Abstract
Drug abuse and obesity are serious public health problems. Dopamine plays a central role in mediating the reinforcing effects of drugs and food. Prolonged use of drugs is known to alter the function and/or sensitivity of many neurotransmitter systems, including dopamine, however, the impact of consuming foods high in fat and/or sugar is less clear. These studies characterized the locomotor effects of acute and repeated cocaine in male and female C57BL/6J mice consuming one of four diets: (1) standard chow + water; (2) standard chow + 10% sucrose solution; (3) high-fat chow + water; or (4) high-fat chow + 10% sucrose solution. The acute locomotor effects of cocaine (3.2–32.0 mg/kg) were evaluated four weeks after initiating dietary conditions; the effects of repeated cocaine administration were evaluated after 5, 6, 7, and 12 weeks. During acute tests, mice consuming a diet high in fat and/or sucrose exhibited greater locomotor responses to cocaine than mice consuming standard chow and water, regardless of sex. Although diet-induced enhancements persisted across repeated cocaine testing, locomotor sensitization developed more rapidly in females drinking sucrose (and consuming either standard or high-fat chow) than in females consuming standard chow and water. In addition to providing evidence that consuming a diet high in fat and/or sugar enhances abuse-related effects of cocaine in ways that might increase vulnerability to abuse cocaine, these studies identified a potentially important sex-related difference in the interaction between nutrition and cocaine effects, with the impacts of sucrose consumption being greater in females than in males.
Keywords: High-Fat Diet, Sucrose, Cocaine, Behavioral Sensitization, Sex-Differences
Introduction
Drug abuse and obesity are serious public health problems worldwide, with recent estimates indicating that combined they cost the United States over $800 billion per year (Bouchery, Harwood, Sacks, Simon, & Brewer, 2011; Cawley & Meyerhoefer, 2010; National Drug Intelligence Center, 2011; United States Department of Health and Human Services, 2014). Dopamine systems play a crucial role in mediating the reinforcing effects of drugs of abuse and food. Prolonged use of drugs or consumption of palatable foods can produce long-lasting changes in the function and sensitivity of several neurotransmitter systems, including dopamine (Nader et al., 2006; Volkow, Wang, Fowler, & Telang, 2008; Collins et al., 2011; Olsen, 2011; Baladi, Daws, & France, 2012). Although it is now well established that food restriction (i.e., the amount of food consumed) increases the sensitivity of dopamine systems (e.g., Collins, Calinski, Newman, Grundt, & Woods, 2008) and enhances the acquisition of drug self-administration (e.g., Carroll, France, & Meisch, 1979; Carroll, France, & Meisch, 1981), less is known about whether the type of food consumed affects dopamine system sensitivity, and ultimately how such changes might alter drug effects.
Early evidence that the consumption of specific types of food could enhance the abuse-related effects of stimulant drugs was provided by Aveana and Hoebel (2003), who showed that rats that intermittently consumed a 10% (w/v) sucrose solution exhibited an enhanced locomotor response to an acute amphetamine challenge. A similar interaction was also reported between sucrose and cocaine, with rats consuming sucrose exhibiting enhanced locomotor responses to both acute and repeated cocaine administrations (Gosnell, 2005). More recent studies in rats have shown that the consumption of a high-fat diet can sensitize rats to the behavioral effects of both direct- and indirect-acting dopamine receptor agonists (Baladi & France, 2009; Baladi & France, 2010; Baladi, Newman, & France, 2011), suggesting that these interactions are not limited to sucrose consumption. Importantly, the behavioral changes observed in rats consuming a high-fat diet are similar to those reported for rats that received repeated cocaine administrations (Collins et al., 2011), further supporting the notion that both food and drugs can impact dopamine systems in ways that might alter the abuse-related effects of drugs.
Although the use of “cafeteria” (e.g., a mix of peanut butter, hazelnut spread, chocolate biscuits, cheese and bacon avored extruded potato snacks, sweetened multi-grain breakfast cereal, ham and chicken avored processed meat, lard and standard rat chow; Ong, Wanasuria, Lin, Hiscock, & Muhlhausler, 2013) or “junk food” (e.g., biscuits, marshmallows, cheese, jam doughnuts, chocolate chip muffins, butter flapjacks, potato crisps and caramel/chocolate bars and standard rat chow; Bayol, Farrington, & Strickland, 2007) diets have become popular in recent years, relatively few studies have systematically compared the effects of consuming a diet high in fat and/or sugar on the abuse-related effects of drugs. Moreover, despite the widespread use of C57BL/6J mice to model diet-induced obesity and/or diabetes (e.g., Winzell & Ahre, 2004), relatively little is known about whether consumption of a diet high in fat and/or sucrose impacts the abuse-related effects of drugs, such as cocaine, in mice (Erhardt, Zibetti, Godinho, Bacchieri, & Barros, 2006; Morales et al., 2012).
In the current studies, adult male and female C57BL/6J mice were maintained with free access to one of four diets: (1) Standard Diet: standard chow and water, (2) High-Fat Diet: high-fat chow and water, (3) Standard Diet + Sucrose: standard chow and a 10% sucrose solution, or (4) High-Fat Diet + Sucrose: high-fat chow and a 10% sucrose solution. The aims were threefold; first, to characterize the effects of consuming diets high in fat and/or sucrose on the sensitivity of mice to the acute locomotor effects of cocaine; second, to determine if the consumption of diets high in fat and/or sucrose alters the development of locomotor sensitization following repeated cocaine administration; and third, to determine if the interactions between nutritional factors and the locomotor effects of cocaine differ as a function of sex.
Materials and Methods
Subjects
Male (n=32) and female (n=32) C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME, USA) received at 8–9 weeks of age were housed 2–3 per cage in a climate-controlled vivarium (24°C; 14/10hr light/dark cycle). Cages of mice were randomly assigned to one of four dietary conditions, with each mouse weighed daily throughout the experiment. All procedures were conducted in accordance with the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio, and the Guide for Care and Use of Laboratory Animals (National Research Council, 2010).
Dietary conditions
The four dietary conditions were Standard Diet, High-Fat Diet, High-Fat Diet + Sucrose, and Standard Diet + Sucrose. Mice from the two standard conditions had free access to a standard chow (Harlan Teklad 7012; 19.1% protein, 44.3% carbohydrate, and 5.8% fat by weight) whereas mice from the two high-fat conditions had free access to a high-fat chow (Harlan Teklad TD.06414; 23.5% protein, 27.3% carbohydrate, and 34.3% fat by weight). Mice from the Standard Diet and High-Fat Diet conditions had free access to tap water, whereas mice from the two sucrose conditions had free access to a 10% (w/v) solution of sucrose dissolved in tap water. The standard chow contained 3.1 kcal/g (25% from protein, 58% from carbohydrate, and 17% from fat) and the high-fat chow contained 5.1 kcal/g (18.4% from protein, 21.3% from carbohydrate, and 60.3% from fat). The sucrose solution contained 0.4 kcal/g. Food and liquid were weighed at the same time each day, with day-to-day difference in food and liquid weights divided by the number of mice in the cage (2 or 3) to obtain a per mouse estimate of intake.
Locomotor apparatus
Locomotor activity was assessed using sixteen (30×15×15 cm) acrylic chambers (Instrumentation Services, University of Texas Health Science Center, San Antonio). Each chamber was enclosed in a sound-attenuating chamber equipped with a ventilation fan (ENV-022M; MED Associates, St. Albans, VT, USA). The chamber floor consisted of parallel stainless steel rods, 2.3 mm in diameter, mounted 6.4 mm apart. Chambers had four pairs of infrared photobeam emitters and detectors, mounted 2 cm above the floor and 6 cm apart. Photobeam occlusions were recorded by commercially available software (Multi-Varimex version 1.00, Columbus Instruments, Columbus, OH, USA), with the total number of beam breaks used as an index of horizontal locomotion; vertical locomotion was not recorded. After each test, chambers were wiped with an alcohol based cleaner.
Locomotor activity tests
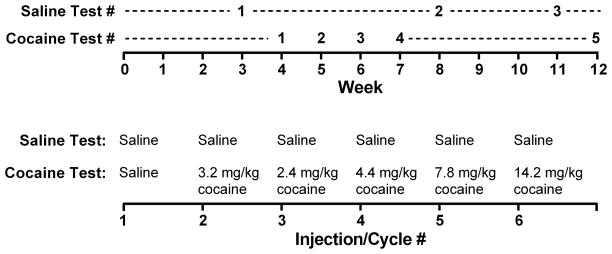
Eight locomotor tests were performed, with each test composed of six 15-min cycles separated by a 3-min period to allow for injections. Locomotor testing began during the third week of the study, with the order and timing of saline (weeks 3, 8 and 11) and cocaine tests (weeks 4, 5, 6, 7, and 12) shown in Figure 1. Saline tests were composed of 6 injections of saline, whereas cocaine tests were composed of a saline injection followed by five injections of cocaine (3.2, 2.4, 4.4, 7.8, and 14.2 mg/kg). This allowed for cumulative cocaine doses of 3.2, 5.6, 10.0, 17.8, and 32.0 mg/kg to be tested within a single session (Figure 1). Testing occurred between 1400 and 1600 hrs, Monday through Friday. Individual mice were always tested on the same day of the week and in the same chamber. Male and female mice were tested in different chambers.
Figure 1.
Experimental timeline (top panel) and schema depicting the order of injections during saline and cocaine tests (bottom panel).
Drugs
Cocaine HCl was purchased from Sigma-Aldrich (St. Louis, MO, USA), dissolved in physiologic saline, and administered IP in a volume of 10 ml/kg.
Data analysis
Body weight and consumption data are presented as the mean ± the standard error of the mean (SEM) for the daily change in body weight (g), food weight (g), liquid weight (g) and the total kcals consumed from food and liquid. Because mice were group housed, cage served as the experimental unit for the analysis for measures of intake (n=3); all other endpoints were analyzed using a single mouse as the experimental unit for the analysis (n=8). A two-way repeated-measures ANOVA with post-hoc Holm-Sidak’s tests was used to determine if weight gain or intake differed as a function of diet (diet and time as factors; within sex) or sex (sex and time as factors; within diet). To control for sex-related differences in size, body weight data were also analyzed by comparing the percent change in body weight between male and female mice (within diet). Finally, the mean proportion of calories obtained from food, relative to sucrose, were determined for each of the 12 weeks, with data from weeks 1 and 12 analyzed with paired, two-tailed t-tests to determine if preference differed as a function of time (within diet and sex; across weeks), and with unpaired, two-tailed t-tests to determine if preference for food versus sucrose differed as a function of sex (within diet and week; across sex).
Locomotor activity data for Saline Test #1 are reported as the mean ± SEM of the total number of locomotor counts during each 15-min cycle. Two-way repeated-measures ANOVAs with post-hoc Holm-Sidak’s tests were used to determine if locomotor activity differed as a function of diet (diet and cycle as factors; within sex), and to determine if locomotor activity differed as a function of sex (sex and cycle as factors; within diet). Absolute numbers of photobeam breaks obtained during the 15-min habituation period (i.e., cycle 1) during Saline Test #1 and Cocaine Tests #1–4 were analyzed by one-way repeated-measures ANOVA with post-hoc Dunnett’s tests to test for the development of a conditioned locomotor response.
Because locomotor activity decreased as a function of cycle number during Saline Test #1, data from Cocaine Tests #1–4 are reported as the mean ± SEM of the difference from saline for each cycle (e.g., activity following 3.2 mg/kg cocaine – activity from 2nd cycle of saline, activity following 5.6 mg/kg cocaine – activity from 3rd cycle of saline, etc.). Two-way repeated-measures ANOVAs with post-hoc Holm-Sidak’s tests were used to determine if locomotor activity differed as a function of diet (diet and dose as factors; within sex) or sex (sex and dose as factors; within diet). For each cocaine test, the maximal locomotor response was used as a measure of effectiveness (Emax), and these values are reported as the mean ± SEM for each group of mice. Individual Emax values were used to construct normalized log dose-response curves (i.e., Emax = 100% response) for each cocaine test. The dose that produced a half maximal response (i.e., ED50) was determined by linear regression using the portion of the log dose-response curve that spanned the 10% to 90% effect levels. In order to assess the development of locomotor sensitization across Cocaine Tests #1–4, measures of potency (ED50), effectiveness (Emax), and area under the cocaine dose-response curve (AUC) were compared using one-way repeated-measures ANOVAs with post-hoc Dunnett’s tests (within sex and diet), and by two-way repeated-measures ANOVAs with post-hoc Holm-Sidak’s tests (across sex and diet). AUC calculations provide a composite measure of the magnitude of sensitization (i.e., accounts for changes in both ED50 and Emax) and facilitated historical comparisons with data from this laboratory on the impact of consuming high-fat chow on cocaine-induced locomotor activity in rats (Baladi, Koek, Aumann, Velasco, & France, 2012). In order to determine if locomotor responses changed following 4 weeks without cocaine, log ED50, Emax, and AUC values for Cocaine Tests #4 and 5 were compared using paired, two-tailed t-tests. Finally, in order to determine if mice developed a conditioned locomotor response to the experimental chamber, locomotor activity from Saline Tests #1–3 were compared using two-way repeated-measures ANOVA with post-hoc Holm-Sidak’s tests (within sex and diet).
Statistical analyses were performed with GraphPad Prism 6 (GraphPad Prism, La Jolla, CA, USA).
Results
Body Weight
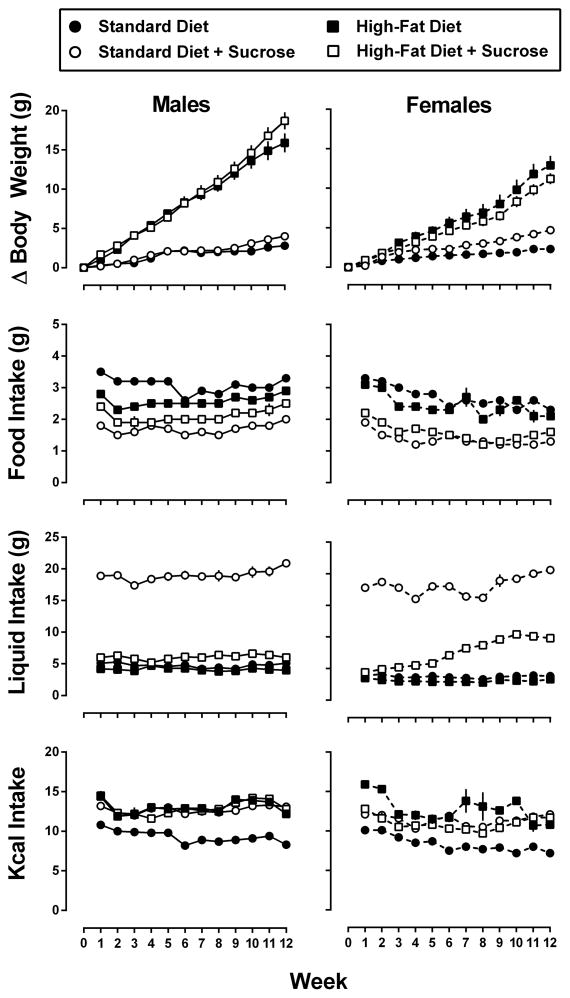
Prior to implementing dietary conditions, male mice (23.4±0.3 grams) weighed significantly more than female mice (18.4±0.2 grams) (p<0.001); however, there were no within-sex differences in body weight among the experimental groups. As shown in Figure 2 (top row), all mice gained weight across the 12-week study, and there were significant main effects of diet and time, as well as significant interactions between these factors in both male and female mice [Male – diet: F(3, 28) = 74.8, p<0.001; time: F(11, 308) = 350.1, p<0.001; interaction: F(33, 308) = 58.3, p<0.001; Female – diet: F(3, 28) = 25.2, p<0.001; time: F(11, 308) = 214.0, p<0.001; interaction: F(33, 308) = 27.0, p<0.001]. Male mice from the High-Fat Diet and High-Fat Diet + Sucrose conditions weighed significantly more than male mice from the Standard Diet condition by week 3 of the study; there were no differences between male mice from the Standard Diet + Sucrose and Standard Diet conditions. Compared to female mice from the Standard Diet condition, females maintained under the High-Fat Diet, High-Fat Diet + Sucrose, and Standard Diet + Sucrose conditions weighed significantly more by weeks 4, 5, and 12, respectively.
Figure 2.
Changes in body weight, food intake, liquid intake, and kcal consumption for male (left panels) and female (right panels) mice consuming one of four experimental diets: standard chow (filled circles), standard chow + 10% sucrose (open circles), high-fat chow (filled squares), or high-fat chow + 10% sucrose (open squares) over the 12 weeks of the study. Data for change in body weight (g) represent the mean ± SEM, n=8 mice/group, however, because mice were group housed, intake data represent the mean ± SEM intake of food (g), liquid (g), or kcals, n=3 cages per group.
Although male mice gained significantly more weight than female mice [High-Fat Diet – sex: F(1, 14) = 6.2, p<0.05; time: F(11, 154) = 174.2, p<0.001; interaction: F(11, 154) = 3.6, p<0.001; High-Fat Diet + Sucrose – sex: F(1, 14) = 23.8, p<0.001; time: F(11, 154) = 262.1, p<0.001; interaction: F(11, 154) = 19.4, p<0.001; Standard Diet + Sucrose – sex: F(1, 14) = 9.9, p<0.05; time: F(11, 154) = 139.7, p<0.001; interaction: F(11, 154) = 2.2, p<0.05], the percent change in body weight was comparable for males and females for each diet (data not shown).
Consumption
As shown in Figure 2, the average amount of food consumed (g) by each group of mice (within diet) did not change significantly across the 12-week study, and a similar rank order was observed for both male and female mice (Standard Diet > High-Fat Diet > High-Fat + Sucrose > Standard Diet + Sucrose). With respect to the amount of liquid consumed, mice from the Standard Diet + Sucrose condition drank significantly more than mice from any other condition (~4x more; p<0.001 for both males and females). As with food consumption, a similar rank order for liquid intake was observed for male and female mice (Standard Diet + Sucrose ≫ High-Fat + Sucrose > Standard Diet = High-Fat Diet). Although male mice from the Standard Diet and High-Fat Diet + Sucrose conditions consumed comparable amounts of liquid over the course of the study, female mice from the High-Fat Diet + Sucrose condition drank significantly more liquid than their Standard Diet counterparts by the third week of the study (p<0.01).
Together, these levels of consumption resulted in male mice from each of the three experimental dietary conditions taking in significantly more calories than male mice from the Standard Diet condition throughout the study [diet: F(3, 8) = 14.9, p<0.01; time: F(11, 88) = 6.7, p<0.001; interaction: F(33, 88) = 2.3; p<0.01]. Although female mice maintained under each of the experimental dietary conditions consumed slightly more calories than female mice maintained under the Standard Diet condition, the differences did not reach significance.
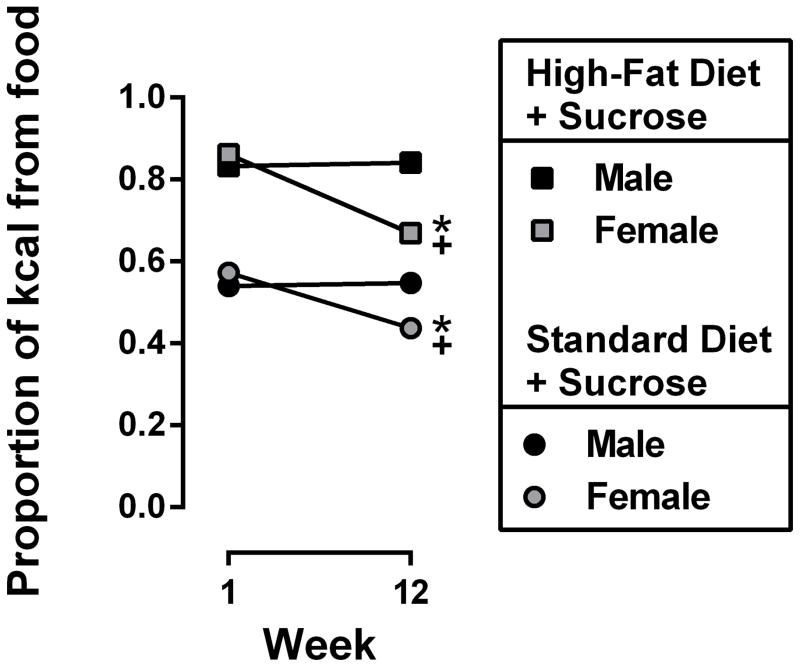
While mice from the Standard Diet and the High-Fat Diet conditions could only obtain calories from chow, mice from the Standard Diet + Sucrose and the High-Fat Diet + Sucrose conditions could choose to obtain calories from either chow or the sucrose solution. Although male and female mice obtained a similar proportion of their calories from the standard (54% and 57%, respectively) and high-fat chows (83% and 86%, respectively) relative to sucrose at the start of the study (Figure 3), sex-related differences in caloric preference developed over the course of the 12-week study. Whereas male mice maintained their initial preferences for chow versus sucrose, a significant shift in caloric preference was observed for female mice, regardless of whether they had access to standard (57% to 43%; p<0.05) or high-fat chows (86% to 66%; p<0.05). Not only did this shift away from chow and towards sucrose result in female mice obtaining significantly more calories from sucrose than they did at the start of the study, but their preference for obtaining calories from sucrose was significantly greater than male mice maintained under the Standard Diet + Sucrose (15% greater; p<0.05) or High-Fat Diet + Sucrose (20% greater; p<0.05) conditions.
Figure 3.
Proportion of calories that were obtained from food, relative to sucrose, for male (black symbols) and female (gray symbols) mice consuming either standard chow + 10% sucrose (circles), or high-fat chow + 10% sucrose (squares). Data represent the mean proportion of calories from food ± SEM, n=3 cages per group, during week 1 and week 12. *, p<0.05; significant difference between week 1 and week 12 (within group) as determined by paired, two-tailed t-tests. +, p<0.05; significant difference between male and female mice (within diet) at week 1 or week 12 as determined by unpaired, two-tailed t-tests.
Locomotor activity
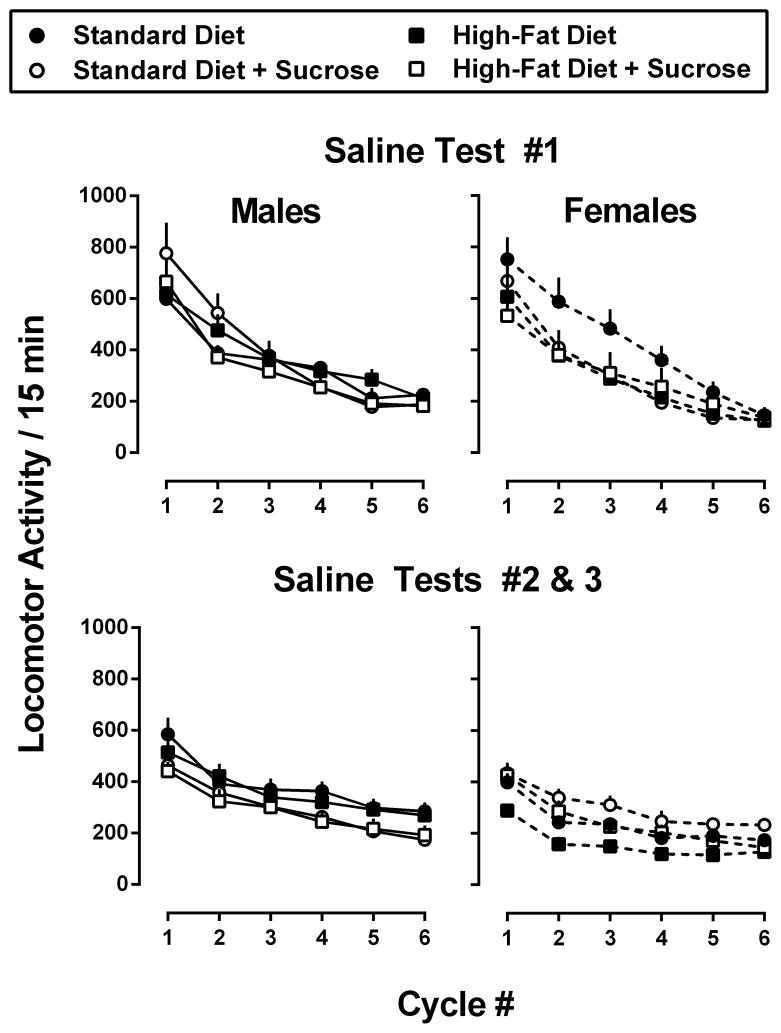
Figure 4 shows the absolute number of locomotor activity counts observed during the pre- (Saline Test #1; top panels) and post-cocaine saline tests (Saline Tests #2 and 3; bottom panels) for male (left panels) and female mice (right panels). Although two-way, repeated-measures ANOVAs revealed a significant main effect of cycle number (i.e., injection number) for both male [F(5,186)=45.0, p<0.001] and female [F(5,186)=34.3, p<0.001] mice, there was no effect of diet in either sex during the pre-cocaine test. Two-way, repeated-measures ANOVAs also failed to identify sex-related differences in the amount of locomotor activity observed following injections of saline. Because there were no significant differences between the amount of activity observed during Saline Tests #2 and 3, these values were collapsed across tests (Figure 4; lower panels). With the exception of female mice from the Standard Diet [injection: F(5,35)=39.9, p<0.0001; test#: F(1,7)=11.1, p<0.05; interaction: F(5,35)=10.1, p<0.0001] and High-Fat Diet conditions [injection: F(5,35)=37.3, p<0.0001; test#: F(1,7)=10.3, p<0.05; interaction: F(5.35)=6.2, p<0.001], there were no significant differences in the locomotor responses observed before and after repeated cocaine testing. Importantly, for both of these groups, post-hoc tests revealed significantly lower levels of activity following the first 2 or 3 saline injections for females maintained on the High-Fat or Standard Diets, respectively (p<0.01 for all). Moreover, comparisons of locomotor activity observed following the habituation injection that preceded each of the weekly tests decreased slightly, but did not differ significantly across the 8 tests (data not shown).
Figure 4.
Total locomotor activity following saline injections in male (left panels) and female (right panels) mice consuming one of four experimental diets: standard chow (filled circles), standard chow + 10% sucrose (open circles), high-fat chow (filled squares), or high-fat chow + 10% sucrose (open squares). Top panels show locomotor activity from the pre-cocaine saline test (week 3), whereas bottom panels show locomotor activity from post-cocaine tests (weeks 8 and 11). Data represent the mean number of photobeam breaks ± SEM, n=8 mice per group during each 15-min cycle.
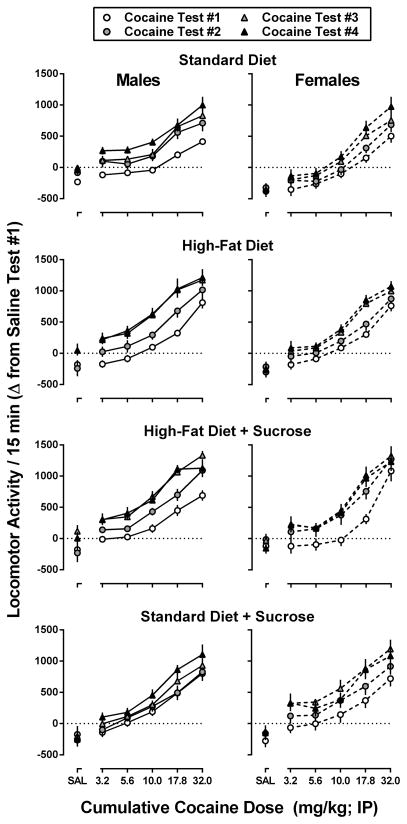
As shown in Figure 5, cocaine dose-dependently increased locomotor activity in each of the eight groups of mice, during each of the four cocaine tests. Upon repeated testing, all mice (with the exception of male mice from the Standard Diet + Sucrose condition) developed sensitization to the locomotor effects of cocaine as demonstrated by progressive leftward and/or upward shifts in the dose-response curves. To better quantify changes in the potency and effectiveness of cocaine to increase locomotor activity, ED50, Emax, and area under the curve (AUC) values were obtained from dose-response curves for each mouse across each of the four weekly tests, as well as the final cocaine test performed on week 12. Data for males are shown in Figure 6, and data for females are shown in Figure 7.
Figure 5.
Dose-response curves for cocaine-induced locomotor activity in male (left panels) and female (right panels) mice consuming one of four experimental diets: standard chow, standard chow + 10% sucrose, high-fat chow, or high-fat chow + 10% sucrose during each of four weekly tests. Data represent the mean ± SEM, n=8 mice per group, difference in the total locomotor activity observed during the 15-min period following saline and each cumulative dose of cocaine (3.2, 5.6, 10.0, 17.8, and 32.0 mg/kg) relative to the total locomotor activity counts observed during the corresponding cycle during saline test #1.
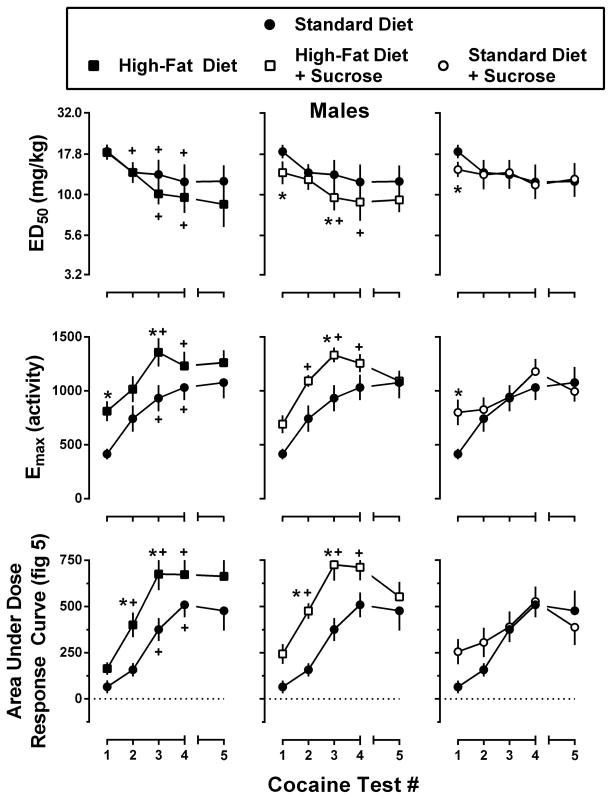
Figure 6.
Potency (top row) and effectiveness (middle row) of cocaine to increase locomotor activity in male mice consuming one of four experimental diets: standard chow (filled circles), standard chow + 10% sucrose (open circles), high-fat chow (filled squares), or high-fat chow + 10% sucrose (open squares). Potency data represent the mean ± 95% CI, n=8 mice per group, of the dose required to produce 50% of the maximal locomotor response during each of the five cocaine tests. Effectiveness data represent the mean ± SEM, n=8, of the maximal locomotor response during each of the five cocaine tests. Area under dose-response curve (AUC) data (bottom row) represent the mean ± SEM, n=8, of the AUC for cocaine tests #1–5 (3.2–32.0 mg/kg cocaine; Figure 5). Data from Standard Diet mice are re-plotted on each panel for comparison. *, p<0.05; significant difference from Standard Diet mice as determined by two-way repeated measures ANOVA with post-hoc Holm-Sidak’s tests. +, p<0.05; significant difference from cocaine test #1 as determined by one-way repeated measures ANOVA with post-hoc Dunnett’s tests.
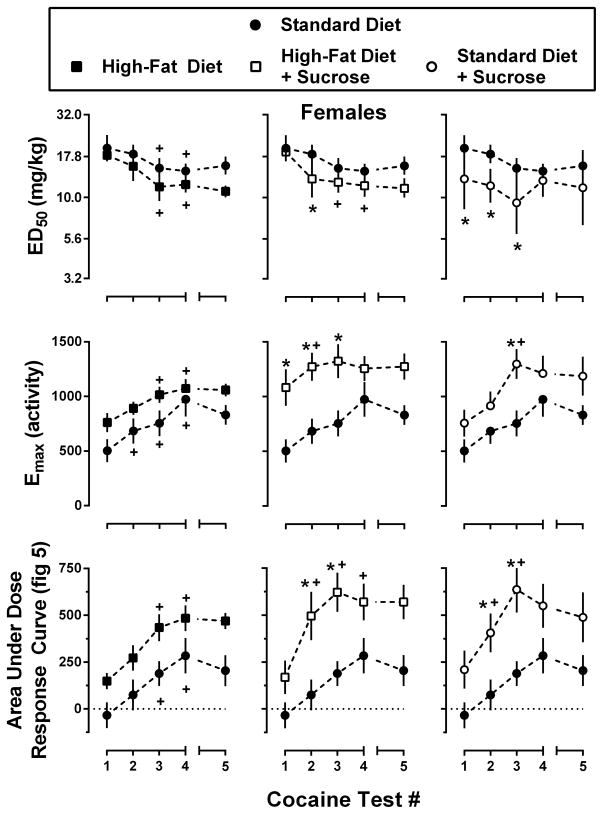
Figure 7.
Potency (top row) and effectiveness (middle row) of cocaine to increase locomotor activity in male mice consuming one of four experimental diets: standard chow (filled circles), standard chow + 10% sucrose (open circles), high-fat chow (filled squares), or high-fat chow + 10% sucrose (open squares). Potency data represent the mean ± 95% CI, n=8 mice per group, of the dose required to produce 50% of the maximal locomotor response during each of the five cocaine tests. Effectiveness data represent the mean ± SEM, n=8, of the maximal locomotor response during each of the five cocaine tests. Area under dose-response curve (AUC) data (bottom row) represent the mean ± SEM, n=8, of the AUC for cocaine tests #1–5 (3.2–32.0 mg/kg cocaine; Figure 5). Data from Standard Diet mice are re-plotted on each panel for comparison. *, p<0.05; significant difference from Standard Diet mice as determined by two-way repeated measures ANOVA with post-hoc Holm-Sidak’s tests. +, p<0.05; significant difference from cocaine test #1 as determined by one-way repeated measures ANOVA with post-hoc Dunnett’s tests.
With regard to the acute locomotor effects of cocaine (Figure 5, open circles; Figure 6, Test 1), male mice consuming sucrose (High-Fat Diet + Sucrose and Standard Diet + Sucrose conditions) were significantly more sensitive (p<0.05) to the locomotor effects of cocaine (i.e., lower ED50) than male mice from the Standard Diet condition. Male mice from the High-Fat Diet and Standard Diet + Sucrose conditions also exhibited a significantly greater maximal locomotor response than male mice from the Standard Diet condition during the acute cocaine test (p<0.05). Significant differences were also observed in the composite AUC values for the acute cocaine dose-response curves for male mice maintained under the Standard Diet + Sucrose, High-Fat Diet, and High-Fat Diet + Sucrose conditions compared to male mice from the Standard Diet condition. Female mice maintained under the Standard Diet + Sucrose condition were significantly more sensitive (lower ED50; p<0.05) than female mice from the Standard Diet condition, and those maintained under the High-Fat Diet + Sucrose condition exhibited a significantly greater maximal locomotor response (p<0.05) during the acute cocaine test than female mice from the Standard Diet condition. Unlike with male mice, significant differences among the AUC values for the acute cocaine tests were only observed between female mice maintained under the High-Fat Diet and Standard Diet conditions.
Analysis of ED50 values obtained in male mice across repeated cocaine tests (Figure 6; top row) identified significant main effects of diet and test number as well as a significant diet x test interaction [diet: F(3, 28) = 3.7, p<0.05; test#: F(3, 84) = 25.7, p<0.001; interaction: F(9, 84) = 2.4, p<0.05], whereas ED50 values obtained in female mice only showed a significant main effect of test number, with a diet x test interaction [test#: F(3, 84) = 33.2, p<0.001; interaction: F(9, 84) = 2.1, p<0.05]. Within diet (across test) analysis of ED50 values revealed significant reductions in ED50 values for male mice from the Standard Diet [F(3, 21) = 5.9, p<0.05], High-Fat Diet [F(3, 21) = 22.7, p<0.001], and High-Fat Diet + Sucrose [F(3, 21) = 10.1, p<0.001] conditions, but not for male mice from the Standard Diet + Sucrose condition. Similar effects were obtained in female mice (Figure 7), with significant reductions in ED50 values observed in the Standard Diet [F(3, 21) = 8.9, p<0.01], High-Fat Diet [F(3, 21) = 12.5, p<0.001], and High-Fat Diet + Sucrose [F(3, 21) = 20.5, p<0.001] conditions, but not the Standard Diet + Sucrose condition. It should be noted, however, that male and female mice from the Standard Diet + Sucrose condition were both significantly more sensitive to the acute effects of cocaine as compared with their Standard Diet counterparts, which might account for the inability to detect further decreases in ED50 values with repeated testing. Changes in ED50 values for cocaine did not differ as a function of sex.
As shown in Figures 6 and 7 (middle rows), repeated testing with cocaine also enhanced the effectiveness of cocaine to increase locomotor activity in a diet- and time-dependent manner [Males – diet: F(3, 28) = 5.3, p<0.001; test#: F(3, 84) = 24.2, p<0.001; Females – diet: F(3, 28) = 3.8, p<0.05; test#: F(3, 84) = 24.9, p<0.001; interaction: F(9, 84) = 2.0, p<0.05]. Further analysis of Emax values (within diet, across test) revealed significant increases in maximal effect for male mice from the Standard Diet [F(3, 21) = 7.0, p<0.05], High-Fat Diet [F(3, 21) = 9.4, p<0.001], and High-Fat Diet + Sucrose [F(3, 21) = 23.9, p<0.001] conditions, but not the Standard Diet + Sucrose condition. Significant increases in Emax were observed for all groups of female mice [Standard Diet – F(3, 21) = 16.7, p<0.01; High-Fat Diet – F(3, 21) = 7.0, p<0.01; High-Fat Diet + Sucrose – F(3, 21) = 4.7, p<0.05; and Standard Diet + Sucrose – F(3, 21) = 6.8, p<0.05]. Changes in Emax values for cocaine did not differ as a function of sex.
Because the development of locomotor sensitization can result from changes in potency (ED50) and/or effectiveness (Emax), the area under the cocaine portion of each of the weekly dose-response curves (Figure 5) was used as a composite index of the magnitude of the sensitization. As shown in Figures 6 and 7 (bottom rows), AUC values increased in a diet- and test-dependent manner for both male [Diet: F(3, 28) = 6.4, p<0.001; Test#: F(3, 84) = 41.0, p<0.001] and female mice [Diet: F(3, 28) = 3.4, p<0.05; Test#: F(3, 84) = 55.6, p<0.001]. Although post-hoc analyses revealed significantly greater AUC values for male mice that consumed the high-fat chow (i.e., High-Fat Diet and High-Fat Diet + Sucrose) relative to male mice from the Standard Diet condition, for female mice significant increases in AUC values were only observed in mice that consumed sucrose (i.e., High-Fat Diet + Sucrose and Standard Diet + Sucrose relative to Standard Diet). Within diet analysis of AUC values showed significant increases in AUC for male mice from the Standard Diet [F(3, 21) = 38.9, p<0.001], High-Fat Diet [F(3, 21) = 13.2, p<0.001], and High-Fat Diet + Sucrose [F(3, 21) = 29.3, p<0.001] conditions, but not the Standard Diet + Sucrose condition; significant increases in AUC were observe for all groups of female mice [Standard Diet – F(3, 21) = 24.3, p<0.001; High-Fat Diet – F(3, 21) = 21.9, p<0.001; High-Fat Diet + Sucrose – F(3, 21) = 20.9, p<0.001; and Standard Diet + Sucrose – F(3, 21) = 8.7, p<0.01]. T-tests used to compare the rate of change for AUC (i.e., slope of function) identified significant increases in the rate of sensitization for female mice from the High-Fat Diet + Sucrose and Standard Diet + Sucrose conditions relative to mice from the Standard Diet condition (p<0.05 for both). Conversely, male mice from the Standard Diet + Sucrose condition exhibited a significantly slower the rate of sensitization than those from the Standard Diet condition (p<0.05). In addition to these effects of diet, there were also significant sex-related differences in the rate of sensitization to cocaine, with male mice from the Standard Diet and High-Fat Diet conditions developing sensitization faster than female mice maintained under identical dietary conditions. Conversely, consumption of the Standard chow and sucrose resulted in a more rapid sensitization in female as compared to male mice.
T-tests used to compare ED50, Emax, and AUC values (within diet and sex) for cocaine tests 4 and 5 failed to identify any significant differences in the effects of cocaine before and after the five week period without cocaine testing.
Discussion
Studies in rats have provided clear evidence that the type and amount of food consumed can dramatically alter sensitivity to the behavioral effects of stimulant drugs, such as cocaine and methamphetamine (Carroll et al., 1981; Avena & Hoebel, 2003; Gosnell, 2005; McGuire, Baladi, & France, 2011; Baladi et al., 2012). The results of the current studies confirm this general finding and extend it by examining the impact of consuming diets high in fat and/or sugar on sensitivity to the acute locomotor effects of cocaine as well as the development of locomotor sensitization upon repeated cocaine administrations in male and female C57BL/6J mice. Not only do the results of these studies provide evidence that consuming diets high in fat and/or sugar can enhance the locomotor effects of acute and repeated cocaine administrations, but they also identify an important sex-related difference in the interaction between sucrose and cocaine’s behavioral effects, with the impact of consuming sugar on cocaine effects being more pronounced in female than in male mice.
Unlike previous studies suggesting that female C57BL/6J mice exhibit more locomotor activity than males in response to cocaine (Reith, Wiener, & Fischette, 1991; Van Swearingen, Walker, & Kuhn, 2013), the current studies did not find any sex-related difference in the potency or effectiveness of acute cocaine injections to stimulate locomotor activity in mice from the Standard Diet condition; however, it’s possible that differences would have emerged if locomotor activity had been assessed for longer periods of time after each injection. Similar to reports in rats (Avena & Hoebel, 2003; Gosnell, 2005; Baladi et al., 2012), the acute locomotor effects of cocaine were enhanced in mice consuming high-fat chow and/or the 10% sucrose solution relative to mice that consumed the standard chow and water. In male mice, consumption of sucrose increased sensitivity to cocaine (i.e., lower ED50) whereas consumption of high-fat chow tended to increase the magnitude of the locomotor response to cocaine (i.e., greater Emax) without altering potency. Although similar effects were generally observed in males and females, the High-Fat Diet + Sucrose condition failed to increase the potency, and the High-Fat Diet condition failed to increase the effectiveness of acute cocaine injections in females as they had in males. Despite these slight differences, it is important to note that the acute locomotor effects of cocaine in male and female mice consuming both the high-fat chow and sucrose were no different than those observed in mice consuming either high-fat chow and water or standard chow and sucrose. Given that similar enhancements of cocaine-induced locomotor activity were observed in mice consuming either high-fat chow alone or sucrose alone, the lack of additivity suggests that the impact of consuming fat or sugar may be quantal rather than graded in nature. However, additional tests with diets containing higher and lower amounts of fat and/or sucrose will be needed to determine the nature of these interactions.
Despite the lack of clear sex-related differences in the effects of consuming a diet high in fat and/or sucrose on the acute locomotor effects of cocaine, differences began to emerge with repeated cocaine testing. In particular, locomotor sensitization to cocaine (i.e., increase in AUC) differed as a function of both sex and dietary condition, with female mice from the Standard Diet or High-Fat Diet conditions developing sensitization more slowly than male mice maintained under identical conditions. Although the rate of sensitization did not differ when male and female mice consumed both high-fat chow and sucrose, significant sex-related differences were observed between male and female mice maintained under the Standard Diet + Sucrose condition, with females developing sensitization faster, and males developing sensitization slower than their sex-matched counterparts maintained under the Standard Diet condition. The apparent lack of interaction between sucrose and cocaine in male mice is somewhat surprising given that sucrose consumption has been shown to enhance the development of locomotor sensitization to either amphetamine or cocaine in both male and female rats (Avena & Hoebel, 2003; Gosnell, 2005). However, it should be noted that in addition to species differences, the current studies also employed a group housing design raising the possibility that social interaction blunted the impact of consuming sucrose on cocaine’s behavioral effects.
Although it is possible that the impact of consuming sucrose on the locomotor effects of cocaine differs between rats and mice, or between intermittent (Avena & Hoebel, 2003; Gosnell, 2005) and continuous access (current study), it should be noted that sex-related differences in the pattern of sucrose (versus food) intake emerged over the course of the study. Consistent with previous reports in rats (Spector & Smith, 1984), male and female mice did not differ in their initial preference for sucrose, obtaining approximately 45% (Standard Diet + Sucrose) and 15% (High-Fat Diet + Sucrose) of their calories from sucrose during the first week of study. However, over the course of the 12-week study female mice gradually increased the proportion of calories obtained from sucrose, an effect that resulted from a concomitant increase in sucrose consumption and decrease in food consumption, and occurred regardless of whether female mice were consuming the standard or high-fat chow. Although the group-housing design limited direct comparisons of sucrose intake and cocaine-induced locomotion for individual mice, it is possible that sex-related differences in the impact of sucrose consumption on cocaine effects resulted from differential levels of sucrose intake rather than a qualitatively different interaction between sucrose consumption and cocaine effects in male and female mice.
In addition, analyses of the ED50, Emax, and AUC functions suggest that sensitization might have plateaued by the fourth cocaine test in male mice consuming the high-fat chow (with or without sucrose), as well as in female mice consuming sucrose (with or without the high-fat chow). Although it is difficult to say whether additional cocaine tests would have resulted in further enhancements of cocaine-induced locomotion in these groups of mice, or if a similar plateau would have been observed in mice consuming the standard chow alone, that the sensitized locomotor response was still apparent after 5 weeks without cocaine suggests that sex and feeding condition did not augment the persistence of the effect. Finally, because the amount of locomotor activity observed during the three saline tests, and the weekly habituation cycle (i.e., cycle 1) did not change over the course of the experiment, the leftward and upward shifts of the cocaine dose-response curves can be attributed to the development of an enhancement of the behavioral effects of cocaine rather than an interaction between the direct effects of cocaine and the conditioned properties of the experimental chamber (Barr et al., 1983; Brown, Robertson, & Meisch, 1992).
In agreement with previous studies investigating high-fat diet-induced obesity in C57BL/6J mice (Winzell & Ahre, 2004; Collins, Martin, Surwit, & Robidoux, 2004), a rapid and consistent weight gain was observed in both male and female mice that were allowed to consume the high-fat chow (with or without the 10% sucrose solution). Similar enhancements of the acute locomotor effects of cocaine were observed in mice maintained under each of the dietary conditions, which suggests that these effects resulted from the prior consumption of fat and/or sugar rather than larger mice (e.g., High-Fat Diet compared to Standard Diet) simply receiving more cocaine on a mg/mouse basis. Although this notion is consistent with previous findings in rats (Baladi et al., 2012; Baladi, Daws, & France, 2012), and is further supported by the lack of an association between body weight and the rate at which locomotor sensitization developed in the current studies (e.g., male Standard Diet = male High-Fat Diet, and female Standard Diet + Sucrose faster than female High-Fat Diet), we cannot rule out the possibility of diet-induced changes in body composition affecting pharmacokinetic properties of cocaine (e.g., absorption, distribution, etc.). However, several lines of evidence suggest that diet-induced enhancements in the effects of cocaine result from pharmacodynamic rather than pharmacokinetic changes. With respect to dopamine transporter (DAT) function and dopamine clearance, consumption of a high-fat diet has been shown to significantly reduce the rate of dopamine clearance (Cone, Chartoff, Potter, Ebner, & Roitman, 2013). In addition, studies in rats have also shown that consuming a high-fat diet (Baladi, Daws, & France, 2012; Baladi et al., 2012) or sucrose (Foley, Fudge, Kavaliers, & Ossenkopp, 2006) can enhance the behavioral effects of direct-acting dopamine receptor agonists. These findings suggest that the diet-induced changes in the behavioral effects of cocaine are likely due to pharmacodynamic alterations (i.e., prolonged elevation in synaptic dopamine levels and/or increased sensitivity/function of dopamine receptors) rather than changes in the pharmacokinetic properties of cocaine.
In summary, the current studies evaluated the impact of consuming a diet high in fat and/or sucrose on the locomotor effects of cocaine. There were four main findings: (1) mice that consumed high-fat chow and/or sucrose exhibited an enhanced locomotor response to cocaine during acute tests, regardless of sex; (2) although male and female mice that consumed high-fat chow both exhibited enhanced locomotor responses to cocaine across repeated tests, the rate at which locomotor sensitization developed was comparable to that observed in mice that consumed standard chow alone; (3) although sucrose consumption enhanced the development of locomotor sensitization in female mice, male mice that consumed standard chow and sucrose developed sensitization more slowly than males that consumed standard chow and water; (4) diet-induced changes in the behavioral effects of cocaine were not related to the amount of calories consumed, or the amount of weight gained, suggesting that the enhanced locomotor response resulted from changes in pharmacodynamic rather than pharmacokinetic properties of cocaine. In conclusion, the results of the current studies provide clear evidence that consuming fat and/or sucrose can sensitize animals to the locomotor effects of cocaine, and that these interactions can differ as a function of sex. However, further studies are needed to identify the mechanism(s) through which these effects are mediated, as well as the degree to which the amount of time an animal has consumed high-fat chow and/or sucrose interacts with the development of cocaine sensitization. Moreover, although the neuroadaptations that underlie locomotor sensitization to cocaine are thought to play an important role in the development of addiction (Robinson & Berridge, 1993; McClung & Nestler, 2008; Steketee & Kalivas, 2011), the impact of consuming a diet high in fat and/or sugar on drug-taking is less clear with remote histories of sugar or fat consumption having no effect, or enhancing cocaine self-administration, respectively (Vendruscolo, Gueye, Darnaudery, Ahmed, & Cador, 2010; Puhl, Cason, Wojnicki, & Grigson, 2011). Future studies will be expanded to include drugs from diverse pharmacologic classes and to investigate the degree to which sex and dietary history (e.g., current versus past, or continuous versus intermittent consumption) impact the reinforcing effectiveness of drugs using intravenous self-administration assays.
Abbreviations
- AUC
area under the curve
- ED50
effective dose 50%
- Emax
maximum effect
Contributor Information
Gregory T. Collins, Department of Pharmacology; University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Dr., San Antonio, TX, 78229. South Texas Veterans Health Care System, 7400 Merton Minter Dr., San Antonio, TX 78229.
Yu Chen, Department of Pharmacology; University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Dr., San Antonio, TX, 78229.
Chris Tschumi, Department of Pharmacology; University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Dr., San Antonio, TX, 78229.
Elise L. Rush, Department of Pharmacology; University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Dr., San Antonio, TX, 78229.
Ayele Mensah, Department of Pharmacology; University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Dr., San Antonio, TX, 78229.
Wouter Koek, Department of Pharmacology; University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Dr., San Antonio, TX, 78229. Department of Psychiatry; University of Texas Health Science Center at San Antonio 7703 Floyd Curl Dr., San Antonio, TX, 78229.
Charles P. France, Department of Pharmacology; University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Dr., San Antonio, TX, 78229. Department of Psychiatry; University of Texas Health Science Center at San Antonio 7703 Floyd Curl Dr., San Antonio, TX, 78229.
References
- Avena NM, Hoebel BG. A diet promoting sugar dependency causes behavioral cross-sensitization to a low dose of amphetamine. Journal of Neuroscience. 2003;122:17–20. doi: 10.1016/S0306-4522(03)00502-5. [DOI] [PubMed] [Google Scholar]
- Baladi MG, Daws LC, France CP. You are what you eat: influence of type and amount of food consumed on central dopamine systems and the behavioral effects of direct- and indirect-acting dopamine receptor agonists. Neuropharmacology. 2012;63:76–86. doi: 10.1016/j.neuropharm.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladi MG, France CP. High fat diet and food restriction differentially modify the behavioral effects of quinpirole and raclopride in rats. European Journal of Pharmacology. 2009;610:55–60. doi: 10.1016/j.ejphar.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladi MG, France CP. Eating high-fat chow increases the sensitivity of rats to quinpirole-induced discriminative stimulus effects and yawning. Behavioural Pharmacology. 2010;21:615–620. doi: 10.1097/FBP.0b013e32833e7e5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladi MG, Koek W, Aumann M, Velasco F, France CP. Eating high fat chow enhances the locomotor-stimulating effects of cocaine in adolescent and adult female rats. Psychopharmacology (Berl) 2012;222:447–457. doi: 10.1007/s00213-012-2663-7. [DOI] [PubMed] [Google Scholar]
- Baladi MG, Newman AH, France CP. Influence of body weight and type of chow on the sensitivity of rats to the behavioral effects of the direct-acting dopamine-receptor agonist quinpirole. Psychopharmacology (Berl) 2011;217:573–585. doi: 10.1007/s00213-011-2320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr GA, Sharpless NS, Cooper S, Schiff SR, Paredes W, Bridger WH. Classical conditioning, decay and extinction of cocaine-induced hyperactivity and stereotypy. Life Sciences. 1983;33:1341–1351. doi: 10.1016/0024-3205(83)90817-2. [DOI] [PubMed] [Google Scholar]
- Bayol SA, Farrington SJ, Stickland NC. A maternal “junk food” diet in pregnancy and lactation promotes an exacerbated taste for “junk food” and a greater propensity for obesity in rat offspring. British Journal of Nutrition. 2007;98:843–851. doi: 10.1017/S0007114507812037. [DOI] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. American Journal of Preventative Medicine. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Brown EE, Robertson GS, Fibiger HC. Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: role of forebrain limbic structures. Journal of Neuroscience. 1992;12:4112–4121. doi: 10.1523/JNEUROSCI.12-10-04112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, France CP, Meisch RA. Food deprivation increases oral and intravenous drug intake in rats. Science. 1979;205:319–321. doi: 10.1126/science.36665. [DOI] [PubMed] [Google Scholar]
- Carroll ME, France CP, Meisch RA. Intravenous self-administration of etonitazene, cocaine and phencyclidine in rats during food deprivation and satiation. Journal of Pharmacology and Experimental Therapeutics. 1981;217:241–247. [PubMed] [Google Scholar]
- Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. National Bureau of Economic Research. 2010 doi: 10.1016/j.jhealeco.2011.10.003. Working Paper 16467. [DOI] [PubMed] [Google Scholar]
- Collins GT, Calinski DM, Newman AH, Grundt P, Woods JH. Food restriction alters N′-Propyl-4,5,6,7-tetrahydrobenzothiazole-2,6-diamine dihydrochloride (Pramipexole) -induced yawning, hypothermia, and locomotor activity in rats: evidence for sensitization of dopamine D2 receptor-mediated effects. Journal of Pharmacology and Experimental Therapeutics. 2008;325:691–697. doi: 10.1124/jpet.107.133181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Truong YN, Levant B, Chen J, Wang S, Woods JH. Behavioral sensitization to cocaine in rats: evidence for temporal differences in dopamine D3 and D2 receptor sensitivity. Psychopharmacology (Berl) 2011;215:609–620. doi: 10.1007/s00213-010-2154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S, Martin TL, Surwit RS, Robidoux J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiology & Behavior. 2004;81:243–248. doi: 10.1016/j.physbeh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Cone JJ, Chartoff EH, Potter DN, Ebner SR, Roitman MF. Prolonged high fat diet reduces dopamine reuptake without altering DAT gene expression. PLoS One. 2013;8:e58251. doi: 10.1371/journal.pone.0058251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt E, Zibetti LCE, Godinho JM, Bacchieri B, Barros HM. Behavioral changes induced by cocaine in mice are modified by a hyperlipidic diet or recombinant leptin. Brazilian Journal of Medical and Biological Research. 2006;39:1625–1635. doi: 10.1590/s0100-879x2006001200014. [DOI] [PubMed] [Google Scholar]
- Foley KA, Fudge MA, Kavaliers M, Ossenkopp KP. Quinpirole-induced behavioral sensitization is enhanced by prior scheduled exposure to sucrose: A multi-variable examination of locomotor activity. Behavioural Brain Research. 2006;167:49–56. doi: 10.1016/j.bbr.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Gosnell BA. Sucrose intake enhances behavioral sensitization produced by cocaine. Brain Research. 2005;1031:194–201. doi: 10.1016/j.brainres.2004.10.037. [DOI] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ. Neuroplasticity mediated by altered gene expression. Neuropsychopharmacology. 2008;33:3–17. doi: 10.1038/sj.npp.1301544. [DOI] [PubMed] [Google Scholar]
- McGuire BA, Baladi MG, France CP. Eating high-fat chow enhances sensitization to the effects of methamphetamine on locomotion in rats. European Journal of Pharmacology. 2011;658:156–159. doi: 10.1016/j.ejphar.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales L, Del Olmo N, Valladolid-Acebes I, Fole A, Cano V, Merino B, Ruiz-Gayo M. Shift of circadian feeding pattern by high-fat diets is coincident with reward deficits in obese mice. PLoS One. 2012;7:e36139. doi: 10.1371/journal.pone.0036139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Mach RH. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nature Neuroscience. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. 8. Washington D.C: National Academy Press; 2010. [Google Scholar]
- Olsen CM. Natural rewards, neuroplasticity, and non-drug addictions. Neuropharmacology. 2011;61:1109–1122. doi: 10.1016/j.neuropharm.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong ZY, Wanasuria AF, Lin MZP, Hiscock J, Muhlhausler BS. Chronic intake of a cafeteria diet and subsequent abstinence. Sex-specific effects on gene expression in the mesolimbic reward system. Appetite. 2013;65:189–199. doi: 10.1016/j.appet.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Puhl MD, Cason AM, Wojnicki FH, Corwin RL, Grigson PS. A history of bingeing on fat enhances cocaine seeking and taking. Behavioral Neuroscience. 2011;125:930–942. doi: 10.1037/a0025759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith MEA, Wiener HL, Fischette CT. Sertraline and cocaine-induced locomotion in mice. Psychopharmacology (Berl) 1991;103:297–305. doi: 10.1007/BF02244282. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- United States Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- National Drug Intelligence Center. The Economic Impact of Illicit Drug Use on American Society. Washington D.C: United States Department of Justice; 2011. [Google Scholar]
- Spector AC, Smith JC. A detailed analysis of sucrose drinking in the rat. Physiology & Behavior. 1984;33:127–136. doi: 10.1016/0031-9384(84)90023-4. [DOI] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacological Reviews. 2011;63:348–365. doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Swearingen AED, Walker QD, Kuhn CM. Sex differences in novelty- and psychostimulant-induced behaviors of C57BL/6 mice. Psychopharmacology (Berl) 2013;225:707–718. doi: 10.1007/s00213-012-2860-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Gueye AB, Darnaudery M, Ahmed SH, Cador M. Sugar overconsumption during adolescence selectively alters motivation and reward function in adult rats. PLoS One. 2010;5:e9296. doi: 10.1371/journal.pone.0009296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler J, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzell MS, Ahre B. The High-Fat Diet–Fed Mouse: A Model for Studying Mechanisms and Treatment of Impaired Glucose Tolerance and Type 2 Diabetes. Diabetes. 2004;53:S215–S219. doi: 10.2337/diabetes.53.suppl_3.s215. [DOI] [PubMed] [Google Scholar]