Abstract
Background
In spite of profound reduction in incidence, cervical cancer claims >275,000 lives annually. Previously we demonstrated efficacy and safety of radioimmunotherapy directed at HPV16 E6 oncoprotein in experimental cervical cancer.
Materials & methods
We undertook a direct comparison of targeting E7 and E6 oncoproteins with specific 188Rhenium-labeled monoclonal antibodies in CasKi subcutaneous xenografts of cervical cancer cells in mice.
Results
The most significant tumor inhibition was seen in radioimmunotherapy-treated mice, followed by the unlabeled monoclonal antibodies to E6 and E7. No hematological toxicity was observed. Immunohistochemistry suggests that the effect of unlabeled antibodies is C3 complement mediated.
Conclusion
We have demonstrated for the first time that radioimmunotherapy directed toward E7 oncoprotein inhibits experimental tumors growth, decreases E7 expression and may offer a novel approach to cervical cancer therapy.
Keywords: 188Rhenium, apoptosis, complement cytotoxicity, E6 and E7 oncoproteins, HPV16 positive cervical cancer, p53 expression, radioimmunotherapy, retinoblastoma
It has been well established and is universally accepted that cervical cancer is caused by Human Papilloma Virus, HPV [1,2]. Molecular epidemiologists have been able to trace strains of HPV back to the prehistoric era noting conservation of the viral epitopes for over 200,000 years [3]. Throughout the course of time the preservation of the active oncogenic portions of the virus, the reliance of malignant transformation on early oncogenes expression has remained the same; namely of the E6 and E7 viral oncogenes [4]. Persistence of HPV infection leads to the malignant change of normal cervical epithelium to cancer. In concert with other factors such as loss of heterozygosity, E6 oncogene deregulates the p53 pathway, while E7 de regulates retinoblastoma gene (Rb) [5]. In spite of the profound reduction in incidence of cervical cancer due to Papanicolaou screening and initiation of HPV vaccination, cervical cancer remains a pandemic with more than 85% of the global burden of disease occurring in developing countries and claims more than 275,000 lives annually (WHO, 2011). In early stages of cervical cancer, surgery can be curative, however, when cancer is metastatic at the time of a patient presentation or is recurrent, treatment options are limited.
Our approach to address this limitation is the development of radioimmuno-therapy (RIT) to target the viral specific oncoproteins of HPV-induced cervical cancer by labeling monoclonal antibodies (mAbs) to E6 or E7 oncoproteins, the products of E6 and E7 expression, with a radionuclide which emits cytotoxic radiation, such as 188Rhenium (188Re) [6]. To access the intranuclearly located target E6 and E7 oncoproteins, this approach initially relies on tumor necrosis that occurs as the tumor outgrows its blood supply causing the release of intranuclear contents into the interstitial spaces where it becomes accessible to mAbs while further damage is being mediated via beta-emission of a radionuclide. Our previous studies focused on RIT targeting E6 oncoprotein. We have demonstrated the ability of a mAb C1P5 which is specific for HPV-16 E6 to target E6 antigen in experimental cervical cancer models with both high and low HPV copy numbers [7,8]. We observed abrogation of xenografted cervical tumors in nude mice by 188Re-labeled C1P5 mAb to E6 and a surprising effect of tumor growth inhibition in the unlabeled C1P5 mAb treatment group.
The molecular arrangement of E7 oncogene is intricately linked to another early gene E2 in the HPV genome and although the relative expression in actual human tumors (and commercially available cell lines) is blunted compared with E6 as shown in [9], the impact of the linkage might render targeting it with RIT equally efficacious. In our previous studies we detected E7 expression by western blot analysis in CasKi, SiHa and HeLa human cervical cancer cell lines with the E7 specific mAb TVG701Y as in [10], however, the potential of E7 as an RIT target in vivo remains unexplored.
This report focuses on the direct comparison of efficacy of targeting E7 and E6 oncoproteins with specific mAbs labeled with 188Re in CasKi subcutaneous xenografts of cervical cancer cells in nude mice. We hypothesized that the effects of RIT directed against E7 oncoprotein will be comparable to those of RIT directed against E6. We also compared the effect of unlabeled mAbs to E6 and E7 on the tumors. To our knowledge, this is the first report on comparative targeting of E6 and E7 oncoproteins with specific mAbs for developing novel i mmunotherapy for cervical cancer.
Materials & methods
Cell lines, antibodies & reagents
The commercially available CasKi human cervical cancer cell line, expressing both E6 and E7 oncoproteins, was purchased from the American Type Culture Collection (VA, USA). Cells were grown in RPMI-1640 medium containing 10% FBS (Sigma) and 1% Penicillin-streptomycin solution (Sigma, penicillin 10,000 U and streptomycin 10 mg/ml) at 37°C in a 5% CO2 incubator. Matrigel, used in development of tumors, was purchased from BD Biosciences (MD, USA). Murine mAbs C1P5 (IgG1) to HPV-16 E6 + HPV-18 E6 and TVG-701Y (IgG2a to HPV-16 E7) were procured from Abcam.
Radiolabeling of antibodies
The beta-emitter 188Re (half-life, 16.9 h) was produced from beta decay of a parent radionuclide 188W (half-life 69 days) using a 188W/188Re generator (ITG Isotope Technologies Garching GmbH, Germany). After 188Re was eluted in the form of sodium perrhenate, the antibodies were labeled with 188Re ‘directly’ through binding of reduced 188Re to the generated sulfhydryl groups on the antibodies, as described previously [11]. The radiolabeling yields were measured by instant thin layer chromatography by developing silica gel (SG) 10 cm strips in saline. In this system the 188Re-labeled antibodies stay at the point of application while free 188Re moves with the solvent front. The typical radiolabeling yields for both C1P5 and TVG-701Y mAbs were 85%. The radiolabeled mAbs were purified by HPLC using TosoHaas size exclusion column with PBS at 1 ml/min as an eluent using Waters HPLC system equipped with UV and radiation (Bioscan) flow-through detectors. The stability of the 188Re-radiolabel on the mAbs was determined by incubating the radiolabeled mAbs in mouse serum for 48 h (~3 physical half-lives for 188Re) at 37°C and analyzing the aliquots on the HPLC size exclusion column described above at 0, 1, 2, 4, 8, 24 and 48 h during the incubation in serum. No loss of 188Re radiolabel from the mAbs was noted.
Tumor model
All animal studies were carried out in accordance with the guidelines of the Institute for Animal Studies at the Albert Einstein College of Medicine. Twenty-five six-week-old female athymic balb/c nude mice were purchased from Charles River Laboratories. 8 × 106 CasKi cells mixed with Matrigel were implanted into the flanks of mice in a subcutaneous fashion and allowed to grow to tumor size of 3–5 mm.
Biodistribution of 188Re-C1P5 mAb in tumor-bearing mice & dosimetry calculations
For biodistribution the CasKi tumor-bearing mice were randomized into the groups of four mice per group and injected intraperitoneally (IP) with 50 μCi 188Re-C1P5. The mice were sacrificed at the preset time points of 24, 48 and 72 h, their tumors and major organs removed, blotted from blood, weighed and counted for radio activity in the gamma counter, and percentage of injected dose per gram organ/tissue was calculated. These radioactive decay-corrected data were used to perform the radiation dosimetry calculations. Each mouse organ or tissue has a uniquely calculated absorbed fraction depending on geometry (and density for tissue, bone and lungs) and complete energy spectrum of the applicable radionuclide. The absorbed fractions for 188Re vary from 0.172 to 0.709 [12]. The effective clearance values for each organ and time point were obtained from the bio-distribution data, then fit each to a single exponential. The correlation coefficients are given in the last column of Table 1 under the heading ‘r2’. The areas under curve were then integrated, multiplied by the equilibrium dose constant and absorbed fraction, and corrected for units. The equilibrium dose constant for 188Re was obtained from the updated radionuclide emission data provided in [13]. The equilibrium dose constant was determined only for electrons (because only negligible photon radiation is absorbed by the small mouse organs). The results were expressed in cGy per unit μCi administered to a typical mouse.
Table 1.
Dosimetry results for 188Rhenium-C1P5 monoclonal antibodies administered to CasKi tumor-bearing mice.
| Organ | cGy/μCi | Label | r2 |
|---|---|---|---|
| Blood | 5.292 | Re-188 | 0.98 |
| Lung | 1.066 | Re-188 | 0.99 |
| Heart | 1.633 | Re-188 | 0.98 |
| Spleen | 0.903 | Re-188 | 0.99 |
| Liver | 1.400 | Re-188 | 0.98 |
| Kidney | 2.138 | Re-188 | 0.99 |
| Stomach | 1.071 | Re-188 | 0.99 |
| Tumor | 0.907 | Re-188 | 0.99 |
| Muscle | 0.712 | Re-188 | 0.96 |
| Bone | 0.698 | Re-188 | 0.98 |
RIT of CasKi tumor-bearing mice with 188Re-labeled mAbs to E6 & E4 oncoproteins
CasKi tumor-bearing mice were randomized to five treatment groups of five mice per group: untreated controls, 300 μCi 188Re-C1P5, 300 μCi 188Re-TVG-701Y, unlabeled TVG-701Y and unlabeled-C1P5 groups. The total amount of an antibody per mouse in all groups was 10 μg. As patients with the metastatic cervical cancer have significant peritoneal disease, we administered the radiolabeled mAbs as IP injections to approximate the clinical situation. After IP injection administered as 200 μl volume, the mice were observed for 33 days and the tumors were measured in three dimensions over the observation period every three days. The volume of the tumors was calculated in the following fashion: Volume (initial) = V0 = (x * y * z)/2. The subsequent measurements (V2) were calculated as % change from initial volume (V2 – V0)/V0. The experiment was performed two times.
Analyses of white blood cells & platelets
Baseline blood samples were taken from the tail vein of five non-tumor bearing mice and compared with the published data for balb/c athymic mice from Charles River Laboratories. Weekly blood samples were obtained in a random fashion from three mice per treatment group by ocular aspiration of blood. An aliquot of 1.5 μl of blood was added to 200 μl of ammonium oxalate solution (1 ml ammonium oxalate mixed with 3 ml of sterile water) and platelets were counted according to the published protocol [14]. White blood cells were counted according to standard protocol after diluting 10 μl of blood to 190 μl with 2% acetic acid solution.
Immunohistochemical analyses
Complement deposition was assessed using the murine mAb 6F64 to C3 (Santa Cruz Biotechnology) with the dilution of 1:1000. In addition, the tumors were stained for apoptosis (TUNEL), p53, Rb, p16 (BD Pharmingen) and CD3 (Dako). The immuno histochemistry (IHC) procedures were performed according to published protocols. The evaluation/scoring of the immunoreactivity was based on published criteria, and by two independent observers. TUNEL assay was performed according to the in situ cell death detection protocol (Roche). Retinoblastoma (Leica Biosystems) and p53 (Dako) assays were performed at a dilution of 1:20. For CD3 staining, the positive control tissue was tonsil using the CD3 antibody and the negative control tissue was tonsil with nonspecific rabbit IgG. For Rb the positive control tissue was tonsil using the Rb antibody and the negative control was tonsil using nonspecific rabbit IgG. Positive control for p16 was metastatic squamous cell carcinoma; and positive control for p53 was endometrial intraepithelial serous carcinoma. The tumors were also stained and evaluated for the expression of E7 and E6 oncoproteins. C1P5 mAb was used for staining E6 oncoprotein at a concentration of 1:200 and a mAb to E7 oncoprotein (Cervimax, Germany) was used for E7 detection according to the Cervimax HPV E7 kit protocol.
Statistical analysis
The differences between the tumor sizes measured in the control and treatment groups were analyzed using Graphpad Prism Software two-way ANOVA. The differences were considered statistically significant when p-values were <0.05. The standard error of the repeated measurements of white blood cells and platelets was derived using Graphpad Prism Software.
Results
Treatment of CasKi derived tumors with TVG701Y mAb to E7 demonstrates tumor growth inhibition comparable to that achieved with C1P5 mAb
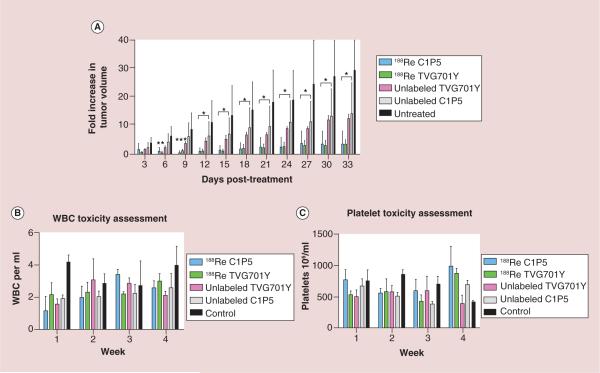
Mice bearing CasKi xenografts were treated with equal activities of the 188Re-labeled mAbs TVG701Y and C1P5 to E7 and E6 oncoproteins, respectively, as well as the matching amounts of the unlabeled mAbs. Statistically significant tumor growth inhibition was observed in all treatment groups by 12 days status post-intraperitoneal injection compared with untreated controls (Figure 1A). In the untreated group, tumors grew aggressively and mice had to be sacrificed when tumors exceed 1 cm3 as per the Institute for Animal Studies protocol. Therefore the absolute number of mice of the control did not remain the same during the observation period. All mice otherwise survived the 33 day observation period. The experiment was performed two times. The most pronounced effect on the tumor growth was seen in mice treated with RIT dose of 300 μCi 188Re-C1P5 or 188Re-TVG701Y. There was not a significant difference with respect to tumor growth abrogation by the radiolabeled mAbs based on the target oncoprotein, E6 or E7. Unlabeled C1P5 and TVG701Y mAbs also inhibited tumor throughout the observation period. The effect of the unlabeled mAbs on the tumor, though less pronounced than that of the radiolabeled mAbs, was nevertheless statistically significant in comparison with the untreated controls (Figure 1A). Similarly to the radiolabeled mAbs, there was no difference between unlabeled C1P5 and TVG701Y mAbs effect on the tumors. This experiment not only demonstrated the potential of E7 oncoprotein as a target for RIT but also, exactly as observed previously with unlabeled C1P5 mAb in [7,8,10], revealed the inhibition of tumor growth by unlabeled TVG701Y mAb to E7. It should be noted that we did not use an irrelevant radiolabeled mAb as a control in this study because to be a proper control such mAb also has to bind to the intracellular antigen and as it was pointed out in [15] describing using a mAb to a different intracellular antigen – a single strand DNA in nonviable tumor cells – such radiolabeled mAb will also have some therapeutic effect on the tumor.
Figure 1. Radioimmunotherapy of CasKi tumors with 188Rhenium-C1P5 and 188Rhenium-TVG-701Y monoclonal antibodies.
(A) Average change in tumor volume (V2 – V0/V0) over time among treatment groups was compared with untreated CasKi control tumors. (B) WBC counts during the observation period. X-axis shows 106 WBC per ml; (C) platelet counts during the observation period.
*p < 0.05; **p < 0.005; ***p < 0.0005.
188Re: 188Rhenium; WBC: White blood cell.
Dosimetry calculations predicted relatively low radiation doses to all major organs
The dosimetry calculations (Table 1) allowed to estimate that the dose to the tumor delivered by 300 μCi 188Re-C1P5 mAb used in this study was approximately 300 cGy. The doses to all major organs were well below the maximum tolerated limits for these organs.
Antibody treatment results in limited toxicity to bone marrow
Weekly assessment of white blood cells (WBCs) and platelet did not demonstrate any depression of counts to the diagnosis of neutropenia or thrombocytopenia throughout the observation period nor were there significant differences in between treatment groups (Figure 1B & C). The evaluation of the WBCs showed that the main effect on WBCs was some decrease in counts in the untreated group while the counts in the treatment groups increased (Figure 1B), however, no infections were noted in any groups. There were no significant differences in platelet counts between the groups (Figure 1C), pointing to the lack of toxicity related to the bone marrow suppression with the 300 μCi doses of RIT.
Radiolabeled & unlabeled TVG701Y & C1P5 mAbs decreased the expression of E7 oncoprotein in the tumors
We evaluated the impact of RIT and unlabeled mAbs on the expression of E7 oncoprotein by IHC in the tumors procured at the completion of the in vivo experiments described above. Positive staining for E7 HPV oncoprotein is characterized by brown intranuclear uptake that should not be confused with nonspecific uptake in the cytoplasm (Figure 2). It can be seen that untreated CasKi cells show intranuclear staining for E7 (Figure 2A) which decreases in the tumors treated with 188Re-TVG701Y (Figure 2B) or unlabeled TVG701Y (Figure 2D). Interestingly, treatment of CasKi tumors with 188Re-C1P5 mAb also resulted in somewhat decreased staining for E7 oncoprotein (Figure 2C). This could be explained by the fact that RIT kills the tumor cells which express both E6 and E7 oncoproteins thus resulting in the decreased expression of either oncoprotein.
Figure 2. Immunohistochemical staining for E7 protein in CasKi tumors.
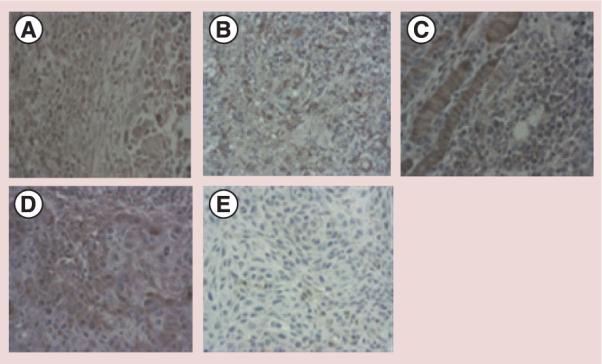
(A) Untreated tumor; (B) treated with 188Rhenium-TVG-701Y monoclonal antibodies (mAb); (C) treated with 188Rhenium-C1P5 mAb; (D) treated with unlabeled TVG701Y mAb; (E) non-HPV expressing, untreated control tumor. Focused brown staining indicates the presence of E7 protein.
Radiolabeled TVG701Y & C1P5 mAbs increased the levels of apoptosis in the CasKi tumors
TUNEL analysis was performed on the untreated tumors (Figure 3A) and the results compared with those obtained from tumors treated with 188Re-C1P5 or 188Re-TVG701Y mAbs (Figure 3B & C). Increased number of apoptotic cells was detected in tumors treated with 188Re-C1P5 or 188Re-TVG701Y mAbs, however, the most pronounced response was noted in the 188Re-C1P5 treatment group (Figure 3B). RIT is known to cause apoptotic cell death which is precipitated by the impact of charged particles on the nucleus and cellular machinery [16].
Figure 3. TUNEL assay for DNA fragmentation.
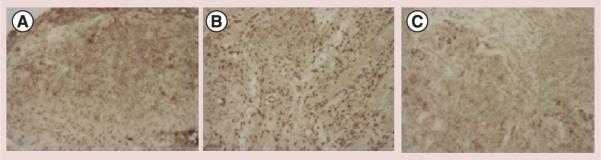
(A) Untreated CasKi tumors; (B) treated with 300 μCi 188Rhenium-C1P5 monoclonal antibodies; (C) treated with 300 μCi 188Rhenium-TVG-701Y. Apoptotic cells stain brown.
Active host immune response is seen in the tumors post-treatment with C1P5 & TVG701Y mAbs
We first sought to determine if there was an increase in C3 complement deposition in the tumors of mice treated with TVG701Y mAb. The significance of C3 complement deposition in the unlabeled C1P5 (Figure 4B) and TVG701Y (Figure 4C) mAbs treated tumors can be seen as compared with the untreated control (Figure 4A). To further investigate the host immune response post-treatment with mAbs to E6 and E7, we stained the tumors from all groups with CD3-specific mAb to evaluate for the presence and proportion of tumor infiltrating lymphocytes. There was a distinct presence of tumor infiltrating lymphocytes in the tumors of treated mice with either C1P5 or TVG-701Y mAbs. Figure 5B shows the presence of CD3 positive cells in the tumors from the mice treated with unlabeled TVG701Y mAb. All tumors across the treatment groups showed diffuse and strong immunoreactivity for p16, supporting their high-risk HPV associated oncogenesis (Figure 5D). None of the tumors stained positive for p53 indicating the absence of protein expression at the IHC level (Figure 5F). There was no consistent pattern of Rb pathway expression in the treatment groups. Figure 5G displays the staining for Rb in the unlabeled TVG701Y mAb treatment group.
Figure 4. Immunohistochemical staining for C3 complement deposition.
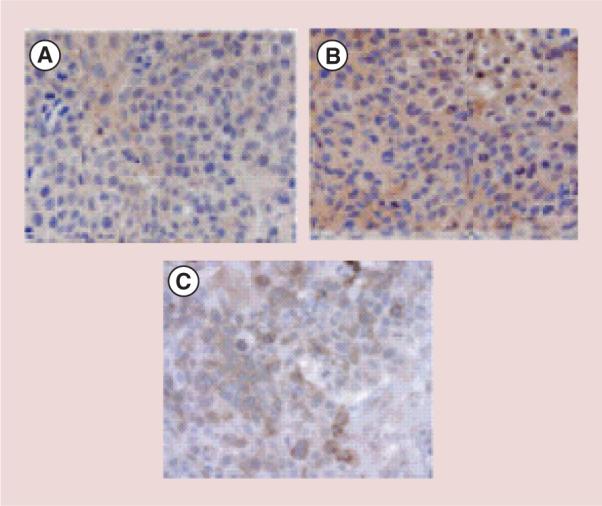
(A) Untreated CasKi tumors; (B) CasKi tumors treated with unlabeled C1P5 monoclonal antibodies; (C) CasKi tumors treated with unlabeled TVG701Y monoclonal antibodies. Brown staining indicates C3 complement presence.
Figure 5. Immunohistochemical analyses of immune system interaction with the antibodies treated CasKi tumors.
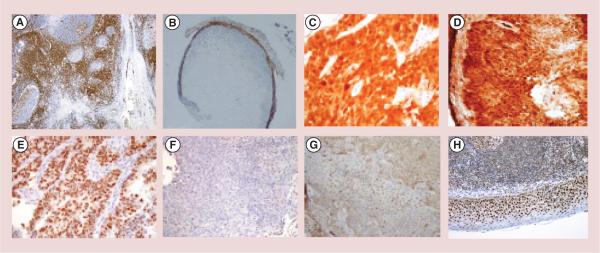
(A) Positive control for CD3 positive cells (tonsil); (B) lymphocytic invasion with CD3 positive cells of the tumors treated with unlabeled TVG701Y monoclonal antibodies (mAb) to E7. CD3 positive cells stain brown; (C) positive control stained for p16 (metastatic squamous cell carcinoma); (D) tumor treated with unlabeled C1P5 mAb stained positive for p16; (E) positive control for p53 (endometrial intraepithelial serous carcinoma); (F) tumor treated with unlabeled C1P5 mAb stained negative for p53; (G) tumor treated with unlabeled TVG701Y mAb stained positive for retinoblastoma; (H) positive control for retinoblastoma (tonsil).
Discussion
In the era of personalized medicine, it is imperative that we look closely at the malignant portions of the well-preserved HPV oncoproteins and seek to develop less toxic, more specific treatment options for women world-wide who still succumb to this disease at an alarming rate. Cervical cancer is the only known curable gynecologic malignancy that remains pandemic in many areas in the world, in spite of the availability of a preventive vaccine and early treatment. RIT is currently clinically approved in the USA and Europe for treatment of B-cell lymphomas with 90Y- and 131I-labeled CD20-binding mAbs and in China – for treatment of advanced lung cancer with 131I-labeled mAb to single strand DNA. However, all previous clinically approved and still experimental RIT applications target human ‘self’ antigens which are also expressed to certain extent on healthy tissues. Our approach of targeting virally associated antigens on tumors is unique as viral antigens have no homology to human antigens, thus allowing for much higher specificity of targeting and less toxicity of the treatment.
We have published previously on the ability of RIT against E6 oncoprotein to inhibit the growth of experimental cervical tumors [7,8,10]. However, in spite of the significance of these results, tumor growth after one intraperitoneal dose administration was maximally inhibited for about 18 days in all models with the subsequent regrowth of tumors while less rapid than that of control groups, still being observed. It was therefore logical to determine if targeting E7 oncoprotein would be comparable to these data or perhaps even be superior to the results obtained with 188Re-C1P5 mAb to E6. Secondarily, due to the rapid clearance from non-target organs as demonstrated in 188Re-C1P5 biodistribution studies in [8], we anticipated that this treatment modality would also have limited toxicity to the bone marrow – non-significant neutropenia or thrombocytopenia as this often is the dose limiting toxicity in radiation-associated therapies. We also hypothesized that the unlabeled mAb TVG701Y to E7 would exert tumor growth inhibition as noted in previous studies with C1P5 mAb to E6. Finally, we studied the potential mechanisms of the unlabeled mAbs to determine if their action is in part related to the restoration of p53 and Rb, stimulation of complement mediated toxicity (CDC) and/or induction of apoptosis based on the actions of US FDA-approved unlabeled mAbs currently in clinical use [17].
During the 33-day observation period and serial measurements of tumor growth, we observed that the abundance of the target oncoproteins did not influence the effect on the tumor growth inhibition as the tumor suppressive effects of both 188Re C1P5 and 188Re-TVG701Y were not statistically significant from each other and no depression of WBCs or platelets was recorded during the observation period. Though 300 cGy dose delivered to the tumors by the radiolabeled mAbs was clearly much lower than the doses routinely delivered to the tumors by external beam radiation therapy, it resulted in significant retardation of the tumor growth as RIT works through different mechanisms than external beam radiation therapy [16,18].
We also observed the profound effects for unlabeled C1P5 and TVG701Y mAbs on the tumor growth. It is important to note that in any preparation of a radiolabeled mAb only one in 1000 to one in 50 mAb molecules are actually combined with a radionuclide, thus, the fact that unlabeled mAbs also mediate a profound effect on the tumor constitutes an important addition to the therapeutic profile of RIT.
To investigate the findings of unlabeled mAbs efficacy, we sought to gain insights into the antitumor activities of these mAb via IHC analyses of complement deposition, patterns of T-cell infiltration, intensity of E6 and E7 oncoprotein staining and presence of p53 and Rb. The classical pathway, triggered by antigen-bound antibody, alternative pathway and the mannose-binding lectin pathway all converge at C3 level which then stimulates subsequent steps that causes the assembly of the membrane attack complex as described in [19] which conceivably can attack the membranes of the nearby cells. We observed C3 complement deposition in the tumors treated with either C1P5 mAb to E6 or TVG701Y mAb to E7 as well as in our previous work when treating experimental melanoma tumors with unlabeled mAb to melanin which is also an intracellular antigen available for antibody binding only in nonviable tumor cells [20]. In this regard, an extensive profile was established via IHC analyses of complement regulatory proteins decay-accelerating factor CD55, membrane cofactor protein, CD46 and CD59 to investigate the comparative expression profiles in the spectrum of clinically relevant samples of normal human uterine cervix, premalignant and malignant disease [21]. The authors noted differential expression patterns in response to adjacent nests of tumor cells, stromal factors and hypothesized that these regulators may have functions to protect against complement. There is a complex interplay in the tumor between the necrosis and the complement as inflammation is a critical component of tumor progression. It has been demonstrated that although complement and TNF-α potentially exert significant antitumor effects, both mediators may also promote tumor progression [22]. For the purposes of this study aimed at demonstrating that E7 oncoprotein could also be an RIT target in cervical cancer we showed that there was much more complement deposition in both C1P5 and TVG-701Y mAbs treated tumors than in untreated tumor (Figure 4) which strongly suggests that the complement was induced by the mAbs rather than necrosis. It is much too early in our investigation to determine if mAbs to E6 and E7 reverse or inhibit some of the local tumor microenvironment effects, however, along with other investigators who have observed sporadic deposition of C3 on tumor cells and stroma prior to treatment with mAbs which subsequently increased twofold after treatment as shown in [23], we are planning further investigations along this avenue.
The finding of peripheral infiltration of T cells in the tumor is consistent with the published literature that demonstrates that T-cell infiltration in cervical cancer is oftentimes non-homogenous, with rare invasion in tumor maintaining either periphery or within stroma [24,25]. Therefore we did not expect to see a differential pattern of staining from the periphery of the tumor versus the center. These CD3 positive cells are most likely intestinal intraepithelial lymphocytes which have been shown to be present in significant amounts in athymic nude mice [26]. Our next steps are to perform double staining analysis to look at the ratios of CD4+ and CD8+ regulatory cells as well as procure tumors at various timepoints to determine if the profiles of regulatory cells are affected by treatment with RIT or unlabeled antibody [27]. Our current findings support the observations that the microenvironments of cervical cancer cells are somehow resisting the infiltration of the cells.
IHC for p53 showed that none of the tumors stained positively for this protein regardless of treatment group. We postulate that the differences in levels of p53 perhaps may not be noted via IHC and would be best analyzed in a quantitative fashion with real-time PCR as the increased deposition of apoptotic bodies noted in the TUNEL assay may correlate directly with radiation-induced damage versus that of antibody-dependent cytotoxicity as a result of the function inhibition of E6 and E7 oncoproteins. On the other hand, the absence of immunoreactivity for p53 may possibly suggest that there is a downstream pathway and perhaps incomplete restoration of the p53 pathway for a sufficient specific protein expression that can be detected at the level of IHC. The absence of a consistent pattern in Rb expression can potentially be related to the actions of E7 in destabilizing Rb by making it a target for proteosome mediated degradation, however, when the host cell recognizes the ongoing proliferation, apoptosis or senescence by p53 activation is triggered.
The RIT approach will have the utility in cervical cancer as all patients are pretreated with chemotherapy such as cisplatin, and chemotherapy kills some cells in the tumors and thus creates the opportunity for the mAbs to E6 and E7 to reach their targets. In fact, in our previous studies we have shown that pretreatment of mice bearing HPV-16 positive cervical and head and neck tumors with cisplatin greatly enhances the efficacy of chemotherapy [28]. Furthermore, we anticipate that this treatment approach will be able to overcome some of the limitations of treatment with chemotherapy as a radiosensitizer. As tumors grow in size and become necrotic, the interstitial pressures noted at a cellular level oftentimes are such that the chemotherapeutic agent is not able to enter the cell, but rather bypass the tumor and is excreted without effect. Factors of better oxygenation and improved drug access from a lower interstitial pressure have best been studied in anal cancers (similar origin of HPV) using chemotherapy prior to definitive treatment or other small molecules to interrupt the natural history of the tumor prior to definitive management [29].
In summary, this report unequivocally demonstrates that the relative low expression of HPV E7 oncoprotein compared with E6 does not negatively affect its therapeutic potential seen with administration of RIT and of unlabeled mAb to E7. In fact, E7 therapeutic potential may indeed be synergistic or additive as we develop multimodal therapy approaches for future study. The limited toxicity to the bone marrow portends a favorable toxicity profile.
Conclusion
We performed direct comparison of targeting E7 and E6 oncoproteins with specific 188Re-labeled mAbs in CasKi subcutaneous xenografts of cervical cancer cells in mice. The most significant tumor inhibition was seen in RIT-treated mice, followed by the unlabeled mAbs to E6 and E7. No hematological toxicity was observed. Immunohistochemistry suggests that the effect of unlabeled antibodies is C3 complement mediated. We have demonstrated for the first time that RIT directed toward E7 oncoprotein inhibits experimental tumors growth, decreases E7 expression and may offer a novel approach to cervical cancer therapy.
Future perspective
In future studies, we will evaluate for potential toxicity to the kidney and liver. Furthermore, combination therapy using both C1P5 and TVG701Y or serial injections in a once a week fashion may result in complete eradication of the tumors in future studies. By taking a closer look at the mechanisms of action of the unlabeled antibodies we are further intrigued by the findings and seek to develop further depths of understanding in order to magnify the effects in a clinically relevant manner. We anticipate that this treatment will be initially offered to patients who have recurrent or metastatic disease, a group who would have more commonly undergone chemotherapy with or without radiation therapy.
Executive summary.
In spite of profound reduction in incidence, cervical cancer claims >275,000 lives annually.
Previously we demonstrated efficacy and safety of radioimmunotherapy (RIT) directed at HPV16 E6 oncoprotein in experimental cervical cancer.
Here we compare for the first time targeting E7 and E6 oncoproteins with specific 188Rhenium-labeled monoclonal antibodies in CasKi subcutaneous xenografts of cervical cancer cells in mice.
The most significant tumor inhibition was seen in RIT-treated mice, followed by the unlabeled monoclonal antibodies to E6 and E7. RIT reduced E7 expression in the tumors. No hematological toxicity was observed.
Immunohistochemical evaluation of the tumors suggested active involvement of the immune system in the process of tumor growth abrogation with C3 complement deposition and lymphocytes infiltration. The levels of p53 and Rb were not affected by the treatment.
We demonstrated for the first time that RIT directed toward E7 oncoprotein inhibits experimental tumor growth, decreases E7 expression and may offer a novel approach to cervical cancer therapy.
Acknowledgments
The study was funded by the K12 HD000849 Reproductive Scientist Development Program from NICHD/NIH to R Phaëton.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as:
• of interest;
•• of considerable interest
- 1.Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F. WHO International Agency for Research on Cancer Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005;6:204–210. doi: 10.1016/s1470-2045(05)70086-3. [DOI] [PubMed] [Google Scholar]
- 2.Kadaja M, Isok-Paas H, Laos T, Ustav E, Ustav M. Mechanisms of genomic instability in cells infected with the high-risk human papillomaviruses. PLoS Pathog. 2009;5(4):e100397. doi: 10.1371/journal.ppat.1000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho L, Chan SY, Burk RD, et al. The genetic drift of human papillomavirus type 16 is a means of reconstructing prehistoric viral spread and the movement of ancient human populations. J. Virol. 1993;67:6413–6423. doi: 10.1128/jvi.67.11.6413-6423.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4••.zur Hausen H. Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis. J. Natl Cancer Inst. 2000;92:690–698. doi: 10.1093/jnci/92.9.690. [The seminal paper on causal role of E6 and E7 in cervical cancer.] [DOI] [PubMed] [Google Scholar]
- 5.Martin AG. Molecular biology of cervical cancer. Clin. Transl. Oncol. 2007;9:347–354. doi: 10.1007/s12094-007-0066-8. [DOI] [PubMed] [Google Scholar]
- 6•.Dadachova E, Wang XG, Casadevall A. Targeting the virus with radioimmunotherapy in virus-assoicated cancer. Cancer Biother. Radiopharm. 2007;7:595–597. doi: 10.1089/cbr.2007.344. [Proof of principle paper showing the feasibility of radioimmunotherapy of cervical cancer targeting E6 oncoprotein.] [DOI] [PubMed] [Google Scholar]
- 7.Phaeton R, Wang XG, Einstein MH, Goldberg GL, Casadevall A, Dadachova E. The influence of proteasome inhibitor MG132, external radiation n and unlabeled antibody on the tumor uptake and biodistribution of 188Re labeled anti-E6 C1P5 antibody in cervical cancer in mice. Cancer. 2010;116(S4):1067–1074. doi: 10.1002/cncr.24794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phaeton R, Harris M, Jiang Z, et al. Radioimmunotherapy with an antibody to the HPV16 E6 oncoprotein is effective in an experimental cervical tumor expressing low levels of E6. Cancer Biol. Ther. 2010;10:1041–1047. doi: 10.4161/cbt.10.10.13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai Q, Lv L, Shao Q, Li X, Dian A. Human papillomavirus early proteins and apoptosis. Arch. Gynecol. Obstet. 2013;287(3):541–548. doi: 10.1007/s00404-012-2665-z. [DOI] [PubMed] [Google Scholar]
- 10.Wang XG, Revskaya E, Bryan RA, et al. Treating cancer as an infectious disease – viral antigens as novel targets for treatment and potential prevention of tumors of viral etiology. PLoS ONE. 2007;2(10):e1114. doi: 10.1371/journal.pone.0001114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dadachova E, Mirzadeh S. The role of tin in the direct labelling of proteins with Rhenium-188. Nucl. Med. Biol. 1997;24:605–608. doi: 10.1016/s0969-8051(97)00079-6. [DOI] [PubMed] [Google Scholar]
- 12.Miller WH, Hartmann-Siantar C, Fisher DR, et al. Evaluation of beta absorbed fractions in a mouse model for 90Y, 188Re, 166Ho, 149Pm, 64Cu, and 177Lu and radionuclides. Cancer Biother. & Radiopharm. 2005;20(4):436–449. doi: 10.1089/cbr.2005.20.436. [DOI] [PubMed] [Google Scholar]
- 13.Eckerman KF, Endo A. MIRD Radionuclide Data and Decay Schemes. 2nd Edition. Society of Nuclear Medicine; Reston, VA, USA: 2008. [Google Scholar]
- 14.Harrison P, Briggs C, Machin SJ. Platelet counting. Methods Mol. Biol. 2004;272:29–46. doi: 10.1385/1-59259-782-3:029. [DOI] [PubMed] [Google Scholar]
- 15.Bryan RA, Jiang Z, Jandl T, et al. Treatment of experimental pancreatic cancer with 213-Bismuth-labeled chimeric antibody to single-strand DNA. Expert Rev. Anticancer Ther. 2014;14:1243–1249. doi: 10.1586/14737140.2014.952285. [DOI] [PubMed] [Google Scholar]
- 16.Pickhard A, Piontek G, Seidl C, et al. 213Bi-anti-EGFR radioimmunoconjugates and X-ray irradiation trigger different cell death pathways in squamous cell carcinoma cells. Nucl. Med. Biol. 2014;41:68–76. doi: 10.1016/j.nucmedbio.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Zhou X, Hu W, Qin X. The role of complement in the mechanism of action of rituximab for B-cell lymphoma: implications for therapy. Oncologist. 2008;13:954–966. doi: 10.1634/theoncologist.2008-0089. [DOI] [PubMed] [Google Scholar]
- 18.Macklis RM. How and why does radioimmunotherapy work? Int. J. Radiat. Oncol. Biol. Phys. 2004;59(5):1269–1271. doi: 10.1016/j.ijrobp.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Morgan BP. Regulation of the complement membrane attack pathway. Crit. Rev. Immunol. 1999;19:173–198. [PubMed] [Google Scholar]
- 20.Jandl T, Revskaya E, Jiang Z, Bryan RA, Casadevall A, Dadachova E. Unlabeled antibody to melanin contributes to the radioimmunotherapeutic efficacy of metastatic melanoma through complement-dependent cytotoxicity. Immunotherapy. 2013;5(4):1–7. doi: 10.2217/imt.13.16. [DOI] [PubMed] [Google Scholar]
- 21.Simpson K, Jones A, Norman S, Holmes CH. Expression of complement regulatory proteins decay accelerating factor (DAF, CD55), membrane cofactor protein (MCP, CD46) and CD 59 in the normal human uterine cervix and in premalignant and malignant cervical disease. Am. J. Pathol. 1997;151:1455–1467. [PMC free article] [PubMed] [Google Scholar]
- 22.Liu L, Li W, Li Z, Kirschfink M. Sublytic complement protects prostate cancer cells from tumour necrosis factor-α-induced cell death. Clin. Exp. Immunol. 2012;169:100–108. doi: 10.1111/j.1365-2249.2012.04596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gelderman KA, Block VT, Flueren GJ, Gorter A. The inhibitory effect of CD46, CD55 and CD59 on complement activation after immunotherapeutic treatment of cervical carcinoma cells with monoclonal antibodies or bispecific monocolonal antibodies. Lab Invest. 2002;82(4):483–493. doi: 10.1038/labinvest.3780441. [DOI] [PubMed] [Google Scholar]
- 24.Loddenkemper C, Hoffmann C, Stanke J, et al. Regulatory (FOXP3+) T cells as target for immune therapy of cervical intraepithelial neoplasia and cancer. Cancer Sci. 2009;100:1112–1117. doi: 10.1111/j.1349-7006.2009.01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu MY, Kuo T, Ho H. Tumor-infiltrating lymphocytes contain a higher proportion of FOXP3+ T lymphocytes in cervical cancer. J. Formosan Med. Association. 2010;110:580–586. doi: 10.1016/j.jfma.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 26.de Geus B1, van den Enden M, Coolen C, Rozing J. Localization and phenotype of CD3 associated gamma/ delta receptor expressing intestinal intraepithelial lymphocytes. Thymus. 1989;14:31–41. [PubMed] [Google Scholar]
- 27.Adurthi S, Krishna S, Mukherjee G, Bafna UD, Devi U, Jayshree RS. Regulatory T cells in a spectrum of HPV-induced cervical lesions: cervicitis, cervical intraepthelial neoplasia and squamous cell carcinoma. Amer. J. Reproductive Immunol. 2008;60:55–65. doi: 10.1111/j.1600-0897.2008.00590.x. [DOI] [PubMed] [Google Scholar]
- 28.Harris M, Wang XG, Jiang Z, et al. Combined treatment of the experimental human papilloma virus-16-positive cervical and head and neck cancers with cisplatin and radioimmunotherapy targeting viral E6 oncoprotein. Br. J. Cancer. 2013;108(4):859–865. doi: 10.1038/bjc.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taghian AG, Abi-Raad R, Asaad SI, et al. Paclitaxel decreases the interstitial fluid pressure and improves oxygenation in breast cancer cancers in patients treated with neoadjuvant chemotherapy: clinical implications. J. Clin. Oncol. 2005;23:1951–1961. doi: 10.1200/JCO.2005.08.119. [DOI] [PubMed] [Google Scholar]