Abstract Abstract
Agaporomorphus julianeae sp. n. is described from the Biological Field Station Panguana, in Huànuco province of central Peru. The new species belongs to the Agaporomorphus knischi-group sensu Miller 2005. Together with Agaporomorphus knischi Zimmermann, 1921 and Agaporomorphus colberti Miller & Wheeler, 2008 this is the third species of the genus with broadly enlarged male antennomeres. The new species can be separated from Agaporomorphus colberti and Agaporomorphus knischi by the smaller please expanded male antennomere VIII, and the form of the median lobe. Important species characters (median lobe, male antennae, metafemur, colour pattern) of the new species and Agaporomorphus knischi are figured, and the habitat, a temporary blackwater forest pond, and its species rich water beetle coenosis are illustrated and described in detail. The Brazilian Agaporomorphus mecolobus Miller, 2001, only known from the type material from Sao Paulo, is here recorded for Minas Gerais. Habitus photos of four additional Agaporomorphus species and Hydrodytes opalinus (Zimmermann, 1921) are provided. Altogether ten species of Agaporomorphus are now known.
Keywords: Dytiscidae, Agaporomorphus, Hydrodytes, new species, new records, habitat, Peru, Panguana, Neotropical region
Introduction
The diving beetle genus Agaporomorphus Zimmermann, 1921 is restricted to the Neotropical region and distributed from central Peru north to Suriname and Venezuela, and south to south-eastern Brazil and northern Argentina (Miller and Wheeler 2008, Libonatti et al. 2011). The genus was taxonomically reviewed by Miller (2001, 2005, 2014), and Miller and Wheeler (2008), who described seven of the nine known species (Nilsson 2015). Phylogenetic analyses of Copelatinae suggest a possible sister group relation of Agaporomorphus with the Malagasy Madaglymbus (Bilton et al. 2015).
Most Agaporomorphus are known from Brazil, Peru and Venezuela (Miller 2014). The new species of the Agaporomorphus knischi-group sensu Miller 2005 described herein is the fifth Peruvian species and the tenth species of Agaporomorphus to date. The biology of Agaporomorphus has remained virtually unknown. Almost all specimens were collected with different light traps (Miller 2001, 2005, Miller and Wheeler 2008) except for Agaporomorphus sharynae Miller, 2014 which was collected among accumulations of fallen leaves in a small backwater of a sandy and slow flowing forest stream in Venezuela (Miller 2014).
The research station Panguana, in the Peruvian lowland rainforest in Province Huànuco of central Peru, is situated close to the western slopes of the Andes on the southern bank of the Rio Llullapichis (= Yuyapichis) an eastern affluent of the Rio Pachitea, some 170 km south of the town of Pucallpa. The station was founded by the German zoologist couple Maria Koepcke and Hans-Wilhelm Koepcke in 1968 and is now operated by Juliane Diller and the Zoologische Staatssammlung in Munich, Germany (Schlüter 2005).
During eight field trips (1982–2013) E.-G. Burmeister spent several months collecting thousands of aquatic insects, including the first specimens of the new Agaporomorphus described herein. In 2013 R. Apenborn travelled to Panguana for eight weeks collecting aquatic beetles including additional specimens of Agaporomorphus and the first Hydrodytes known from that area. In an unpublished report Apenborn (2013) gave a first overview about the water beetle fauna of Panguana, listing 122 species in ten families.
Besides the description of the tenth species of the genus we present a detailed description of an Agaporomorphus habitat, which housed a stable population for many years, and its species rich water beetle coenosis. Furthermore, habitus photos of four additional Agaporomorphus species and Hydrodytes opalinus (Zimmermann, 1921) are provided for the first time.
Material and methods
Beetles were studied with a Leica MZ 12.5 microscope at 10–100×. Habitus photographs were taken with a digital photo imaging system, composed of a Leica Z 6 APO and a Nikon V1 camera. The genitalia images were produced with a Mitutoyo M Plan Apo ELWD lens attached via a bellows to a Nikon D3 camera. Image stacks were produced by moving the camera with a StackShot macrorail. Image stacks were aligned and assembled with the computer software Helicon Focus 4.77TM.
Label data of type material are cited in quotation marks. All type specimens of the herein described species are provided with red labels. The terminology to denote the orientation of the genitalia follows Miller and Nilsson (2003). The following abbreviations were used: TL (total length), TL-H (total length without head), and MW (maximum width). Exact label data are cited for the type material. Additional remarks are found in square brackets.
We used Google Earth (http://earth.google.com) to locate localities and the coordinates are given in decimal notation. Our map bases on “MICROSOFT ENCARTA World-Atlas 2000”.
The specimens included in this study are deposited in the following collections:
MUSM
MNHN
NMPC
SMTD
UFMT
ZSM
The descriptive style follows Miller (2014).
Checklist of Agaporomorphus
Agaporomorphus colberti Miller & Wheeler, 2008 Venezuela
Agaporomorphus dolichodactylus Miller, 2001 Brazil, Bolivia
Agaporomorphus grandisinuatus Miller, 2001 Brazil, Peru
Agaporomorphus julianeae sp. n. Peru
Agaporomorphus knischi Zimmermann, 1921 Brazil, Peru, Bolivia
Agaporomorphus mecolobus Miller, 2001 Brazil
Agaporomorphus pereirai Guignot, 1957 Brazil, Surinam
Agaporomorphus sharynae Miller, 2014 Venezuela
Agaporomorphus silvaticus Miller, 2005 Peru
Agaporomorphus tambopatensis Miller, 2005 Peru
Taxonomy and faunistics
Agaporomorphus julianeae sp. n.
http://zoobank.org/50D5990E-3D52-4592-82AA-B06D7B885E2B
Figs 1 , 3 , 6 , 8 , 10 , 11 , 12–14
Figure 1.
Habitus of Agaporomorphus julianeae sp. n., paratype, male, 3.5 mm.
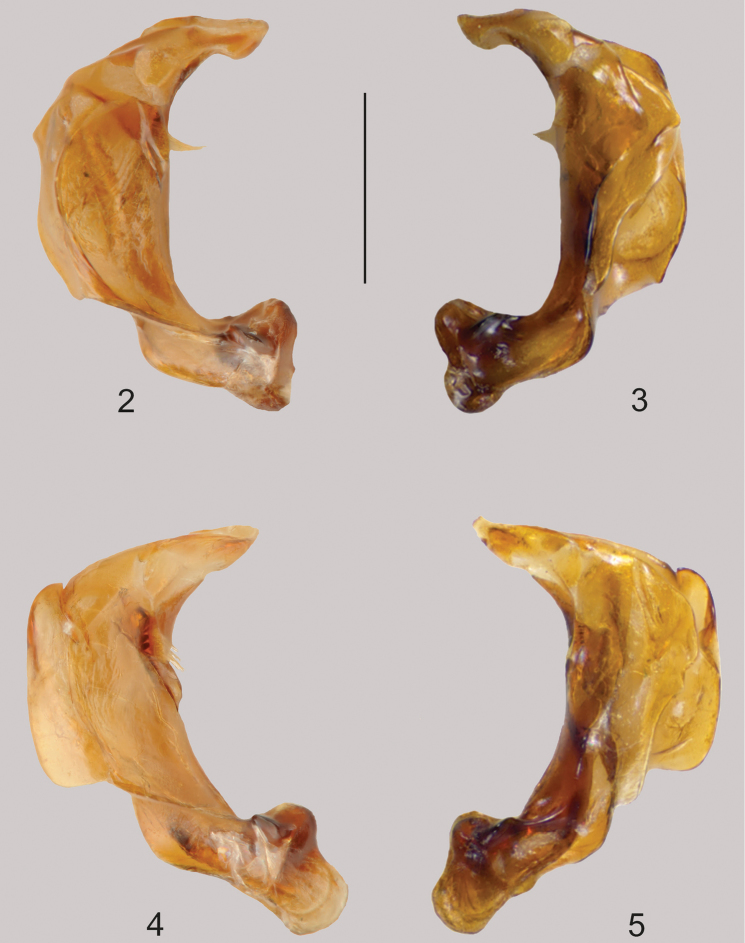
Figures 2–5.
Aedeagus of Agaporomorphus julianeae sp. n., 2–3 median lobe in lateral view, right and left side; Agaporomorphus knischi 4–5 median lobe in lateral view, right and left side. Scale bar = 0.4 mm.
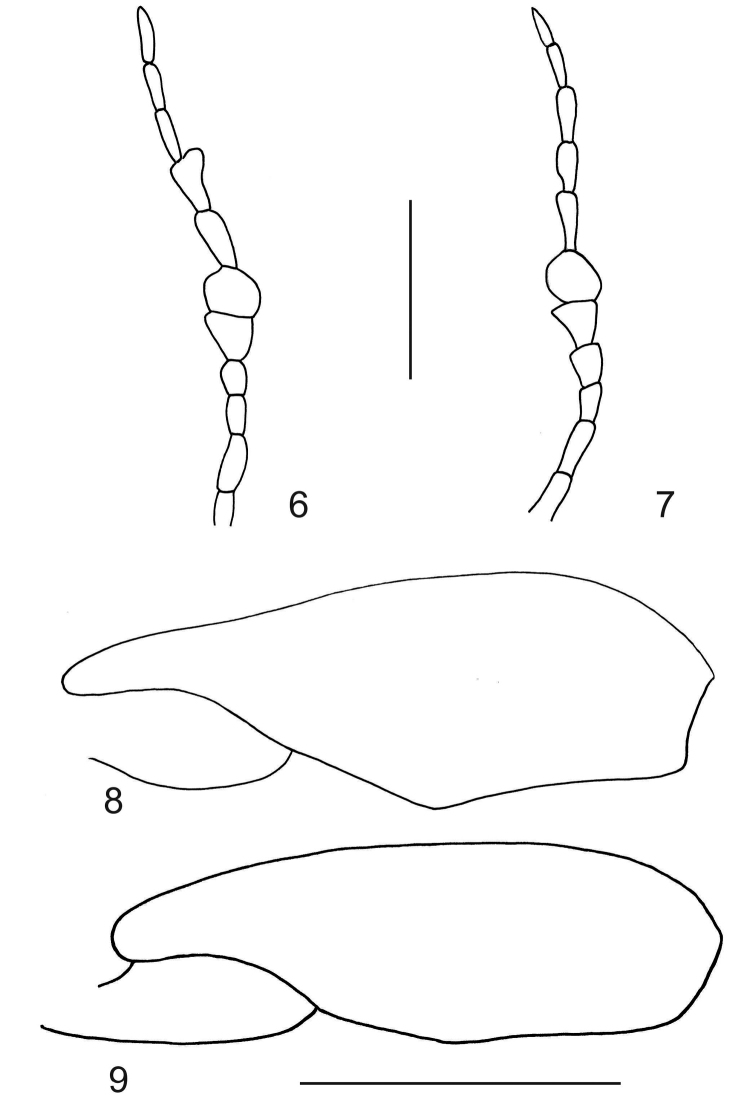
Figures 6–9.
Right male antennae of Agaporomorphus julianeae sp. n. (6) and Agaporomorphus knischi (7); right metatrochanter and metafemur, anterior aspect of Agaporomorphus julianeae sp. n. (8) and Agaporomorphus knischi (9). Scale bars = 0.5 mm.
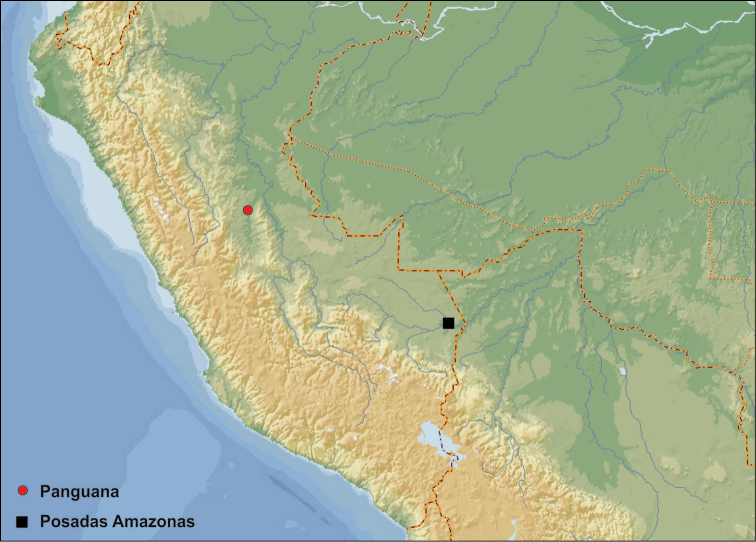
Figure 10.
Records of Agaporomorphus species in Peru. Agaporomorphus julianeae sp. n. and Agaporomorphus tambopatensis (Panguana, red dot); Agaporomorphus grandisinuatus, Agaporomorphus knischi, Agaporomorphus silvaticus and Agaporomorphus tambopatensis (Posadas Amazonas, black square).
Figure 11.
Biological Field Station Panguana, Huànuco province of central Peru: Aguajal forest pond, habitat of Agaporomorphus julianeae sp. n.
Figures 12–14.
Biological Field Station Panguana, Huànuco province of central Peru: Estanque forest pond (12, 13), Shallow puddles and accumulations of fallen wet leaves at the edge of Aguajal (14), habitat of Agaporomorphus julianeae sp. n. and Agaporomorphus tambopatensis, and Hydrodytes opalinus.
Type locality.
Peru, Huànuco province, Rio Yuyapichis, Biological Field Station Panguana, 260 m [9°37'S, 74°56'W], temporary forest pond.
Type material.
Holotype ♂: “Peru, Prov. Huànuco, Rio Yuyapichis, Biol. Stat. Panguana östl. Ort, 9°37'S, 74°56'W 6–17. April 2003 leg. H.J. u. E.-G. Burmeister”; “HOLOTYPE Agaporomorphus julianeae sp. nov. Hendrich, Apenborn, Balke & Burmeister des. 2013 [red label, printed]” (MUSM). Paratypes: 2 ♂♂ 5 ♀♀, same label data as holotype (ZSM); 3 ♂♂ 8 ♀♀, “Peru, Dept. Huànuco, ACP Panguana, Rio Yuyapichis, östl. Ort, 9°37'S, 74°56'W, 230m, 10.05.–25.7.2013, leg. R. Apenborn” (NMPC, ZSM). Each paratype is provided with the respective red printed label.
Description of male holotype.
Measurements. Holotype: TL = 3.5 mm, TL-H = 3.2 mm, MW = 1.65 mm. Paratypes: TL = 3.3–3.5 mm, TL-H = 3.0–3.2 mm, MW = 1.6–1.7 mm.
Coloration (Fig. 1). Head yellowish-brown to brown. Pronotum yellowish-brown medially and lighter laterally. Elytra with most of surface yellowish-brown to brown, with broad, yellow basal band. Ventral surfaces and appendages yellow except abdominal ventrites yellowish-brown.
Sculpture and structure. Head and pronotum with microreticulation consisting of fine cells, with few very fine punctures interspersed; pronotum with narrow lateral pronotal margin. Prosternum medially strongly carinate, carina extending onto prosternal process; prosternal process medially with a rounded longitudinal carina extending to apex, laterally with strongly beaded margins, apex pointed. Elytron covered with extremely fine, evenly spaced, short striae, striae more punctiform laterally and apically. Metafemur moderately broad, length about 2.8 × greatest width (Fig. 8). Metacoxae smooth, impunctate; metacoxal lines closely approximated.
Male genitalia. Median lobe in lateral aspect robust and strongly curved medially; apex elongate, with distinct dorsally-directed lobe on right side medially and very broad, angular region sub-basally, with linear series of fine setae on each side of dorsal midline (Figs 2, 3). Parameres broad, strongly curved, apex strongly curved, with series of long setae medially along internal membrane.
Sexual dimorphism. Male protarsal claws unmodified; pro- and mesotarsal claws about half length of mesotarsomere V; without apical lobe on mesotarsomere V; protarsomeres I and II broadened, protarsomere I with two large adhesive setae, protarsomere II without adhesive setae; mesotarsomeres I and II slightly broadened, mesotarsomere I with one large, medial adhesive seta and two large, apical adhesive setae, mesotarsomere II with two moderately sized apical sucker disks. Male with small but distinct triangular, posteriorly-directed tooth-like prominence medially along posterior margin of visible abdominal ventrite V and with broad and shallow depression medially on abdominal ventrite VI. Male with vague parallel series of rugulosities on each side of midline on abdominal ventrite III. Antennomeres V, VI and VIII modified; V broadly triangular, VIII broad with large posterior emargination (as in Fig. 6). Pro- and mesotarsomeres of female unmodified. Shallow depression medially on abdominal ventrite VI and parallel series of rugulosities on each side of midline on abdominal ventrite III absent. Antennomeres and femur of female unmodified.
Measurements. TL = 3.3–3.5 mm, TL-H = 3.0–3.2 mm, MW = 1.6–1.7 mm.
Etymology.
The new species is named after Juliane Diller, deputy director of the Zoologische Staatssammlung in Munich, and head and owner of the Biological Field Station Panguna, in recognition of her longstanding efforts in biological research and nature conservation in Peru.
Affinities.
The new species can be clearly placed in the Agaporomorphus knischi species-group sensu Miller 2005, characterized by distinctly modified male genitalia and expanded male antennomeres. Within this group, Agaporomorphus julianeae sp. n. is most similar to Agaporomorphus knischi, but differs from that species in the shape of the median lobe (Figs 2–5), expanded male antennomere VIII (Figs 6, 7) and different form of the metafemur (Figs 8, 9). Furthermore, the posteromedial triangular spine on abdominal ventrite V is slightly larger in Agaporomorphus julianeae sp. n. than in Agaporomorphus knischi (see Miller 2001, Fig. 32).
Distribution.
Only known from the type locality in Panguana, Peru. The occurrence in other parts of Peru is likely (Fig. 10).
Habitat.
Collected from two mainly shaded forest ponds, seasonally flooded during the rainy season from October to April, and with high fluctuation level. The ponds are rainwater fed and located in a primary tropical lowland rainforest surrounded by Aguaje palm trees (Figs 11–14). The muddy bottom is covered by broad layers of fallen and rotten leaves and twigs. In the dry season when the surface of the water goes down, a huge, wet area of these leaves and twigs remains. There, and at the edge of the ponds, in small isolated puddles (Fig. 12) of the shallow water zone (less than 10 cm), Agaporophus julianeae sp. n. was collected with a dip net, among accumulations of fallen leaves. The species was associated with Agaporomorphus tambopatensis Miller, 2005, Hydrodytes opalinus (Zimmermann, 1921), Vatellus grandis Buquet, 1840, several unidentified species of Copelatus, Hydaticus subfasciatus Laporte, 1835 (all Dytiscidae), Tropisternus chalybeus Laporte, 1840 and several unidentified species of Helochares (all Hydrophilidae). In general specimens of Agaporomorphus julianeae sp. n. were collected rarely but continuously in the time of observation from May to July (Apenborn 2013).
Type material examined for comparison
Agaporomorphus knischi Zimmermann, 1921
Fig. 15
Figures 15–18.
Habitus of Agaporomorphus knischi, male, paralectotype (15); Agaporomorphus mecolobus, male (16); Agaporomorphus pereirai, male, paratype (17) and Agaporomorphus tambopatensis, male (18). Scale = 1 mm.
Lectotype. ♂, “Brasilien”, “Mato Grosso Corumba”, “Type” [blue handwritten label], “Lectotype Agaporomorphus knischi Zimmermann, 1921 des. K.B. Miller 2001 [red printed label] (ZSM).
Paralectotypes. 12 ♂ ♂, 28 ♀♀, same data as lectotype (ZSM); 10 ♂ ♂, 10 ♀♀, “Corumba [Brazil], Matt [Matto] Grosso”, “W.M. Muche Radebeul Ankauf”, “Staatl. Museum für Tierkunde Dresden” (SMTD).
Agaporomorphus pereirai Guignot, 1957
Fig. 17
Paratypes. 3 ♂♂, 5 ♀♀, “Brasilien, Para Cachimbo X 1955 Pereira”, printed genus label, “Paratype” [red printed label], “Museum Paris 1960 Coll. F. Guignot” [light blue printed label] (MNHN).
Faunistic notes
Agaporomorphus mecolobus Miller, 2001
Fig. 16
Agaporomorphus mecolobus Miller, 2001a: 527 (orig. descr.); Miller 2005: 49 (system., catal.); Miller andWheeler 2008: 64 (catal.); Miller 2014: 181 (system., catal.); Nilsson 2015: 46 (catal.).
Material studied. 20 ♂♂, 40 ♀♀, “Brasil/Minas Gerais Cordisburgo, Faz. Potinha, XII.1993 [at light] Vaz de Mello leg.” (NMPC, UFMT, ZSM).
Remarks. This species was only known after the few type specimens from Sao Paulo (Miller 2001). It is here recorded for the first time for Minas Gerais in Brazil.
Agaporomorphus tambopatensis Miller, 2005
Fig. 18
Agaporomorphus tambopatensis Miller, 2005: 52 (orig. descr.); Miller and Wheeler 2008: 64 (system., catal.); Miller 2014: 181 (system., catal.); Nilsson 2015: 46 (catal.).
Material studied. 1 ♂ and 1 ♀, “Peru, Dept. Huànuco, ACP Panguana, Rio Yuyapichis, östl. Ort, 9°37'S, 74°56'W, 230 m, 10.05.–25.7.2013, leg. R. Apenborn” (ZSM).
Remarks. Described from Madre de Dios, Rio Tambopata in Peru and just known from the type material (Miller 2005). This is the second record of the species in Peru.
Hydrodytes opalinus (Zimmermann, 1921)
Fig. 19
Figure 19.

Habitus of Hydrodytes opalinus, male (7). Scale = 1 mm.
Agaporomorphus opalinus Zimmermann, 1921: 204 (orig. descr.); Guéorguiev 1968: 37 (system., catal.).
Hydrodytes opalinus (Zimmermann, 1921); Miller 2001b: 77 (system.); Miller 2002: 6 (system.); Nilsson 2015: 95 (catal.).
Material studied. 1 ♂ 3 ♀♀, “Peru, Dept. Huànuco, ACP Panguana, Rio Yuyapichis, östl. Ort, 9°37'S, 74°56'W, 230m, 10.05.–25.7.2013, leg. R. Apenborn” (ZSM).
Remarks. Described from Mato Grosso, Brazil and widespread in northern South America (Miller 2002). This is the third record of the species in Peru.
Supplementary Material
Acknowledgements
Rico Apenborn and Ernst-Gerhard Burmeister are obliged to Juliane and Erich Diller (ZSM, Munich) for the possibility to work at the Panguana field station and to the Módena family for their advice and steady support during the field work. The senior author warmly thanks Fernando Zagury Vaz-de-Mello (Cuiabá, Brazil) for providing interesting material. Special thanks to Katja Neven (ZSM, Munich, Germany) for preparation of the habitus photos and to Garth Foster (Ayr, United Kingdom) for critically reviewing the manuscript. Finally, R. Apenborn thanks Katrin Lammers (Berlin, Germany) for her companionship, and Christa M. Heidger (Hochschule Zittau/Görlitz, Germany) for the possibility to work on this topic.
Citation
Hendrich L, Apenborn R, Burmeister E-G, Balke M (2015) A new species of Agaporomorphus Zimmermann, 1921 from Peru (Coleoptera, Dytiscidae, Copelatinae). ZooKeys 512: 63–76. doi: 10.3897/zookeys.512.9505
References
- Apenborn R. (2013) Die Wasserkäferfauna von Panguana - Artenspektrum und Habitatansprüche. Unpublished thesis (Praxissemesterarbeit), Hochschule Zittau/Görlitz, 80 pp. [Google Scholar]
- Bilton DT, Turner C, Toussaint E, Balke M. (2015) Capelatus prykei gen. n., sp. n. (Coleoptera: Dytiscidae: Copelatinae) – a phylogenetically isolated diving beetle from the Western Cape of South Africa. Systematic Entomology 40(3): 520–531. doi: 10.1111/syen.12128 [Google Scholar]
- Guéorguiev VB. (1968) Essai de classification des coléoptères Dytiscidae. I. Tribus Copelatini (Colymbetinae). Izvestija na Zoologitjeskija Institut s Musei Sofia 28: 5–45. [Google Scholar]
- Libonatti ML, Michat MC, Torres PLM. (2011) Key to the subfamilies, tribes and genera of adult Dytiscidae of Argentina (Coleoptera: Adephaga). Revista de la Sociedad Entomológica Argentina 70(3–4): 317–336. [Google Scholar]
- Miller KB. (2001a) Revision of the genus Agaporomorphus Zimmermann (Coleoptera: Dytiscidae). Annals of the Entomological Society of America 94: 520–529. doi: 10.1603/0013-8746(2001)094[0520:ROTGAZ]2.0.CO;2 [Google Scholar]
- Miller KB. (2001b) On the phylogeny of the Dytiscidae (Coleoptera) with emphasis on the morphology of the female reproductive tract. Insect Systematic & Evolution 32(1): 45–92. doi: 10.1163/187631201X00029 [Google Scholar]
- Miller KB. (2002) Revision of the subfamily Hydrodytinae Miller (Coleoptera: Dytiscidae) with description of a new genus. Insect Systematic & Evolution 33(1): 45–92. doi: 10.1163/187631202x00019 [Google Scholar]
- Miller KB. (2005) Two new species of Agaporomorphus Zimmermann (Coleoptera: Dytiscidae) from Peru. Zootaxa 1059: 49–59. [Google Scholar]
- Miller KB. (2014) Agaporomorphus sharynae, a new species of diving beetle (Coleoptera: Dytiscidae: Copelatinae) from Venezuela. Zootaxa 3790: 177–184. doi: 10.11646/zootaxa.3790.1.8 [DOI] [PubMed] [Google Scholar]
- Miller KB, Nilsson AN. (2003) Homology and terminology: Communicating information about rotated structures in water beetles. Latissimus 17: 1–4. [Google Scholar]
- Miller KB, Wheeler QD. (2008) A new species of Agaporomorphus Zimmermann from Venezuela, and a review of the A. knischi species group (Coleoptera: Dytiscidae: Copelatinae). Zootaxa 1859: 63–68. [Google Scholar]
- Nilsson AN. (2015) A world catalogue of the family Dytiscidae or the diving beetles (Coleoptera, Adephaga). Version 1.I.2015, 298 pp Available from: http://www2.emg.umu.se/projects/biginst/andersn/World%20catalogue%20of%20Dytiscidae%202015.pdf, being a corrected and updated version of the printed catalogue: Nilsson AN. (2001) Dytiscidae (Coleoptera). World catalogue of insects. Vol. 3 Apollo Books, Stenstrup, 395 pp [last access on 15.4.2015] [Google Scholar]
- Schlüter A. (2005) Amphibien an einem Stillgewässer in Peru: Mit einer illustrierten Checklist der Amphibien und Reptilien des Unteren Río Llullapichis / Amphibians of an Amazonian Blackwater Pond in Peru: With illustrated Checklist of the Amphibians and Reptiles of the Lower Río Llullapichis Frankfurter Beiträge zur Naturkunde, 22, Edition Chimaira, Frankfurt am Main, 347 pp. [Google Scholar]
- Zimmermann A. (1921) Beiträge zur Kenntnis der südamerikanischen Schwimmkäferfauna nebst 41 Neubeschreibungen. Archiv für Naturgeschichte (A) 87(3): 181–206. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.