Abstract
BACKGROUND/OBJECTIVES
The prevalence of nonalcoholic fatty liver disease (NAFLD) has increased worldwide in parallel with overnutrition characterized by high-fat and high-carbohydrate intake. Our objective was to establish, in 16 weeks, a model of NAFLD in Wistar pathogen-free rats following four dietary types.
MATERIALS/METHODS
Forty (6 weeks old) healthy Wistar male rats, weighing an average of 150 g were randomly divided into four groups of ten and assigned a diet with the same quantity (15 g/rat/day), but with different composition. The moderate-fat (MF) group was fed a moderate-fat diet (31.5% fat and 50% carbohydrates), the high-fat (HF) group was fed a fat-rich diet (51% fat), the high-sucrose (HS) group and the high-fructose (HFr) group were fed a carbohydrate-rich diet (61%). The carbohydrate contents of the HS group was composed of 60.3% sucrose while that of the HFr group was composed of 59.3% fructose.
RESULTS
At week 16, the HF group had the highest percentage of cells enriched in fat (40%) and the highest weight and liver weight (P < 0.05). The HFr group showed significantly higher levels of serum triglycerides, alanine aminotransferase and adiponectin at week 16 as compared to week 1 (P < 0.05).
CONCLUSIONS
The 15 g/rat/day diet composed of 51% fat or 61% carbohydrates enriched mainly in fructose may induce characteristics of NAFLD in rats.
Keywords: Adiponectin, sucrose, triglycerides, fructose, Wistar rats
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is defined as the accumulation of triglycerides in liver hepatocytes of patients who do not consume large quantities of alcohol (less than one standard drink/day for women and less than two standard drinks of ethanol/day for men) [1]. It includes nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH). Steatosis is determined by estimating the proportion of hepatocytes containing fat droplets. The suggested lower threshold is 5% of hepatocytes [1]. Fat accumulation could be microvesicular, characterized by numerous small cytoplasmic lipid droplets around the central nucleus, or macrovacuolar where each fatty hepatocyte contains a large droplet of fat that displaces the nucleus to the periphery [2]. This corresponds to the first hit hypothesis proposed by Day and James in 1998 [7] to describe the evolution of NAFL into NASH [1,3,4,5,6,7,8]. NASH, which determines the second hit hypothesis, is characterized by cellular necrosis or apoptosis due to continuous cell exposure to changes in environmental or genetic factors. This is accompanied by an increase in inflammatory cytokines/adipokines such as tumor necrosis factor alpha (TNF-α), interleukin (IL) 6 and 8, mitochondrial dysfunction and oxidative stress which leads to fibrosis [8].
NAFL and NASH have recently been recognized as hepatic manifestations of the metabolic syndrome [3], paralleling the epidemic of obesity, which is characterized by at least three of the following criteria: obesity, hyperlipidemia, hyperglycemia, and hypertension [9]. The current increase in incidence of this disorder may reflect changes in environmental factors, particularly diet and physical activity [10]. With an increase in high-fat food intake, diagnosis of NAFLD has increased substantially over the past decade in parallel with obesity and type 2 diabetes [11].
Along with an increase in consumption of fat in industrialized countries, consumption of fructose, due to intake of high fructose fruit drinks, carbonated-beverages, candies, and baked goods, is increasing significantly. In studies using experimental animals, fructose-enriched diets have been shown to induce the parameters of metabolic syndromes [10] and an increase in fatty liver, fibrosis, and inflammatory markers [12].
The main hypothesis of the current study, is that diets given in strict quantities (15 g/rat/day) but rich in fat (at least 51% of the total energy) or carbohydrates (at least 61% of the total energy), enriched in sucrose or fructose (60.3% and 59.3% of total carbohydrates) induce NAFLD and its characteristics.
MATERIALS AND METHODS
Experimental design and animals
Forty male (6 weeks old) Wistar pathogen-free rats (Arab University, Beirut, Lebanon) weighing between 150 and 180 g were used. They were housed individually in chip-bedded 50 × 50 cm plastic cages at room temperature (23 ± 2℃), humidity (60 ± 10%) in a 12:12 h diffuse light/dark cycle at Saint Joseph University, research sciences laboratory for 16 weeks. Rat cages conformed to the Animal Research Review Panel (ARRP) guidelines 20, for rat housing in scientific institutions according to the floor, area, shape, materials, ventilation, bedding, and nesting [13]. All rats received humane care and were allowed visual, auditory, and olfactory contact with each other. The rats were issued from pure Wistar crossing and they were clear from any mutation regarding the ob/ob genes.
Diets and blood extraction
At week 1, all rats received a chow diet (16% proteins, 3% fat and 60% carbohydrates) ad libitum; the caloric content of the diet was 12.1 kJ/g. At the end of week 1, food was removed from all cages at 4 o'clock in the afternoon of the day preceding the anesthesia. Rats were then starved for 16 hours before extraction of blood from the jugular vein under general anesthesia (0.2 ml/rat, 10 ml of ketamine 50 mg/ml, 2 ml of xylazine 25 mg/ml, injected intramuscularly). 2 ml of blood sample/rat was collected. At week 2, the 40 rats were divided randomly into 4 groups of 10: (1) moderate-fat (MF) group, (2) high-fat (HF) group, (3) high-sucrose (HS) group, and (4) high-fructose (HFr) group.
The same quantity of food (15 g/rat/day) with different nutrient composition was given to each group. The 40 rats had free access to drinking water in a feeding cup on a daily basis until the end of the study. The MF group received a moderatefat diet until the end of the study (17.05 kJ/g; 18.5% proteins, 31.5% fat and 50% carbohydrates). The HF group received a high-fat diet (19.59 kJ/g; 16.2% proteins, 51% fat and 32.8% carbohydrates) and the third and fourth groups were fed on average the same quantity/rat/day of a carbohydrate-rich diet (61%) of which 60.3% of carbohydrate contents was sucrose (16.25 kJ/g) and 59.3% was fructose (16.15 kJ/g) for the same period of time. The fat used in the four diets consisted of butter (51% saturated fatty acid, 21% monounsaturated fatty acid, and 3% polyunsaturated fatty acid) and soya bean oil (16% saturated fatty acid, 23% monounsaturated fatty acid, and 58% polyunsaturated fatty acid). The food was prepared every month following AIN-93G formula [14] at the American University of Beirut pilot plant and stored at a temperature of 3-4℃. The food intake consumed/rat/day and rats weight were measured on a daily and weekly basis. The amount of food consumed/rat/day (absolute food consumption) was calculated by subtracting the 15 g of food given/rat/day from the amount left or spilled in the cage. This was converted to energy intake (kJ/rat/week) (Table 1). At week 16, all rats were starved for 16 hours, similar to week 1, before excising and weighing their liver. Blood samples (2 ml of blood sample/ rat) were obtained simultaneously by the inferior vena cava.
Table 1. Composition of the MF, HF, HS and HFr diets.
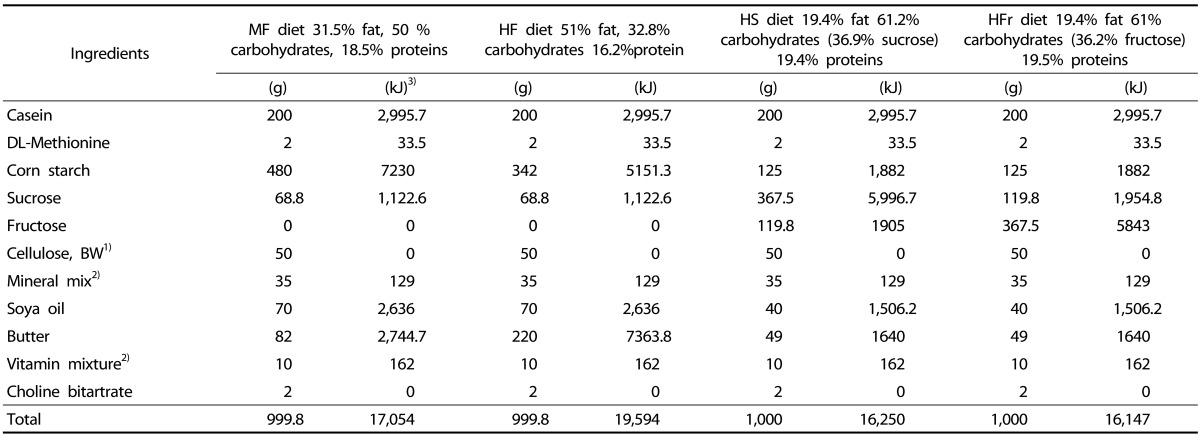
1) Alpha cellulose grade BW-200. Maple Biotech pvt. Ltd.
2) Composition of Vitamin mixture (AIN-93-VX; Clea, Tokyo, Japan) and Mineral mixture (AIN-93G-MX; Clea) [14].
3) Energy values (kJ) of the corresponding items (g) (energy values were calculated according to the typical caloric density values for commonly used diet ingredients for laboratory animals, Dyets, Inc)
Sample preparation, histological examination and liver lipid determination
Blood samples were separated by centrifugation (1000 ×g; 5 min) and blood serum and liver fragments were stored at -80℃ until analysis. A fragment of liver was removed from all rats and sent to the pathology laboratory, and submitted for histological examination. Liver fragments were fixed in formalin at 10%, routinely processed and embedded in paraffin. Sections of 3 µm were cut and stained with hematoxylin and eosin (H&E). Picrosirius staining was perfomed to evaluate the degree of portal and peri-sinusoidal fibrosis. Frozen fragments of liver tissue from the 40 rats were sectioned in order to perform Oil Red O staining.
The slides were examined under optic microscopy (Axioskop, Zeiss, Oberkochen, Germany). The percentage of steatosis was determined by evaluating the number of hepatocytes enriched in fat, multiplied by 100 over the total number of cells in ten randomly chosen different medium-power fields (×200). The pattern of lipid accumulation in hepatocytes (micro or macrovacuolar) was also evaluated. Micro and macrovacuolar patterns, necro inflammation and perisinusoidal fibrosis were scored as absent (0), mild (1), moderate (2) or severe (3) depending on the percentage of fatty hepatocytes, the amount of inflammatory infiltrates, and the extent of extracellular matrix deposition. Mild necro inflammation referred to a few lobular aggregates of inflammatory cells with or without apoptotic bodies. Necro inflammation was considered moderate when at least one lobular area contained two or more of such aggregates. Ballooning degeneration was scored (0) if absent, (1) if few and (2) if many hepatocytes were involved. Nonalcoholic fatty liver disease activity score (NAS) was calculated using the scoring system of Kleiner et al. [15].
Forty frozen fragments of liver tissue from the 40 rats were also sectioned for determinatione of total liver lipid content for each rat. Lipid was extracted using the Soxhlet method according to the official methods of analysis of AOAC INTERNATIONAL, method 960.39 [16]. This method, which uses an organic solvent such as petroleum ether, is suitable for extraction of mainly unbound, neutral lipid such as triglycerides, free fatty acids, and cholesterol esters [17]. Thus, the total hepatic lipid determined on the 40 liver rats was mainly a determination of non-polar lipid.
Method of lipid extraction
A liver fragment of each rat (approximately 3 g) was weighed using an analytical balance (Adam, RE 6801425, Milton Keynes, England), which records weight to nearest 0.0001 g. The sample was then placed in a cellulose thimble containing sea sand, dried for removal of excess moisture, and ground. The lipid was extracted by continuous refluxing of solvent (Petroleum ether) through the sample. The extraction procedure lasted around 6 hours. At the end, the solvent containing the extracted lipid was placed in a cup, evaporated and the fatty residue was dried to a constant weight, then cooled and weighed. The lipid liver content of each rat was calculated according to the formula: lipid liver weight/liver sample (mg/g) = F-T/S, where F = weight of cup + lipid residue (g); T = weight of empty cup (g); S = liver sample wet weight (g). The results were expressed in mg/g.
Serum chemistry
The serum level of fasting glucose was measured using a spectrophotometer, using a commercially available kit (Trinder method, Biolabo SA, Maizy, France). Serum alanine aminotransferases (ALT) and triglycerides were also measured using a spectrophotometer, using commercially available kits (Wrobleski and LaDue method, Biolabo SA, Maizy, France and Fossati and Prencipe method, Biolabo SA, Maizy, France, respectively). Serum TNF-α and adiponectin were analyzed using Elisa kits (rat High Sensitive TNF-α, R&D, UK and rat adiponectin,Xceltis, Mannheim, Germany). Serum insulin concentration was also analyzed using Elisa kits (rat insulin, Xceltis, Germany).
Ethical considerations
All experiments were performed at the Surgical Research Laboratory of Saint Joseph University Medical School (Beirut, Lebanon) in accordance with the "Guide for care and use of laboratory animals" (Department of Health and Human Services. Public Health Service, National Institutes of Health. NIH Publication No. 86-23, Revised 1985). The protocol was accepted by the ethical committee of the Medical School of Saint Joseph University, Beirut, Lebanon (CEHDF 351).
Statistical analyses
The sample size of 40 rats corresponds to the minimal size recommended for experimental animal studies for detection of significant difference between the four groups with a 95% confidence interval. Results were expressed as mean and standard deviation. Statistical analyses were performed using student's paired t-test or Wilcoxon signed-rank test for each group. The Kruskal-Wallis test for non-parametric data or one-way between-groups analysis of variance (ANOVA) over week 1 and week 16 for normally distributed data was also performed, followed by the Bonferroni multiple comparisons test. Significance level was set at P < 0.05. Statistical analysis was performed using SPSS 20 for windows release (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY, USA).
RESULTS
Body weights (g), energy intake (kJ/week) and amount of food (g/rat/day)
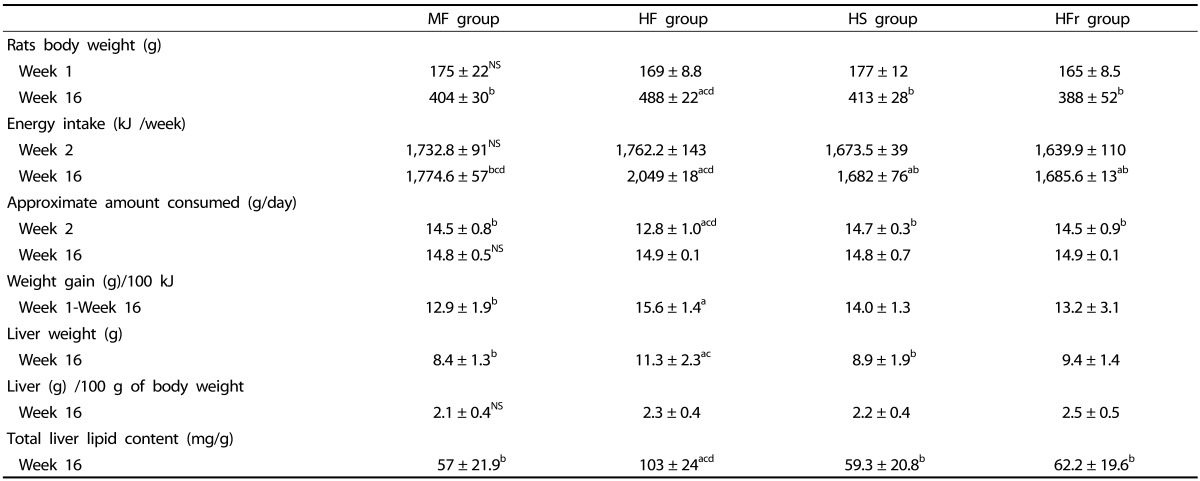
At the end of week 1, there were no significant differences in the rats' body weight. Difference in energy intake (kJ/week) was reported between groups at the end of weeks 16 (P < 0.05). At week 16, the amount of food consumed (g/day) did not differ significantly between groups. Body weight of the HF group was significantly higher than that of the other three groups (P < 0.05). The HF diet group showed a significant body weight gain/100 kJ as compared to the MF group.
Organ weights (g)
At week 16, a significant increase in liver weight was observed in the HF group compared to the other groups, and the lowest increase was observed in the MF and HS groups (P < 0.05). The highest liver weight/100 g of body weight was observed in the HFr group, with no significant difference compared with the other groups (Table 2).
Table 2. Compared of the physique change, liver weight and energy intake in MF, HF, HS and HFr diets.
Data are means ± SD, n = 10 rats/group.
NS, not significant. MF, Moderate-fat; HF, High-fat; HS, High-sucrose; HFr, High-fructose.
"a,b,c,d," refer to differences between groups (P < 0.05). Small "a" refers to the MF group, small "b" refers to the HF group, small "c" refers to the HS group, small "d" refers to the HFr group. The Kruskal-Wallis test for non-parametric data or one-way Anova between-groups over week 1 and week 16 for normally distributed data was also performed followed by the Bonferroni multiple comparisons test.
Liver histopathology
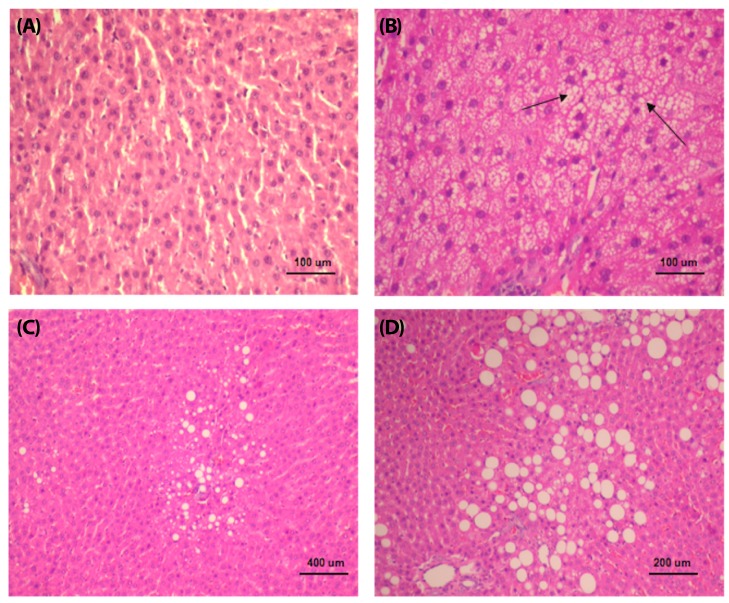
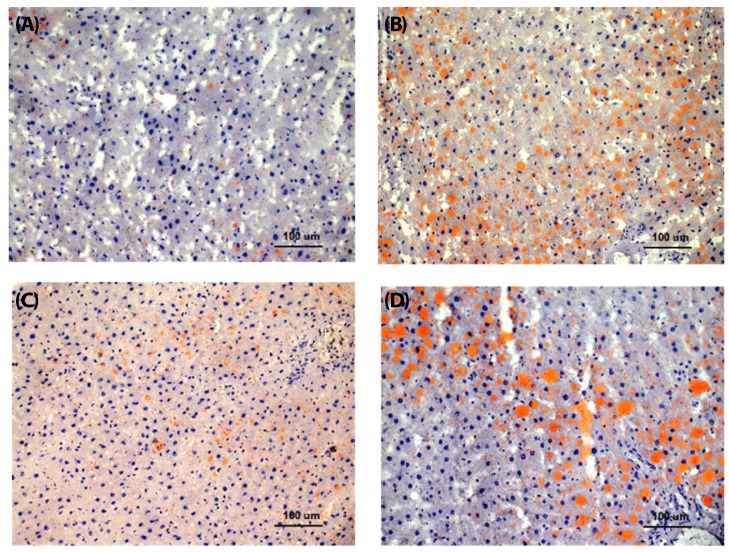
At week 16, steatosis was observed in three studied groups of rats (HF, HS, and HFr groups). The HF group showed the highest percentage of steatosis (40%), whereas the HFr and HS rats presented mild steatosis (15% and 9%, respectively). The MF group did not show significant steatosis (less than 5%) (Table 3, Fig. 1A, 2A). Both micro and macrovacuolar patterns of steatosis were observed in the three studied groups, with variable percentages. While microvesicular steatosis was more prominent in the HF group (Fig. 1B, 2B), macrovacuolar steatosis was mild in the HS group and moderate in the HFr group (Fig. 1C, 2C, 1D, 2D). Necro inflammation was observed mainly in the HF group and to a lesser extent in the HFr group compared to the MF and HS groups. Periportal fibrosis was absent in all groups. No significant difference with respect to perisinusoidal fibrosis, which was absent or mild, was observed between the groups. None of the four rat groups presented a NAS of 5 and above (Table 3). Representative liver specimens from the four experimental groups are shown in Fig. 1A, 1B, 1C, and 1D, and Fig. 2A, 2B, 2C, and 2D (Oil red O staining).
Table 3. Pathological features of liver between groups at week 16.
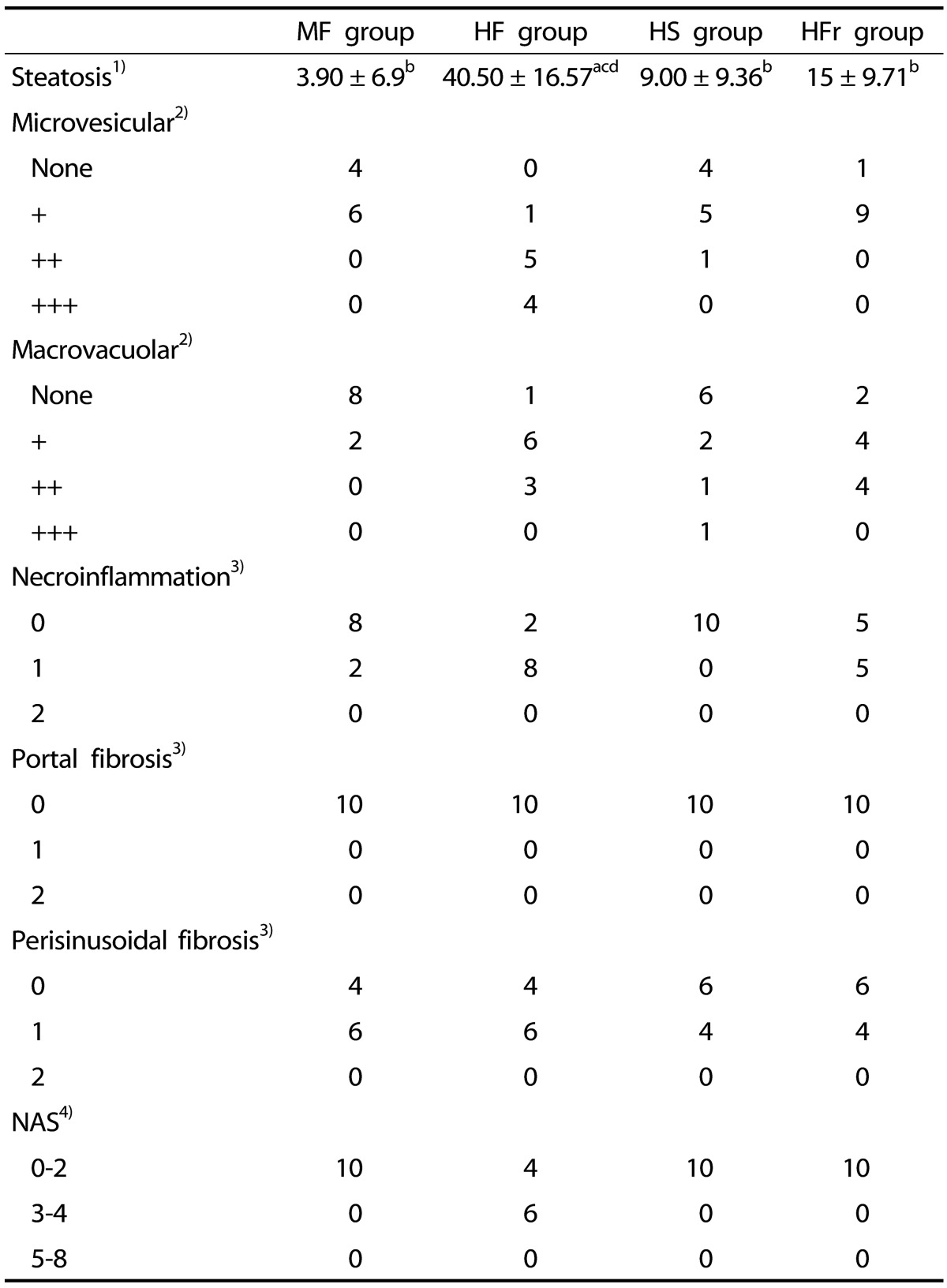
1) Results for steatosis are means ± SD.
MF, Moderate-fat; HF, High-fat; HS, High-sucrose; HFr, High-fructose. "a,b,c,d," refer to differences between groups (P < 0.05). Small "a" refers to the MF group, small "b" refers to the HF group, small "c" refers to HS group, small "d" refers to the HFr group.
2) Results for macrovacuolar and microvesicular steatosis are given as number of rats presenting mild + (<33% of hepatocytes), moderate++ (33-66% of hepatocytes) or severe +++ (>66% of hepatocytes).
3) Necroinflammation and fibrosis are given as number of rats presenting a score of 0, 1 or 2 respectively.
4) Results for NAS are given as number of rats presenting scores of 0-2, 3-4 or 5-8 respectively. NAS, Non alcoholic fatty liver disease activity score.
Fig. 1. Sections of liver in (A) a rat fed the moderate-fat diet (H&E, ×200), (B) area of microvesicular steatosis in a rat fed a high-fat diet (H&E, ×200), and liver of a rat fed a diet rich in (C) sucrose (H&E, ×50), and (D) fructose (H&E, ×100), showing, mild and moderate macrovacuolar steatosis, respectively.
Fig. 2. Oil red O stains in red. (A) rare vesicles of steatosis in a rat fed the moderate-fat diet, (B) numerous small vesicles of steatosis in a rat fed the high-fat diet, (C) mild and (D) moderate steatosis in a rat fed a diet rich in sucrose and fructose, respectively (×200).
Total hepatic lipid content (mg/g)
Total hepatic lipid content was significantly higher in the HF group compared with the other three groups (P < 0.05). Among the three groups, the MF group showed the lowest total hepatic lipid content (Table 2).
Serum chemistry
At the end of week 1, no significant differences in blood parameters were observed between groups except for the HF group. The latter showed a significantly higher mean of ALT and triglycerides compared to the other groups (P < 0.05). At week 16, a significant increase in fasting serum glucose insulin concentration and ALT was observed in all groups between weeks 1 and 16. A significant increase in serum TNF-α concentration was observed in the HFr group compared to the MF group (P < 0.05). The HFr group also showed the highest serum triglycerides concentration at week 16 and was significantly different from week 1. Finally, serum adiponectin concentration showed a significant decrease between weeks 1 and 16 in all groups except for the HFr group, which showed an abrupt increase in serum adiponectin concentration between the two time periods (Table 4).
Table 4. Serum chemistry of rats between and within groups (week 1-week 16).
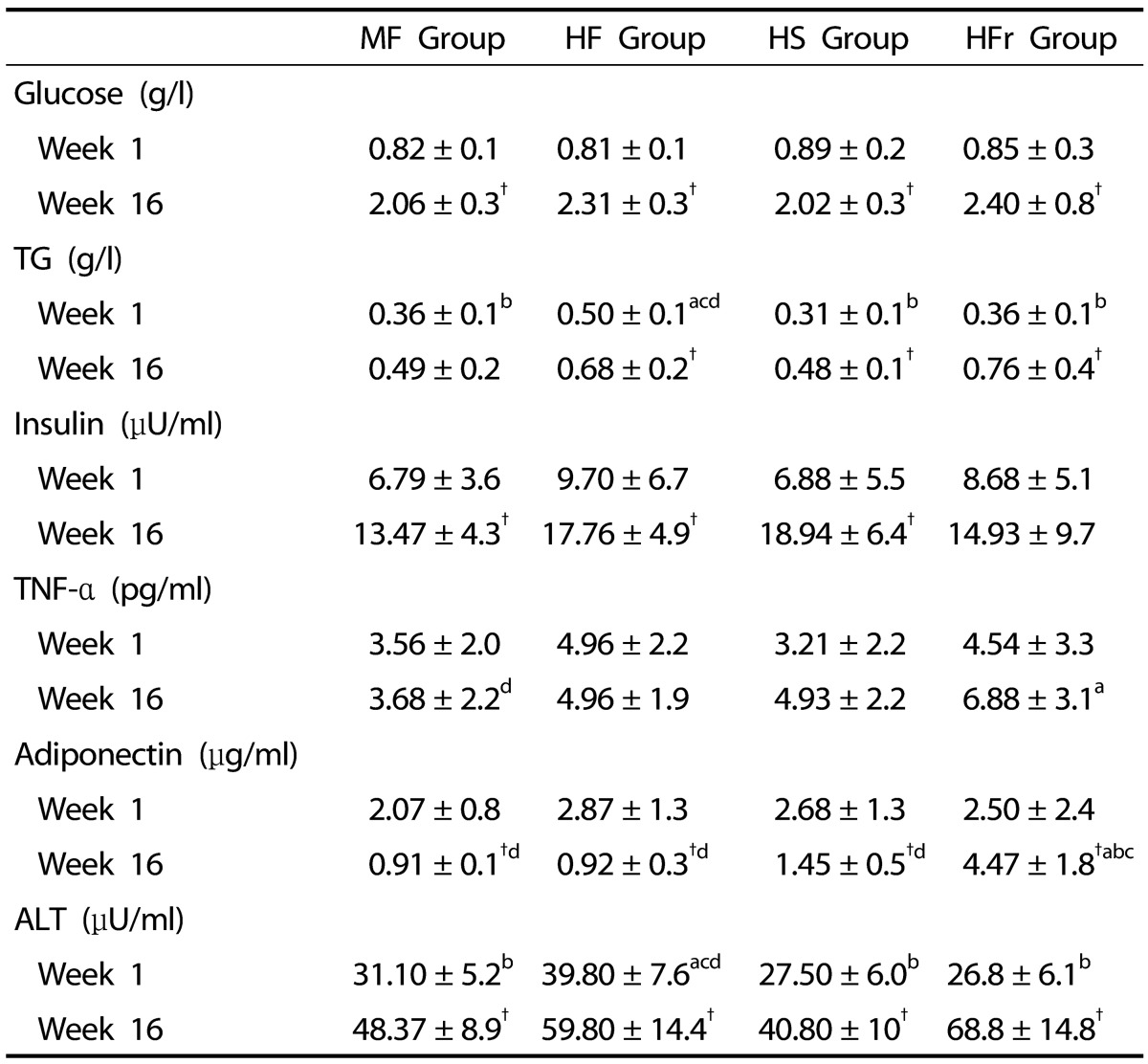
Data are means ± SD, n = 10 rats group.
MF, Moderate-fat; HF, High-fat; HS, High-sucrose; HFr, High-fructose.
† Significant differences within groups (week 1-week 16).
"a,b,c,d," refer to differences between groups (P < 0.05). Small "a" refers to the MF group, small "b" refers to the HF group, small "c" refers to the HS group, small "d" refers to the HFr group.
Statistical analyses were performed using student's paired t-test or Wilcoxon signed-rank test for each group. The Kruskal-Wallis test for non-parametric data or one-way between groups ANOVA over week 1 and week 16 for normally distributed data was also performed followed by the Bonferroni multiple comparisons test.
DISCUSSION
In the current study, we attempted to imitate the diet composition of patients diagnosed with NAFLD. In order to eliminate any nutritional deficiency, we also followed the AIN-93G formula during development of the different diets.
To the best of our knowledge, our study is the first one to restrict the amount usually consumed to 15 g/rat/day in order to highlight the impact of food composition on induction of the disease, especially that food consumption (g/day) was almost the same between groups during the entire experimental period. The difference in energy intake/week at weeks 2 and 16 between groups is related to the difference in energetic composition of the different rats' diets. Of note, the dietary intake of 15 g/rat/day was chosen in compliance with the nutrient requirements of laboratory animals [18] which is considered the minimal amount required per rat/day to ensure normal growth of rats. In addition, one feature of our study is that it assessed the blood parameters at two time points.
The model reproduced the pathogenic factors related to the disease. The significant increase in body and liver weight observed in HF rats at week 16 was reported in other studies [3,11,19]. It reproduced the increase or accumulation of lipid in both body and liver due to the high-fat diet [19]. In addition, the high total hepatic lipid content at week 16 was mainly the result of the high-fat feeding [3,20] and was confirmed by the high percentage of steatosis in this group (40.5%). The increase in total hepatic content in this group was approximately 2-fold those of the HS and MF groups. Significantly higher body weight gain (g)/100 kJ at week 16 was observed in the HF group as compared to the MF group, highlighting the impact of a high-fat diet, even in strict quantities on body weight gain.
The highest liver weight (g)/100 g of body weight observed in the HFr group at week 16 indicated the hepatic role of fructose in de novo lipogenesis (DNL) through its rapid conversion to pyruvate, bypassing the phosphofructokinase regulatory step. Another hepatic role for fructose is its stimulation of both carbohydrate response element binding protein (CHREBP) and sterol regulatory element binding protein (SREBP-1C) which further accentuate DNL enzymatic activity [21]. On the other hand, higher total hepatic lipid content was observed in the HFr group than in the HS and MF groups. Compared to fat, fructose seems to act mainly on hepatic lipid deposition while fat acts on hepatic and body fat deposition (an increase in both liver and body weight). Our results will allow us to suppose that even with a 36.2% fructose restricted diet, the risk of incidence of NAFLD is high and experimentally comparable to results seen with higher percentage of fructose and food was given ad libitum. In the study by Kawasaki et al., the percentage of fructose used in the experimental study to induce hepatic change and damage in Wistar rats was 70% of total energy [10], while in the study by Ackerman et al., the amount of fructose used to induce hepatic damage in Sprague-Dawley rats was 60% of total energy [22].
Regarding steatosis, the highest increase in its percentage in both HF and HFr groups (40.5% and 15% respectively) and the high prevalence of microvesicular and macrovacuolar steatosis indicated the accumulation or recapture of fatty acids in hepatocytes and their esterification as numerous fat droplets around the central nucleus or as unique fatty vacuole. In our study, the MF group had not developed microvesicular or macrovesicular steatosis at week 16. This is a new finding, considering that Wistar rats are very sensitive to fat diets. In studies by Zheng-Jie et al. and Kucera et al. [11,23], the same percentage of fat in the diet induced significant microvesicular steatosis in Wistar and Sprague-Dawley rats at weeks 3 and 4. One explanation is that food was given ad libitum in these two studies as compared to our strict amount of food given per rat/day. Another explanation could be a difference in rat strains. In studies by Lieber et al. and Ahmed et al. [3,24], Sprague-Dawley rats did not experience hepatic change while on the MF diet (considered standard diet in the study by Lieber et al.). However, rats were exposed to the experimental diets for a shorter period of time as compared to our study (3 weeks versus 16 weeks).
In our study severe inflammation or fibrosis was not induced. The presence of mild necroinflammation in both the HF and HFr groups may be attributable to the increase in percentage of steatosis as well as the presence of microvesicular and/or macrovacuolar steatosis. Periportal fibrosis was not observed in any group at week 16, suggesting that more time was needed or a higher percentage of fat or fructose in diets was necessary for development of the final pathway of hepatic damage preceding cirrhosis. The mild perisinusoidal fibrosis observed in all groups may be due to an excess in the quantity of vitamin A in all diets. The concentration of vitamin A (trans-retinyl palmitate) in the AIN-93-VX vitamin mix used was 0.8 g/kg while the nutritional requirement for trans-retinyl palmitate according to the nutritional requirement of laboratory animals is 1.3 mg/kg [18]. Increase in hepatic stellate cells, which are vitamin-A storing cells located in Disse's space around the hepatic sinusoids, can lead to induction of perisinusoidal fibrosis [25].
NAFLD is considered the hepatic component of metabolic syndrome. Biological parameters such as fasting hypertryglicerimia, hyperglycemia, and hyperinsulinemia are the main parameters studied for assessment of metabolic syndrome status. In our study, higher fasting serum glucose concentrations were observed in all groups at week 16 (Table 4). This may be attributable to the anesthesia, ketamine, and xylazine (KX) used during the two time periods of the study. In fasted rats, KX-induced hyperglycemia may result from increased glucose output secondary to an increase in glucose production [26]. Furthermore, the time of blood collection, 20 minutes after anesthesia at week 16 as compared to 10 minutes at week 1, can justify the absence of hyperglycemia at week 1. This difference in time during blood collection was due to the fact that rats were operated and blood collected from the inferior vena cava before sacrifice at week 16 while at week 1, blood was collected from the external jugular in order to minimize the stress related to a surgical procedure. In a study by Kawasaki et al., the authors reported the same increase in plasma glucose concentrations with ether anesthesia before sacrifice [10].
Higher serum insulin concentrations were observed in all groups at week 16 as compared to week 1 with an increase of almost 3-fold in the HS group (Table 4). This is in accordance with other studies, indicating the presence of an insulin resistance status [27,28]. Increase in lipid metabolites and presence of free fatty acids released from adipose tissue have been implicated as interfering with insulin signaling through inhibition of insulin receptor activity and modulation of insulin receptor substrate-2 (IRS-2) phosphorylation [8]. The high availability of serum free fatty acid leading to an increase in insulin is related to the exogenous fat or the high-fat content diet of the HF group. The MF group could be considered high-fat diet [11] when compared to the rats standard pellet diet contatinings 3% lipids and 60% carbohydrates. The increase in serum insulin following high-sucrose ingestion (HS group) is inherent to the role of insulin in facilitating glucose deposit in liver as glycogen by activating glycogen synthase or by increasing SREBP-1C, thus contributing to the process of DNL [21]. Fructose also contributes to an increase in insulin by interfering with insulin signaling and inhibition of insulin receptor activity [21].
Reduced circulating and secretion levels of adiponectin are generally observed in patients with NAFLD [29,30]. In our model, the HFr group showed the highest serum adiponectin concentrations at week 16 as compared to week 1. In contrast, other groups showed a significant decrease in serum adiponectin concentrations between the two time periods (P < 0.05). A significant increase in adiponectin levels in rats consuming a high-fructose diet was reported in other studies [31]. This may be explained by the fact that a diet rich in fructose induces adiponectin resistance leading to a momentary increase in the serum hormonal concentration [31]. The duration of the study was 16 weeks. Increasing the time period might have led to a drastic decrease in this hormone in the HFr group.
TNF-α is a well-known inflammatory marker. High serum level of ALT and AST is also markers of liver damage. AST as a marker was not considered in this study due to the small amount of blood collected at weeks 1 and 16. At week 1, significantly higher serum concentration of triglycerides and ALT was observed in the HF group compared to other groups. However, these values were in the normal range for rats in the HF group and were not significantly different from those of other groups at week 16. On the other hand, at week 16, the HFr group showed the highest serum ALT, TNF-α, and triglycerides concentrations. Findings suggest that this is due to the unique properties of the fructose responsible for hepatic damage and lipogenesis [10,21,32]. De facto, fructose is rapidly converted to acetyl-CoA, which leads to lipogenesis. This rapid accumulation of fatty acids in hepatocytes leads to peroxidation of fatty acids and development of oxidative stress. Consequently, serum levels of ALT and TNF-α increased. These results indicated an inflammatory and profibrogenic response to injury [33]. Furthermore, the attachment of hepatic triglycerides to apolipoprotein B 100 by microsomal triglyceride transfer protein (MTP) promotes triacylglycerol synthesis and very-low-density lipoprotein (VLDL) production [34].
The interesting finding is that the high-fat diet did not induce an increase in serum TNF-α which usually characterizes a status of NASH. This suggests that a longer duration of the experimental study, another rat strain or a higher percentage of fat (more than 51%) may be needed to increase inflammatory markers in rats 51% fat enriched diets. Genetic modifiers could also play a role in resisting disease progression in rats [24]. In the study by Zheng-Jie et al., 24 weeks were required to induce a significant increase in serum TNF-α in Sprague-Dawley rats fed a 30% fat diet [11].
Our study included some limitations; first, the difference in soybean oil concentrations in the rats' diets, (70 g/kg diet in the HF and MF groups versus 40 g/kg diet in the HS and HFr groups). The 70 g/kg diet of soybean oil is a recommended amount for young growing rats, while 40 g/kg diet is a recommended amount for maintenance [14]. Forty g/kg diet of soybean oil was given for both HS and HFr groups and was complemented with butter in order to keep more or less the same composition of fat (saturated versus unsaturated fat) in the four diets, particularly between MF, HS, and HFr groups. Equal amount of soybean oil (g) in different rats' diets should be considered in future studies. Another limitation is the total hepatic lipid (mg/g wet tissues) determined in the four rats groups. This is finally the extractable hepatic lipid determined, mainly the unbound, neutral lipid. This neutral lipid is made up mainly of triglycerides and less of free fatty acids and cholesterol esters [35]. According to Habeck et al., who compared fat extraction from meat according to different analytical methods (acid hydrolysis, soxhlet and chloroform-methanol extraction), these techniques provide similar, acceptable results within and between methods of fat extraction [16]. Thus, they consider the soxhlet-method an acceptable technique for fat extraction in all kinds of meat.
In conclusion, we validated our main hypothesis. Our experimental results showed that, among diets restricted to 15 g/rat/day, the high-fat diet composed of 51% fat, 19.59 kJ/g, and the high-carbohydrate diet composed mainly of 36.2% fructose, 16.15 kJ/g, could induce characteristics of NAFLD in Wistar rats as compared to a calorically equivalent diet 17.05 kJ/g composed of 31.5% fat for a period of 16 weeks. Little pathology developed with a 36.9% sucrose diet. In fact, fructose had the most deleterious effects on hepatic and metabolic parameters of Wistar rats. In close, our findings suggest that a lower threshold of fructose and not sucrose in the diet is necessary to prevent potential risks in animals. Finally, further studies are needed to determine the exact percentage of this sugar in the diet in order to avoid physiological adverse outcomes.
ACKNOWLEDGEMENTS
We also thank Phoenix Clinical Research, Lebanon for their assistance in medical writing.
References
- 1.Paschos P, Paletas K. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia. 2009;13:9–19. [PMC free article] [PubMed] [Google Scholar]
- 2.Amacher DE. Strategies for the early detection of drug-induced hepatic steatosis in preclinical drug safety evaluation studies. Toxicology. 2011;279:10–18. doi: 10.1016/j.tox.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Lieber CS, Leo MA, Mak KM, Xu Y, Cao Q, Ren C, Ponomarenko A, DeCarli LM. Model of nonalcoholic steatohepatitis. Am J Clin Nutr. 2004;79:502–509. doi: 10.1093/ajcn/79.3.502. [DOI] [PubMed] [Google Scholar]
- 4.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 5.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 6.Basaranoglu M, Kayacetin S, Yilmaz N, Kayacetin E, Tarcin O, Sonsuz A. Understanding mechanisms of the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol. 2010;16:2223–2226. doi: 10.3748/wjg.v16.i18.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 8.Dowman JK, Tomlinson JW, Newsome PN. Pathogenesis of nonalcoholic fatty liver disease. QJM. 2010;103:71–83. doi: 10.1093/qjmed/hcp158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118:829–838. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawasaki T, Igarashi K, Koeda T, Sugimoto K, Nakagawa K, Hayashi S, Yamaji R, Inui H, Fukusato T, Yamanouchi T. Rats fed fructose-enriched diets have characteristics of nonalcoholic hepatic steatosis. J Nutr. 2009;139:2067–2071. doi: 10.3945/jn.109.105858. [DOI] [PubMed] [Google Scholar]
- 11.Xu ZJ, Fan JG, Ding XD, Qiao L, Wang GL. Characterization of high-fat, diet-induced, non-alcoholic steatohepatitis with fibrosis in rats. Dig Dis Sci. 2010;55:931–940. doi: 10.1007/s10620-009-0815-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdelmalek MF, Suzuki A, Guy C, Unalp-Arida A, Colvin R, Johnson RJ, Diehl AM Nonalcoholic Steatohepatitis Clinical Research Network. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1961–1971. doi: 10.1002/hep.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Animal Research Review Panel (AU) Guideline 20: Guidelines for the Housing of Rats in Scientific Institutions. Sydney: Animal Research Review Panel; 2004. [Google Scholar]
- 14.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition Ad Hoc Writing Committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 15.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 16.AOAC International (US) Official Methods of Analysis of AOAC International. 18th ed. Gaithersburg (MD): AOAC International; 2005. [Google Scholar]
- 17.Smedes F. Determination of total lipid using non-chlorinated solvents. Analyst. 1999;124:1711–1718. [Google Scholar]
- 18.Subcommittee on Laboratory Animal Nutrition; Committee on Animal Nutrition; Board on Agriculture; Institute for Laboratory Animal Research; National Research Council (US) Nutrient Requirements of Laboratory Animals. 4th rev [Internet] Washington, D.C.: National Academies Press; 1995. [cited 2014 Aug 11]. Available from: http://www.nap.edu/ [Google Scholar]
- 19.Akiyama T, Tachibana I, Shirohara H, Watanabe N, Otsuki M. High-fat hypercaloric diet induces obesity, glucose intolerance and hyperlipidemia in normal adult male Wistar rat. Diabetes Res Clin Pract. 1996;31:27–35. doi: 10.1016/0168-8227(96)01205-3. [DOI] [PubMed] [Google Scholar]
- 20.Gauthier MS, Favier R, Lavoie JM. Time course of the development of non-alcoholic hepatic steatosis in response to high-fat dietinduced obesity in rats. Br J Nutr. 2006;95:273–281. doi: 10.1079/bjn20051635. [DOI] [PubMed] [Google Scholar]
- 21.Lustig RH. Fructose: metabolic, hedonic, and societal parallels with ethanol. J Am Diet Assoc. 2010;110:1307–1321. doi: 10.1016/j.jada.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Ackerman Z, Oron-Herman M, Grozovski M, Rosenthal T, Pappo O, Link G, Sela BA. Fructose-induced fatty liver disease: hepatic effects of blood pressure and plasma triglyceride reduction. Hypertension. 2005;45:1012–1018. doi: 10.1161/01.HYP.0000164570.20420.67. [DOI] [PubMed] [Google Scholar]
- 23.Kučera O, Garnol T, Lotková H, Staňková P, Mazurová Y, Hroch M, Bolehovská R, Roušar T, Červinková Z. The effect of rat strain, diet composition and feeding period on the development of a nutritional model of non-alcoholic fatty liver disease in rats. Physiol Res. 2011;60:317–328. doi: 10.33549/physiolres.932022. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed U, Redgrave TG, Oates PS. Effect of dietary fat to produce non-alcoholic fatty liver in the rat. J Gastroenterol Hepatol. 2009;24:1463–1471. doi: 10.1111/j.1440-1746.2009.05870.x. [DOI] [PubMed] [Google Scholar]
- 25.Lanthier N, Horsmans Y, Leclercq IA. The metabolic syndrome: how it may influence hepatic stellate cell activation and hepatic fibrosis. Curr Opin Clin Nutr Metab Care. 2009;12:404–411. doi: 10.1097/MCO.0b013e32832c7819. [DOI] [PubMed] [Google Scholar]
- 26.Saha JK, Xia J, Grondin JM, Engle SK, Jakubowski JA. Acute hyperglycemia induced by ketamine/xylazine anesthesia in rats: mechanisms and implications for preclinical models. Exp Biol Med (Maywood) 2005;230:777–784. doi: 10.1177/153537020523001012. [DOI] [PubMed] [Google Scholar]
- 27.Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, Ponti V, Pagano G, Ferrannini E, Rizzetto M. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48:634–642. doi: 10.1007/s00125-005-1682-x. [DOI] [PubMed] [Google Scholar]
- 28.Utzschneider KM, Kahn SE. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006;91:4753–4761. doi: 10.1210/jc.2006-0587. [DOI] [PubMed] [Google Scholar]
- 29.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 30.Pagano C, Soardo G, Esposito W, Fallo F, Basan L, Donnini D, Federspil G, Sechi LA, Vettor R. Plasma adiponectin is decreased in nonalcoholic fatty liver disease. Eur J Endocrinol. 2005;152:113–118. doi: 10.1530/eje.1.01821. [DOI] [PubMed] [Google Scholar]
- 31.Kamari Y, Grossman E, Oron-Herman M, Peleg E, Shabtay Z, Shamiss A, Sharabi Y. Metabolic stress with a high carbohydrate diet increases adiponectin levels. Horm Metab Res. 2007;39:384–388. doi: 10.1055/s-2007-976534. [DOI] [PubMed] [Google Scholar]
- 32.Bray GA. How bad is fructose? Am J Clin Nutr. 2007;86:895–896. doi: 10.1093/ajcn/86.4.895. [DOI] [PubMed] [Google Scholar]
- 33.Tetri LH, Basaranoglu M, Brunt EM, Yerian LM, Neuschwander-Tetri BA. Severe NAFLD with hepatic necroinflammatory changes in mice fed trans fats and a high-fructose corn syrup equivalent. Am J Physiol Gastrointest Liver Physiol. 2008;295:G987–G995. doi: 10.1152/ajpgi.90272.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fried SK, Rao SP. Sugars, hypertriglyceridemia, and cardiovascular disease. Am J Clin Nutr. 2003;78:873S–880S. doi: 10.1093/ajcn/78.4.873S. [DOI] [PubMed] [Google Scholar]
- 35.Getz GS, Bartley W, Stirpe F, Notton BM, Renshaw A. The lipid composition of rat liver. Biochem J. 1961;80:176–181. doi: 10.1042/bj0800176. [DOI] [PMC free article] [PubMed] [Google Scholar]