Abstract
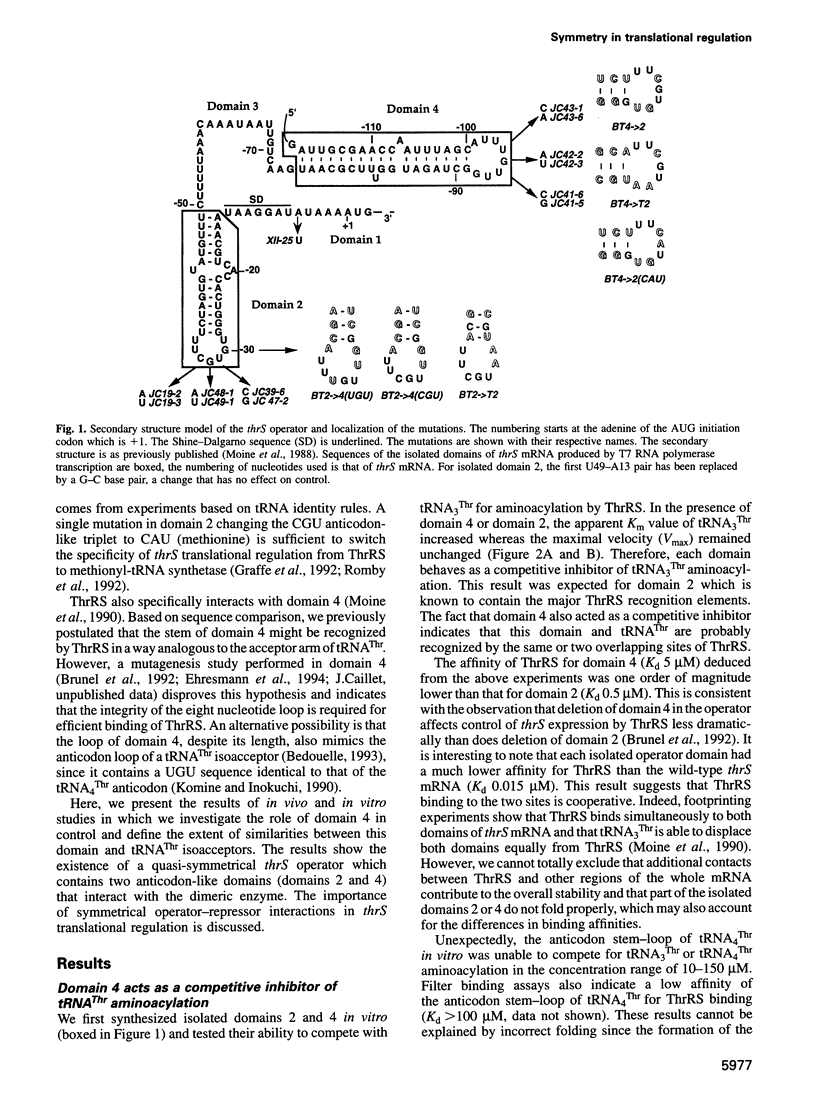
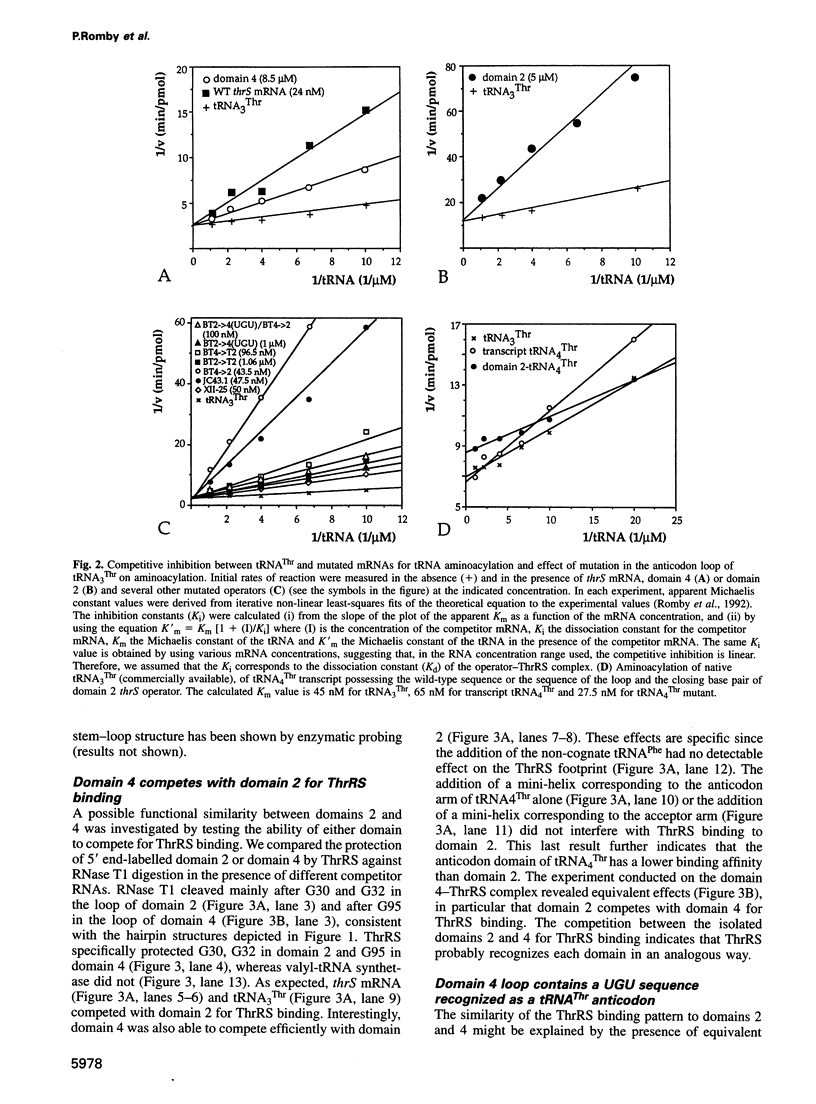
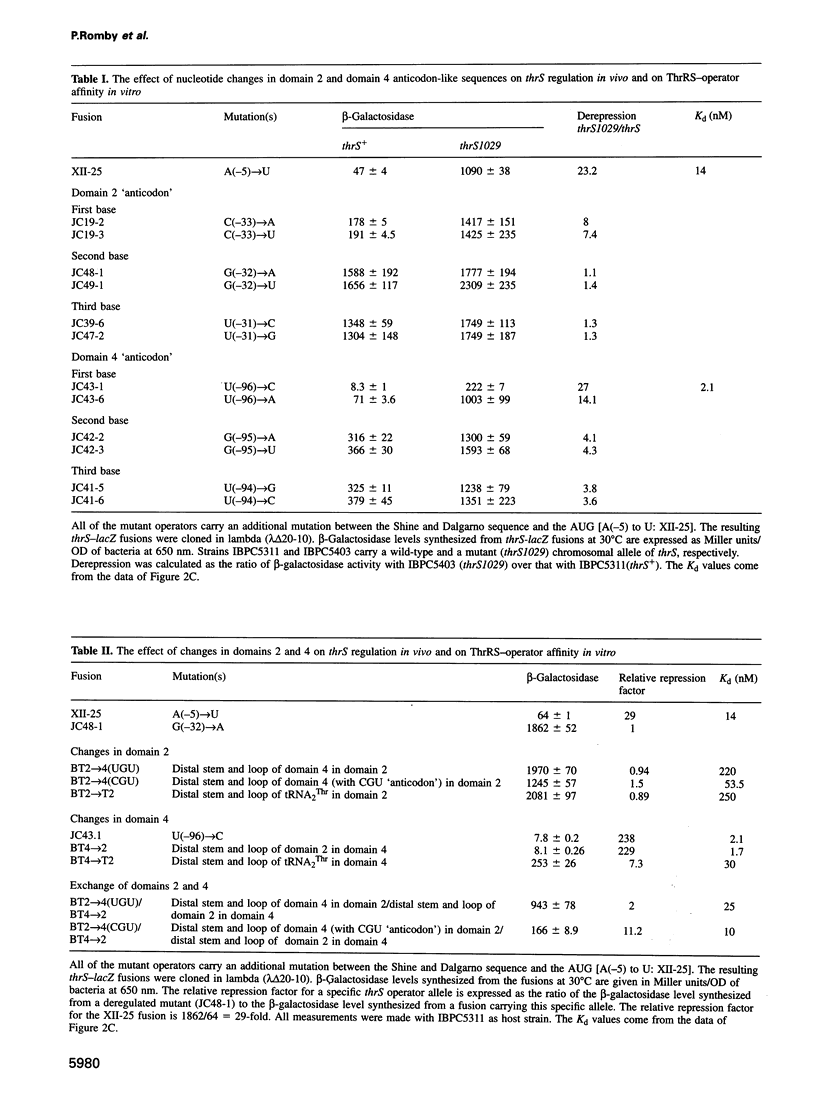
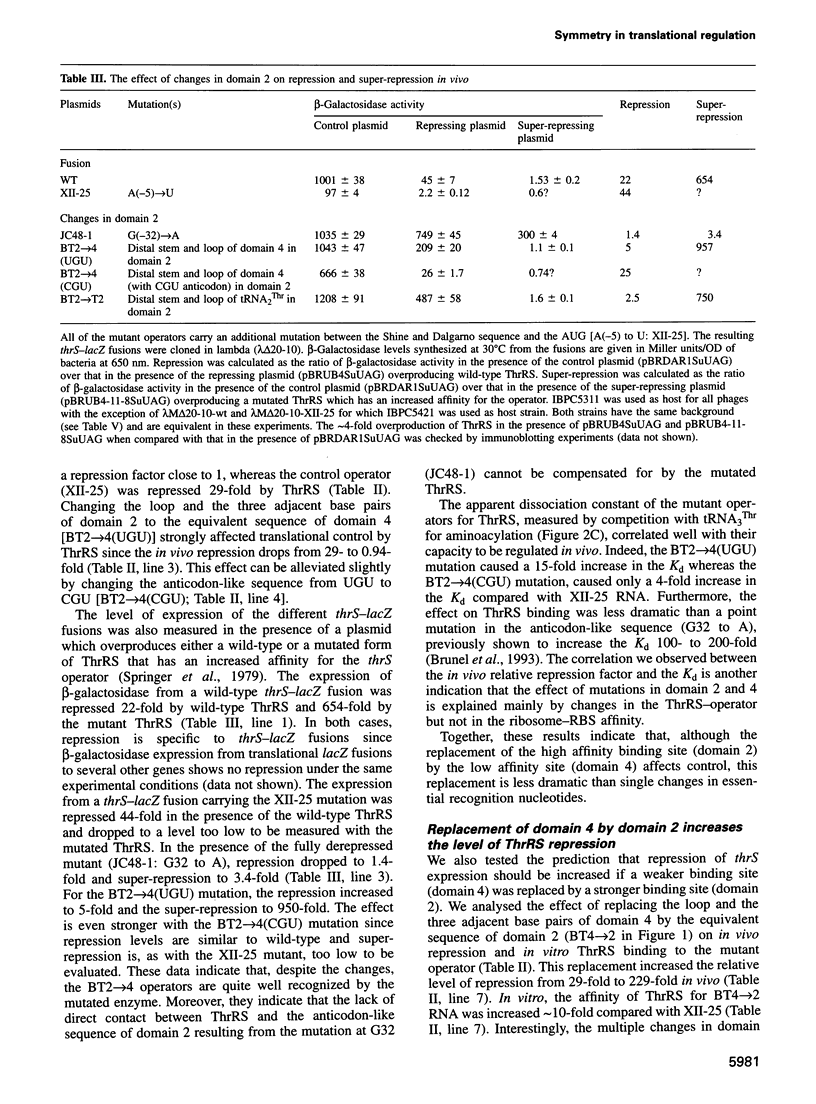
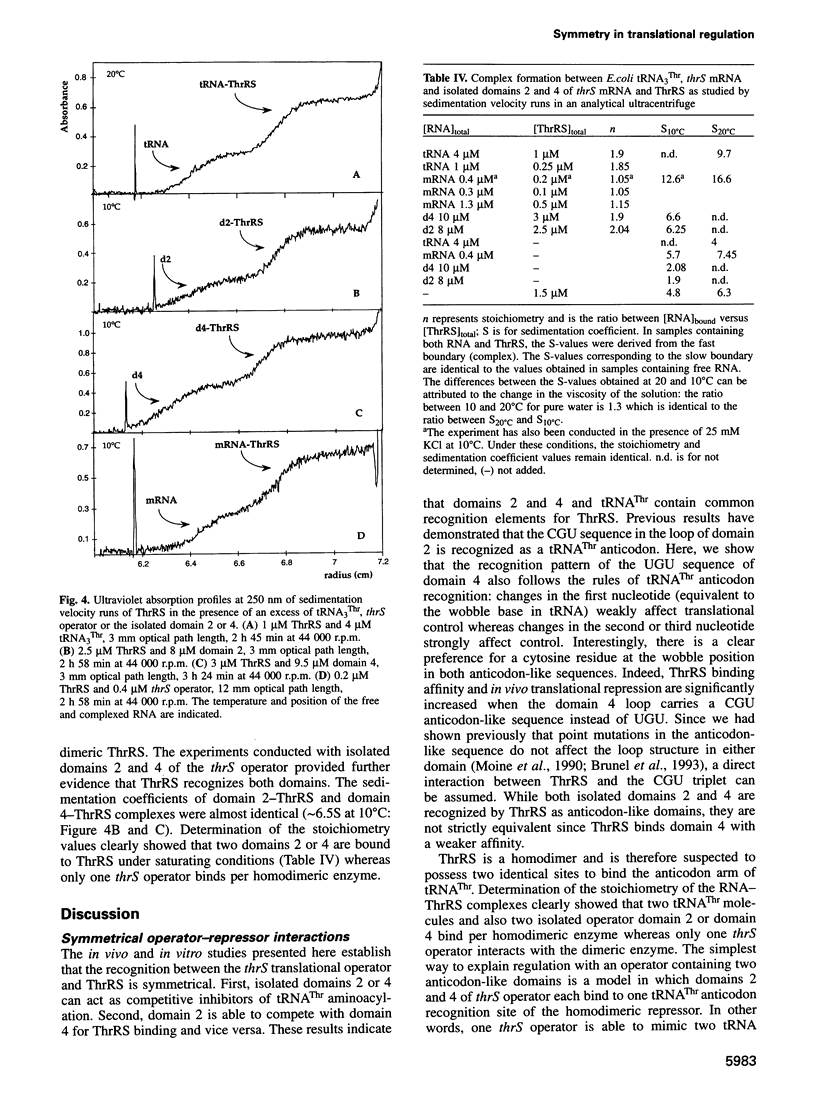
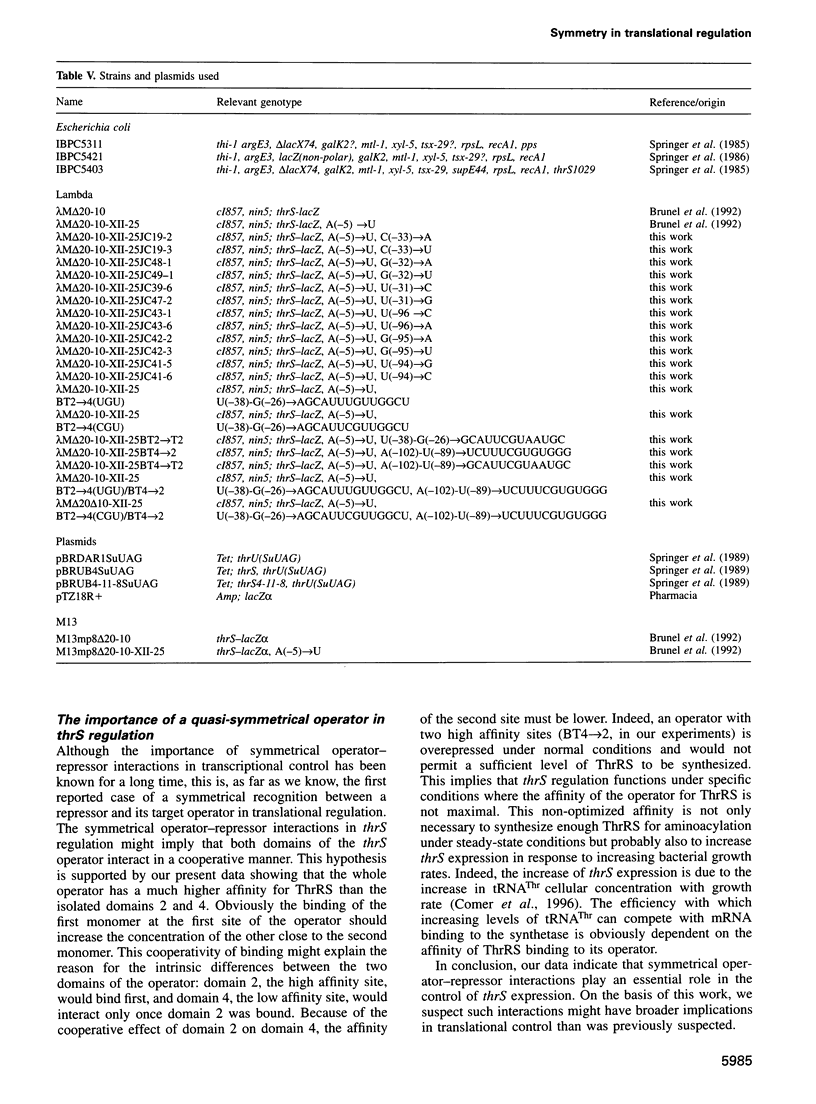
Threonyl-tRNA synthetase from Escherichia coli represses the translation of its own mRNA by binding to the operator region located upstream from the ribosome binding site. The operator contains two stemloop structures which interact specifically with the homodimeric enzyme. Here, we provide in vitro and in vivo evidence that these two stem-loop structures are recognized by the enzyme in an analogous way and mimic the anticodon arm of E.coli tRNA(Thr). Determination of the stoichiometry of the different RNA-threonyl-tRNA synthetase complexes reveals that two tRNA(Thr) molecules bind to the enzyme whereas only one thrS operator interacts with the homodimeric enzyme. A model is presented in which the two anticodon-like domains of the operator bind symmetrically to the two tRNA(Thr) anticodon recognition sites (one per subunit) of the dimeric threonyl-tRNA synthetase. Although symmetrical operator-repressor interactions in transcriptional control are widespread, this report stresses the importance of such interactions in translational regulation of gene expression.
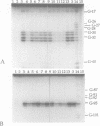
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnez J. G., Harris D. C., Mitschler A., Rees B., Francklyn C. S., Moras D. Crystal structure of histidyl-tRNA synthetase from Escherichia coli complexed with histidyl-adenylate. EMBO J. 1995 Sep 1;14(17):4143–4155. doi: 10.1002/j.1460-2075.1995.tb00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett D., Uhlenbeck O. C. Ribonucleoprotein complexes of R17 coat protein and a translational operator analog. J Mol Biol. 1988 Dec 20;204(4):927–938. doi: 10.1016/0022-2836(88)90052-6. [DOI] [PubMed] [Google Scholar]
- Bedouelle H. Symmetrical interactions between the translational operator of the thrS gene and dimeric threonyl transfer RNA synthetase. J Mol Biol. 1993 Apr 5;230(3):704–708. doi: 10.1006/jmbi.1993.1190. [DOI] [PubMed] [Google Scholar]
- Brunel C., Romby P., Moine H., Caillet J., Grunberg-Manago M., Springer M., Ehresmann B., Ehresmann C. Translational regulation of the Escherichia coli threonyl-tRNA synthetase gene: structural and functional importance of the thrS operator domains. Biochimie. 1993;75(12):1167–1179. doi: 10.1016/0300-9084(93)90016-l. [DOI] [PubMed] [Google Scholar]
- Brunel C., Romby P., Sacerdot C., de Smit M., Graffe M., Dondon J., van Duin J., Ehresmann B., Ehresmann C., Springer M. Stabilised secondary structure at a ribosomal binding site enhances translational repression in E. coli. J Mol Biol. 1995 Oct 20;253(2):277–290. doi: 10.1006/jmbi.1995.0552. [DOI] [PubMed] [Google Scholar]
- Comer M. M., Dondon J., Graffe M., Yarchuk O., Springer M. Growth rate-dependent control, feedback regulation and steady-state mRNA levels of the threonyl-tRNA synthetase gene of Escherichia coli. J Mol Biol. 1996 Aug 16;261(2):108–124. doi: 10.1006/jmbi.1996.0445. [DOI] [PubMed] [Google Scholar]
- Cusack S. Eleven down and nine to go. Nat Struct Biol. 1995 Oct;2(10):824–831. doi: 10.1038/nsb1095-824. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriani G., Delarue M., Poch O., Gangloff J., Moras D. Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature. 1990 Sep 13;347(6289):203–206. doi: 10.1038/347203a0. [DOI] [PubMed] [Google Scholar]
- Fasiolo F., Remy P., Pouyet J., Ebel J. P. Yeast phenylalanyl-tRNA synthetase. Molecular weight and interaction with tRNA Phe and phenylalanine. Eur J Biochem. 1974 Dec 16;50(1):227–236. doi: 10.1111/j.1432-1033.1974.tb03891.x. [DOI] [PubMed] [Google Scholar]
- Francklyn C., Schimmel P. Enzymatic aminoacylation of an eight-base-pair microhelix with histidine. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8655–8659. doi: 10.1073/pnas.87.21.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graffe M., Dondon J., Caillet J., Romby P., Ehresmann C., Ehresmann B., Springer M. The specificity of translational control switched with transfer RNA identity rules. Science. 1992 Feb 21;255(5047):994–996. doi: 10.1126/science.1372129. [DOI] [PubMed] [Google Scholar]
- Hasegawa T., Miyano M., Himeno H., Sano Y., Kimura K., Shimizu M. Identity determinants of E. coli threonine tRNA. Biochem Biophys Res Commun. 1992 Apr 15;184(1):478–484. doi: 10.1016/0006-291x(92)91219-g. [DOI] [PubMed] [Google Scholar]
- Hennecke H., Böck A., Thomale J., Nass G. Threonyl-transfer ribonucleic acid synthetase from Escherichia coli: subunit structure and genetic analysis of the structural gene by means of a mutated enzyme and of a specialized transducing lambda bacteriophage. J Bacteriol. 1977 Sep;131(3):943–950. doi: 10.1128/jb.131.3.943-950.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Tomizawa J. Formation of an RNA primer for initiation of replication of ColE1 DNA by ribonuclease H. Proc Natl Acad Sci U S A. 1980 May;77(5):2450–2454. doi: 10.1073/pnas.77.5.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komine Y., Inokuchi H. Importance of the G27-A43 mismatch at the anticodon stem of Escherichia coli tRNA(Thr2). FEBS Lett. 1990 Oct 15;272(1-2):55–57. doi: 10.1016/0014-5793(90)80447-q. [DOI] [PubMed] [Google Scholar]
- Krauss G., Pingoud A., Boehme D., Riesner D., Peters F., Maas G. Equivalent and non-equivalent binding sites for tRNA on aminoacyl-tRNA synthetases. Eur J Biochem. 1975 Jul 15;55(3):517–529. doi: 10.1111/j.1432-1033.1975.tb02189.x. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Li H., Nicholson A. W. Defining the enzyme binding domain of a ribonuclease III processing signal. Ethylation interference and hydroxyl radical footprinting using catalytically inactive RNase III mutants. EMBO J. 1996 Mar 15;15(6):1421–1433. [PMC free article] [PubMed] [Google Scholar]
- Logan D. T., Mazauric M. H., Kern D., Moras D. Crystal structure of glycyl-tRNA synthetase from Thermus thermophilus. EMBO J. 1995 Sep 1;14(17):4156–4167. doi: 10.1002/j.1460-2075.1995.tb00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy J. E., Kollmus H. Cytoplasmic mRNA-protein interactions in eukaryotic gene expression. Trends Biochem Sci. 1995 May;20(5):191–197. doi: 10.1016/s0968-0004(00)89006-4. [DOI] [PubMed] [Google Scholar]
- Moine H., Romby P., Springer M., Grunberg-Manago M., Ebel J. P., Ehresmann B., Ehresmann C. Escherichia coli threonyl-tRNA synthetase and tRNA(Thr) modulate the binding of the ribosome to the translational initiation site of the thrS mRNA. J Mol Biol. 1990 Nov 20;216(2):299–310. doi: 10.1016/S0022-2836(05)80321-3. [DOI] [PubMed] [Google Scholar]
- Moine H., Romby P., Springer M., Grunberg-Manago M., Ebel J. P., Ehresmann C., Ehresmann B. Messenger RNA structure and gene regulation at the translational level in Escherichia coli: the case of threonine:tRNAThr ligase. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7892–7896. doi: 10.1073/pnas.85.21.7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Pedersen S., Reeh S. Chemical measurement of steady-state levels of ten aminoacyl-transfer ribonucleic acid synthetases in Escherichia coli. J Bacteriol. 1977 Jan;129(1):378–387. doi: 10.1128/jb.129.1.378-387.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M., Strzelecka T., Dorner L. F., Schildkraut I., Aggarwal A. K. Structure of restriction endonuclease BamHI and its relationship to EcoRI. Nature. 1994 Apr 14;368(6472):660–664. doi: 10.1038/368660a0. [DOI] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Transcription factors: structural families and principles of DNA recognition. Annu Rev Biochem. 1992;61:1053–1095. doi: 10.1146/annurev.bi.61.070192.005201. [DOI] [PubMed] [Google Scholar]
- Predki P. F., Nayak L. M., Gottlieb M. B., Regan L. Dissecting RNA-protein interactions: RNA-RNA recognition by Rop. Cell. 1995 Jan 13;80(1):41–50. doi: 10.1016/0092-8674(95)90449-2. [DOI] [PubMed] [Google Scholar]
- Puglisi J. D., Tinoco I., Jr Absorbance melting curves of RNA. Methods Enzymol. 1989;180:304–325. doi: 10.1016/0076-6879(89)80108-9. [DOI] [PubMed] [Google Scholar]
- Romby P., Brunel C., Caillet J., Springer M., Grunberg-Manago M., Westhof E., Ehresmann C., Ehresmann B. Molecular mimicry in translational control of E. coli threonyl-tRNA synthetase gene. Competitive inhibition in tRNA aminoacylation and operator-repressor recognition switch using tRNA identity rules. Nucleic Acids Res. 1992 Nov 11;20(21):5633–5640. doi: 10.1093/nar/20.21.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff M., Krishnaswamy S., Boeglin M., Poterszman A., Mitschler A., Podjarny A., Rees B., Thierry J. C., Moras D. Class II aminoacyl transfer RNA synthetases: crystal structure of yeast aspartyl-tRNA synthetase complexed with tRNA(Asp). Science. 1991 Jun 21;252(5013):1682–1689. doi: 10.1126/science.2047877. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer M., Graffe M., Butler J. S., Grunberg-Manago M. Genetic definition of the translational operator of the threonine-tRNA ligase gene in Escherichia coli. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4384–4388. doi: 10.1073/pnas.83.12.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer M., Graffe M., Dondon J., Grunberg-Manago M. tRNA-like structures and gene regulation at the translational level: a case of molecular mimicry in Escherichia coli. EMBO J. 1989 Aug;8(8):2417–2424. doi: 10.1002/j.1460-2075.1989.tb08372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer M., Graffe M., Grunberg-Manago M. Genetic organization of the E. coli chromosome around the structural gene for initiation factor IF3 (infC). Mol Gen Genet. 1979 Feb 1;169(3):337–343. doi: 10.1007/BF00382279. [DOI] [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theobald A., Springer M., Grunberg-Manago M., Ebel J. P., Giege R. Tertiary structure of Escherichia coli tRNA(3Thr) in solution and interaction of this tRNA with the cognate threonyl-tRNA synthetase. Eur J Biochem. 1988 Aug 15;175(3):511–524. doi: 10.1111/j.1432-1033.1988.tb14223.x. [DOI] [PubMed] [Google Scholar]
- Valegård K., Murray J. B., Stockley P. G., Stonehouse N. J., Liljas L. Crystal structure of an RNA bacteriophage coat protein-operator complex. Nature. 1994 Oct 13;371(6498):623–626. doi: 10.1038/371623a0. [DOI] [PubMed] [Google Scholar]
- Witherell G. W., Gott J. M., Uhlenbeck O. C. Specific interaction between RNA phage coat proteins and RNA. Prog Nucleic Acid Res Mol Biol. 1991;40:185–220. doi: 10.1016/s0079-6603(08)60842-9. [DOI] [PubMed] [Google Scholar]
- Zengel J. M., Lindahl L. Diverse mechanisms for regulating ribosomal protein synthesis in Escherichia coli. Prog Nucleic Acid Res Mol Biol. 1994;47:331–370. doi: 10.1016/s0079-6603(08)60256-1. [DOI] [PubMed] [Google Scholar]