ABSTRACT
Many plant-pathogenic bacteria utilize type II secretion (T2S) systems to secrete degradative enzymes into the extracellular milieu. T2S substrates presumably mediate the degradation of plant cell wall components during the host-pathogen interaction and thus promote bacterial virulence. Previously, the Xps-T2S system from Xanthomonas campestris pv. vesicatoria was shown to contribute to extracellular protease activity and the secretion of a virulence-associated xylanase. The identities and functions of additional T2S substrates from X. campestris pv. vesicatoria, however, are still unknown. In the present study, the analysis of 25 candidate proteins from X. campestris pv. vesicatoria led to the identification of two type II secreted predicted xylanases, a putative protease and a lipase which was previously identified as a virulence factor of X. campestris pv. vesicatoria. Studies with mutant strains revealed that the identified xylanases and the protease contribute to virulence and in planta growth of X. campestris pv. vesicatoria. When analyzed in the related pathogen X. campestris pv. campestris, several T2S substrates from X. campestris pv. vesicatoria were secreted independently of the T2S systems, presumably because of differences in the T2S substrate specificities of the two pathogens. Furthermore, in X. campestris pv. vesicatoria T2S mutants, secretion of T2S substrates was not completely absent, suggesting the contribution of additional transport systems to protein secretion. In line with this hypothesis, T2S substrates were detected in outer membrane vesicles, which were frequently observed for X. campestris pv. vesicatoria. We, therefore, propose that extracellular virulence-associated enzymes from X. campestris pv. vesicatoria are targeted to the Xps-T2S system and to outer membrane vesicles.
IMPORTANCE The virulence of plant-pathogenic bacteria often depends on TS2 systems, which secrete degradative enzymes into the extracellular milieu. T2S substrates are being studied in several plant-pathogenic bacteria, including Xanthomonas campestris pv. vesicatoria, which causes bacterial spot disease in tomato and pepper. Here, we show that the T2S system from X. campestris pv. vesicatoria secretes virulence-associated xylanases, a predicted protease, and a lipase. Secretion assays with the related pathogen X. campestris pv. campestris revealed important differences in the T2S substrate specificities of the two pathogens. Furthermore, electron microscopy showed that T2S substrates from X. campestris pv. vesicatoria are targeted to outer membrane vesicles (OMVs). Our results, therefore, suggest that OMVs provide an alternative transport route for type II secreted extracellular enzymes.
INTRODUCTION
Many Gram-negative plant-pathogenic bacteria utilize specialized protein secretion systems to deliver virulence factors, including DNA or bacterial effector proteins, into plant cells (1). The efficient trans-kingdom transport of bacterial effector proteins is often hindered by the rigid plant cell wall, which mainly consists of cellulose, hemicellulose, and pectin. Bacteria, therefore, often secrete cell wall-degrading enzymes, which contribute to bacterial virulence and include, e.g., cellulases, xylanases, polygalacturonases, and amylases (2–6). It is assumed that the degradation of plant cell wall components by bacterial enzymes facilitates the acquisition of nutrients as well as the translocation of virulence factors into the host cell (7–14).
Bacterial extracellular enzymes are often secreted by a type II secretion (T2S) system, which is a major virulence factor of many Gram-negative plant-pathogenic bacteria, including Ralstonia solanacearum and species of Erwinia and Xanthomonas (8, 15–18). T2S is a two-step process that often requires the Sec system for protein transport across the inner membrane (IM) (18, 19). Sec-dependent transport of proteins depends on an N-terminal signal peptide with an average length of 20 amino acids that is usually cleaved by a peptidase during or after Sec-dependent transport (20, 21). The subsequent delivery of T2S substrates across the outer membrane (OM) is mediated by the T2S apparatus, which consists of 12 to 15 proteins that are located in the IM, the periplasm, and the OM (18, 19, 22). T2S substrates are recognized in the periplasm by a yet-unknown signal and are presumably pushed through the OM secretin channel by the continuous assembly and disassembly of a periplasmic pseudopilus (18, 19).
T2S systems and their cognate substrates have been intensively studied in several bacterial model organisms, including bacteria of the genus Xanthomonas, which belong to the gamma subdivision of proteobacteria and comprise economically important plant pathogens (23, 24). Many Xanthomonas spp. contain two T2S systems that are encoded by homologous xps and xcs gene clusters (25). While Xcs-T2S systems appear to be dispensable for virulence, a virulence function has been reported for Xps-T2S systems (7–9, 11–14, 26–29). In agreement with their contribution to virulence, xps-T2S genes from Xanthomonas spp. are often activated in planta and coregulated with genes encoding components of the type III secretion (T3S) system (8, 10, 13, 14, 30, 31). T3S systems are essential pathogenicity factors of many Gram-negative plant- and animal-pathogenic bacteria and serve as delivery systems for bacterial effector proteins into eukaryotic host cells (32). Given the coregulation of T2S and T3S genes, it has been postulated that the local degradation of the plant cell wall by T2S substrates facilitates the formation of the extracellular T3S pilus, which serves as a transport channel for effector proteins to the host plasma membrane (8, 33). A contribution of the T2S system to T3S-mediated effector protein delivery was previously shown for Xanthomonas campestris pv. vesicatoria, which is the causal agent of bacterial spot disease in pepper and tomato. The only known T2S substrate from X. campestris pv. vesicatoria to date is the xylanase XCV0965, which contributes to virulence and is required for the extracellular xylanase activity of X. campestris pv. vesicatoria (8). In contrast, additional tested candidate proteins with homology to type II secreted extracellular enzymes from other pathogens are secreted T2S independently in X. campestris pv. vesicatoria (8). This finding contradicts the anticipated conservation of T2S substrate repertoires and suggests pathogen-specific differences in T2S substrate specificities, which might reflect bacterial adaptations to certain environments or host plants.
The aim of the present study was the identification of type II secreted virulence factors from X. campestris pv. vesicatoria and the analysis of their potential contribution to the host-pathogen interaction. Secretion assays with candidate T2S substrates led to the identification of two type II secreted xylanases (XCV4358 and XCV4360) and a putative protease (XCV3671) that contribute to the virulence of X. campestris pv. vesicatoria. Furthermore, we identified a previously described virulence-associated lipase (XCV0536) as a substrate of the Xps-T2S system. Secretion of several T2S substrates from X. campestris pv. vesicatoria was T2S independent in the related pathogen Xanthomonas campestris pv. campestris, suggesting differences in the T2S substrate specificities of the two pathovars. Interestingly, immunoelectron microscopy showed that type II secreted proteins from X. campestris pv. vesicatoria are also present in outer membrane vesicles (OMVs), which might provide an alternative transport route for secreted virulence factors across the OM.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli cells were grown at 37°C in lysogeny broth (LB) medium. X. campestris pv. vesicatoria and X. campestris pv. campestris strains were cultivated at 30°C in nutrient-yeast extract-glycerol (NYG) medium (34). Plasmids were introduced into E. coli by electroporation and into X. campestris pv. vesicatoria and X. campestris pv. campestris by electroporation or conjugation, using pRK2013 as the helper plasmid in triparental matings (35). Antibiotics were added to the media at the following final concentrations: ampicillin, 100 μg ml−1; gentamicin, 15 μg ml−1; kanamycin, 25 μg ml−1; rifampin, 100 μg ml−1; spectinomycin, 100 μg ml−1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Reference or source |
|---|---|---|
| Xanthomonas strains | ||
| 85-10 | X. campestris pv. vesicatoria wild-type strain; pepper race 2; Rifr | 63 |
| 85* | 85-10 derivative containing hrpG* | 49 |
| 85-10ΔxpsE | xpsE deletion mutant of strain 85-10 | 8 |
| 85-10ΔxpsD | xpsD deletion mutant of strain 85-10 | 8 |
| 85-10ΔxcsD | xcsD deletion mutant of strain 85-10 | 8 |
| 85-10ΔxpsDΔxcsD | xpsD xcsD double deletion mutant of strain 85-10 | 8 |
| 85-10Δ0965 | Derivative of strain 85-10 with XCV0965 deleted | 8 |
| 85-10ΔEEE | Triple deletion mutant of strain 85-10 lacking xpsE, xcsE, and XCV4312 | 8 |
| 85-10Δ4358 | Derivative of strain 85-10 with XCV4358 deleted | This study |
| 85-10Δ4360 | Derivative of strain 85-10 with XCV4360 deleted | This study |
| 85-10Δ4358Δ4360 | XCV4358 XCV4360 double deletion mutant of strain 85-10 | This study |
| 85-10Δ3671 | Derivative of strain 85-10 with codons 266 to 360 of XCV3671 deleted and containing a frameshift mutation after codon 265 | This study |
| 85-10Δeps | Derivative of strain 85-10 deficient in EPS production | Bonas et al., unpublished |
| LMG 568 | X. campestris pv. campestris wild-type strain | |
| LMG 568ΔxpsD | Derivative of strain LMG 568 with xpsD deleted | M. Lautier, unpublished |
| LMG 568ΔxcsD | Derivative of strain LMG 568 with xcsD deleted | M. Lautier, unpublished |
| LMG 568ΔxpsDΔxcsD | Derivative of strain LMG 568 with xpsD and xcsD deleted | M. Lautier, unpublished |
| E. coli strains | ||
| DH5α | F− recA hsdR17(rk− mk+) ϕ80dlacZΔM15 | Bethesda Research Laboratories |
| DH5α λpir | F− recA hsdR17(rk− mk+) ϕ80dlacZΔM15 [λpir] | 64 |
| Plasmids | ||
| pBluescript(II) KS | Phagemid, pUC derivative; Apr | Stratagene |
| pBRM | Golden Gate-compatible derivative of pBBR1MCS-5, lacP, 3× c-Myc epitope-encoding sequence; Gmr | 8 |
| pBRM-P | Derivative of pBRM lacking lac promoter | 8 |
| pBRM0007 | pBRM derivative encoding XCV0007–c-Myc | This study |
| pBRM0024 | pBRM derivative encoding XCV0024–c-Myc | This study |
| pBRM0027 | pBRM derivative encoding XCV0027–c-Myc | This study |
| pBRM0536 | pBRM derivative encoding XCV0536–c-Myc | This study |
| pBRM-P0536 | pBRM-P derivative encoding XCV0536–c-Myc under control of native promoter | This study |
| pBRM-P0536Δ2–29 | pBRM-P derivative encoding XCV0536Δ2–29–c-Myc under control of native promoter | This study |
| pBRM0583 | pBRM derivative encoding XCV0583–c-Myc | This study |
| pBRM0670 | pBRM derivative encoding XCV0670–c-Myc | This study |
| pBRM0673 | pBRM derivative encoding XCV0673–c-Myc | This study |
| pBRM0730 | pBRM derivative encoding XCV0730–c-Myc | This study |
| pBRM0805 | pBRM derivative encoding XCV0805–c-Myc | This study |
| pBRM0845 | pBRM derivative encoding XCV0845–c-Myc | This study |
| pBRM0889 | pBRM derivative encoding XCV0889–c-Myc | This study |
| pBRM0961 | pBRM derivative encoding XCV0961–c-Myc | This study |
| pBRM0965Δ2–24 | pBRM derivative encoding XCV0965Δ2–24–c-Myc | This study |
| pBRM1033 | pBRM derivative encoding XCV1033–c-Myc | This study |
| pBRM1064 | pBRM derivative encoding XCV1064–c-Myc | This study |
| pBRM1075 | pBRM derivative encoding XCV1075–c-Myc | This study |
| pBRM1202 | pBRM derivative encoding XCV1202–c-Myc | This study |
| pBRM1311 | pBRM derivative encoding XCV1311–c-Myc | This study |
| pBRM1335 | pBRM derivative encoding XCV1335–c-Myc | This study |
| pBRM1372 | pBRM derivative encoding XCV1372–c-Myc | This study |
| pBRM2747 | pBRM derivative encoding XCV2747–c-Myc | This study |
| pBRM2993 | pBRM derivative encoding XCV2993–c-Myc | This study |
| pBRM3283 | pBRM derivative encoding XCV3283–c-Myc | This study |
| pBRM3425 | pBRM derivative encoding XCV3425–c-Myc | This study |
| pBRM3639 | pBRM derivative encoding XCV3639–c-Myc | This study |
| pBRM3671 | pBRM derivative encoding XCV3671–c-Myc | This study |
| pBRM-P3671 | pBRM-P derivative encoding XCV3671–c-Myc under control of XCV4361 promoter | This study |
| pBRM4096 | pBRM derivative encoding XCV4096–c-Myc | This study |
| pBRM4355 | pBRM derivative encoding XCV4355–c-Myc | This study |
| pBRM4358 | pBRM derivative encoding XCV4358–c-Myc | This study |
| pBRM4358Δ2–22 | pBRM derivative encoding XCV4358Δ2–22-c-Myc | This study |
| pBRM-P4358 | pBRM-P derivative encoding XCV4358–c-Myc under control of XCV4361 promoter | This study |
| pBRM4360 | pBRM derivative encoding XCV4360–c-Myc | This study |
| pBRM-P4360 | pBRM-P derivative encoding XCV4360–c-Myc under control of XCV4361 promoter | This study |
| pBRM4437 | pBRM derivative encoding XCV4437–c-Myc | This study |
| pBRMhrpB1 | pBRM derivative encoding HrpB1–c-Myc | 45 |
| pBRMhrpB1Stop | pBRM derivative encoding HrpB1 | 45 |
| pBRMXCC0857 | pBRM derivative encoding XCC0857–c-Myc | 47 |
| pBRMXCC4115 | pBRM derivative encoding XCC4115–c-Myc | 47 |
| pBRMXCC4118 | pBRM derivative encoding XCC4118–c-Myc | 47 |
| pBRMXc0705 | pBRM derivative encoding Xc0705–c-Myc (PghAxc-c-Myc) | 8 |
| pBRMXc1849 | pBRM derivative encoding Xc1849–c-Myc (PghBxc-c-Myc) | 8 |
| pBRMxopJ | pBRM derivative encoding XopJ–c-Myc | D. Büttner, unpublished |
| pDGW0965 | pDGW4 M derivative encoding XCV0965–c-Myc | 8 |
| pDSK602 | Broad-host-range vector; contains triple lacUV5 promoter; Spr | 65 |
| pDSK604 | Derivative of pDSK602 with modified polylinker | 66 |
| pDxpsE | pDSK604 derivative carrying xpsE | This study |
| pOK1 | Suicide vector; sacB sacQ mobRK2 oriR6K; Spr | 40 |
| pOGG2 | Golden Gate-compatible derivative of pOK1 | 52 |
| pOGG4358 | Derivative of pOGG2 containing flanking regions of XCV4358 | This study |
| pOGG4360 | Derivative of pOGG2 containing flanking regions of XCV4360 | This study |
| pOGG3671 | Derivative of pOGG2 containing first 265 and last 268 codons of XCV3671 | This study |
| pRK2013 | ColE1 replicon; TraRK+ Mob+ Kmr | 35 |
| pUC119 | ColE1 replicon; Apr | 67 |
Ap, ampicillin; Gm, gentamicin; Km, kanamycin; Rif, rifampin; Sp, spectinomycin; r, resistant.
Infection studies.
For infection studies, X. campestris pv. vesicatoria strains were inoculated with a needleless syringe into the intercellular spaces of leaves of the near-isogenic pepper lines Early Cal Wonder (ECW) and ECW-10R (36) at concentrations of 1 × 108 CFU/ml in 1 mM MgCl2 if not stated otherwise. Disease symptoms were scored over a period of 10 days postinoculation (dpi). For in planta growth curves, bacteria were inoculated at a density of 1 × 104 CFU/ml into leaves of ECW pepper plants. Bacterial growth was determined as described previously (37). Experiments were repeated at least twice.
Protease and xylanase activity assays.
For the analysis of extracellular protease and xylanase activities, bacteria were grown overnight in liquid NYG medium, adjusted to an optical density at 600 nm (OD600) of 1.0, and incubated at 30°C for 1 to 2 days on NYG plates with 1% agar and containing 1% skimmed milk (AppliChem; for analysis of protease activity) or 0.1% remazol brilliant blue (RBB) xylan (Sigma-Aldrich; for analysis of xylanase activity) (38). Bacteria were inoculated into holes in the agar and removed before the documentation of the halos. Experiments were repeated at least twice.
Generation of expression constructs.
Gene fragments were amplified by PCR from X. campestris pv. vesicatoria strain 85-10 or X. campestris pv. campestris strain LMG 568 and cloned into plasmid pBRM in a one-step restriction/ligation reaction using the type IIS restriction enzyme BsaI and ligase (39). For fragments with an internal BsaI site, the reaction mixture was incubated with ligase for an additional 20 min at 37°C after heat inactivation (39). Alternatively, internal BsaI sites were removed by PCR using primers that introduced silent mutations. For the generation of a pBRM-P0536 construct, XCV0536 including a 530-bp upstream region was amplified by PCR and cloned into plasmid pBRM-P using BsaI and ligase. pBRM-P is a derivative of pBRM which lacks the lac promoter (Table 1). Similarly, XCV3671, XCV4358, and XCV4360 were amplified by PCR and cloned individually together with a 494-bp fragment spanning the predicted promoter of XCV4361 into pBRM-P in a single restriction/ligation reaction. Alternatively, XCV3671, XCV4358, and XCV4360 were cloned into the Golden Gate-compatible suicide vector pLAND-P downstream of the predicted promoter of XCV4361 by using BsaI and ligase. To obtain an expression construct encoding XCV0536Δ2–29–c-Myc, the 530-bp upstream regions of XCV0536 and XCV0536 lacking codons 2 to 29 were amplified by PCR and cloned into pBRM-P in a single restriction/ligation reaction. All primer sequences are listed in Table S1 in the supplemental material.
Generation of deletion constructs.
To delete XCV4358 and XCV4360 from the genome of strain 85-10, 700- to 800-bp flanking regions of both genes were amplified by PCR using genomic DNA from X. campestris pv. vesicatoria strain 85-10 as the template. The amplicons were introduced into the Golden Gate-compatible suicide vector pOGG2 in a single restriction/ligation reaction using BsaI and ligase. For the deletion of XCV3671, the first 265 and the last 268 codons of XCV3671 were amplified by PCR and cloned into the suicide vector pOGG2. The resulting construct contained a deletion of codons 266 to 360 of XCV3671 and an additional frameshift mutation after codon 266. Deletion constructs were introduced into the genome of X. campestris pv. vesicatoria strains by homologous recombination as described previously (40). Double-crossover events resulted in the deletion mutant strains that are described in Table 1.
Secretion experiments and protein analysis.
For in vitro T2S assays, bacteria were grown overnight in NYG medium, resuspended at a cell density of 1.5 × 108 CFU/ml, and incubated on a rotary shaker at 30°C for 1 h (41). Culture supernatants were separated from bacterial cells by filtration, and secreted proteins were precipitated by trichloroacetic acid as described previously (41). Equal protein amounts of bacterial cell extracts and culture supernatants (adjusted according to cell density) were analyzed by SDS-PAGE and immunoblotting using an antibody specific for the c-Myc epitope (Roche) or a polyclonal XopA-specific antibody (42). Secondary antibodies were horseradish peroxidase-labeled anti-mouse or anti-rabbit antibodies (Amersham Pharmacia Biotech). Antibody reactions were visualized by enhanced chemiluminescence. Experiments were repeated at least twice.
For experiments with proteinase K, bacteria were resuspended at a cell density of 1.5 × 108 CFU/ml in NYG medium and incubated on a rotary shaker at 30°C for 1 h or overnight. Two-milliliter aliquots of bacterial culture supernatants were incubated with 200 μg proteinase K (Thermo Scientific) for 2 h at 37°C. As a control, supernatants were incubated without proteinase K for 2 h on ice or at 37°C. To degrade cellular proteins, bacteria were grown overnight in 200 ml NYG medium, and the cell pellet after centrifugation was dissolved in 3 ml 1× phosphate-buffered saline (PBS) and lysed with a French press. Ten microliters of a 1:10 dilution of the cell lysate was incubated with 1 μg proteinase K for 2 h at 37°C. Proteins were analyzed by SDS-PAGE and immunoblotting using c-Myc epitope-specific antibodies. Experiments were repeated at least twice.
RNA analysis.
For transcript analyses via quantitative reverse transcription-PCR (qRT-PCR), bacteria were grown overnight in NYG medium, adjusted to an OD600 of 0.15, and incubated at 30°C until the culture reached an OD600 of 0.6 to 0.8. RNA was extracted using a TRIzol-based protocol, and cDNA was synthesized using 2 μg RNA in a RevertAid H Minus first-strand cDNA synthesis kit (Fermentas). XCV0536, XCV4358, XCV4360, XCV3671, and 16S rRNA were amplified with a CFX Connect real-time PCR detection system (Bio-Rad) using Absolute Blue SYBR green fluorescein mix (Thermo Scientific) and a 1:100 dilution of cDNA solution. The efficiency of each PCR was determined by standard curves based on dilution series of template and melting curve analysis. Mean transcript levels were determined based on values obtained from technical triplicates and the levels of constitutively expressed 16S rRNA transcripts as described elsewhere (ABI user bulletin 2; Applied Biosystems). Individual experiments were performed with three different RNA preparations for each strain and were repeated twice with similar results.
Immunoelectron microscopy.
For immunoelectron microscopy, bacteria were cultivated overnight at 30°C in NYG medium and rapidly frozen with a high-pressure freeze fixation apparatus (HPM 010; BAL-TEC, Liechtenstein). The material was cryosubstituted with 0.25% glutaraldehyde (Sigma) and 0.1% uranyl acetate (Chemapol) in acetone for 2 days at −80°C using cryosubstitution equipment (FSU; BAL-TEC) and embedded in HM20 (Polysciences Europe) at −20°C. For immunolabeling, ultrathin sections (80 nm) were treated with a monoclonal c-Myc epitope-specific antibody (generous gift of U. Conrad) and a secondary anti-mouse antibody conjugated with 10-nm gold particles (Sigma-Aldrich). The sections were poststained with uranyl acetate and lead citrate in an EM-Stain apparatus (Leica) and subsequently observed with a Zeiss Libra 120 tranmission electron microscope (TEM) operating at 120 kV (Carl Zeiss Microscopy). Images were obtained as described above.
For negative staining of isolated OMVs, 4-μl aliquots of isolated OMVs were placed on copper grids covered with a Formvar film for 1 min. Excess liquid was removed with a filter paper, and the grids were air dried for 10 min, washed three times with H2O, and stained with 2% aqueous uranyl acetate solution. The samples were inspected with an EM 900 TEM (Zeiss SMT). Micrographs were taken with an slow-scan charge-coupled-device SM-1k-120 camera (TRS). For the analysis of OMV formation in planta, infected plant tissue was analyzed as described previously (43).
Isolation of OMVs.
For the isolation of OMVs, bacteria were incubated overnight in 10 ml NYG medium at 30°C. At an OD600 of 0.8 to 1.0, cells were pelleted by centrifugation (10,000 × g for 10 min at 4°C), and culture supernatants were passed through a filter with a pore size of 0.45 μm and subsequently through a filter with a pore size of 0.20 μm to remove residual cells. Filtrates were centrifuged at 38,000 × g for 3 h at 4°C, supernatants were removed, and the samples were centrifuged again at 41,000 × g for 1 h at 4°C. The pellet was resuspended in PBS and centrifuged at 100,000 × g for 6 h at 4°C. After removal of the supernatant, the pellet was resuspended in 60 μl PBS and analyzed by immunoblotting and electron microscopy.
RESULTS
Secretion assays with T2S candidate substrates from X. campestris pv. vesicatoria strain 85-10.
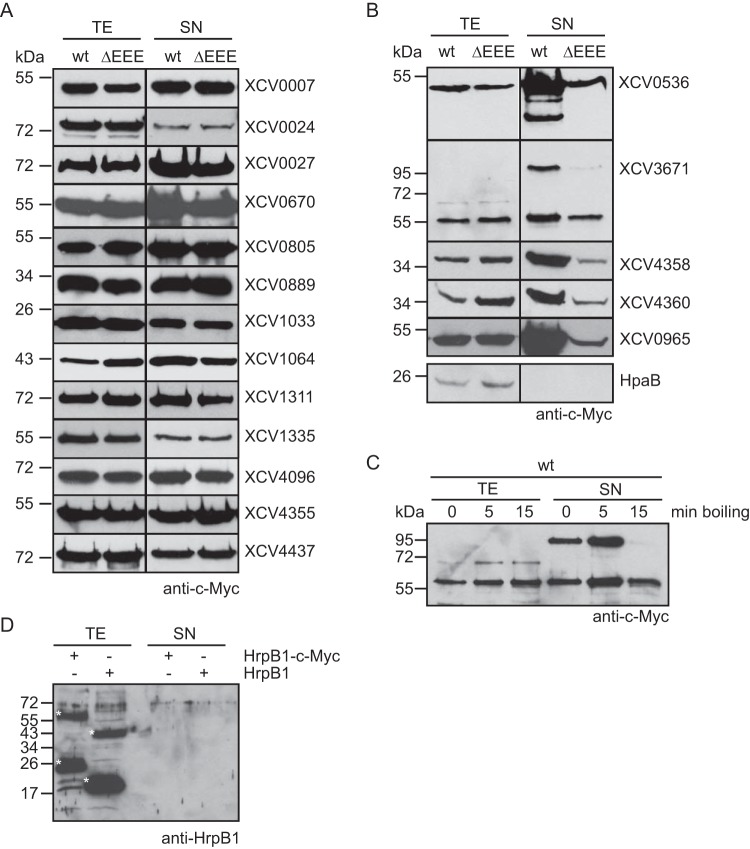
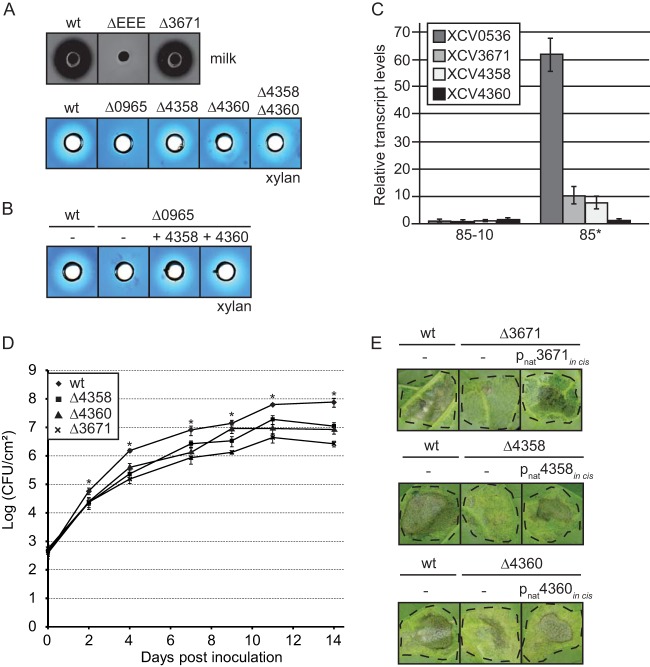
To identify type II secreted virulence factors from X. campestris pv. vesicatoria strain 85-10, we analyzed the secretion of 25 individual candidate substrates that were mainly selected based on homologies to known T2S substrates and/or virulence factors from Xanthomonas spp. (Table 2). The corresponding genes were expressed in fusions with a C-terminal triple c-Myc epitope-encoding sequence in X. campestris pv. vesicatoria strain 85-10 and the T2S mutant 85-10ΔEEE, from which the putative ATPase-encoding genes of the xps- and xcs-T2S clusters and the homologous XCV4312 gene had been deleted (8). The analysis of bacterial culture supernatants by immunoblotting revealed that eight of the C-terminally c-Myc epitope-tagged proteins were not, or were not efficiently, secreted by the wild-type and the T2S mutant strain when the bacteria were incubated in complex NYG medium (Table 2; see also Fig. S1 in the supplemental material). However, a negative influence of the C-terminal c-Myc epitope on secretion cannot be excluded. Seventeen proteins were secreted, including putative proteases, xylanases, and the lipase XCV0536 (also referred to as LipA), which was previously identified as a virulence factor of X. campestris pv. vesicatoria (44) (Fig. 1A and B). Reduced amounts of XCV0536–c-Myc, the putative protease XCV3671–c-Myc, and the predicted xylanases XCV4358–c-Myc and XCV4360–c-Myc were present in the culture supernatant of the T2S mutant strain 85-10ΔEEE compared with strain 85-10, suggesting that the efficient secretion of these proteins depends on the T2S systems (Fig. 1B). A similar finding was observed for the previously identified T2S substrate XCV0965 when the blot was overexposed (Fig. 1B). We observed a size shift for XCV3671–c-Myc, which has a molecular mass of approximately 70 kDa. The predominant signal detected by the c-Myc epitope-specific antibody corresponded to a protein of approximately 55 kDa and thus presumably to a degradation product of XCV3671–c-Myc. However, an additional signal at approximately 100 kDa was detected in the culture supernatants (Fig. 1B). When the samples were boiled for 15 min instead of 5 min, the intensity of this signal decreased (Fig. 1C). Notably, we previously observed a similar size shift for the secreted predicted proteases XCV0959 and XCV3669, which share sequence similarities with XCV3671 (Table 2) (8).
TABLE 2.
Candidate T2S substrates from X. campestris pv. vesicatoria strain 85-10
| Candidate T2S substrate and no.a | Signal peptideb | Secretionc | Homolog(s) in X. campestris pv. vesicatoria | Homologous T2S substrate(s) or virulence factor(s) (amino acid identity)d | Reference(s) |
|---|---|---|---|---|---|
| Cellulase XCV0031 | + | + | XCV0029, XCV0033, XCV0358, XCV1826 | Virulence factor XOO0282 from X. oryzae pv. oryzae strain PXO99 (87%) | 8, 9 |
| Proteases | |||||
| XCV0007 | + | + | − | This study | |
| XCV0024 | + | + | XCV0730 | This study | |
| XCV0027 | + | + | XCV0673, XCV4096, XCV3425 | T2S substrate Lpg0032 from Legionella pneumophila (27%) | 68; this study |
| XCV0583 | + | − | XCV0845 | T2S substrate XAC0552 from X. axonopodis pv. citri (96%) | 14, 30; this study |
| XCV0961 | + | − | XCV0960, XCV3671, XCV3669 | T2S substrate XCV3671 from X. campestris pv. vesicatoria (58%) | This study |
| XCV1064 | + | + | XCV3406, XCV0965e | 8; this study | |
| XCV1311 | + | + | XCV4437 | This study | |
| XCV2993 | + | − | XCV3669, XCV3671 | T2S substrate XAC2831 from X. axonopodis pv. citri (62%) | 14; this study |
| XCV3425 | + | − | XCV4096 | 68; this study | |
| XCV3669 | + | + | XCV3671, XCV0959, XCV0960, XCV0961, XCV2993 | T2S substrate XCV3671 from X. campestris pv. vesicatoria (57%) | 8 |
| XCV3671 | + | T2S | XCV3669, XCV0959, XCV0960, XCV0961, XCV2993 | This study | |
| XCV4096 | + | + | XCV0673, XCV0027, XCV3425 | T2S substrate Lpg0032 from L. pneumophila (30%) | 68; this study |
| XCV4437 | + | + | XCV1311 | This study | |
| Xylanases | |||||
| XCV0965 | + | T2S | XCV1202 | T2S substrate XCC0857 from X. campestris pv. campestris (93%) | 8; this study |
| XCV1202 | + | − | XCV0965 | This study | |
| XCV1335 | + | + | − | This study | |
| XCV4355 (XynA) | − | + | XCV4358, XCV4360 | T2S substrates XCV4358 (30%) and XCV4360 (32%) from X. campestris pv. vesicatoria | This study |
| XCV4358 (XynB2) | + | T2S | XCV4355, XCV4360 | Extracellular xylanases XOO4428 (XynB; 94%) from X. oryzae pv. oryzae and XCV4360 (57%) from X. campestris pv. vesicatoria | 11; this study |
| XCV4360 (XynB3) | + | T2S | XCV4355, XCV4358 | T2S substrate XCV4358 (57%) from X. campestris pv. vesicatoria | This study |
| Endoglucanase XCV0670 | + | + | XCV1823 | T2S substrates XOO4019 (ClsA) (88%) from X. oryzae pv. oryzae and Lpg1918 from L. pneumophila (22%) | 12, 68; this study |
| Oxidoreductase XCV2747 | + | − | XCV2904 | This study | |
| Hydrolase XCV0889 | + | + | XCV1606 | This study | |
| Lipases | |||||
| XCV0536 | + | T2S | − | XOO0526 (LipA) from X. oryzae pv. oryzae (86%) | 7; this study |
| XCV0805 | + | + | − | Virulence factor XOO3849 (accession no. YP_202488) from X. oryzae pv. oryzae KACC 10331 (94%) | 9; this study |
| XCV3283 | − | − | − | T2S substrate Lpg1157 from L. pneumophila | 69; this study |
| Decarboxylase XCV1075 | − | − | − | T2S substrate Lpg0406 from L. pneumophila (21%) | 68; this study |
| Peptidyl isomerase XCV1033 | − | + | XCV2946 | T2S substrate Lpg1962 from L. pneumophila (54%) | 69; this study |
All candidate T2S substrates are conserved in Xanthomonas spp.
+, there is a predicted Sec signal; −, no predicted Sec signal. The presence of a putative Sec signal was analyzed using the SignalP program (www.cbs.dtu.dk/services/SignalP/).
+, secreted in wild-type and T2S mutant strains; −, not detectable in culture supernatants; T2S, reduced secretion in strains with mutations in T2S genes.
The percent amino acid identity with the homologous candidate T2S substrate from X. campestris pv. vesicatoria listed in the first column.
The homology is restricted to the N-terminal region of XCV1064.
FIG 1.
The T2S systems from X. campestris pv. vesicatoria strain 85-10 contribute to the secretion of the lipase XCV0536, the predicted protease XCV3671, and the putative xylanases XCV4358 and XCV4360. (A) In vitro secretion assays with individual candidate T2S substrates. Strains 85-10 (wt) and 85-10ΔEEE (ΔEEE) carrying expression constructs encoding C-terminally c-Myc epitope-tagged candidate T2S substrates as indicated were incubated in NYG medium. Total cell extracts (TE) and culture supernatants (SN) were analyzed by immunoblotting using a c-Myc epitope-specific antibody. The c-Myc epitope-specific antibody did not detect unspecific proteins in the cell extracts or culture supernatants, as shown in Fig. S1 in the supplemental material. (B) Secretion levels of the lipase XCV0536, the predicted protease XCV3671, and the putative xylanases XCV4358, XCV4360, and XCV0965 were reduced in strain 85-10ΔEEE. X. campestris pv. vesicatoria strains 85-10 and 85-10ΔEEE containing expression constructs encoding C-terminally c-Myc epitope-tagged derivatives of XCV0536, XCV3671, XCV4358, XCV4360, and XCV0965, respectively, as indicated were incubated in NYG medium. TE and SN were analyzed as described for panel A. For the detection of XCV0965–c-Myc in the SN, the blot was overexposed. (C) Secreted XCV3671–c-Myc was detected as part of a protein complex that dissolved upon prolonged boiling of protein extracts. Strain 85-10 containing XCV3671–c-Myc was incubated in NYG medium, and TE and SN were analyzed by immunoblotting. Samples were boiled for 0, 5, or 15 min as indicated, and the blot was probed with a c-Myc epitope-specific antibody. (D) The periplasmic HrpB1 protein was not detected in culture supernatants. Strain 85-10 containing HrpB1–c-Myc and HrpB1, as indicated, was incubated in NYG medium. TE and SN were analyzed by immunoblotting using an HrpB1-specific antibody. HrpB1 and HrpB1–c-Myc have the expected molecular masses of approximately 16 and 21 kDa, respectively, and formed oligomeric complexes as described previously (45). HrpB1-specific signals are indicated by white asterisks.
The detection of proteins in the culture supernatants was presumably not caused by cell lysis or a general release of periplasmic proteins into the extracellular medium, because the cytoplasmic T3S chaperone HpaB–c-Myc and the periplasmic HrpB1 protein, which were analyzed as a C-terminally c-Myc epitope-tagged protein and an untagged protein, respectively, were detected in the cell extracts but not in the culture supernatants (Fig. 1B and D) (45, 46). Furthermore, the detection of proteins in the culture supernatants did not result from unspecific binding of the c-Myc epitope-specific antibody to bacterial proteins (see Fig. S1 in the supplemental material). As additional controls, we analyzed the secretion of XCV0536–c-Myc and XCV4358–c-Myc in X. campestris pv. vesicatoria strain 85-10Δeps, which is deficient in the production of extracellular polysaccharides (EPS). Comparable amounts of XCV0536–c-Myc and XCV4358–c-Myc were detected in the culture supernatants of X. campestris pv. vesicatoria strains 85-10 and 85-10Δeps (see Fig. S1 in the supplemental material), suggesting that the secretion of both proteins was not affected by a possible attachment of the proteins to EPS. Taken together, we conclude from these data that the predicted protease XCV3671, the lipase XCV0536, and the putative xylanases XCV4358 and XCV4360 are at least partially secreted by one or both T2S systems.
Efficient secretion of XCV0536, XCV3671, XCV4358, and XCV4360 depends on a functional Xps-T2S system and the predicted Sec signals.
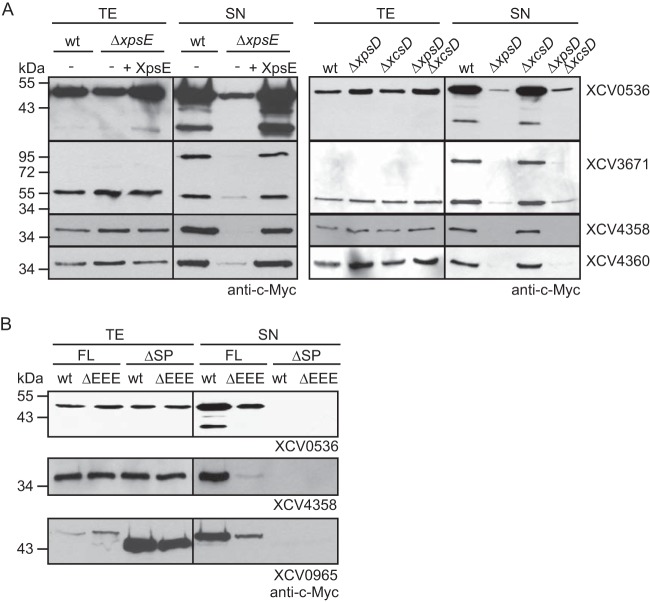
To investigate whether secretion of XCV0536, XCV3671, XCV4358, and XCV4360 is mediated by the Xps-T2S system, we performed secretion assays with X. campestris pv. vesicatoria strain 85-10ΔxpsE, which lacks the putative ATPase-encoding gene of the Xps-T2S system. Reduced amounts of XCV0536–c-Myc, XCV3671–c-Myc, XCV4358–c-Myc, and XCV4360–c-Myc were detected in the culture supernatants of strain 85-10ΔxpsE compared with those of strain 85-10 (Fig. 2A). Wild-type levels of secretion in strain 85-10ΔxpsE were restored upon ectopic expression of xpsE, suggesting that the observed secretion deficiency was specifically caused by the lack of xpsE (Fig. 2A). Similarly to strain 85-10ΔxpsE, reduced secretion of XCV0536–c-Myc, XCV3671–c-Myc, XCV4358–c-Myc, and XCV4360–c-Myc was observed in strains 85-10ΔxpsD and 85-10ΔxpsDΔxcsD, from which the secretin-encoding gene of the xps T2S gene cluster had been deleted. In contrast, wild-type secretion levels were detected in strain 85-10ΔxcsD (Fig. 2A).
FIG 2.
Efficient secretion of XCV0536, XCV3671, XCV4358, and XCV4360 depends on the Xps-T2S system. (A) Efficient secretion of XCV0536, XCV3671, XCV4358, and XCV4360 depends on xpsE and xpsD. Strains 85-10 (wt), 85-10ΔxpsE (ΔxpsE), 85-10ΔxpsD (ΔxpsD), 85-10ΔxcsD (ΔxcsD), and 85-10ΔxpsDΔxcsD (ΔxpsDΔxcsD) without expression constructs (−) or carrying XpsE and/or individual T2S substrates as indicated were incubated in NYG medium. Total cell extracts (TE) and culture supernatants (SN) were analyzed by immunoblotting using a c-Myc epitope-specific antibody. (B) Secretion of the T2S substrates XCV0536, XCV4358, and XCV0965 depends on the N-terminal regions, which contain predicted Sec signal peptides (SP). Strains 85-10 (wt) and 85-10ΔEEE (ΔEEE) carrying full-length XCV0536–c-Myc (XCV0536; FL), XCV0536Δ2–29–c-Myc (XCV0536; ΔSP), XCV4358–c-Myc (XCV4358; FL), XCV4358Δ2–22–c-Myc (XCV4358; ΔSP), XCV0965–c-Myc (XCV0965; FL), or XCV0965Δ2–24–c-Myc (XCV0965; ΔSP) as indicated were incubated in NYG medium. TE and SN were analyzed as described for panel A.
Sequence analysis revealed that most tested candidate T2S substrates contained an N-terminal predicted Sec signal (Table 2) that presumably targets the proteins for Sec-dependent transport across the IM. To analyze the contribution of the predicted N-terminal Sec signals to the secretion of selected T2S substrates, we generated expression constructs encoding N-terminal deletion derivatives of the type II secreted predicted xylanases XCV0965 and XCV4358 and the lipase XCV0536. When XCV0965Δ2–24–c-Myc, XCV4358Δ2–22–c-Myc, and XCV0536Δ2–29–c-Myc were analyzed for secretion in strains 85-10 and 85-10ΔEEE, they were not detectable in the culture supernatants (Fig. 2B), suggesting that the predicted N-terminal Sec signals are required for the efficient secretion of XCV0965, XCV4358, and XCV0536 into the extracellular milieu.
Secretion of XCV0965, XCV4358, and XCV4360 is type II independent in X. campestris pv. campestris.
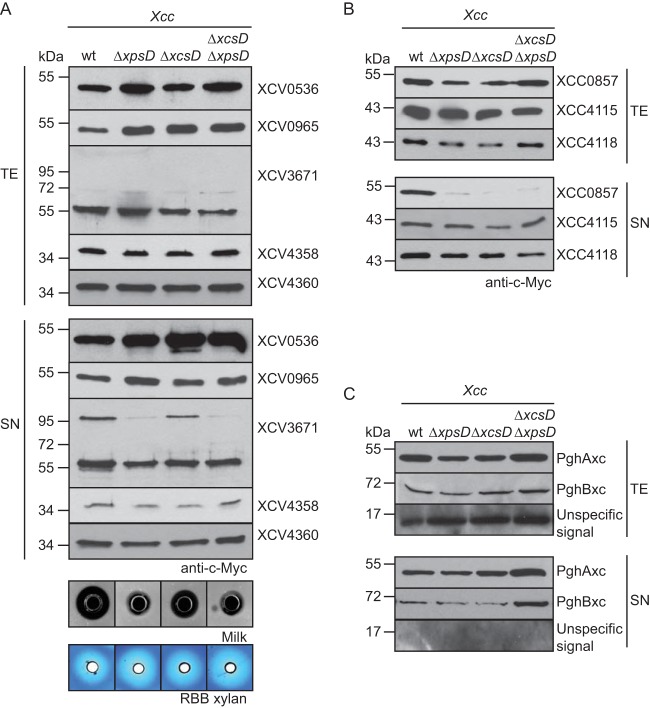
We previously reported that the secretion of the T2S substrates PghBxc (Xc1849) and PghAxc (Xc0705) from X. campestris pv. campestris is type II independent in X. campestris pv. vesicatoria (8). To further investigate possible differences in T2S substrate specificities, we analyzed the secretion of T2S substrates from X. campestris pv. vesicatoria in X. campestris pv. campestris strain LMG 568 and T2S mutant derivatives thereof with deletions of xpsD and/or xcsD. When X. campestris pv. campestris strains were incubated in complex NYG medium, similar amounts of XCV0965–c-Myc, XCV0536–c-Myc, XCV4358–c-Myc, and XCV4360–c-Myc were detected in the culture supernatants of wild-type and T2S mutant strains, suggesting that they were secreted independently of the T2S systems in X. campestris pv. campestris (Fig. 3A). In contrast, secretion of XCV3671–c-Myc was reduced in X. campestris pv. campestris xpsD deletion mutants (Fig. 3A). As was observed for X. campestris pv. vesicatoria, we detected an XCV3671-specific signal at approximately 100 kDa in the culture supernatant (see above) (Fig. 3A). We conclude from these findings that the recognition of T2S substrates can vary in different pathovars of X. campestris. This hypothesis is supported by the finding that the T2S systems from X. campestris pv. campestris are not essential for the extracellular protease and xylanase activities, as was observed for X. campestris pv. vesicatoria (Fig. 3A) (8). Protease activity of X. campestris pv. campestris, however, was reduced in xpsD mutant strains compared with the wild-type strain (Fig. 3A).
FIG 3.
Secretion assays with X. campestris pv. vesicatoria and X. campestris pv. campestris strains revealed differences in T2S substrate specificities. (A) Secretion of XCV0536, XCV0965, XCV4358, and XCV4360 from X. campestris pv. vesicatoria was type II independent in X. campestris pv. campestris. X. campestris pv. campestris strains LMG 568 (wt), LMG 568ΔxpsD (ΔxpsD), LMG 568ΔxcsD (ΔxcsD), and LMG 568ΔxpsDΔxcsD (ΔxpsDΔxcsD) carrying XCV0536–c-Myc, XCV0965–c-Myc, XCV3671–c-Myc, XCV4358–c-Myc, or XCV4360–c-Myc as indicated were incubated in NYG medium. Total cell extracts (TE) and culture supernatants (SN) were analyzed by immunoblotting using a c-Myc epitope-specific antibody. For the analysis of extracellular protease and xylanase activities, bacteria were incubated on NYG agar plates containing milk proteins or RBB xylan. Halo formation was documented 2 dpi. (B) Efficient secretion of the predicted xylanase XCC0857 in X. campestris pv. campestris depends on xpsD and xcsD. X. campestris pv. campestris strain LMG 568 and deletion derivatives thereof lacking xpsD (ΔxpsD), xcsD (ΔxcsD), or both xpsD and xcsD (ΔxpsDΔxcsD) and carrying XCC0857–c-Myc, XCC4115–c-Myc, or XCC4118–c-Myc as indicated were incubated in NYG medium. TE and SN were analyzed as described for panel A. (C) PghAxc and PghBxc were secreted independently of the T2S systems of X. campestris pv. campestris strain LMG 568. X. campestris pv. campestris strain LMG 568 and T2S mutants thereof carrying PghAxc–c-Myc and PghBxc–c-Myc as indicated were incubated in NYG medium. TE and SN were analyzed as described for panel A. The blot was reprobed with an antibody directed against the putative translocon protein XopA, which unspecifically detects intracellular proteins in X. campestris pv. campestris. One representative signal is shown.
The Xps-T2S system from X. campestris pv. campestris secretes the predicted xylanase XCC0857 but is dispensable for the secretion of the putative xylanases XCC4115 and XCC4118.
Next, we analyzed the secretion of putative xylanases from X. campestris pv. campestris that share homology with predicted xylanases from X. campestris pv. vesicatoria. The analyzed proteins include XCC0857 (93% amino acid sequence identity with XCV0965), XCC4118 (62% and 83% amino acid sequence identities with XCV4358 and XCV4360, respectively), and XCC4115 (84% amino acid sequence identity with XCV4355) (Table 2). XCC0857, XCC4115, and XCC4118 were previously shown to be secreted by X. campestris pv. campestris (47). Secretion assays with X. campestris pv. campestris wild-type and T2S mutant strains revealed that secretion of XCC0857–c-Myc was reduced in ΔxpsD and ΔxcsD deletion mutants (Fig. 3B). In contrast, similar amounts of XCC4115–c-Myc and XCC4118–c-Myc were detected in the culture supernatants, suggesting that they were secreted independently of the T2S systems from X. campestris pv. campestris (Fig. 3B). XCC4118 was previously shown to be required for the extracellular xylanase activity of X. campestris pv. campestris (47). The T2S-independent secretion of XCC4118 is, therefore, in agreement with the finding that the extracellular xylanase activity of X. campestris pv. campestris is unaffected in T2S mutant strains (see above). When analyzed in X. campestris pv. vesicatoria, secretion of XCC0857 was reduced in ΔxpsD and almost not detectable in ΔxcsD ΔxpsD deletion mutant strains, whereas XCC4115 and XCC4118 were secreted independently of the T2S systems (see Fig. S2 in the supplemental material).
We also performed secretion assays with X. campestris pv. campestris strain LMG 568 and corresponding T2S mutant strains encoding C-terminally c-Myc epitope-tagged derivatives of PghAxc and PghBxc, which were previously identified as T2S substrates of X. campestris pv. campestris strain 8004 (10). Interestingly, we did not observe T2S-dependent secretion of either protein in X. campestris pv. campestris strain LMG 568 (Fig. 3C), suggesting differences in the T2S substrate specificities of X. campestris pv. campestris strains 8004 and LMG 568.
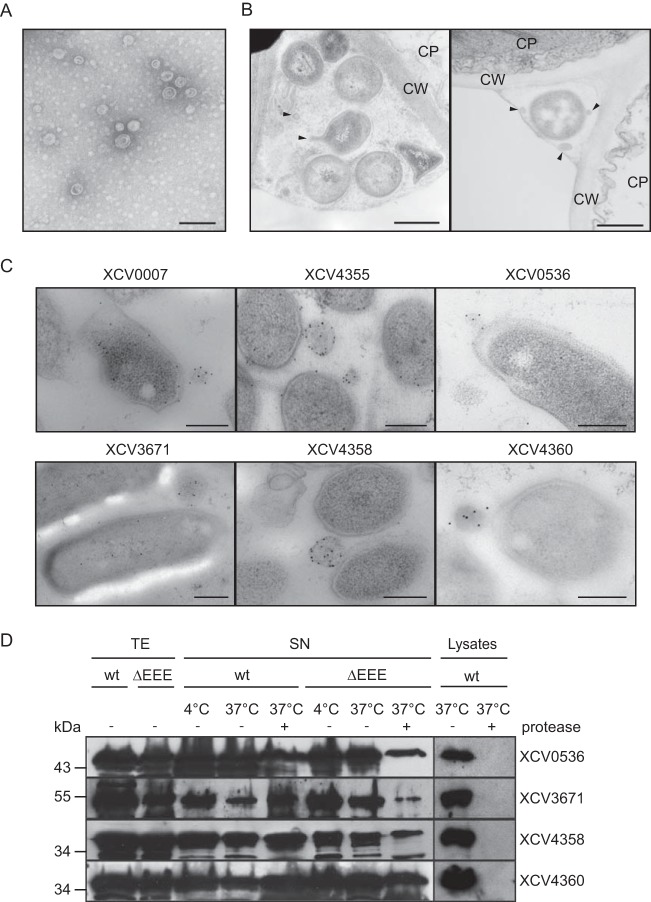
Secreted proteins from X. campestris pv. vesicatoria are detectable in OMVs.
Given the finding that T2S substrates from X. campestris pv. vesicatoria were still partially secreted by T2S mutant strains, we investigated whether extracellular proteins from X. campestris pv. vesicatoria can be secreted by OMVs, which often contribute to the delivery of bacterial virulence factors across the OM (48). Electron microscopy analysis revealed that X. campestris pv. vesicatoria strain 85-10 frequently formed OMVs, which were isolated from bacterial culture supernatants (Fig. 4A; see also Fig. S3 in the supplemental material). OMVs were also observed in infected plant tissue (Fig. 4B) and after in vitro cultivation of the bacteria (Fig. 4C; see also Fig. S4 in the supplemental material). To investigate the presence of secreted proteins in OMVs, we performed immunoelectron microscopy with strain 85-10, which contained the putative protease XCV0007–c-Myc, the predicted xylanase XCV4355–c-Myc, and the T2S substrates XCV0536–c-Myc, XCV3671–c-Myc, XCV4358–c-Myc, and XCV4360–c-Myc. Binding of c-Myc epitope-specific antibodies was detected by secondary antibodies that were coupled to gold particles. Interestingly, all analyzed proteins were detected at the bacterial membranes and inside vesicle-like structures (Fig. 4C). In contrast, almost no gold particles were present in the surrounding milieu or inside the bacterial cells, suggesting that the analyzed proteins were preferentially targeted to the bacterial membranes and to vesicles (Fig. 4C). As gold particles were present in the lumens of the OMVs but not inside bacterial cells, we assume that these structures were vesicles and not sections of bacteria (Fig. 4C). For steric reasons, sections through the tips of bacteria, which were located in a perpendicular orientation, would not be visible in close proximity to another bacterial cell. The labeling was c-Myc epitope specific because only a very few gold particles were detected outside the vesicle structures in samples of strain 85-10 containing plasmid pBRM (see Fig. S4 in the supplemental material). The analyzed T2S substrates were also detected in OMVs of strain 85-10ΔEEE (see Fig. S4). This is in agreement with the observation that XCV0536–c-Myc, XCV3671–c-Myc, XCV4358–c-Myc, and XCV4360–c-Myc were still detectable in the culture supernatant of the T2S-deficient strain 85-10ΔEEE, albeit in reduced amounts (see above).
FIG 4.
Extracellular proteins from X. campestris pv. vesicatoria detected in OMVs. (A) Electron microscopy analysis of isolated OMVs from X. campestris pv. vesicatoria strain 85-10 cultivated in NYG medium. Bar, 100 nm. (B) OMV formation by X. campestris pv. vesicatoria in infected pepper leaves. X. campestris pv. vesicatoria strain 85-10Δeps was inoculated at a bacterial density of 3.6 × 108 into leaves of susceptible ECW plants, and samples were taken 6 h postinfiltration for electron microscopy analysis. Bar, 500 nm; arrow heads, OMVs. CW, plant cell wall; CP, plant cell cytoplasm. (C) Predicted extracellular enzymes were detectable in OMVs. Strain 85-10 containing XCV0007–c-Myc, XCV4355–c-Myc, XCV0536–c-Myc, XCV3671–c-Myc, XCV4358–c-Myc, or XCV4360–c-Myc as indicated was analyzed by immunoelectron microscopy using a c-Myc epitope-specific antibody and a secondary antibody coupled to gold particles. Bar, 250 nm. (D) Secreted proteins were protected from degradation by proteinase K. Strains 85-10 (wt) and 85-10ΔEEE (ΔEEE) containing expression constructs for XCV0536–c-Myc, XCV3671–c-Myc, XCV4358–c-Myc, or XCV4360–c-Myc as indicated were incubated overnight in NYG medium. Culture supernatants (SN) and bacterial lysates were incubated on ice or at 37°C in the presence (+) or absence (−) of proteinase K as indicated. Total cell extracts (TE), SN, and lysates were analyzed by immunoblotting, using c-Myc epitope-specific antibodies. For the detection of proteins in the culture supernatants, the blots were overexposed.
To further test the hypothesis that the analyzed proteins were present in OMVs, we added proteinase K to the supernatants of bacterial cultures that were incubated overnight in complex NYG medium. Immunoblot analysis showed that the amounts of the T2S substrates XCV0536–c-Myc, XCV3671–c-Myc, XCV4358–c-Myc, and XCV4360–c-Myc were only slightly affected after incubation with proteinase K (Fig. 4D). As all proteins were degraded by proteinase K when analyzed in bacterial cell lysates (Fig. 4D), we assume that they were protected from degradation in the culture supernatant. Notably, however, when bacteria were incubated for 1 h in NYG medium, significantly reduced amounts of XCV0536–c-Myc, XCV3671–c-Myc, XCV4358–c-Myc, and XCV4360–c-Myc were detected in the presence of proteinase K (see Fig. S5 in the supplemental material). This was not the case for XCV0007–c-Myc or XCV4355–c-Myc, which were protected against degradation by proteinase K (see Fig. S5). We assume that XCV0536–c-Myc, XCV3671–c-Myc, XCV4358–c-Myc, and XCV4360–c-Myc were not efficiently targeted to OMVs during the 1-h incubation time and were therefore degraded by proteinase K.
Ectopic expression of XCV4358 or XCV4360 restores extracellular xylanase activity in strain 85-10Δ0965.
Next, we analyzed a possible contribution of the identified T2S substrates to extracellular enzyme activities. For this, XCV3671, XCV4358, and XCV4360 were individually deleted from the genome of X. campestris pv. vesicatoria strain 85-10. For the analysis of extracellular protease and xylanase activities, wild-type and T2S substrate mutant strains were cultivated on NYG agar plates containing milk proteins or RBB xylan. On these media, the extracellular protease or xylanase activity of X. campestris pv. vesicatoria results in a cleared halo around the bacterial inoculation zone. Halo formation on milk and RBB xylan plates was severely reduced in strains 85-10ΔEEE and 85-10Δ0965, respectively, as was observed previously (8) (Fig. 5A). In contrast, a wild-type halo on milk plates was detected for strain 85-10Δ3671, suggesting that XCV3671 does not significantly contribute to the degradation of milk proteins (Fig. 5A). Similarly, the extracellular xylanase activities of strains 85-10Δ4358 and 85-10Δ4360 were unaltered compared with those of the wild-type strain 85-10 (Fig. 5A). Wild-type xylanase activity was also observed for a double deletion mutant strain lacking both XCV4358 and XCV4360 (Fig. 5A). As halo formation on RBB xylan plates depends on the type II secreted xylanase XCV0965 (8) (Fig. 5A), we assumed that XCV4358 and XCV4360 did not significantly contribute to the extracellular xylanase activity. However, XCV4358 and XCV4360 restored halo formation when ectopically expressed under the control of the lac promoter in strain 85-10Δ0965 (Fig. 5B), suggesting that they encode active xylanases. The expression levels of the native XCV4358 and XCV4360 genes might therefore not be sufficient to confer detectable xylanase activity in strain 85-10.
FIG 5.
XCV4358, XCV4360, and XCV3671 contribute to virulence. (A) The predicted protease XCV3671 and the predicted xylanases XCV4358 and XCV4360 do not significantly contribute to the extracellular protease and xylanase activities, respectively, of X. campestris pv. vesicatoria. For the analysis of extracellular protease activity, strains 85-10 (wt), 85-10ΔEEE (ΔEEE), and 85-10Δ3671 (Δ3671) were incubated on NYG agar plates containing milk proteins. To study xylanase activity, X. campestris pv. vesicatoria strains 85-10 (wt), 85-10Δ0965 (Δ0965), 85-10Δ4358 (Δ4358), 85-10Δ4360 (Δ4360), and 85-10Δ4358Δ4360 (Δ4358Δ4360) were grown on NYG agar plates containing RBB xylan. Halo formation was documented 2 dpi. (B) Ectopic expression of XCV4358 or XCV4360 in strain 85-10Δ0965 restores extracellular xylanase activity. Strains 85-10 and 85-10Δ0965 without an expression construct (−) or carrying an expression construct encoding XCV4358–c-Myc or XCV4360–c-Myc as indicated were grown on NYG agar plates containing RBB xylan. Halo formation was documented 2 dpi. (C) T2S substrate genes from X. campestris pv. vesicatoria were induced by HrpG. XCV0536, XCV3671, XCV4358, and XCV4360 transcripts were amplified from cDNA derived from X. campestris pv. vesicatoria strains 85-10 and 85*. Transcript amounts were adjusted based on the transcript levels of the 16S rRNA. Data points refer to mean values of relative expression levels, and error bars show standard deviations derived from technical triplicates. (D) In planta growth of T2S substrate mutants. X. campestris pv. vesicatoria strains 85-10, 85-10Δ4358, 85-10Δ4360, and 85-10Δ3671 were inoculated into leaves of susceptible ECW pepper plants, and bacterial growth was analyzed over a period of 14 days. Values are the mean bacterial colony counts from three samples taken from three different plants. Error bars represent standard deviations. The asterisks indicate significant differences between the population sizes of the wild-type and the individual mutant strains (P < 0.05) based on the results of an unpaired Student t test. The experiment was repeated twice with similar results. (E) The predicted protease XCV3671 and the putative xylanases XCV4358 and XCV4360 contribute to bacterial virulence. Strains 85-10, 85-10Δ3671, 85-10Δ4358, and 85-10Δ4360 without an expression construct (−) or encoding XCV3671–c-Myc, XCV4358–c-Myc, or XCV4360–c-Myc under the control of the native XCV4361 promoter (pnat) in the genome as indicated were inoculated into leaves of susceptible ECW pepper plants. Disease symptoms were photographed at 6 to 8 dpi. Dashed lines indicate the inoculated areas.
HrpG induces the expression of XCV0536, XCV3671, and XCV4358 but not of XCV4360.
To analyze the expression levels of T2S substrate genes in X. campestris pv. vesicatoria, we performed transcript studies with strains 85-10 and 85-10hrpG* (85*), which contains HrpG*, a constitutively active version of HrpG (49). HrpG is the key regulator of the T3S genes and also activates the expression of T2S genes (8, 50–52). Yet, the expression of the T2S substrate gene XCV0965 is suppressed by HrpG, as was shown in our previous study (8). When bacteria were grown in NYG medium, transcript amounts of XCV0536 were significantly increased in strain 85* compared with strain 85-10 (Fig. 5C). This is in agreement with the previous finding that expression of XCV0536 is induced by the transcriptional activator HrpX, which is required for the expression of many HrpG-regulated genes and is itself activated by HrpG (53, 54). Slight increases of XCV3671 and XCV4358 transcript accumulation were observed in strain 85* compared with strain 85-10. In contrast, comparable basal transcript levels of XCV4360 were detected in both strains (Fig. 5C). We, therefore, assumed that transcription of the T2S substrate genes XCV0536, XCV3671, and XCV4358 but not of XCV4360 is induced by HrpG. The observed HrpG-dependent expression is in agreement with the presence of a plant-inducible promoter (PIP) motif (consensus sequence TTCG-N16-TTCG) in the promoter region of XCV0536 and a PIP box-like motif (TTCG-N9-TTCG) upstream of XCV3671. The PIP box motif is present in the promoter regions of many HrpX-regulated genes and presumably serves as a binding site for HrpX (53, 54).
XCV3671, XCV4358, and XCV4360 contribute to bacterial virulence.
The HrpG-dependent expression of T2S substrate genes suggests that the corresponding gene products contribute to bacterial virulence. To investigate a virulence function of XCV3671, XCV4358, and XCV4360, the corresponding X. campestris pv. vesicatoria mutant strains were inoculated into leaves of susceptible ECW pepper plants for the analysis of in planta bacterial growth. Strain 85-10 grew to approximately 108 CFU ml−1 at 14 dpi, whereas growth of XCV4358 and XCV4360 mutant strains was decreased approximately 8- to 10-fold (Fig. 5D). An even more pronounced reduction in bacterial multiplication was observed for strain 85-10Δ3671 (Fig. 5D). We also analyzed the in planta phenotypes of wild-type and T2S substrate mutant strains. For this, bacteria were inoculated at a density of 1 × 108 CFU/ml into leaves of susceptible pepper plants. As expected, the wild-type strain 85-10 induced disease symptoms in the form of water-soaked lesions that became necrotic 4 to 5 dpi (Fig. 5E). Compared with the wild-type strain, strains 85-10Δ3671, 85-10Δ4358, and 85-10Δ4360 induced reduced disease symptoms in susceptible pepper plants, which was visible as a reduction in the area of infected necrotic plant tissue (Fig. 5E). The reduced virulence of T2S substrate mutants was in agreement with the observed reduction in their in planta growth (Fig. 5D).
For complementation assays, we introduced expression constructs encoding XCV3671–c-Myc, XCV4358–c-Myc, and XCV4360–c-Myc under the control of the lac promoter into strains 85-10, 85-10Δ3671, 85-10Δ4358, and 85-10Δ4360, respectively. Infection experiments revealed that the ectopic expression of T2S substrate genes in both wild-type and deletion mutant strains led to reduced water-soaked lesions in susceptible plants (see Fig. S6 in the supplemental material), suggesting that elevated levels of the analyzed T2S substrates interfere with bacterial virulence. Similar results were obtained when the genes were expressed without a C-terminal c-Myc epitope tag-encoding sequence (data not shown) or under the control of the native promoter of XCV4361, which presumably drives the expression of XCV4358 and/or XCV4360 (see Fig. S6 in the supplemental material). Ectopic expression of T2S substrate genes might have an effect on the transport efficiency of the T2S system and thus negatively affect bacterial virulence. To avoid dominant-negative effects, we inserted the genes under the control of the XCV4361 promoter into the genome of the corresponding deletion mutants by using the suicide plasmid pLAND-P, which allows the integration of genes into the hpaFG region next to the T3S gene cluster (55). As the in cis expression of XCV3671–c-Myc, XCV4358–c-Myc, and XCV4360–c-Myc restored wild-type plant reactions in the corresponding deletion mutant strains (Fig. 5E), we assume that the observed phenotypes were specifically caused by the deletion of the T2S substrate genes. We, therefore, conclude that XCV3671, XCV4358, and XCV4360 contribute to virulence and bacterial multiplication in planta.
DISCUSSION
In this study, we identified novel substrates of the Xps-T2S system from X. campestris pv. vesicatoria, including the lipase XCV0536, the predicted protease XCV3671, and the xylanases XCV4358 and XCV4360. Studies with mutants revealed that XCV3671, XCV4358, and XCV4360 contribute to disease symptoms and in planta bacterial growth (Fig. 5). Similar findings were previously reported for the lipase XCV0536 and the type II secreted xylanase XCV0965 (8, 44). The contribution of T2S substrates to the host-pathogen interaction highlights the important function of the T2S system as a delivery pathway for extracellular virulence-associated enzymes, which might degrade plant cell components. Until now, enzyme activities of T2S substrates were described for the lipase XCV0536 and the xylanase XCV0965 (8, 44). In contrast to XCV0965, the predicted xylanases XCV4358 and XCV4360 do not significantly contribute to the extracellular xylanase activity of X. campestris pv. vesicatoria (Fig. 5). However, ectopic expression of XCV4358 and XCV4360 restored enzyme activity in the XCV0965 deletion mutant, suggesting that both genes encode active xylanases (Fig. 5). It is possible that the contribution of XCV4358 and XCV4360 to the extracellular xylanase activity is restricted to specific environmental conditions and can therefore not be monitored in vitro. Furthermore, the observed wild-type xylanase activity of X. campestris pv. vesicatoria XCV4358 and XCV4360 deletion mutants could also be explained by the weak expression of both genes under the experimental conditions, as was revealed by quantitative RT-PCR analyses (Fig. 5). Taken together, the secretion of at least three xylanases by the Xps-T2S system from X. campestris pv. vesicatoria illustrates the potential importance of xylan degradation for the plant-pathogen interaction. Xylan, which is part of the plant cell wall, is a major component of hemicellulose and the second most abundant polysaccharide on earth. The degradation of xylan by microbial xylanases has been the subject of numerous studies, mainly because of its potential application in the production of paper and biofuel (33, 56). However, until now, only little was known about the virulence functions of xylanases.
In addition to xylanases, X. campestris pv. vesicatoria secretes at least nine predicted proteases, including the Xps-T2S substrate XCV3671 (Fig. 1) (8). Enzyme activity assays revealed that XCV3671 is dispensable for the extracellular protease activity of X. campestris pv. vesicatoria on milk plates (Fig. 5). The predicted protease activity of XCV3671, therefore, still remains to be experimentally confirmed. Efficient halo formation by X. campestris pv. vesicatoria on milk plates was previously shown to depend on xps genes and an inactive HrpG protein (Fig. 5) (8, 51). Thus, the Xps-T2S system from X. campestris pv. vesicatoria likely secretes at least one additional, yet-unknown protease, which is presumably repressed by HrpG. XCV3671, however, is induced by HrpG, as was revealed by quantitative RT-PCR studies (Fig. 5). Similar findings were observed for the lipase gene XCV0536, which was previously shown to be induced during the early stages of infection and regulated by the HrpG-dependent transcriptional regulator HrpX (44, 53). In contrast to XCV3671 and XCV0536, transcript levels of the xylanase XCV4358 gene were only slightly elevated by HrpG, whereas transcript levels of XCV4360 were not affected. Given our previous finding that HrpG suppresses the transcript levels of the xylanase gene XCV0965 (8), we assume that genes encoding type II secreted xylanases of X. campestris pv. vesicatoria are differentially regulated by HrpG. It is conceivable that the individual expression patterns of xylanase genes reflect differences in their contributions to bacterial virulence at certain stages of the infection process (33).
Comparative sequence analyses revealed that homologs of T2S substrates from X. campestris pv. vesicatoria are also present in other Xanthomonas spp., suggesting that different pathogens possess a conserved repertoire of extracellular virulence factors. However, we previously observed that T2S substrates from X. campestris pv. campestris are secreted type II independently in X. campestris pv. vesicatoria (8). Vice versa, we showed here that the secretion of several T2S substrates from X. campestris pv. vesicatoria is type II independent in X. campestris pv. campestris (Fig. 3). Furthermore, the extracellular xylanase activity of X. campestris pv. campestris is independent of the T2S system, which is in contrast to the type II-dependent xylanase activity of X. campestris pv. vesicatoria (Fig. 3) (8). We, therefore, assume that T2S systems from different Xanthomonas pathovars differ in their substrate specificities. To understand the observed differences in T2S secretomes, future studies will have to focus on the identification of T2S signals as well as on the mechanisms underlying substrate recognition by the T2S system.
Secretion of the identified T2S substrates from X. campestris pv. vesicatoria was not completely abolished in the absence of functional Xcs- and Xps-T2S systems (Fig. 1), suggesting that these proteins were partially secreted by an alternative Sec-dependent transport system. Electron microscopy showed that X. campestris pv. vesicatoria frequently forms OMVs in vivo and in vitro and that T2S substrates as well as T2S-independent putative extracellular enzymes were present inside the vesicles (Fig. 4). As OMVs from X. campestris pv. vesicatoria were even observed in the absence of expression constructs (Fig. 4A and B; see also Fig. S4 in the supplemental material), vesicle formation was presumably not induced by a possible accumulation of proteins in the periplasm as a result of ectopic gene expression (57). In agreement with their localization in OMVs, the analyzed proteins were partially protected from degradation by proteinase K in the culture supernatant when the bacteria were incubated overnight in NYG medium (Fig. 4). Taken together, we conclude from these observations that XCV0536, XCV3671, XCV4358, and XCV4360 are either secreted by the T2S system or by OMVs. A similar finding was previously reported for T2S substrates from Vibrio cholerae cultures (58, 59). To date, OMVs have been intensively studied in animal-pathogenic bacteria and shown to contribute to the secretion of virulence factors (48, 60). OMV cargo proteins are protected against degradation and can be transported over long distances in a concentrated manner (57, 60). While OMVs from animal-pathogenic bacteria presumably fuse with the host cell membrane to deliver virulence factors (61), the access of OMVs from plant pathogens to the host cell membrane is presumably hindered by the plant cell wall. To date, OMVs from plant-pathogenic bacteria have only been reported for X. campestris pv. campestris, where they appear to contain virulence-associated proteins, including predicted enzymes, type III effector proteins and, surprisingly, also components of the T3S system (62). OMVs might, therefore, provide an alternative transport route for different virulence factors, including T2S substrates from Xanthomonas spp., to the extracellular milieu. The mechanisms that control T2S- or OMV-mediated export of virulence factors remain to be investigated.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to M. Jordan and S. Fraas for technical assistance and to U. Bonas for comments on the manuscript.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (BU 2145/6-1; CRC 648, Molecular mechanisms of information processing in plants) to D.B. and G.H.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00322-15.
REFERENCES
- 1.Gerlach RG, Hensel M. 2007. Protein secretion systems and adhesins: the molecular armory of Gram-negative pathogens. Int J Med Microbiol 297:401–415. doi: 10.1016/j.ijmm.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 2.Faulkner C, Robatzek S. 2012. Plants and pathogens: putting infection strategies and defence mechanisms on the map. Curr Opin Plant Biol 15:699–707. doi: 10.1016/j.pbi.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Hématy K, Cherk C, Somerville S. 2009. Host-pathogen warfare at the plant cell wall. Curr Opin Plant Biol 12:406–413. doi: 10.1016/j.pbi.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Gibson DM, King BC, Hayes ML, Bergstrom GC. 2011. Plant pathogens as a source of diverse enzymes for lignocellulose digestion. Curr Opin Microbiol 14:264–270. doi: 10.1016/j.mib.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Hamann T. 2012. Plant cell wall integrity maintenance as an essential component of biotic stress response mechanisms. Front Plant Sci 3:77. doi: 10.3389/fpls.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarkar P, Bosneaga E, Auer M. 2009. Plant cell walls throughout evolution: towards a molecular understanding of their design principles. J Exp Bot 60:3615–3635. doi: 10.1093/jxb/erp245. [DOI] [PubMed] [Google Scholar]
- 7.Rajeshwari R, Jha G, Sonti RV. 2005. Role of an in planta-expressed xylanase of Xanthomonas oryzae pv. oryzae in promoting virulence on rice. Mol Plant Microbe Interact 18:830–837. doi: 10.1094/MPMI-18-0830. [DOI] [PubMed] [Google Scholar]
- 8.Szczesny R, Jordan M, Schramm C, Schulz S, Cogez V, Bonas U, Büttner D. 2010. Functional characterization of the Xps and Xcs type II secretion systems from the plant pathogenic bacterium Xanthomonas campestris pv. vesicatoria. New Phytol 187:983–1002. doi: 10.1111/j.1469-8137.2010.03312.x. [DOI] [PubMed] [Google Scholar]
- 9.Wang JC, So BH, Kim JH, Park YJ, Lee B-M, Kang HW. 2008. Genome-wide identification of pathogenicity genes in Xanthomonas oryzae pv. oryzae by transposon mutagenesis. Plant Pathol 57:1136–1145. doi: 10.1111/j.1365-3059.2008.01884.x. [DOI] [Google Scholar]
- 10.Wang L, Rong W, He C. 2008. Two Xanthomonas extracellular polygalacturonases, PghAxc and PghBxc, are regulated by type III secretion regulators HrpX and HrpG and are required for virulence. Mol Plant Microbe Interact 21:555–563. doi: 10.1094/MPMI-21-5-0555. [DOI] [PubMed] [Google Scholar]
- 11.Ray SK, Rajeshwari R, Sonti RV. 2000. Mutants of Xanthomonas oryzae pv. oryzae deficient in general secretory pathway are virulence deficient and unable to secrete xylanase. Mol Plant Microbe Interact 13:394–401. doi: 10.1094/MPMI.2000.13.4.394. [DOI] [PubMed] [Google Scholar]
- 12.Jha G, Rajeshwari R, Sonti RV. 2007. Functional interplay between two Xanthomonas oryzae pv. oryzae secretion systems in modulating virulence on rice. Mol Plant Microbe Interact 20:31–40. doi: 10.1094/MPMI-20-0031. [DOI] [PubMed] [Google Scholar]
- 13.Furutani A, Tsuge S, Ohnishi K, Hikichi Y, Oku T, Tsuno K, Inoue Y, Ochiai H, Kaku H, Kubo Y. 2004. Evidence for HrpXo-dependent expression of type II secretory proteins in Xanthomonas oryzae pv. oryzae. J Bacteriol 186:1374–1380. doi: 10.1128/JB.186.5.1374-1380.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamazaki A, Hirata H, Tsuyumu S. 2008. HrpG regulates type II secretory proteins in Xanthomonas axonopodis pv. citri. J Gen Plant Pathol 74:138–150. doi: 10.1007/s10327-008-0075-7. [DOI] [Google Scholar]
- 15.Tsujimoto S, Nakaho K, Adachi M, Ohnishi K, Kiba A, Hikichi Y. 2008. Contribution of the type II secretion system in systemic infectivity of Ralstonia solanacearum through xylem vessels. J Gen Plant Pathol 74:71–75. doi: 10.1007/s10327-007-0061-5. [DOI] [Google Scholar]
- 16.Barras F, van Gijsegem F, Chatterjee AK. 1994. Extracellular enzymes and pathogenesis of soft-rot Erwinia. Annu Rev Phytopathol 32:201–234. doi: 10.1146/annurev.py.32.090194.001221. [DOI] [Google Scholar]
- 17.Cianciotto NP. 2005. Type II secretion: a protein secretion system for all seasons. Trends Microbiol 13:581–588. doi: 10.1016/j.tim.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Korotkov KV, Sandkvist M, Hol WG. 2012. The type II secretion system: biogenesis, molecular architecture and mechanism. Nat Rev Microbiol 10:336–351. doi: 10.1038/nmicro2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Douzi B, Filloux A, Voulhoux R. 2012. On the path to uncover the bacterial type II secretion system. Philos Trans R Soc Lond B Biol Sci 367:1059–1072. doi: 10.1098/rstb.2011.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Natale P, Bruser T, Driessen AJ. 2008. Sec- and Tat-mediated protein secretion across the bacterial cytoplasmic membrane: distinct translocases and mechanisms. Biochim Biophys Acta 1778:1735–1756. doi: 10.1016/j.bbamem.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Yuan J, Zweers JC, van Dijl JM, Dalbey RE. 2010. Protein transport across and into cell membranes in bacteria and archaea. Cell Mol Life Sci 67:179–199. doi: 10.1007/s00018-009-0160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nivaskumar M, Francetic O. 2014. Type II secretion system: a magic beanstalk or a protein escalator. Biochim Biophys Acta 1843:1568–1577. doi: 10.1016/j.bbamcr.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 23.Leyns F, De Cleene M, Swings J, De Ley J. 1984. The host range of the genus Xanthomonas. Bot Rev 50:305–355. [Google Scholar]
- 24.Chan JWYF, Goodwin PH. 1999. The molecular genetics of virulence of Xanthomonas campestris. Biotechnol Adv 17:489–508. doi: 10.1016/S0734-9750(99)00025-7. [DOI] [PubMed] [Google Scholar]
- 25.Lu H, Patil P, Van Sluys MA, White FF, Ryan RP, Dow JM, Rabinowicz P, Salzberg SL, Leach JE, Sonti R, Brendel V, Bogdanove AJ. 2008. Acquisition and evolution of plant pathogenesis-associated gene clusters and candidate determinants of tissue-specificity in Xanthomonas. PLoS One 3:e3828. doi: 10.1371/journal.pone.0003828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian W, Jia Y, Ren SX, He YQ, Feng JX, Lu LF, Sun Q, Ying G, Tang DJ, Tang H, Wu W, Hao P, Wang L, Jiang BL, Zeng S, Gu WY, Lu G, Rong L, Tian Y, Yao Z, Fu G, Chen B, Fang R, Qiang B, Chen Z, Zhao GP, Tang JL, He C. 2005. Comparative and functional genomic analyses of the pathogenicity of phytopathogen Xanthomonas campestris pv. campestris. Genome Res 15:757–767. doi: 10.1101/gr.3378705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dow JM, Milligan DE, Jaison L, Barber CE, Daniels MJ. 1987. A gene cluster in Xanthomonas campestris required for pathogenicity controls the excretion of polygalacturonate lyase and other enzymes. Physiol Mol Plant Pathol 31:261–271. doi: 10.1016/0885-5765(87)90070-1. [DOI] [Google Scholar]
- 28.Baptista JC, Machado MA, Homem RA, Torres PS, Vojnov AA, do Amaral AM. 2010. Mutation in the xpsD gene of Xanthomonas axonopodis pv. citri affects cellulose degradation and virulence. Genet Mol Biol 33:146–153. doi: 10.1590/S1415-47572009005000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun QH, Hu J, Huang GX, Ge C, Fang RX, He CZ. 2005. Type-II secretion pathway structural gene xpsE, xylanase- and cellulase secretion and virulence in Xanthomonas oryzae pv. oryzae. Plant Pathol 54:15–21. doi: 10.1111/j.1365-3059.2004.01101.x. [DOI] [Google Scholar]
- 30.Guo Y, Figueiredo F, Jones J, Wang N. 2011. HrpG and HrpX play global roles in coordinating different virulence traits of Xanthomonas axonopodis pv. citri. Mol Plant Microbe Interact 24:649–661. doi: 10.1094/MPMI-09-10-0209. [DOI] [PubMed] [Google Scholar]
- 31.He SY, Nomura K, Whittam TS. 2004. Type III protein secretion mechanism in mammalian and plant pathogens. Biochim Biophys Acta 1694:181–206. doi: 10.1016/j.bbamcr.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 32.Büttner D. 2012. Protein export according to schedule: architecture, assembly and regulation of type III secretion systems from plant and animal pathogenic bacteria. Microbiol Mol Biol Rev 76:262–310. doi: 10.1128/MMBR.05017-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belien T, Van Campenhout S, Robben J, Volckaert G. 2006. Microbial endoxylanases: effective weapons to breach the plant cell-wall barrier or, rather, triggers of plant defense systems? Mol Plant Microbe Interact 19:1072–1081. doi: 10.1094/MPMI-19-1072. [DOI] [PubMed] [Google Scholar]
- 34.Daniels MJ, Barber CE, Turner PC, Sawczyc MK, Byrde RJW, Fielding AH. 1984. Cloning of genes involved in pathogenicity of Xanthomonas campestris pv. campestris using the broad host range cosmid pLAFR1. EMBO J 3:3323–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Figurski D, Helinski DR. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A 76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Minsavage GV, Dahlbeck D, Whalen MC, Kearny B, Bonas U, Staskawicz BJ, Stall RE. 1990. Gene-for-gene relationships specifying disease resistance in Xanthomonas campestris pv. vesicatoria-pepper interactions. Mol Plant Microbe Interact 3:41–47. doi: 10.1094/MPMI-3-041. [DOI] [Google Scholar]
- 37.Bonas U, Schulte R, Fenselau S, Minsavage GV, Staskawicz BJ, Stall RE. 1991. Isolation of a gene-cluster from Xanthomonas campestris pv. vesicatoria that determines pathogenicity and the hypersensitive response on pepper and tomato. Mol Plant Microbe Interact 4:81–88. doi: 10.1094/MPMI-4-081. [DOI] [Google Scholar]
- 38.Vroemen S, Heldens J, Boyd C, Henrissat B, Keen NT. 1995. Cloning and characterization of the bgxA gene from Erwinia chrysanthemi D1 which encodes a beta-glucosidase/xylosidase enzyme. Mol Gen Genet 246:465–477. doi: 10.1007/BF00290450. [DOI] [PubMed] [Google Scholar]
- 39.Engler C, Kandzia R, Marillonnet S. 2008. A one pot, one step, precision cloning method with high throughput capability. PLoS One 3:e3647. doi: 10.1371/journal.pone.0003647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huguet E, Hahn K, Wengelnik K, Bonas U. 1998. hpaA mutants of Xanthomonas campestris pv. vesicatoria are affected in pathogenicity but retain the ability to induce host-specific hypersensitive reaction. Mol Microbiol 29:1379–1390. doi: 10.1046/j.1365-2958.1998.01019.x. [DOI] [PubMed] [Google Scholar]
- 41.Rossier O, Wengelnik K, Hahn K, Bonas U. 1999. The Xanthomonas Hrp type III system secretes proteins from plant and mammalian pathogens. Proc Natl Acad Sci U S A 96:9368–9373. doi: 10.1073/pnas.96.16.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noël L. 2001. Utilisation de la technique de cDNA-AFLP pour l'étude d'un transcriptome procaryote: identification et caractérisation du régulon hrp chez Xanthomonas campestris pv. vesicatoria. Ph.D. thesis Université de Paris-Sud, Paris, France. [Google Scholar]
- 43.Gürlebeck D, Jahn S, Gürlebeck N, Szczesny R, Szurek B, Hahn S, Hause G, Bonas U. 2009. Visualization of novel virulence activities of the Xanthomonas type III effectors AvrBs1, AvrBs3 and AvrBs4. Mol Plant Pathol 10:175–188. doi: 10.1111/j.1364-3703.2008.00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamir-Ariel D, Rosenberg T, Navon N, Burdman S. 2012. A secreted lipolytic enzyme from Xanthomonas campestris pv. vesicatoria is expressed in planta and contributes to its virulence. Mol Plant Pathol 13:556–567. doi: 10.1111/j.1364-3703.2011.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hausner J, Hartmann N, Lorenz C, Büttner D. 2013. The periplasmic HrpB1 protein from Xanthomonas spp. binds to peptidoglycan and to components of the type III secretion system. Appl Environ Microbiol 79:6312–6324. doi: 10.1128/AEM.01226-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Büttner D, Gürlebeck D, Noël LD, Bonas U. 2004. HpaB from Xanthomonas campestris pv. vesicatoria acts as an exit control protein in type III-dependent protein secretion. Mol Microbiol 54:755–768. doi: 10.1111/j.1365-2958.2004.04302.x. [DOI] [PubMed] [Google Scholar]
- 47.Dejean G, Blanvillain-Baufume S, Boulanger A, Darrasse A, de Bernonville TD, Girard AL, Carrere S, Jamet S, Zischek C, Lautier M, Sole M, Büttner D, Jacques MA, Lauber E, Arlat M. 2013. The xylan utilization system of the plant pathogen Xanthomonas campestris pv. campestris controls epiphytic life and reveals common features with oligotrophic bacteria and animal gut symbionts. New Phytol 198:899–915. doi: 10.1111/nph.12187. [DOI] [PubMed] [Google Scholar]
- 48.Lloubes R, Bernadac A, Houot L, Pommier S. 2013. Nonclassical secretion systems. Res Microbiol 164:655–663. doi: 10.1016/j.resmic.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 49.Wengelnik K, Rossier O, Bonas U. 1999. Mutations in the regulatory gene hrpG of Xanthomonas campestris pv. vesicatoria result in constitutive expression of all hrp genes. J Bacteriol 181:6828–6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wengelnik K, Van den Ackerveken G, Bonas U. 1996. HrpG, a key hrp regulatory protein of Xanthomonas campestris pv. vesicatoria is homologous to two-component response regulators. Mol Plant Microbe Interact 9:704–712. doi: 10.1094/MPMI-9-0704. [DOI] [PubMed] [Google Scholar]
- 51.Noël L, Thieme F, Nennstiel D, Bonas U. 2001. cDNA-AFLP analysis unravels a genome-wide hrpG-regulon in the plant pathogen Xanthomonas campestris pv. vesicatoria. Mol Microbiol 41:1271–1281. doi: 10.1046/j.1365-2958.2001.02567.x. [DOI] [PubMed] [Google Scholar]
- 52.Schulze S, Kay S, Büttner D, Egler M, Eschen-Lippold L, Hause G, Krüger A, Lee J, Müller O, Scheel D, Szczesny R, Thieme F, Bonas U. 2012. Analyses of new type III effectors from Xanthomonas uncover XopB and XopS as suppressors of plant immunity. New Phytol 195:894–911. doi: 10.1111/j.1469-8137.2012.04210.x. [DOI] [PubMed] [Google Scholar]
- 53.Koebnik R, Krüger A, Thieme F, Urban A, Bonas U. 2006. Specific binding of the Xanthomonas campestris pv. vesicatoria AraC-type transcriptional activator HrpX to plant-inducible promoter boxes. J Bacteriol 188:7652–7660. doi: 10.1128/JB.00795-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wengelnik K, Bonas U. 1996. HrpXv, an AraC-type regulator, activates expression of five of the six loci in the hrp cluster of Xanthomonas campestris pv. vesicatoria. J Bacteriol 178:3462–3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lorenz C, Hausner J, Büttner D. 2012. HrcQ provides a docking site for early and late type III secretion substrates from Xanthomonas. PLoS One 7:e51063. doi: 10.1371/journal.pone.0051063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Collins T, Gerday C, Feller G. 2005. Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol Rev 29:3–23. doi: 10.1016/j.femsre.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 57.Manning AJ, Kuehn MJ. 2013. Functional advantages conferred by extracellular prokaryotic membrane vesicles. J Mol Microbiol Biotechnol 23:131–141. doi: 10.1159/000346548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Altindis E, Fu Y, Mekalanos JJ. 2014. Proteomic analysis of Vibrio cholerae outer membrane vesicles. Proc Natl Acad Sci U S A 111:E1548–E1556. doi: 10.1073/pnas.1403683111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sikora AE, Zielke RA, Lawrence DA, Andrews PC, Sandkvist M. 2011. Proteomic analysis of the Vibrio cholerae type II secretome reveals new proteins, including three related serine proteases. J Biol Chem 286:16555–16566. doi: 10.1074/jbc.M110.211078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bonnington KE, Kuehn MJ. 2014. Protein selection and export via outer membrane vesicles. Biochim Biophys Acta 1843:1612–1619. doi: 10.1016/j.bbamcr.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.MacDonald IA, Kuehn MJ. 2012. Offense and defense: microbial membrane vesicles play both ways. Res Microbiol 163:607–618. doi: 10.1016/j.resmic.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sidhu VK, Vorholter FJ, Niehaus K, Watt SA. 2008. Analysis of outer membrane vesicle associated proteins isolated from the plant pathogenic bacterium Xanthomonas campestris pv. campestris. BMC Microbiol 8:87. doi: 10.1186/1471-2180-8-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Canteros BI. 1990. Diversity of plasmids and plasmid-encoded phenotypic traits in Xanthomonas campestris pv. vesicatoria. Ph.D. thesis University of Florida, Gainesville, FL. [Google Scholar]
- 64.Ménard R, Sansonetti PJ, Parsot C. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol 175:5899–5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murillo J, Shen H, Gerhold D, Sharma A, Cooksey DA, Keen NT. 1994. Characterization of pPT23B, the plasmid involved in syringolide production by Pseudomonas syringae pv. tomato PT23. Plasmid 31:275–287. doi: 10.1006/plas.1994.1029. [DOI] [PubMed] [Google Scholar]
- 66.Escolar L, Van den Ackerveken G, Pieplow S, Rossier O, Bonas U. 2001. Type III secretion and in planta recognition of the Xanthomonas avirulence proteins AvrBs1 and AvrBsT. Mol Plant Pathol 2:287–296. doi: 10.1046/j.1464-6722.2001.00077.x. [DOI] [PubMed] [Google Scholar]
- 67.Vieira J, Messing J. 1987. Production of single-stranded plasmid DNA. Methods Enzymol 153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- 68.DebRoy S, Dao J, Soderberg M, Rossier O, Cianciotto NP. 2006. Legionella pneumophila type II secretome reveals unique exoproteins and a chitinase that promotes bacterial persistence in the lung. Proc Natl Acad Sci U S A 103:19146–19151. doi: 10.1073/pnas.0608279103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cianciotto NP. 2009. Many substrates and functions of type II secretion: lessons learned from Legionella pneumophila. Future Microbiol 4:797–805. doi: 10.2217/fmb.09.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.