within the 12-week feeding period. (C) Time-dependent energy intake. (D) Cumulative energy intake: open bars depict energy intake originating from chow intake whereas shaded bars represent energy intake from chocolate/cookie bars. Means ± SEM, n = 11–14. *P < 0.05. †Intake of chocolate/cookie bars: P < 0.05 versus SDCD. ‡Chow intake: P < 0.05 versus SDCD.
Figure S3 Histological findings of livers from control- or CD-fed SD and TG rats. Means ± SEM, n = 11–14. *P < 0.05 versus control. Tissue specimens were evaluated, without knowledge of treatments, based on and scored for hepatocytes with steatosis.
Figure S4 mRNA levels of components of the ACE2, Mas, and AT1A and AT1b receptors in hypothalami of rats of protocol 2 (control- or CD-fed SD and TG rats) and protocol 3, respectively [CD-fed SD rats that were treated with telmisartan (TEL; 8 mg·kg−1·day−1) or telmisartan plus A779 (24 or 72 μg·kg−1·day−1) while controls received vehicle + saline]. Means ± SEM.
Figure S5 mRNA levels of MAS and ACE2 in different metabolic tissues of rats of protocol 2 (control- or CD-fed SD and TG rats) and protocol 3, respectively [CD-fed SD rats that were treated with telmisartan (TEL; 8 mg·kg−1·day−1) or telmisartan plus A779 (24 or 72 μg·kg−1·day−1) while controls received vehicle + saline]. Means ± SEM.
Figure S6 Insulin response is impaired by CD feeding in SD but not in TG rats (protocol 2). (A) Fasting glucose levels; (B) glucose plasma concentrations after insulin injections (0.6 IU insulin·kgbw−1, s.c.). The AUC (C), the minimal glucose levels (D), the time points of minimal glucose levels (E) and the half-life of glucose decline (F) were higher in SDCD than in SDcontrol, indicating impaired glucose control. A strain difference could be observed for all parameters. Means ± SEM, n = 11–14, *P < 0.05.
Figure S7 Systolic blood pressure (SBP, A), heart rate (HR, B), left ventricular weight (C), and AngII plasma concentration (D) in CD-fed SD rats that were treated for 4 weeks with telmisartan (TEL; 8 mg·kg−1·day−1) or telmisartan plus A779 (14 or 72 μg·kg−1·day−1, by osmotic minipumps). Controls received vehicle and saline. Means ± SEM, n = 11–12. *P < 0.05 versus vehicle + saline.
Figure S8 Energy expenditure (EE; A–C) respiratory ratio (RER, D–F), locomotion (G–I) and energy intake (K–M) during indirect calorimetry measurements. Animals were housed for 3 days in calorimetry cages, but only the data of the 3rd day are depicted. Mean values during light and dark periods were calculated for EE (B, C), RER (E, F) and locomotion (H, I), whereas total energy intake was depicted specifically considering light (L) and dark periods (M). Means ± SEM, n = 12, *P < 0.05 versus vehicle + saline.
Table S1 Nutrition composition of chocolate and cookie bars.
Table S2 mRNA levels of orexigenic (PPO, NPY, MCH, AgRP) and anorexigenic peptides (POMC, CART, corticotropin-releasing hormone, CRH) in hypothalami of Sprague-Dawley (SD) rats or transgenic rats (TG) overexpressing Ang(1–7) that received control diet or cafeteria diet (CD).
Table S3 mRNA levels of orexigenic (PPO, NPY, MCH, AgRP) and anorexigenic peptides (POMC, CART, CRH) in hypothalami of rats of CD-fed SD rats that were treated with telmisartan (8 mg·kg−1·day−1) or telmisartan plus A779 (24 or 72 μg·kg−1·day−1) while controls received vehicle + saline.
Appendix S1 Supplementary methodical information to OGTT, ITT, LTT, RNA isolation, cDNA synthesis and quantification of mRNA as well as supplementary discussion on hypothalamic expression of (an-)orexigenic peptides.