Abstract
We previously reported that NR2E3, an orphan nuclear receptor, plays an important role in maintaining the basal expression of estrogen receptor α (ER) and that the NR2E3 level is highly correlated with the relapse-free survival of breast cancer patients. Here, we investigated the role of NR2E3 in benzo(a)pyrene (BaP)-mediated cell injury. BaP treatment reduced NR2E3 homo-dimer formation and expression and subsequently decreased ER expression. The chromatin immunoprecipitation assay results showed that the treatment of MCF-7 breast cancer cells and the mouse liver with BaP released NR2E3 from the ER promoter to transform the transcriptionally active histone modification status into a repressive state. NR2E3 depletion in MCF-7 cells also induced a similar inactive epigenetic status in the ER promoter region, indicating that NR2E3 is an essential epigenetic player that maintains basal ER expression. Interestingly, these negative effects of BaP on the expression levels of NR2E3 and ER were rescued by antioxidant treatment. Collectively, our study provides novel evidence to show that BaP-induced oxidative stress decreases ER expression, in part by regulating NR2E3 function, which modulates the epigenetic status of the ER promoter. NR2E3 is likely an essential epigenetic player that maintains basal ER expression to protect cells from BaP-induced oxidative injury.
Keywords: Polycyclic aromatic hydrocarbon, an orphan nuclear receptor, epigenetics, histone demethylase, antioxidant
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are environmental pollutants that possess strong teratogenic, carcinogenic and mutagenic properties (Pahlman and Pelkonen, 1987). Typically, they are metabolized into highly mutagenic and carcinogenic compounds. Among them, BaP is the most ubiquitous environmental carcinogen that present in diesel, coal drinking water and tobacco and diet. In particular, BaP is present in cooked food such as well-done red meat, fried poultry with skin, and fried eggs (Kazerouni et al., 2001).
NR2E3 is an orphan nuclear receptor that is a vital player in retina development, differentiation and survival (Kobayashi et al., 1999; Akhmedov et al., 2000; Haider et al., 2001; Milam et al., 2002). In photoreceptor cells, NR2E3 is highly expressed and plays a role as both a transcriptional activator and repressor, depending on the gene promoter context; NR2E3 activates rod-specific genes and suppresses cone-specific genes (Cheng et al., 2004; Chen et al., 2005; Peng et al., 2005; Webber et al., 2008). This dual role of NR2E3 contributes to photoreceptor cell development and maintenance. NR2E3 consist of 410 amino acids, an N-terminal DNA binding domain and a C-terminal LBD domain, although ligands of this receptor have not yet been identified (Qin et al., 2005). Mutations of the NR2E3 gene are strongly associated with the development of several degenerative retina diseases, such as Goldman-Favre disease and retinitis pigmentosa (Sharon et al., 2003; Schorderet and Escher, 2009; Gire et al., 2007; Pachydaki et al., 2009). Recently, an analysis of the NR2E3 LBD crystal structure showed that NR2E3 forms a homo-dimer by interacting with LBD. Mutations of the LBD domain disrupted NR2E3 homo-dimerization and compromised its transcriptional regulative function, suggesting that NR2E3 function is in part regulated by this NR2E3 homo-dimerization activity (Tan et al., 2013). NR2E3 has primarily been studied in retina-related human diseases, and its potential role in other tissues or diseases has not yet been investigated.
We previously reported a novel significant association of NR2E3 with increased breast cancer patient survival. Breast cancer patients who express more NR2E3 survive longer and experience fewer recurrences than those who express low levels of NR2E3 (Park et al., 2012). The molecular basis for the strong clinical association of NR2E3 partly lies in the NR2E3-dependent basal expression of the estrogen receptor α (ER), a critical guideline for the prognosis and treatment of breast cancer patients (Fisher et al., 1989; Allred et al., 1998; Harvey et al., 1999; Paik et al., 2006). Our differential gene expression and signaling pathway analyses further indicated that the NR2E3 gene network is associated with endocrine system development and function, small molecule biochemistry and drug metabolism (Park et al., 2012). Moreover, a recent report showed that NR2E3 serves another function in cancer. NR2E3 directly interacts with p53, a tumor suppressor, and promotes its function, leading to cell death in several cancer cell lines (Wen et al., 2012). These reports suggest that NR2E3 likely plays a role in endocrine system regulation and environmental-induced cell injury responses.
In this study, we investigated the effect of benzo(a)pyrene, an ubiquitous environmental carcinogen, on the level and homo-dimerization activity of NR2E3 using ER-positive breast cancer cell lines as a model. Our results demonstrated that BaP treatment disrupt NR2E3 homo-dimerization at early time point and subsequently leads to decrease in NR2E3 expression level. These events consequently induced epigenetic status changes in histone acetylation and methylation. Interestingly, treatment with antioxidants, such as Glutathione ethyl ester (GSH) or NAC, reversed the effects of BaP on the expression levels of NR2E3 and ER, a downstream target gene of NR2E3. Taken together, these results demonstrate that NR2E3 is a novel epigenetic regulator that helps to maintain a normal epigenetic status in response to BaP-mediated toxic injury. Thus, NR2E3 may be a potential target for cancer prevention.
2. Materials and Methods
2.1. Cell lines, Reagents and Chemicals
HepG2, HuH7, T47D, and MCF-7 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA) and were not further tested or authenticated by the authors. The cell lines were maintained at 37°C in the presence of 5% CO2 in DMEM or RPMI medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin solution (Sigma Aldrich, St Louis). The cells (2.5 × 104 per well) were seeded, and the FBS content of the medium was reduced to 5%. Antibodies against β-actin, AHR, RNA Pol II and ER-α were purchased from Santa Cruz Biotechnology (Santa Cruz, CA); NR2E3 was obtained from Aviva Systems Biology (San Diego, CA); Sin3A and LSD1 were purchased from Cell Signaling (Danvers, MA); H4Ac and H3K4me2 were procured from Active Motif, Carlsbad, CA). Benzo[a]pyrene (BaP) was dissolved in DMSO, and the stock solutions were directly added to the culture media. Control cells were treated with DMSO only.
2.2. Animals and treatment
Male C57BL/6 mice weighing 23–25 g were obtained from Jackson Laboratory (Bar harbor, ME). The animals were used in compliance with the guidelines established by the Animal Care Committee of University of Cincinnati. The animals were acclimated to temperature- and humidity-controlled rooms with a 12-h light/dark cycle for 1 week prior to use. The mice had access to laboratory chow and tap water ad libitum. The mice were allocated for treatment with corn oil (n=6) or BaP (125 mg/kg) (n=6) dissolved in corn oil. Two doses were injected intraperitoneally (i.p.) every 48 h until sacrifice, which occurred 48 h after the final dose of BaP.
2.3. Western Blotting
The livers were quickly removed and homogenized in lysis buffer. Whole cellular lysates were harvested, and equal amounts of total cellular protein were resolved by SDS-PAGE and transferred onto PVDF membranes. After blocking, the membranes were incubated with the ER-α or NR2E3 antibody. A horseradish peroxidase-conjugated secondary antibody was used to visualize the immunoreactivity with the ECL western blot detection system. The protein level was compared to that of the loading control, β-actin.
2.4. Chromatin Immunoprecipitation Assay
The livers were quickly removed, and 300 mg of liver was minced. A chromatin immunoprecipitation (ChIP) assay was then carried out using the ChiP-IT Express Enzymatic Magnetic Chromatin Immunoprecipitation Kit (Active Motif, Carlsbad, CA) according to the manufacturer’s protocol. The following Chip primer sets were used for the Chip assay: hESR1 II (forward): 5′-GCT GGA GCC CCT GAA CCG TCC GC-3′, hESR1 II (reverse): 5′-GGC CCA GAC TCC GAC GCC GCA-3′ (Park et al, 2012); mESR1 II (forward): 5′-CCT CCC GCC TTC TAC AGG T-3′, mESR1 II (reverse): 5′-CAC ACG GCA CAG TAG CGA G-3′. Chip-PCR was performed using the 7300HT Real-Time PCR System with a 96-well block module (Applied Biosystems) and Primer II. The cycling conditions were 56°C for 30 min and 95°C for 10 min, followed by 50 cycles of 95°C for 25 s and 60°C for 60 s.
2.5. Real-Time Quantitative RT-qPCR
The total RNA was extracted, the cDNAs were amplified, quantitative real-time PCR was performed as previously described [11], and the data were normalized to GAPDH. The relative expression levels were calculated using the Ct method, where Ct = (CtGene – CtActin)Exp – (CtGene – CtActin)Control. The primer set sequence for ER and NR2E3 detection: ER (forward): 5′-AGC ACC CTG AAG TCT CTG GA-3′, ER (reverse): 5′-GAT GTG GGA GAG GAT GAG GA-3′; NR2E3 (forward): 5′-AGC AGC GGG AAG CAC TAT G-3′, NR2E3 (reverse): 5′-CCT GGC ACC TGT AGA TGA GC-3′.
2.6. Protein Co-immunoprecipitation (Co-IP)
MCF-7 cells were cotransfected with Flag-NR2E3 and pcDNA 4.0 Xpress-tag NR2E3 expression plasmids (Park et al., 2012). After 24 hr, cells were treated with/without BaP 5μM for 90 min and then cell lysate were collected. The samples (2 mg of total protein) were incubated overnight with 3 μg of primary antibody at 4°C, after which 20 μL of protein A agarose beads was added to the mixture, which was then incubated for 3 h at 4°C. The immunoprecipitated protein complexes were washed three times with Co-IP buffer. After the supernatant was discarded, the antibody/protein complexes were re-suspended in 40 μL of loading buffer and boiled for 5 min. The entire sample was separated with SDS-PAGE and assayed via protein immunoblotting.
2.7. Immunofluorescence
The cells were fixed with 4% paraformaldehyde for 30 min at 4°C followed by permeabilization using PBS containing 1% Triton X-100 and 0.5% NP-40 for 30 min. The cells were blocked with 1% BSA in PBS for 30 min on the blotter and then incubated with diluted primary antibody (1:100) in 1% BSA in PBS in a humidified chamber overnight at 4°C. The cells were washed three times and then incubated with anti-rabbit IgG antibodies conjugated with Alexa Fluor 647 (1:1000) in 1% BSA for 3 h at room temperature. The cells were mounted with Prolong antifade reagent with DAPI (Life Technologies). The images were acquired using a Zeiss Axiovert 200M Fluorescence/Live cell Imaging Microscope with AxiocamMR3.
2.8. Luciferase Assay
A reporter luciferase construct linked to the ER gene promoter region (−2756/+212) was used (Park et al, 2012). The transfected cells were lysed and re-suspended in 40 μL of loading buffer as previously described (Park et al., 2012). The luciferase activity was normalized to the renilla activity. The GAL4DBD-NR2E3 LBD and VP16-NR2E3 LBD (192–410 aa) constructs were kindly provided by Dr. Eric Xu (Grand Rapids, MI).
2.9. Statistical analysis
A one-way analysis of variance (ANOVA) was used to determine the significance of the differences between treatment groups. The Newman–Keuls test was used for multi-group comparisons. Statistical significance was accepted for P values < 0.05.
3. Results
3.1. Down-regulation of NR2E3 by BaP treatment
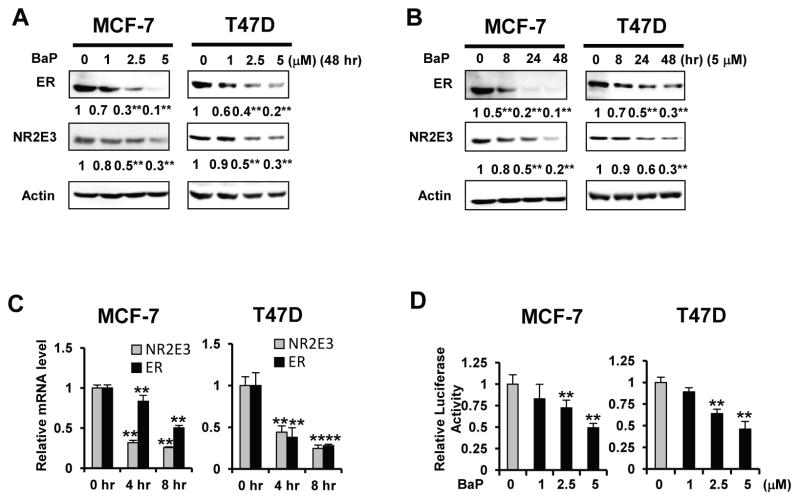
Our initial studies showed that treating MCF-7 and T47D breast cancer cells with 1, 2.5, and 5 μM of BaP for 48 hours significantly decreased the protein expression levels of NR2E3 and ER, a known NR2E3 downstream target gene (Park et al., 2012) (Fig. 1A). This effect was time-dependent (0, 8, 24, 48 hr) at 5 μM of BaP (Fig. 1B). The expression of NR2E3 protein is likely decreased at the transcriptional level because BaP reduced the levels of both NR2E3 and ER mRNA (Fig. 1C). This observation was further corroborated by the activity of luciferase linked to ER gene promoter region (−2756/+212). BaP treatment of MCF-7 and T47D cells for 16 hr markedly decreased the ER-luciferase activity in both cell lines. This effect was not limited ER-positive cell lines: a BaP-mediated NR2E3 protein level reduction was observed in other ER-negative cancer cell lines, including MCF-10A normal mammary epithelial cells, HepG2 cells and Huh7 hepatocellular carcinoma cells (Suppl. Fig. 1). However, downregulation of NR2E3 at lower BaP concentrations and early time point is marginal in comparison to the decrease in ER levels, suggesting that the decreased expression of ER is likely dependent on other mechanisms relevant to NR2E3 activity such as perturbation of NR2E3 homo-dimerization and disturbance of NR2E3 nucleus-cytoplasm shuttling, in which events could occur in early time point of BaP exposure.
Fig. 1. Effect of BaP on NR2E3 and ER expression in MCF-7 and T47D cells.
(A) Effect of BaP on NR2E3 and ERα protein expression. MCF-7 and T47D cells were treated with BaP (1, 2.5 or 5 μM) for 48 h, and the NR2E3 and ER protein level was analyzed on immunoblots of cell lysates probed with an anti-NR2E3 and anti-ER antibody. (B) MCF-7 and T47D cells were cultured with 5 μM BaP for 8, 24, or 48 h, and the NR2E3 and ER protein level was analyzed on immunoblots of cell lysates probed with an anti-NR2E3 and anti-ER antibody. (C) The effect of BaP on NR2E3 and ER mRNA expression. MCF-7 and T47D cells were treated with 5 μM BaP for 4 or 8 h. The cells were lysed, and the total RNA was prepared for the PCR analysis of NR2E3 and ER mRNA expression relative to GAPDH expression. (D) The effect of BaP on the ERα promoter activity. MCF-7 and T47D cells transfected with ER-Luc were treated with BaP (1, 2.5 or 5 μM) for 24 h, harvested, and assayed for luciferase activity. Results are means ± SE for at least 3 replicated determinations, and significantly (P < .05) decreased (**) responses are indicated.
3.2. BaP-mediated disruption of NR2E3 homo-dimerization
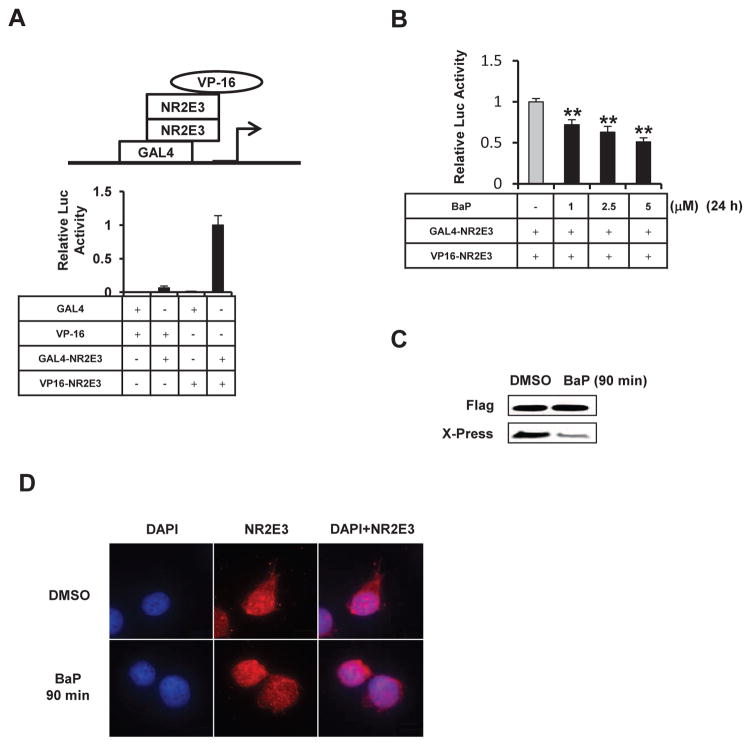
A previous study indicated that NR2E3 can form dimer via the LBD domain and that this dimerization activity mediates its transcriptional repressive function (Tan et al., 2013). To determine whether BaP perturbs NR2E3 homo-dimerization, we employed a mammalian two-hybrid assay using GAL4DBD-NR2E3 LBD and VP16-NR2E3 LBD constructs that contain the LBD domain (192–410 aa). A significant increase in the GAL4 reporter luciferase activity was in MCF-7 cells was only observed when the GAL4DBD-NR2E3 LBD and VP-16-NR2E3 LBD constructs were co-transfected into cells, but not when GAL4DBD-NR2E3 LBD or VP-16-NR2E3 LBD were transfected alone, which is consistent with a previous report (Tan et al., 2013) (Fig. 2A). BaP markedly disrupted this NR2E3 homo-dimerization activity in a dose-dependent manner (Fig. 2B). A co-immunoprecipitation assay was used to further examine whether the disruption of NR2E3 homo-dimer occurs early. The cells were transfected with a flag-tagged NR2E3 expression plasmid for 24 hr. After treatment with 5 μM BaP for 90 min, the endogenous NR2E3 protein bound to flag-tagged NR2E3 was detected by western blotting. The result showed that NR2E3 dimer formation was markedly decreased at 90 min (Fig. 2C). BaP treatment did not alter the localization of NR2E3 in the nucleus (Fig. 2D).
Fig. 2. Effects of BaP on NR2E3 homo-dimerization and localization.
(A) Detection of NR2E3-NR2E3 homo-dimer formation by a mammalian two-hybrid assay in MCF-7 cells. The cells were co-transfected with different combinations of GAL4 empty (GAL4), VP16 empty (VP16), GAL4-NR2E3 LBD and VP16-NR2E3 LBD constructs plus GAL4-reporter luciferase. (B) BaP treatment disrupts NR2E3 homo-dimerization in a mammalian two-hybrid assay in MCF-7 cells. Cells were treated with BaP (1, 2.5 or 5 μM) for 24 h. (C) Detection of BaP effect (5 μM, 90 min) on NR2E3 homo-dimerization activity by co-immunoprecipitaion assay using cells cotransfected with Flag- and Xpress-tagged NR2E3 expression plasmids. (D) The nuclear localization of NR2E3 with/without BaP treatment. Results are means ± SE for at least 3 replicated determinations, and significantly (P < .05) decreased (**) responses are indicated.
3.3. BaP treatment induced the similar epigenetic changes in both in vitro and in vivo
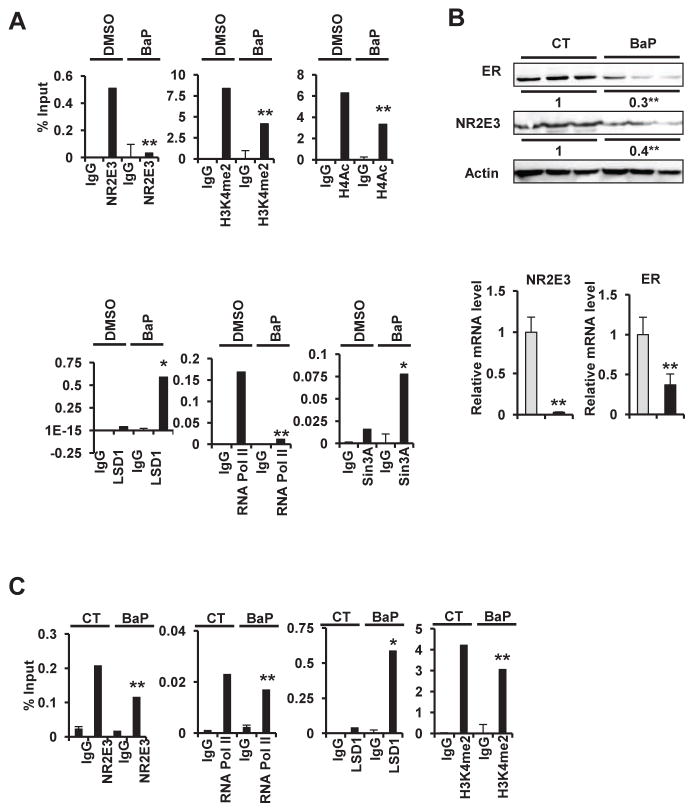
The transcriptional regulatory role of NR2E3 is pleiotropic: it acts as a repressor and activator by interacting with a histone acetylase and histone deacetylase depending on the gene promoter context (Cheng et al., 2004; Chen et al., 2005; Peng et al., 2005; Webber et al., 2008). We employed a Chip assay to determine whether the histone methylation/acetylation status and coactivator/corepressor recruitment to the ER gene promoter region changes in response to BaP. This specific ER region was previously defined as a NR2E3 binding site (Park et al., 2012). The Chip assay was performed after the MCF-7 cells were treated with BaP for 90 min, and the result showed that NR2E3 was rapidly released from the ER promoter. The levels of histone acetylation markers in the actively transcribed promoter region, such as H4Ac and H3K4me2, were significantly decreased. In contrast, the recruitment of Sin3A, a co-repressor protein, was increased in this promoter region, and LSD1, a histone demethylase of H3K4me2, was also recruited (Fig. 3A). We employed the mouse liver as a model to further validate the role of NR2E3 in BaP-mediated epigenetic changes in vivo because the mouse liver also expresses both ER and NR2E3. Exposing the mouse liver to BaP drastically reduced both the NR2E3 and ER protein and mRNA levels (Fig. 3B top and 3B bottom). To further analyze the transcriptional and epigenetic status changes induced by BaP in vivo, we performed a Chip assay using mouse liver tissue with/without BaP exposure. The mouse ER gene specific primer set that covers a region similar to the ER 5′ proximal promoter region in humans (−206/+49) was employed. Notably, a similar pattern of epigenetic modification was observed in response to BaP exposure in vivo. The levels of NR2E3, RNA pol II and the active histone marker H3K4me2 decreased, whereas LSD1 recruitment was enhanced in this promoter region (Fig. 3C). Collectively, these results indicate that NR2E3 plays a role in BaP-mediated epigenetic changes in the ER promoter.
Fig. 3. Effects of BaP treatment on transcriptional and epigenetic status of the ER gene proximal promoter region.
(A) BaP treatment significantly decreased the binding of NR2E3 relative to the control. MCF-7 cells were treated with BaP (5 μM) for 90 min and determined by ChIP-PCR. (B) The mice were injected twice with BaP (125 mg/kg) and sacrificed 96 h later. The livers were homogenized, and the NR2E3 and ER protein levels were analyzed on immunoblots of cell lysates probed with anti-NR2E3 and anti-ERα antibodies. The livers were homogenized and lysed, and the total RNA was prepared for the PCR analysis of NR2E3 and ERα mRNA expression relative to the GAPDH expression. (C) BaP treatment significantly decreased the binding of NR2E3 relative to the control, as determined by ChIP-PCR. Results are means ± SE for at least 3 replicated determinations, and significantly (P < .05) increased/attenuated (*) or decreased (**) responses are indicated.
3.4. NR2E3 depletion induces the similar histone modification pattern in the ER promoter
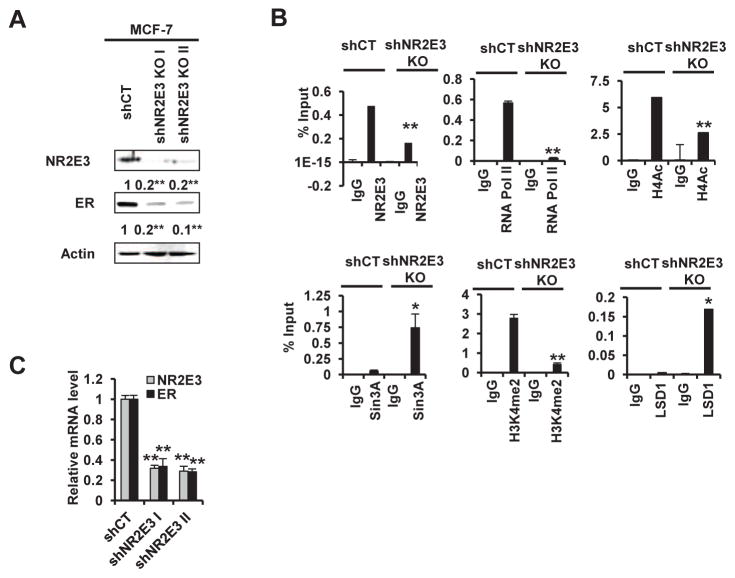
Consistent with our previous report (Park et al., 2012), NR2E3 silencing in MCF-7 cell using NR2E3-specific small hairpin RNAs (shRNA) resulted in decrease in the NR2E3 and ER protein and mRNA levels, the latter of which was confirmed by qRT-PCR (Fig. 4A top and 4A bottom). To further identify the histone modification changes induced by NR2E3 silencing on the ER promoter, we carried out a Chip assay using MCF-7 control (shCT) and NR2E3-depleted MCF-7 cell lysate (shNR2E3 KO). Consistently, NR2E3 and RNA pol II binding to ER promoter markedly decreased, and this decrease was accompanied by reduced levels of active histone markers, such as H4Ac and H3K4me2. In contrast, the recruitment of Sin3A and LSD1 to the ER promoter increased. Overall, these changes were similar to the epigenetic status change induced by BaP exposure (Fig. 3A and 3C). The results strongly supported that NR2E3 is an important epigenetic regulator that helps ER status in the face of BaP-induced injury.
Fig. 4. Effects of NR2E3 loss on transcriptional and epigenetic status of the ER gene proximal promoter region.
MCF-7 cells were transfected with lentiviral-based small hairpin RNA (shRNA). MCF-7 cells were transfected with NR2E3 shRNA or a non-specific control shRNA according to the manufacturer’s instructions. (A) Effect of NR2E3 shRNA on NR2E3 and ER protein expression. The NR2E3 and ERα protein levels were analyzed on immunoblots of cell lysates probed with anti-NR2E3 and anti-ERα antibodies. (B) NR2E3 loss significantly altered the transcriptional and epigenetic status of the ER proximal promoter region. Results are means ± SE for at least 3 replicated determinations, and significantly (P < .05) increased/attenuated (*) or decreased (**) responses are indicated.
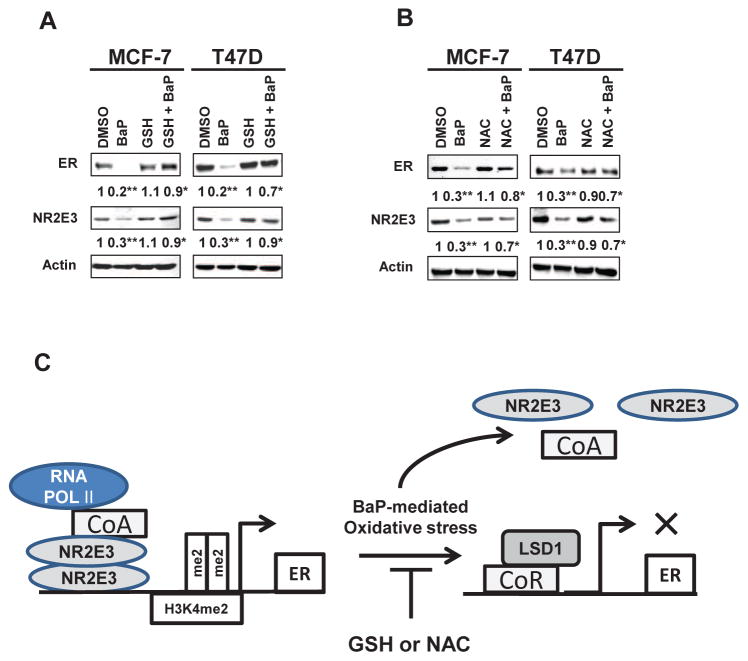
3.5. Effects of antioxidant on BaP-mediated NR2E3 down-regulation
CYP1A1 was expressed in breast tissues and tumors (Huang et al., 1996). Previous studies demonstrated that BaP treatment of MCF-7 cells greatly induced CYP1A1 expression (Androutsopoulos and Tsatsakis, 2014; Takemura et. al., 2010). BaP is pro-oxidant that can generates reactive oxygen species (ROS) via CYP1A1 metabolism (Burczynski et al., 1999; Burczynski and Penning, 2000; Safe, 1995). These ROS and metabolites can cause oxidative damage, ultimately attributing to tumor initiation (Pelicano et al., 2004; Trachootham et al., 2009). To test whether BaP-generated oxidative stress involves the express ion of NR2E3, MCF-7 and T47D cells were pretreated with either Glutathione ethyl ester (GSH 5 mM), which is permeable to cell membrane or NAC (10 mM) for 3 h. These antioxidant pretreatments rescued the downregulation of NR2E3 and ER induced by BaP exposure (Fig. 5A and 5B). The interactive model between BaP exposure and NR2E3 activity change that turn off the basal ER expression was illustrated (Fig. 5C). BaP-mediated oxidative stress disrupted NR2E3 homo-dimer formation and released NR2E3 and RNA pol II from the ER promoter and at the same time facilitates the recruitment of corepressor Sin3A and LSD1 a histone demethylase for H3K4me2. Thus, these events results in significant decrease of active histone mark H3K4me2 and subsequently reduced ER gene expression but can be prevented by pretreatment of antioxidants. These results strongly suggest that the NR2E3 level or activity is partly regulated by BaP-induced oxidative stress and that NR2E3 is likely a key molecule that links BaP-induced oxidative stress to altered histone modifications.
Fig. 5. Inhibition of NR2E3 and ERα downregulation by antioxidant treatments and a model for the role of NR2E3 in BaP-mediated oxidative injury.
(A) MCF-7 and T47D cells were pre-treated with GSH for 3 h, and BaP (5 μM) was then added for 48 h. The NR2E3 and ERα protein levels were analyzed on immunoblots of cell lysates probed with anti-NR2E3 and anti-ERα antibodies. (B) MCF-7 and T47D cells were pre-treated with NAC (10 mM) for 3 h, and BaP (5 μM) was then added for 48 h. The NR2E3 and ER protein levels were analyzed. (C) A model of the effects of BaP on the epigenetic status of the ER promoter. Results are means ± SE for at least 3 replicated determinations, and significantly (P < .05) increased/attenuated (*) or decreased (**) responses are indicated.
4. Discussion
Polycyclic aromatic hydrocarbons (PAHs) are environmental toxicants that are significantly associated with various human cancers (Boffetta et al., 1997). Among PAHs, benzo(a)pyrene is the most ubiquitous environmental carcinogen and is a byproduct of the incomplete combustion of organic matter, including cigarette smoke, gasoline, wood and meat (Gelboin, 1980). Via metabolic activation in the human body, BaP produces reactive oxygen species (ROS) that can cause harmful DNA adduct formation and subsequent genetic mutation. This DNA damage has been considered a crucial genetic risk factor for BaP-mediated carcinogenesis (Pelkonen and Nebert, 1982; Hecht, 2002). However, increasing evidence has indicated that the harmful effects of BaP are not only associated with genetic mutations but also with epigenetic aberrations; BaP exposure also mediates abnormal epigenetic changes that can promote carcinogenesis (Yoon et al., 2001; Sadikovic et al., 2008; Teneng et al., 2011). However, the mechanistic basis for the harmful epigenetic effects of BaP remains largely unclear.
In the current study, we have shown that BaP exposure altered the activity and level of NR2E3. BaP treatment disrupted NR2E3 homo-dimerization (Fig. 2A and 2B) and decreased the expression of ER, a known NR2E3 downstream target gene, at both the protein and mRNA levels (Fig. 1A, 1B and 1C). The luciferase reporter activity linked to the ER promoter region was also markedly decreased (Fig. 1D). These results indicate that the disruption of NR2E3 homo-dimerization or NR2E3 abolished the basal level of ER expression. NR2E3 reportedly functions as a transcriptional regulator by forming a protein complex with either histone acetylase or histone deacetylase, depending on the gene context or developmental stage during photoreceptor cell development. We used the known NR2E3 target gene ER as a model to further investigate whether NR2E3 plays a role in BaP-induced epigenetic status changes. Treating cells with BaP induces NR2E3 release from the ER promoter region while reducing RNA pol II binding and active histone markers of acetylation (H4Ac) and methylation (H3K4me2). In contrast, a co-repressor, Sin3A, and a histone demethylase for H3K4me2, LSD1, were recruited to this region, which also indicated that BaP treatment altered the ER epigenetic status from an active to a repressive state (Fig. 3A). Moreover, the in vivo animal study results using the mouse liver as a model further validated the decreased expressions of NR2E3 and ER at both the protein and mRNA levels (Fig. 3B). The subsequent in vivo Chip assay showed that BaP induced a similar pattern of epigenetic status change: the release of NR2E3 and RNA pol II, decreased H3K4me2 histone marker levels and the recruitment of LSD1 (Fig. 3C). Interestingly, LSD1 is a histone demethylase that functions as a transcriptional repressor (Shi et al., 2004). LSD1 overexpression has been observed in ER-negative breast cancer, and LSD1 can serve as a biomarker for increased breast cancer cell aggression (Lim et al., 2010). Our results suggest that the recruitment of LSD1 to the ER promoter by either BaP treatment or NR2E3 depletion may be a molecular pathway responsible for ER loss and that BaP exposure may promote breast cancer progression.
Our previous report indicated that NR2E3 depletion decreased ER expression (Fig. 4A) (Park et al., 2012). We tested whether NR2E3 loss induces transcriptional and epigenetic changes in the ER promoter similar to those mediated by BaP. A similar pattern of NR2E3 and Pol II release was detected, and the recruitment of Sin3A and LSD1 was observed (Fig. 4B). The levels of active histone markers, such as H4Ac and H3K4me2, were equally decreased. The results strongly indicated that NR2E3 depletion induced similar transcriptional and epigenetic status on the ER promoter, substantiating a bridging role of NR2E3 as an epigenetic regulator in BaP-induced ER down-regulation.
ER has been a major guideline for both the prognosis and treatment of breast cancer patients (Fisher et al., 1989; Allred et al., 1998). ER loss is a frequent event during breast cancer development that promotes the development of drug resistance and the acquisition of an aggressive phenotype (Kuukasjärvi et al., 1996; Ottaviano et al., 1994). Interestingly, exposure to PAHs has been acknowledged to correlate with breast cancer incidence (Li et al., 1996; Petralia et al., 1999; Xiong et al., 2001), and BaP exposure has been shown to induce oncogenic transformation in normal mammary epithelial cells (Stampfer and Bartley, 1985; Burdick et al., 2003; Pluchino and Wang, 2014), which agrees with the malignant cell phenotypic change that results from ER loss. Nevertheless, the potential molecular link between PAH exposure and ER loss has not yet been well defined. As we previously reported, NR2E3 is a strong prognostic marker of breast cancer patient survival, and NR2E3 is a major player that helps maintain the basal level of ER expression. This study provides insights into the role of NR2E3 as a novel molecular link that likely connects BaP-induced oxidative injury to aberrant epigenetic changes, resulting in decreased ER expression.
6. Conclusion
Our study established for the first time that NR2E3 is an epigenetic regulator that responds to BaP-mediated oxidative injury. BaP exposure decreased the homo-dimerization activity and expression of NR2E3, which likely perturbs the histone modification status of the ER promoter. This alteration subsequently leads to decreased basal ER expression. ER loss is a major molecular event that promotes breast cancer progression. Similarly, BaP exposure has been consistently acknowledged as a major risk factor for breast cancer development. These results present a novel pathway that shows how BaP-mediated oxidative stress mediates epigenetic changes by regulating NR2E3 actions on the ER promoter. Moreover, the reversal of NR2E3 loss by antioxidant treatment indicated that NR2E3 may be a target to prevent cancer due to BaP-induced oxidative injury. Our future studies will focus on the role of NR2E3 in other environmental toxicant-induced epigenetic changes and other downstream target gene promoters. We are also investigating the regulative mechanisms responsible for the decreased homo-dimerization and expression of NR2E3 induced by environmental toxicant-generated oxidative stress.
Supplementary Material
Highlights.
BaP-mediated oxidative stress perturbs NR2E3 homo-dimerization and expression.
NR2E3 is an essential epigenetic player for basal ER expression.
NR2E3 links BaP-mediated oxidative injury to altered ER epigenetic status.
Antioxidants prevent BaP-induced NR2E3 functional alteration.
Acknowledgments
Funding:
This research was supported by NIEHS Grant (P30-ES006096) from Center Environmental Genetics, CCTST (Center for Clinical & Translational Science & Training) Junior T1 grant and starting fund from College of Medicine in University of Cincinnati.
Abbreviations
- BaP
benzo(a)pyrene
- LSD1
lysine specific demethylase 1
- H3K4me2
histone 3 lysine 27 dimethylation
- H4ac
histone 4 acetylation
- GSH
Glutathione
- NAC
N Acetylcysteine
- Chip
Chromatin immunoprecipitation
- LBD
ligand binding domain
Footnotes
Disclosure of Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akhmedov NB, Piriev NI, Chang B, Rapoport AL, Hawes NL, Nishina PM, Farber DB. A deletion in a photoreceptor-specific nuclear receptor mRNA causes retinal degeneration in the rd7 mouse. Proc Natl Acad Sci USA. 2000;97:5551–5556. doi: 10.1073/pnas.97.10.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- Androutsopoulos VP, Tsatsakis AM. Benzo[a]pyrene sensitizes MCF7 breast cancer cells to induction of G1 arrest by the natural flavonoid eupatorin-5-methyl ether, via activation of cell signaling proteins and CYP1-mediated metabolism. Toxicol Lett. 2014;230:304–313. doi: 10.1016/j.toxlet.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Boffetta P, Jourenkova N, Gustavsson P. Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes Control. 1997;8:444–472. doi: 10.1023/a:1018465507029. [DOI] [PubMed] [Google Scholar]
- Burczynski ME, Lin HK, Penning TM. Isoform-specific induction of a human aldo-keto reductase by polycyclic aromatic hydrocarbons (PAHs), electrophiles, and oxidative stress: implications for the alternative pathway of PAH activation catalyzed by human dihydrodiol dehydrogenase. Cancer Res. 1999;59:607–614. [PubMed] [Google Scholar]
- Burczynski ME, Penning TM. Genotoxic polycyclic aromatic hydrocarbon ortho-quinones generated by aldo-keto reductases induce CYP1A1 via nuclear translocation of the aryl hydrocarbon receptor. Cancer Res. 2000;60:908–915. [PubMed] [Google Scholar]
- Burdick AD, Davis JW, 2nd, Liu KJ, Hudson LG, Shi H, Monske ML, Burchiel SW. Benzo(a)pyrene quinones increase cell proliferation, generate reactive oxygen species, and transactivate the epidermal growth factor receptor in breast epithelial cells. Cancer Res. 2003;63:7825–33. [PubMed] [Google Scholar]
- Chen J, Rattner A, Nathans J. The rod photoreceptor-specific nuclear receptor Nr2e3 represses transcription of multiple cone-specific genes. J Neurosci. 2005;25:118–129. doi: 10.1523/JNEUROSCI.3571-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Khanna H, Oh ECT, Hicks D, Mitton KP, Swaroop A. Photoreceptor-specific nuclear receptor NR2E3 functions as a transcriptional activator in rod photoreceptors. Hum Mol Gene t. 2004;13:1563–1575. doi: 10.1093/hmg/ddh173. [DOI] [PubMed] [Google Scholar]
- Fisher B, Costantino J, Redmond C, Poisson R, Bowman D, Couture J, Dimitrov NV, Wolmark N, Wickerham DL, Fisher ER, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989;23:479–484. doi: 10.1056/NEJM198902233200802. [DOI] [PubMed] [Google Scholar]
- Gire AI, Sullivan LS, Bowne SJ, Birch DG, Hughbanks-Wheaton D, Heckenlively JR, Daiger SP. The Gly56Arg mutation in NR2E3 accounts for 1–2% of autosomal dominant retinitis pigmentosa. Mol Vis. 2007;13:1970–1975. [PubMed] [Google Scholar]
- Gelboin HV. Benzo[alpha]pyrene metabolism, activation and carcinogenesis: role and regulation of mixed-function oxidases and related enzymes. Physiol Rev. 1980;60:1107–1166. doi: 10.1152/physrev.1980.60.4.1107. [DOI] [PubMed] [Google Scholar]
- Haider NB, Naggert JK, Nishina PM. Excess cone cell proliferation due to lack of a functional NR2E3 causes retinal dysplasia and degeneration in rd7/rd7 mice. Hum Mol Genet. 2001;10:1619–1626. doi: 10.1093/hmg/10.16.1619. [DOI] [PubMed] [Google Scholar]
- Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- Hecht SS. Tobacco smoke carcinogens and breast cancer. Environ Mol Mutagen. 2002;39:119–126. doi: 10.1002/em.10071. [DOI] [PubMed] [Google Scholar]
- Huang Z, Fasco MJ, Figge HL, Keyomarsi K, Kaminsky LS. Expression of cytochromes P450 in human breast tissue and tumors. Drug Metab Dispos. 1996;24:899–905. [PubMed] [Google Scholar]
- Kazerouni N, Sinha R, Hsu CH, Greenberg A, Rothman N. Analysis of 200 food items for benzo[a]pyrene and estimation of its intake in an epidemiologic study. Food Chem Toxicol. 2001;39:423–436. doi: 10.1016/s0278-6915(00)00158-7. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Takezawa S, Hara K, Yu RT, Umesono Y, Agata K, Umesono K. Identification of a photoreceptor cell-specific nuclear receptor. Proc Natl Acad Sci USA. 1999;96:4814–4819. doi: 10.1073/pnas.96.9.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuukasjärvi T, Kononen J, Helin H, Holli K, Isola J. Loss of estrogen receptor in recurrent breast cancer is associated with poor response to endocrine therapy. J Clin Oncol. 1996;14:2584–9. doi: 10.1200/JCO.1996.14.9.2584. [DOI] [PubMed] [Google Scholar]
- Li D, Wang M, Dhingra K, Hittelman WN. Aromatic DNA adducts in adjacent tissues of breast cancer patients: clues to breast cancer etiology. Cancer Res. 1996;56:287–293. [PubMed] [Google Scholar]
- Lim S, Janzer A, Becker A, Zimmer A, Schüle R, Buettner R, Kirfel J. Lysine-specific demethylase 1 (LSD1) is highly expressed in ER-negative breast cancers and a biomarker predicting aggressive biology. Carcinogenesis. 2010;31:512–520. doi: 10.1093/carcin/bgp324. [DOI] [PubMed] [Google Scholar]
- Milam AH, Rose L, Cideciyan AV, Barakat MR, Tang WX, Gupta N, Jacobson SG. The nuclear receptor NR2E3 plays a role in human retinal photoreceptor differentiation and degeneration. Proc Natl Acad Sci USA. 2002;99:473–478. doi: 10.1073/pnas.022533099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviano YL, Issa JP, Parl FF, Smith HS, Baylin SB, Davidson NE. Methylation of the estrogen receptor gene CpG island marks loss of estrogen receptor expression in human breast cancer cells. Cancer Res. 1994;54:2552–2555. [PubMed] [Google Scholar]
- Pachydaki SI, Klaver CC, Barbazetto IA, Roy MS, Gouras P, Allikmets R, Yannuzzi LA. Phenotypic Features of Patients With NR2E3 Mutations. Arch Ophthalmol. 2009;127:71–75. doi: 10.1001/archophthalmol.2008.534. [DOI] [PubMed] [Google Scholar]
- Pahlman R, Pelkonen O. Mutagenicity studies of different polycyclic aromatic hydrocarbons: the significance of enzymatic factors and molecular structure. Carcinogenesis. 1987;8:773–778. doi: 10.1093/carcin/8.6.773. [DOI] [PubMed] [Google Scholar]
- Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, Cronin M, Baehner FL, Watson D, Bryant J, Costantino JP, Geyer CE, Jr, Wickerham DL, Wolmark N. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;10:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- Park YY, Kim K, Kim SB, Hennessy BT, Kim SM, Park ES, Lim JY, Li J, Lu Y, Gonzalez-Angulo AM, Jeong W, Mills GB, Safe S, Lee JS. Reconstruction of nuclear receptor network reveals that NR2E3 is a novel upstream regulator of ESR1 in breast cancer. EMBO Mol Med. 2012;4:52–67. doi: 10.1002/emmm.201100187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia SA, Vena JE, Freudenheim JL, Dosemeci M, Michalek A, Goldberg MS, Brasure J, Graham S. Risk of premenopausal breast cancer in association with occupational exposure to polycyclic aromatic hydrocarbons and benzene. Scand J Work Environ Health. 1999;25:215–221. doi: 10.5271/sjweh.426. [DOI] [PubMed] [Google Scholar]
- Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Updat. 2004;7:97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Pelkonen O, Nebert DW. Metabolism of polycyclic aromatic hydrocarbons: etiologic role in carcinogenesis. Pharmacol Rev 1982. 1982;34:189–222. [PubMed] [Google Scholar]
- Peng GH, Ahmad O, Ahmad F, Liu J, Chen S. The photoreceptor-specific nuclear receptor Nr2e3 interacts with Crx and exerts opposing effects on the transcription of rod versus cone genes. Hum Mol Genet. 2005;14:747–764. doi: 10.1093/hmg/ddi070. [DOI] [PubMed] [Google Scholar]
- Pluchino LA, Wang HC. Chronic exposure to combined carcinogens enhances breast cell carcinogenesis with mesenchymal and stem-like cell properties. PLoS One. 2014;9:e108698. doi: 10.1371/journal.pone.0108698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Q, Knapinska A, Dobri N, Madoux F, Chase P, Hodder P, Petrukhin K. In pursuit of synthetic modulators for the orphan retina-specific nuclear receptor NR2E3. J Ocul Pharmacol Ther. 2013;29:298–309. doi: 10.1089/jop.2012.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadikovic B, Andrews J, Carter D, Robinson J, Rodenhiser DI. Genome-wide H3K9 histone acetylation profiles are altered in benzopyrene-treated MCF7 breast cancer cells. J Biol Chem. 2008;283:4051–60. doi: 10.1074/jbc.M707506200. [DOI] [PubMed] [Google Scholar]
- Safe SH. Modulation of gene expression and endocrine response pathways by 2,3,7,8-tetrachlorodibenzo-p-dioxin and related compounds. Pharmacol Ther. 1995;67:247–281. doi: 10.1016/0163-7258(95)00017-b. [DOI] [PubMed] [Google Scholar]
- Schorderet DF, Escher P. NR2E3 mutations in enhanced S-cone sensitivity syndrome (ESCS), Goldmann-Favre syndrome (GFS), clumped pigmentary retinal degeneration (CPRD), and retinitis pigmentosa (RP) Hum Mutat. 2009;30:1475–1485. doi: 10.1002/humu.21096. [DOI] [PubMed] [Google Scholar]
- Sharon D, Sandberg MA, Caruso RC, Berson EL, Dryja TP. Shared mutations in NR2E3 in enhanced S-cone syndrome, Goldmann-Favre syndrome, and many cases of clumped pigmentary retinal degeneration. Arch Ophthalmol. 2003;121:1316–1323. doi: 10.1001/archopht.121.9.1316. [DOI] [PubMed] [Google Scholar]
- Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA, Shi Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Stampfer MR, Bartley JC. Induction of transformation and continuous cell lines from normal human mammary epithelial cells after exposure to benzo[a]pyrene. Proc Natl Acad Sci USA. 1985;82:2394–2398. doi: 10.1073/pnas.82.8.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura H, Nagayoshi H, Matsuda T, Sakakibara H, Morita M, Matsui A, Ohura T, Shimoi K. Inhibitory effects of chrysoeriol on DNA adduct formation with benzo[a]pyrene in MCF-7 breast cancer cells. Toxicology. 2010;274:42–48. doi: 10.1016/j.tox.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Tan MH, Zhou XE, Soon FF, Li X, Li J, Yong EL, Melcher K, Xu HE. The crystal structure of the orphan nuclear receptor NR2E3/PNR ligand binding domain reveals a dimeric auto-repressed conformation. PLoS One. 2013;12:e74359. doi: 10.1371/journal.pone.0074359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teneng I, Montoya-Durango DE, Quertermous JL, Lacy ME, Ramos KS. Reactivation of L1 retrotransposon by benzo(a)pyrene involves complex genetic and epigenetic regulation. Epigenetics. 2011;6:355–367. doi: 10.4161/epi.6.3.14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- Webber AL, Hodor P, Thut CJ, Vogt TF, Zhang T, Holdere DJ, Petrukhina K. Dual role of Nr2e3 in photoreceptor development and maintenance. Exp Eye Res. 2008;87:35–48. doi: 10.1016/j.exer.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Wen Z, Pyeon D, Wang Y, Lambert P, Xu W, Ahlquist P. Orphan nuclear receptor PNR/NR2E3 stimulates p53 functions by enhancing p53 acetylation. Mol Cell Biol. 2012;32:26–35. doi: 10.1128/MCB.05513-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong P, Bondy ML, Li D, Shen H, Wang LE, Singletary SE, Spitz MR, Wei Q. Sensitivity to benzo(a)pyrene diol-epoxide associated with risk of breast cancer in young women and modulation by glutathione S-transferase polymorphisms: a case-control study. Cancer Res. 2001;61:8465–8469. [PubMed] [Google Scholar]
- Yoon JH, Smith LE, Feng Z, Tang M, Lee CS, Pfeifer GP. Methylated CpG dinucleotides are the preferential targets for G-to-T transversion mutations induced by benzo[a]pyrene diol epoxide in mammalian cells: similarities with the p53 mutation spectrum in smoking-associated lung cancers. Cancer Res. 2001;61:7110–7117. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.