Abstract
To determine whether human immunodeficiency virus type 1 (HIV-1) strains in the lungs of infected individuals are derived from proviral forms contemporaneously present in the peripheral blood or whether they evolve independently as an autonomous pool of viral quasispecies, HIV-1 envelope V3 domain structures at these sites were analyzed and compared. The V3 loop proviral nucleotide and inferred amino acid sequences from lung bronchoalveolar lavage, where HIV-1 is primarily found in macrophages, were more homogeneous within individuals than those from unseparated peripheral blood mononuclear cells, where virus is predominantly in T cells. Comparison between individuals revealed that strains from bronchoalveolar lavage, but not from peripheral blood mononuclear cells, contained V3 domain nucleotide sequences with a great degree of homogeneity in the C-terminal region and a highly conserved, negatively charged amino acid motif. This V3 loop C-terminal structure could be important in the ability of HIV-1 to infect alveolar macrophages. Phylogenetic analyses of V3 domain nucleotide sequences in cells of monocyte/macrophage lineage at both sites revealed the strains in lung macrophages to have evolved further from a presumed ancestral species than those in blood monocytes and to differ considerably in the inferred V3 loop amino acid structures. These results show that, as disease progression occurs, viral strains in monocyte/macrophage lineage cells within the lung and blood microenvironments are not in a state of unrestricted bidirectional traffic but, instead, evolve independently.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agostini C., Trentin L., Zambello R., Semenzato G. HIV-1 and the lung. Infectivity, pathogenic mechanisms, and cellular immune responses taking place in the lower respiratory tract. Am Rev Respir Dis. 1993 Apr;147(4):1038–1049. doi: 10.1164/ajrccm/147.4.1038. [DOI] [PubMed] [Google Scholar]
- Chayt K. J., Harper M. E., Marselle L. M., Lewin E. B., Rose R. M., Oleske J. M., Epstein L. G., Wong-Staal F., Gallo R. C. Detection of HTLV-III RNA in lungs of patients with AIDS and pulmonary involvement. JAMA. 1986 Nov 7;256(17):2356–2359. [PubMed] [Google Scholar]
- Clarke J. R., Krishnan V., Bennett J., Mitchell D., Jeffries D. J. Detection of HIV-1 in human lung macrophages using the polymerase chain reaction. AIDS. 1990 Nov;4(11):1133–1136. doi: 10.1097/00002030-199011000-00012. [DOI] [PubMed] [Google Scholar]
- Dwyer E., Itescu S., Winchester R. Characterization of the primary structure of T cell receptor beta chains in cells infiltrating the salivary gland in the sicca syndrome of HIV-1 infection. Evidence of antigen-driven clonal selection suggested by restricted combinations of V beta J beta gene segment usage and shared somatically encoded amino acid residues. J Clin Invest. 1993 Jul;92(1):495–502. doi: 10.1172/JCI116593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch W. M., Margoliash E. Construction of phylogenetic trees. Science. 1967 Jan 20;155(3760):279–284. doi: 10.1126/science.155.3760.279. [DOI] [PubMed] [Google Scholar]
- Fouchier R. A., Groenink M., Kootstra N. A., Tersmette M., Huisman H. G., Miedema F., Schuitemaker H. Phenotype-associated sequence variation in the third variable domain of the human immunodeficiency virus type 1 gp120 molecule. J Virol. 1992 May;66(5):3183–3187. doi: 10.1128/jvi.66.5.3183-3187.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Hwang S. S., Boyle T. J., Lyerly H. K., Cullen B. R. Identification of the envelope V3 loop as the primary determinant of cell tropism in HIV-1. Science. 1991 Jul 5;253(5015):71–74. doi: 10.1126/science.1905842. [DOI] [PubMed] [Google Scholar]
- Itescu S., Brancato L. J., Winchester R. A sicca syndrome in HIV infection: association with HLA-DR5 and CD8 lymphocytosis. Lancet. 1989 Aug 26;2(8661):466–468. doi: 10.1016/s0140-6736(89)92085-0. [DOI] [PubMed] [Google Scholar]
- Itescu S., Dalton J., Zhang H. Z., Winchester R. Tissue infiltration in a CD8 lymphocytosis syndrome associated with human immunodeficiency virus-1 infection has the phenotypic appearance of an antigenically driven response. J Clin Invest. 1993 May;91(5):2216–2225. doi: 10.1172/JCI116448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980 Dec;16(2):111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Korber B., Wolinsky S., Haynes B., Kunstman K., Levy R., Furtado M., Otto P., Myers G. HIV-1 intrapatient sequence diversity in the immunogenic V3 region. AIDS Res Hum Retroviruses. 1992 Aug;8(8):1461–1465. doi: 10.1089/aid.1992.8.1461. [DOI] [PubMed] [Google Scholar]
- Koyanagi Y., Miles S., Mitsuyasu R. T., Merrill J. E., Vinters H. V., Chen I. S. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987 May 15;236(4803):819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- Looney D. J., Fisher A. G., Putney S. D., Rusche J. R., Redfield R. R., Burke D. S., Gallo R. C., Wong-Staal F. Type-restricted neutralization of molecular clones of human immunodeficiency virus. Science. 1988 Jul 15;241(4863):357–359. doi: 10.1126/science.3388046. [DOI] [PubMed] [Google Scholar]
- Meltzer M. S., Skillman D. R., Gomatos P. J., Kalter D. C., Gendelman H. E. Role of mononuclear phagocytes in the pathogenesis of human immunodeficiency virus infection. Annu Rev Immunol. 1990;8:169–194. doi: 10.1146/annurev.iy.08.040190.001125. [DOI] [PubMed] [Google Scholar]
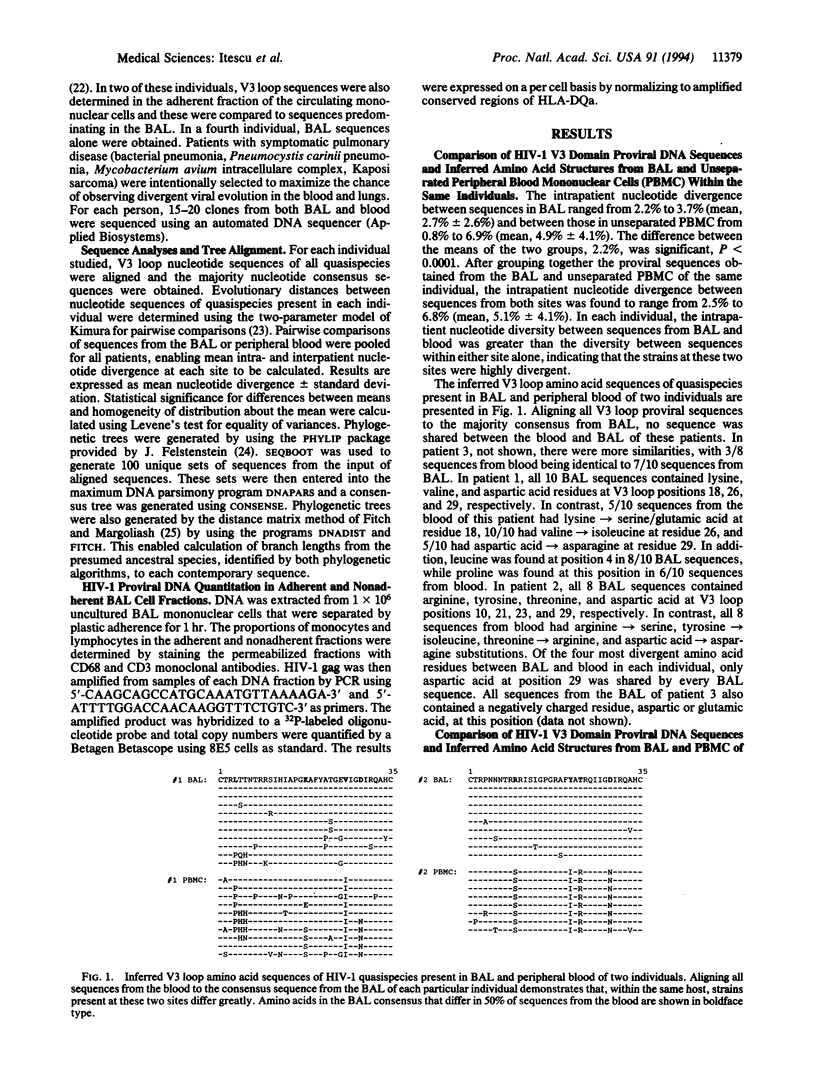
- Nelson J. A., Ghazal P., Wiley C. A. Role of opportunistic viral infections in AIDS. AIDS. 1990 Jan;4(1):1–10. doi: 10.1097/00002030-199001000-00001. [DOI] [PubMed] [Google Scholar]
- O'Brien W. A., Koyanagi Y., Namazie A., Zhao J. Q., Diagne A., Idler K., Zack J. A., Chen I. S. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature. 1990 Nov 1;348(6296):69–73. doi: 10.1038/348069a0. [DOI] [PubMed] [Google Scholar]
- Plata F., Autran B., Martins L. P., Wain-Hobson S., Raphaël M., Mayaud C., Denis M., Guillon J. M., Debré P. AIDS virus-specific cytotoxic T lymphocytes in lung disorders. Nature. 1987 Jul 23;328(6128):348–351. doi: 10.1038/328348a0. [DOI] [PubMed] [Google Scholar]
- Rich E. A., Chen I. S., Zack J. A., Leonard M. L., O'Brien W. A. Increased susceptibility of differentiated mononuclear phagocytes to productive infection with human immunodeficiency virus-1 (HIV-1). J Clin Invest. 1992 Jan;89(1):176–183. doi: 10.1172/JCI115559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg Z. F., Fauci A. S. The immunopathogenesis of HIV infection. Adv Immunol. 1989;47:377–431. doi: 10.1016/s0065-2776(08)60665-3. [DOI] [PubMed] [Google Scholar]
- Rusche J. R., Javaherian K., McDanal C., Petro J., Lynn D. L., Grimaila R., Langlois A., Gallo R. C., Arthur L. O., Fischinger P. J. Antibodies that inhibit fusion of human immunodeficiency virus-infected cells bind a 24-amino acid sequence of the viral envelope, gp120. Proc Natl Acad Sci U S A. 1988 May;85(9):3198–3202. doi: 10.1073/pnas.85.9.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuitemaker H., Koot M., Kootstra N. A., Dercksen M. W., de Goede R. E., van Steenwijk R. P., Lange J. M., Schattenkerk J. K., Miedema F., Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J Virol. 1992 Mar;66(3):1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds P., Balfe P., Peutherer J. F., Ludlam C. A., Bishop J. O., Brown A. J. Human immunodeficiency virus-infected individuals contain provirus in small numbers of peripheral mononuclear cells and at low copy numbers. J Virol. 1990 Feb;64(2):864–872. doi: 10.1128/jvi.64.2.864-872.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Cohen J., Hosmalin A., Cease K. B., Houghten R., Cornette J. L., DeLisi C., Moss B., Germain R. N., Berzofsky J. A. An immunodominant epitope of the human immunodeficiency virus envelope glycoprotein gp160 recognized by class I major histocompatibility complex molecule-restricted murine cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1988 May;85(9):3105–3109. doi: 10.1073/pnas.85.9.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfs T. F., de Jong J. J., Van den Berg H., Tijnagel J. M., Krone W. J., Goudsmit J. Evolution of sequences encoding the principal neutralization epitope of human immunodeficiency virus 1 is host dependent, rapid, and continuous. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9938–9942. doi: 10.1073/pnas.87.24.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky S. M., Wike C. M., Korber B. T., Hutto C., Parks W. P., Rosenblum L. L., Kunstman K. J., Furtado M. R., Muñoz J. L. Selective transmission of human immunodeficiency virus type-1 variants from mothers to infants. Science. 1992 Feb 28;255(5048):1134–1137. doi: 10.1126/science.1546316. [DOI] [PubMed] [Google Scholar]
- Zhu T., Mo H., Wang N., Nam D. S., Cao Y., Koup R. A., Ho D. D. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993 Aug 27;261(5125):1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- Zwart G., Langedijk H., van der Hoek L., de Jong J. J., Wolfs T. F., Ramautarsing C., Bakker M., de Ronde A., Goudsmit J. Immunodominance and antigenic variation of the principal neutralization domain of HIV-1. Virology. 1991 Apr;181(2):481–489. doi: 10.1016/0042-6822(91)90880-k. [DOI] [PubMed] [Google Scholar]