Abstract
Exercise reduces the risk of developing a number of neurological disorders and increases the efficiency of cellular energy production. However, overly strenuous exercise produces oxidative stress. Proper oxygenation is crucial for the health of all tissues, and tight regulation of cellular oxygen is critical to balance O2 levels and redox homeostasis in the brain. Hypoxia Inducible Factor (HIF)1α and HIF2α are transcription factors regulated by cellular oxygen concentration that initiate gene regulation of vascular development, redox homeostasis, and cell cycle control. HIF1α and HIF2α contribute to important adaptive mechanisms that occur when oxygen and ROS homeostasis become unbalanced. It has been shown that preconditioning by exposure to a stressor prior to a hypoxic event reduces damage that would otherwise occur. Previously we reported that three months of exercise protects SNpc DA neurons from toxicity caused by Complex I inhibition. Here, we identify the cells in the SNpc that express HIF1α and HIF2α and show that running exercise produces hypoxia in SNpc DA neurons, and alters the expression of HIF1α and HIF2α. In mice carrying a conditional knockout of Hif1α in postnatal neurons we observe that exercise alone produces SNpc TH+ DA neuron loss. Loss of HIF1α also abolishes exercise-induced neuroprotection. In mice lacking Hif2α in postnatal neurons, the number of TH+ DA neurons in the adult SNpc is diminished, but three months of exercise rescues this loss. We conclude that HIF1α is necessary for exercise-induced neuroprotection and both HIF1α and HIF2α are necessary for the survival and function of adult SNpc DA neurons.
Keywords: Hypoxia, Exercise, substantia nigra, neuroprotection, preconditioning, oxidative stress
1. Introduction
Exercise is beneficial for cognitive health and reduces the risk of developing a number neurological disorders including stroke (Curry et al., 2010, Liebelt et al., 2010), Alzheimer’s (Briones, 2006, Stranahan et al., 2012) and Parkinson’s disease (Petzinger et al., 2013, Murray et al., 2014) but the factors that underlie this neuroprotection are not entirely clear. Cellular hypoxia that occurs during aerobic exercise may induce responses that precondition the cell and are later protective during periods of oxidative stress. The activity of Hypoxia Inducible Factor is modulated by the relative levels of antioxidant and reactive oxygen species (ROS) in the microenvironment as well as the O2 environment of the cells (Wang et al., 1995, Chandel et al., 1998, Tajima et al., 2009, Radak et al., 2013); each of these are factors modulated by exercise (Radak et al., 2008, Radak et al., 2013).
Oxidative phosphorylation, the production of ATP, and oxygen homeostasis are critical to cell survival. Within the cell, molecular oxygen is tightly regulated, and a key component of this oxygen-sensing pathway is the transcription factor Hypoxia Inducible Factor (HIF) (Wang and Semenza, 1993, 1995). HIF is important for the adaptation of the cell during periods of reduced oxygen availability and its activation promotes the expression of numerous genes, including those involved in erythropoiesis (Semenza and Wang, 1992), vascular development and angiogenesis (Carmeliet et al., 1998, Iyer et al., 1998), and glycolysis (Semenza et al., 1994). HIF is a heterodimer consisting of two subunits, a constitutively present HIF1β (Aryl Hydrocarbon Nuclear Receptor Translocator, ARNT) (Wang et al., 1995), and HIF1α, a subunit that is expressed in hypoxic conditions (Wang and Semenza, 1993). In the presence of O2, iron and 2-oxoglutarate, HIF1α is hydroxylated by prolyl hydroxylases (PHDs) for proteasomal destruction (Epstein et al., 2001). However, when cells become hypoxic, PHD’s are inhibited and HIF1α is stabilized (Bruick and McKnight, 2001, Epstein et al., 2001, Ivan et al., 2001, Jaakkola et al., 2001). Although much has been published concerning the stabilization of HIF protein, HIF biological activity and expression is known to be regulated at multiple levels including mRNA expression, nuclear localization, and transactivation as well as protein stabilization (Wang et al., 1995, Semenza, 2000, Tajima et al., 2009). The induction and accumulation of HIF1α requires an intact mitochondrial respiratory chain (Chandel et al., 1998, Agani et al., 2000, Agani et al., 2002). It has also been shown that ROS and antioxidant levels in the microenvironment modulate Hif mRNA expression (Chandel et al., 1998).
HIF2α, encoded by Epas1, is also a member of the bHLH-PAS subfamily, binds to HIF1β, is regulated by O2, and has 48% sequence similarity to HIF1α (Tian et al., 1997). Although they share biochemical characteristics, the expression patterns of HIF1α and HIF2α do not completely overlap (Tian et al., 1997, Jain et al., 1998) and it has been shown that HIF1α and HIF2α have unique and sometimes antagonistic functions (Yuan et al., 2013). HIF2α is also involved in catecholamine synthesis and the regulation of cardiac function (Tian et al., 1998). Previous studies have also shown that complete loss of hif1α (Iyer et al., 1998) or epas1 (Peng et al., 2000, Scortegagna et al., 2003) in mice results in embryonic/perinatal lethality.
Here we identify the cells in the SNpc that express HIF1α and HIF2α and show that exercise induces hypoxia in DA neurons of the substantia nigra pars compacta (SNpc), and modulates HIF expression in the SN. Reduction of neuronal Hif1α results in loss of DA neurons with exercise, while Hif2α is necessary for survival of DA neurons in standard conditions. Therefore, while HIF1α and HIF2α are both necessary for DA neuron survival, they play different roles in the subsistence of these neurons.
2. Experimental Procedures
2.1 Animals
All of the experimental animal procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and were approved by the St Jude Children’s Research Hospital IACUC (protocol 364). Experiments were carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for animal experiments. Mice were maintained on a 12:12 light:dark cycle and with food and water ad libetum. Equal numbers of male and female mice were used in each condition in all of the experiments.
Hif1a and Epas1-CKO mice were generated by mating floxed Hif1a (B6.129-Hif1atm3Rsjo/J; stock # 007561) or Hif2α (Epas1tm1Mcs/J; stock # 008407) (Jackson Laboratories, Bar Harbor, ME) with a transgenic mouse expressing Cre Recombinase driven by a CaMKII promoter (L7ag#13cre)(Dragatsis and Zeitlin, 2000, Baranova et al., 2007) backcrossed to a C57BL/6 background. Control mice were hif1α or epas1 non-recombined floxed mice (referred to as WT) or C57BL/6J stock mice (Stock number 000664, Jackson Laboratories).
2.2 Voluntary exercise: wheel running
At 3 months of age, mice were placed into individual monitored running cages (Lafayette Instruments, Cat#80820, Lafayette, IN) and allowed unrestricted access to the running wheel. Wheel revolutions were recorded using Animal Wheel Monitor (AWM) software (Lafayette Instruments, Lafayette, IN, Cat# 86065). Littermate mice were used in standard housing (SH) conditions.
2.3 Genotyping
Genotyping of transgenic mice was performed using PCR primers and protocols suggested by Jackson Laboratories. Primers used to identify hif1α transgenic mice were: Primer 1, 5′ to 3′:TGCTCATCAGTTGCCACTT and Primer 2, 5′ to 3′:GTTGGGGCAGTACTGGAAAG; epas1: 5′ to 3′:GAGAGCAGCTTCTCCTGGAA and 5′ to 3′:TGTAGGCAAGGAAACCAAGG. L7ag#13cre were genotyped using 5′ to 3′:CTGCCACGACCAAGTGACAGC and 5′ to 3′:CTTCTCTACACCTGCGGTGCT. PCR products were run on 3% (hif1α and epas1) or 2% (L7ag#13cre) agarose gels.
2.4 MPTP treatment
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is a xenobiotic that is metabolized by MAO-B to MPP+, which readily crosses the blood-brain-barrier, enters DA neurons through the dopamine transporter and blocks Complex I (Smeyne and Jackson-Lewis, 2005). Here, mice were injected using the acute MPTP protocol that is 4 ip injections of 20mg/kg MPTP-HCl spaced 2 hours apart (Hamre et al., 1999). After 7 days, some mice were transcardially perfused with 1X PBS followed by 2% paraformaldehyde for anatomical analysis.
2.5 β-Galactosidase Histochemical Staining
To detect cells within the SNpc that expressed CaMKII, the L7ag#13 CRE mouse was crossed with a ROSA26-CRE reporter mouse. Adult mice from this cross were sectioned at 40μm through the SN and stained for β-Galactosidase histochemistry as previously described (Oberdick et al., 1994).
2.6 Immunocytochemistry
Mice were transcardially perfused with 2% paraformaldehyde (PFA) after clearing with phosphate buffered saline (PBS). Brains were removed from the calvaria and postfixed in 2% PFA overnight. After postfixation, brains were placed in 30% sucrose/PBS until they were no longer floating. Once cryoprotected, the brains were blocked and rapidly frozen in TFM™ Tissue Freezing Medium (Fisher), maintained at −18°C and sectioned at 40μm. Serial sections were collected in 1X TBS and stored at 4°C until processed.
Sections containing the SN (Paxinos and Franklin, 2001) were selected and treated for antigen retrieval by incubation in 2N HCl for 10 min (37–39°C) and then in 0.5 M boric acid for 10 mins at RT. Sections were rinsed in 1X PBS, blocked and permeabalized in 10% goat serum, 0.3% Triton X-100 for 60 mins. at RT and incubated in primary antibodies to HIF1α (H-206, 1:100, Santa Cruz, Dallas, TX) or Hif2α (GTX30114, 1:100, GeneTex, Irvine, CA ) overnight at 4°C. On day 2 sections were rinsed thoroughly in 1X PBS and double labeled for tyrosine hydroxylase (TH, Sigma #T1299, 1:200, St. Louis, MO), Glial Fibrillary Acidic Acid (GFAP, Sigma #G3893, 1:500), CD31 (BD#550274, BD Bioscience, San Jose, CA), or EAAC1, Millipore #AB1520 1:2000, Temecula, CA) for 1 hr. at RT. Sections were rinsed and secondary antibodies (AlexaFluor ™, Life Technologies, Carlsbad, CA ) were applied for 1 hr. at RT in the dark. NOTE: When sections were treated with more than 1 antibody secondary antibodies were applied in succession. After secondary antibody application, sections were rinsed with 1X PBS and mounted with Vectashield mounting media for fluorescence (Vector Labs, Burlingame, CA) or Vectashield plus DAPI mounting media. Sections used for immunofluorescence were alternate sections from the same mice used for TH cell counts and were processed 7 days after administration of MPTP, a time when the toxin –induced cell death was maximal (Boyd et al., 2007). Confocal images were collected on a Zeiss LSM 510 microscope and analyzed with LSM Image Browser software (Carl Zeiss, Germany). HIF1α or HIF2α positive or negative immunostaining illustrated by confocal images in each cell type were seen in SN sections from at least 3–5 mice in each condition. Following processing, the 40μm sections shrink to approximately 22 μm. Sections were scanned at 1 micron through the entire z-place, and images were collected.
2.7 Nissl immunohistochemistry
Sections previously stained for TH+ immunoreactivity were rehydrated and incubated in Cresyl violet stain for 2 hrs at RT. Sections were then rinsed in water, dehydrated to 95% ethanol, differentiated with glacial acetic acid in 95% ethanol, fully dehydrated in 100% ethanol, cleared in xylene and coverslipped with Permount ™.
2.8 EF-5 visualization
Three-month old C57Bl/6N mice (Harlan, Indianapolis, IN) were placed into running or SH cages. One to two hours into the second evening, mice were removed, lightly anesthetized with 2,2,2-Tribromoethanol (Avertin) and tail veins injected (following manufacturers suggested protocol or 10mM in 1% volume of body mass) with EF-5 solution (Hypoxia Imaging, Philadelphia, PA) (Bergeron et al., 1999) or 0.9% saline. Once mice recovered from the anesthesia, they were returned to their home cages for an additional 3 hrs, after which they were deeply anesthetized with Avertin and decapitated. Brains were rapidly removed and sliced while chilled on dry ice, and a tissue block containing the midbrain was rapidly frozen in cryoprotective media and stored at −80°C until sectioned. Twenty micron sections were cut on a cryostat and fixed in 3% PFA on polyionic slides for 1 hr. in the dark. Sections were then processed for detection of both EF-5 using an ELK3-51 cy3-conjugated antibody (Bergeron et al., 1999) and TH to identify dopaminergic neurons (Baquet et al., 2009). Confocal images were collected on a Zeiss LSM 510 microscope and analyzed with LSM Image Browser software. Individual mice were scored as positive or negative for ELK3-51 antibody staining after 2–3 sections were examined using the confocal microscope to focus through the full z plane of the SN in each section. Images shown in figure 4 are generated from a single 1μm z plane.
Fig. 4.
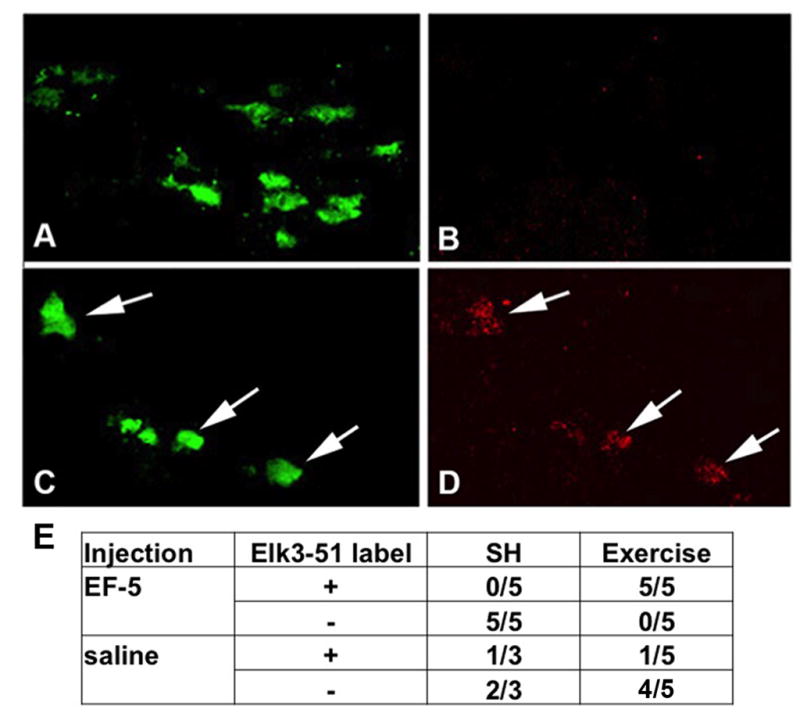
Exercise induces hypoxia in SNpc DA neurons. (A, B) C57BL/6 mice in the SH condition taken during the evening active period show DA neurons labeled with TH (A) but not Elk3-51 (B). (C, D) SNpc from C57BL/6 mice in wheel cages taken during day 2 in the active period (running) show DA neurons colabeled with TH (C) and Elk3-51 (D). (E) All of the mice in exercise conditions injected with EF-5 (5/5) show Elk3-51 labeling of DA neurons, while none of the mice in SH injected with EF-5 (0/5) show Elk3-51+ DA neurons.
2.9 Removal of SN for mRNA and protein samples
Mice were anesthetized with Avertin and brains were removed and placed in a brain matrix (Model BS-AL-5000C, Braintree Scientific, Braintree. MA), chilled on dry ice, ventral side up. Two millimeter coronal slices including the SN were isolated using anatomical landmarks for the midbrain/SN (Jang et al., 2012) and placed immediately in Eppendorf tubes in dry ice. Samples were stored in dry ice until transfer to −80°C. All exercise samples and SH cohorts were collected during the active running period of the mice, while MPTP samples were collected 2 hours after the final injection of MPTP. This time point was chosen based upon the peak of oxidative stress after MPTP as indicated by changes in expression of GSTpi (Smeyne et al., 2007).
2.10 Reverse-transcriptase quantitative PCR
mRNA was isolated from individual SN using a MELT™ kit (Invitrogen, Life Technologies, Carlsbad, CA), following manufacturers suggested protocol. Taqman Gene Expression assays (Life Technologies, Carlsbad, CA ) following manufacturers suggested protocol were used for Hif1a (#Mm00468875_m1), Epas1 (#Mm01236112_m1), VegfA (#Mm01281449_m1), Egln1 (#Mm00459770_m1), and RPS18S (#Mm02601778_g1) (n=5–8 for each exercise condition, n=18 for SH). Samples were processed on an Eppendorf RealPlex2 Mastercycler and analyzed using included Realplex software (Fisher Scientific, Pittsburgh, PA). Statistical analysis of differences between conditions was analyzed by ANOVA followed by Fisher’s LSD post hoc analysis (Prism V.6, GraphPad Software, LaJolla, CA).
2.11 Analysis of HIF1a and HIF2a Protein Levels in SN
Western blot analysis of HIF1α and HIF2α were performed using protein extracts from individual SN. Protein was isolated by mechanical dissociation using RIPA buffer supplemented with protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN) at 4°C, incubated for 1 hr. at 4°C, and separated by centrifugation at 13kG for 30 mins. at 4°C. Protein concentration was determined by Lowry assay with Dc™ protein assay kit (BioRad, Hercules, CA). Lysates were aliquotted and stored at −80°C until use. Electrophoresis was performed using standard protocol and Tris-HCl precast gels (7.5%, BioRad), wells were loaded with 20 micrograms per lane, from individual SN samples (n= 4 per condition). Proteins were transferred to PVDF membrane, rinsed, incubated in blocking buffer and incubated overnight 4°C in primary antibody: mouse anti-HIF1α (1:500, R&D Systems, Minneapolis, MN) or goat anti-HIF2α (1:300, R&D Systems). Blots were rinsed and incubated with peroxidase linked secondary antibody (HIF1a; ECL Amersham, GE Healthcare, Pittsburgh, PA, HIF2α; Santa Cruz, Dallas, TX) 1 hr. RT, rinsed and reacted with DuraWest Super Signal (ECL Pierce, Thermoscientific, Rockford, IL), and visualized with an Odyssey Fc system. Blots were then stripped (Restore buffer, Pierce), rinsed, and incubated with rabbit anti-β-actin (Abcam, Cambridge, MA) for 1 hr. RT, rinsed and incubated with peroxidase labeled anti-Rabbit IgG (ECL Amersham, GE Healthcare Biosciences, Pittsburgh, PA), and processed as above. To reduce experimental variability, the electrophoresis and transfer of each set of HIF1α and HIF2 α immunoblots were run concurrently with the same protein samples. Western blots were analyzed with Image Studiolite software (LI-COR Biotechnology, Lincoln, NE) and compared to SH condition after normalization to β-actin. COS-7 cell extracts (in normoxic and hypoxic conditions) (Novus Biologicals, Littleton, CO), and HIF2α recombinant protein (Novus Biologicals) were loaded as controls. Bands corresponding to approximately 120kD (the expected MW of antibody-labeled HIF1α and HIF2α) and the hypoxia induced COS7 band (HIF1α and HIF2α) and HIF2α recombinant protein (HIF2α) were used for analysis. We saw no cross-reaction of the HIF1α antibodies with the HIF2α recombinant protein (results not shown). Statistical analysis of differences between conditions was analyzed by unpaired non-parametric comparisons (Kolmogorov-Smirnov analysis, Prism V.6, GraphPad Software, LaJolla, CA).
2.12 Stereology of TH+ SNpc neurons and Nissl stained cells
Forty micron floating sections spaced 200μm apart were immunostained for tyrosine hydroxylase followed by a cresyl violet counterstain. The number of these TH+ neurons were estimated using optical fractionator stereological methods as described in Baquet et al (Baquet et al., 2009). Similar parameters were used for estimating the number of cresyl violet stained cells in the SN.
Since daily wheel running activity varies between (Lightfoot et al., 2004) and within (Coletti et al., 2013) mouse strains, to determine whether running is protective for DA neurons, mice running an average of 16k or greater revolutions per day over 3 months were used in this analysis, since this amount of exercise has been previously shown to be neuroprotective in C57BL/6 mice (Gerecke et al., 2010).
3.0 Results
3.1 Cellular localization of HIF1α and HIF2α in the Substantia Nigra pars compacta
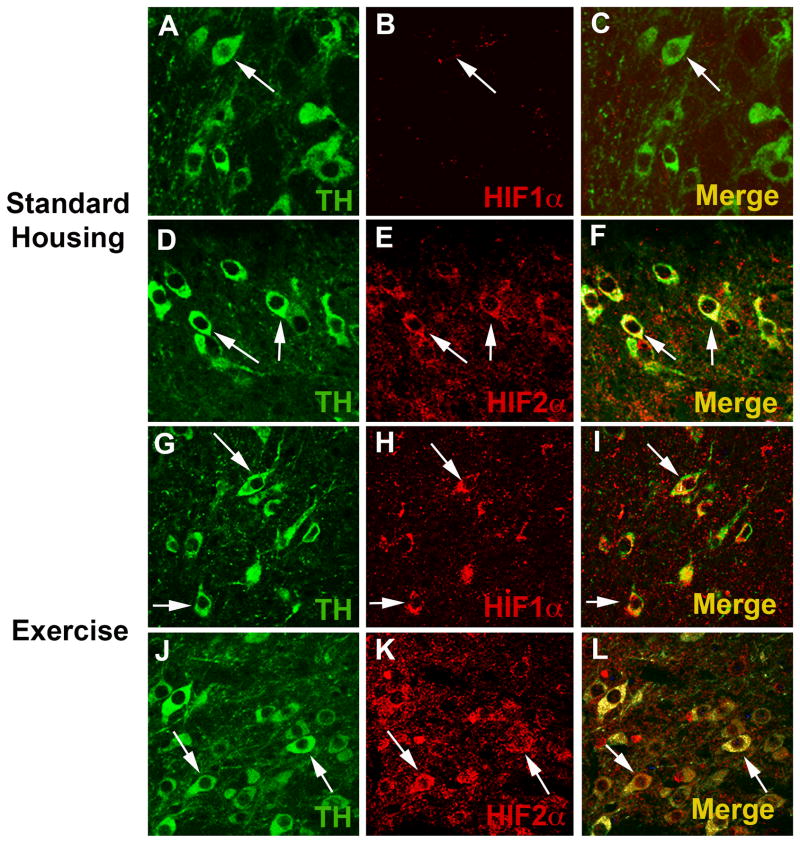
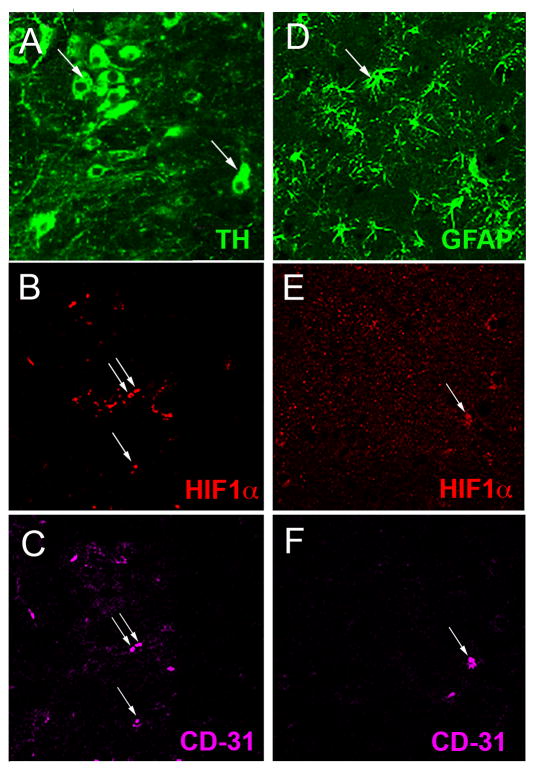
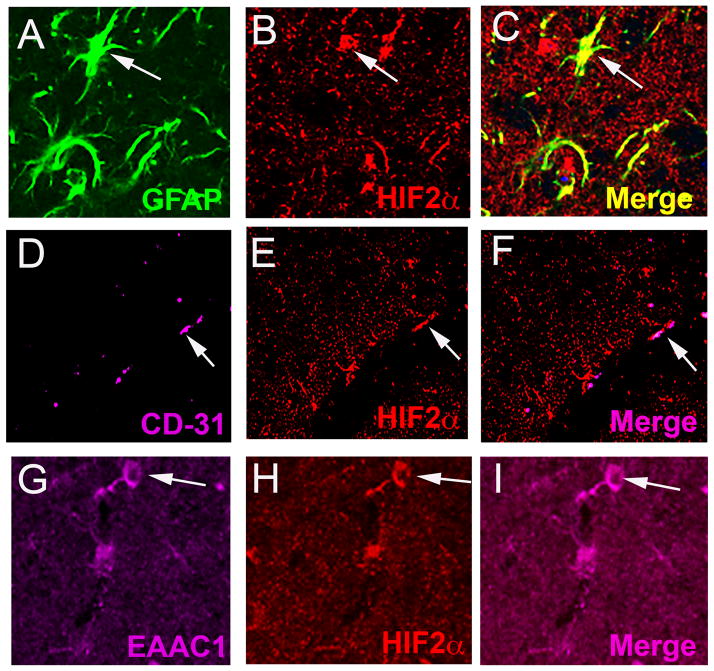
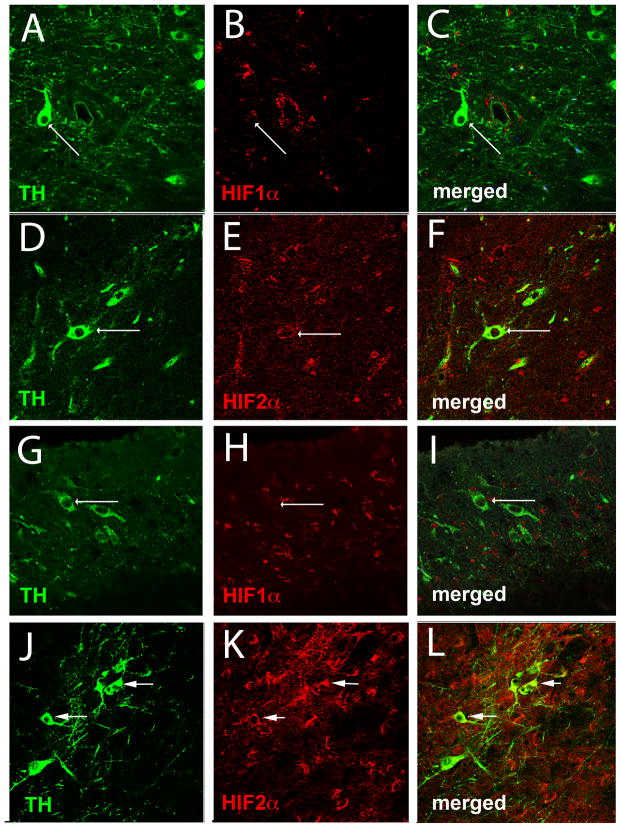
Although the substantia nigra (SN) contains a number of cell types including dopaminergic (DA), glutamatergic, and GABAergic neurons, astrocytes, microglia, and endothelial cells of the vasculature, it is the DA neurons of the SNpc that are highly sensitive to oxidative stress and are lost during the progression of Parkinson’s disease. The presence of HIF1α and HIF2α and their cell specificity in the SNpc was investigated to explore the possible role of HIF during exercise-induced neuroprotection and oxidative stress generated by the administration of MPTP. In the WT SNpc, HIF1α is expressed at barely detectable levels in DA neurons (Fig. 1A–C) and more abundantly in endothelial cells (Fig. 2A–C). HIF1α does not appear to be expressed in astrocytes or glutamatergic neurons (Fig. 2D–F and not shown). HIF2α is expressed in DA neurons (Fig. 1D–F), astrocytes, endothelial cells and glutamatergic neurons (Fig. 3A–I), and in all conditions is more consistently discernable than HIF1α (Fig 1D–F, J–L). Exercise enhances HIF immunostaining of DA neurons in the SNpc (Fig. 1G–L).
Fig. 1.
Exercise enhances HIF1α and HIF2α immunostaining of dopaminergic cells. (A–F): SNpc from WT mice in standard housing conditions. An arrow identifies a TH+ dopaminergic (DA) neuron (A, C, green). (B, C) HIF1α (red) is barely detectable in these neurons. (D) TH+ dopaminergic neurons (arrows) are also labeled with HIF2α antibody (E, F). (G–L): SNpc from WT mice housed in wheel cages and allowed voluntary access to running for 3 months. Exercise does not alter the appearance of TH+ neurons in the SNpc (G, J) (arrows), but HIF1α (H,I) and HIF2α (K,L) immunostaining is more evident in SNpc DA neurons (arrows). Each image is taken from a single 1μm z-plane to insure that any co-localization of immunofluorescence is from the same cell.
Fig. 2.
Cellular localization of HIF1α in the SNpc. (A–C): A section immunostained with TH (A, arrows), HIF1α (B, arrows) and the endothelial cell marker CD-31 (C, arrows) shows HIF1α present in endothelial cells but absent in TH+ DA neurons. GFAP+ astrocytes (D, arrow) are not labeled with HIF1α (E, arrow) that colocalizes with the endothelial marker CD31 (F, arrow).
Fig. 3.
HIF2α is present in several cell types in addition to DA neurons in the SN. HIF2α antibody (B, C, E, F, H, I, red) colocalizes with astrocytes (GFAP, A, C, green), endothelial cells (CD31, D, F, magenta), and glutamatergic neurons (EAAC1, G, I, magenta).
3.2 Exercise produces hypoxic conditions in SN dopaminergic neurons
Numerous forms of exercise in humans and rodents have been used to demonstrate the benefits of exercise, and both moderate and more intensive exercise regimes have been shown to be effective (Cotman and Berchtold, 2002, Zigmond and Smeyne, 2014). Neuroprotection from oxidative stress produced by MPTP treatment in mice, that is afforded by wheel running, requires 3 months of running at daily averages greater than 12k wheel revolutions (Gerecke et al., 2010). Given the substantial nature of this exercise, we hypothesize that it generates aerobic conditions in the brain and induces the activity of hypoxia sensitive factors. To assess the state of oxygenation of neurons during exercise, C57BL/6N mice were injected with the compound 2-(2-nitro-1H-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoropropyl) acetamide (EF-5) during the period of active running, as were cohorts of mice housed in standard cages (SH). The immunovisualization of EF-5 by the ELK3-51 antibody has been used to label hypoxic cells (Bergeron et al., 1999) in the brain. EF-5 is a pentafluorinated derivative of the drug etanidazole and is used to detect reduced oxygenation in tissues (Koch et al, 1995). Additionally, when the cell is hypoxic EF-5 is reduced by a redox dependent mechanism, and forms stable adducts with cellular macromolecules by covalent binding to protein thiols (Evans et al., 1995, Marques de Olivera et al., 2010); an indication that hypoxic cells may be experiencing oxidative stress (Chandel and Budinger, 2007). EF-5 binding is inhibited as oxygen concentration rises and increases as oxygen concentration is lowered (Koch et al., 1995, Chavez and LaManna, 2002). The monoclonal antibody, Elk3-51 binds specifically to these adducts and may be used to detect and localize low oxygenation at the cellular level (Koch et al., 1995). Here, C57BL/6J mice were exposed to 2 days of voluntary exercise (9,000 to 39,000 running wheel revolutions daily, with 7/10 mice averaging greater than 20,000 wheel revolutions daily) or standard housing (SH), during which they were injected with EF-5 or saline via the tail vein. Of the cohorts injected with EF-5, ELK3-51+ DA neurons were observed in none of the mice that were housed in standard conditions (0/5) (Fig. 4A–B, E) while ELK3-51+ DA neurons were observed in the SNpc of all of the mice in exercise cages (5/5) (Fig. 4C–E). Mice exposed to exercise (n=5) or SH (n=3) conditions injected with saline showed absent (6/8) or very low (2/8) ELK3-51+ staining.
3.3 HIF expression in the SN is modulated with voluntary wheel running
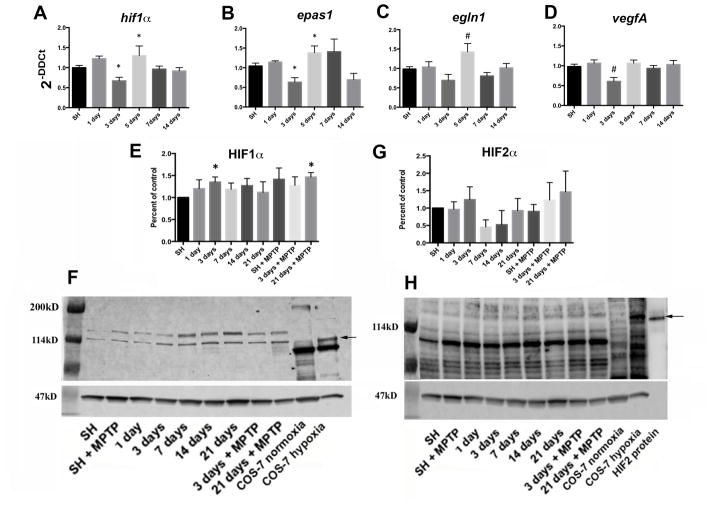
Previous studies have shown that HIF1α is induced in the brain during initial exposure to hypoxia, although with chronic hypoxia HIF1α levels decline unless another hypoxic challenge occurs (Chavez et al., 2000). To determine whether exercise produces changes in the expression of hypoxia sensitive molecules in the SN, hif1α, epas1, egl nine homologue1 (egln1), and vascular endothelial growth factor A (vegfA) levels were measured. mRNA was isolated from the SN of C57BL/6J mice housed in SH conditions or after 1,3,5,7, or 14 days of voluntary exercise (during the active period) in cages with monitored running wheels. Exercise induces a significant reduction in hif1α expression (p≤0.01) at 3 days, is elevated with 5 days of exercise (p≤0.05), and returns to SH levels at 7 and 14 days of exercise (Fig. 5A). Epas1 expression follows a pattern similar to hif1α (p≤0.05 at 3 and 5 days) but remains somewhat elevated through 7 days of exercise before decreasing to approximate SH levels at 14 days of exercise (Fig. 5B). The expression of egln1, the gene that encodes the PHD2 protein that functions in the hydroxylation and degradation of HIF1α (Epstein et al., 2001), follows an expression pattern similar to hif1α levels after exercise, with 5 days of exercise promoting a significant increase in egln1 expression (p≤0.01), compared to all other conditions (Fig. 5C). VegfA expression decreases after 3 days of exercise (p≤0.01) but returns to baseline levels after 5 days of exercise (Fig. 5D).
Fig. 5.
Expression of hypoxia sensitive molecules in the SN is modulated by wheel running exercise. hif1a (A), epas1 (B), egln1 (C), and vegfA (D) expression from SN mRNA isolated at 1, 3, 5, 7, and 14 days of running show significant reduction at 3 days (A,B,D) and increase at 5 days (A,C) of running compared to controls in SH. *=p≤0.05, #=p≤0.01. N=17–18 for SH, 5–8 for each exercise condition. HIF1α protein from the SN (E, F) is significantly increased at running day 3 and after 21 days of running plus MPTP administration (SN taken at 2 hrs. after the last MPTP injection). *=p≤0.05. SN HIF2α protein (G, H) shows no statistically significant change with running. Error bars =± SEM. Arrows indicate 114kD, expected bands for these HIF1α and HIF2α antibodies are ~120kD. Lysate from COS-7 cells in normoxic and hypoxic conditions are used as negative and positive controls (F, H), and HIF2α human recombinant protein (H) as a positive control (~120kD). Values are expressed as percent of control relative to SH after normalization to β-actin (F, H, ~47kD) for individual samples. N= 4 SN for each condition.
The effect of exercise on the accumulation of HIF protein was measured in SN collected from C57BL/6J mice during their active periods at 1,3,7,14, and 21 days. HIF1α protein increases an average of 20–30% after 3 days of running (p≤0.05), but by day 7 returns to levels similar to mice in SH (Fig 5E, F). Following MPTP administration, mice housed in SH conditions show no significant change in HIF1α compared to the control mice. In mice allowed 3 days of exercise, little change in HIF1α expression was observed, however, after 21 days of exercise MPTP treatment results in a significant increase in HIF1α protein in the SN, (p≤0.05) compared to mice in SH conditions (Fig. 5E, F). The HIF1α increase seen in mice allowed 21 days of exercise and treated with MPTP is similar to HIF1α levels in mice after 3 days of exercise, and greater than HIF1α after 21 days of exercise. The MPTP induced increase in HIF1α protein after 3 weeks of exercise may involve processes similar to the rebound effect of HIF expression reported in other brain areas (Chavez et al., 2000). Lysate from COS7 cells exposed to normoxic and hypoxic conditions are used as negative and positive controls for comparison and analysis of the HIF1α response to exercise and MPTP (Fig 5F). The presence of differentially labeled bands at approximately 120kD when comparing normoxic and hypoxic COS7 lysates were used to identify the analytes.
Immunoblot results of HIF2α levels in the SN reveal no significant differences between any of the observed conditions. HIF2α bands corresponding to COS7 cell hypoxic response and HIF2α recombinant protein are barely detectable, however, there is a trend where HIF2α rises early in a running regime (3 days), then falls (7–14 days), before returning to approximate baseline levels (21 days). MPTP does not appear to affect HIF2α levels in mice housed in SH conditions, or in animals allowed exercise (Fig. 5G,H). As in the HIF1α blots, COS7 cell lysate (normoxic and hypoxic) and HIF2α recombinant protein were used for comparison and analysis of HIF2α response to exercise and MPTP (Fig 5H).
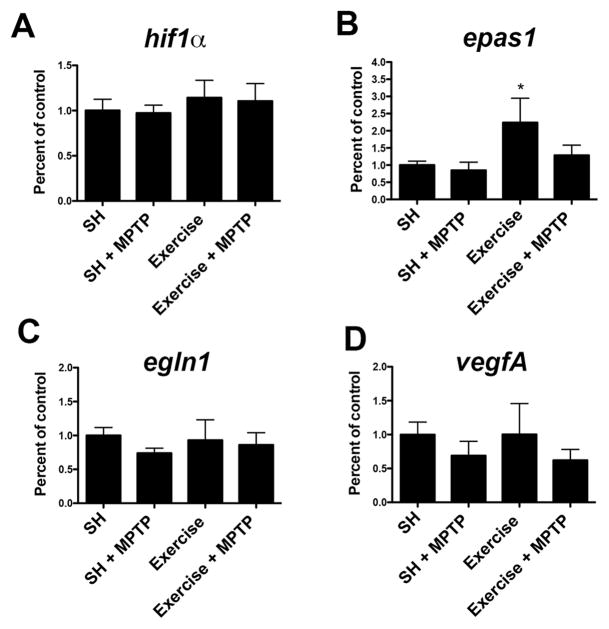
After 3 months of running, examination of mRNA levels show no significant differences in the expression of hif1α, egln1, or vegfA from SN samples obtained from WT mice in SH, MPTP treatment, 3 months of exercise, or 3 months of exercise plus MPTP treatment conditions (Fig 6A, C, D). Exercise increases epas1 expression in WT mice (124%, p≤0.02), but when MPTP is administered after 3 months of exercise this increase is not seen (Fig 6B).
Fig. 6. Epas1 (Hif2α).
is increased in the SN with 3 months of wheel running exercise. The expression of hif1α (A), epas1 (B), egln1 (C), and vegfA (D) in the SN from WT mice after 3 months of exercise or SH was measured by qPCR. Epas1 (B) in the SN of exercised mice is significantly elevated (*= p≤0.02). mRNA levels are normalized to 18S ribosomal RNA and compared to expression in SH. Epas1 expression from mice in SH+MPTP and exercise + MPTP showed no changes, neither did hif1α, egln1, or vegfA expression in any condition.
3.4 Conditional deletion of HIF1α and HIF2α reduces their expression in postnatal SN DA neurons
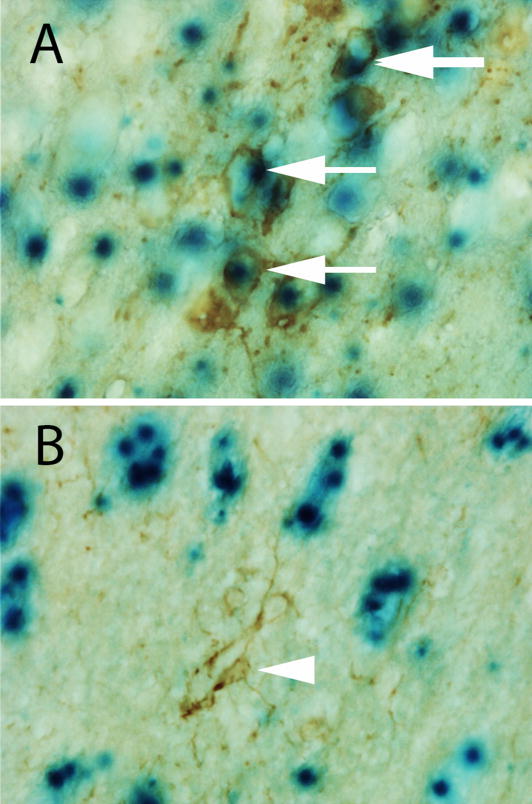
To examine the role of hif1α and hif2α during oxidative stress in the SN, we administered MPTP to mice in SH or exercise conditions carrying a conditional deletion of hif1α or hif2α based on the expression of Ca2/calmodulin-dependent protein kinase II (CaMKII) using the L7ag#13 CRE driver mouse. Targeting of postnatal neurons for hif1α and hif2α deletion was chosen to examine the reduction of these factors in this specific population of cells. CaMKII is expressed in subsets of postnatal neurons in cerebellum, hippocampus, cortex, and basal ganglia (Dragatsis and Zeitlin, 2000). Using a ROSA26-CRE reporter, we empirically determined that CaMKII is expressed in the DA neurons of the SN (Fig 7A), but not in GFAP-positive astrocytes (Fig. 7B). Mice carrying the deletion of hif1α in postnatal neurons are referred to as hif1α-CKO and epas1 (hif2α) deletion from postnatal neurons as epas1-CKO.
Fig. 7.
CamKII is expressed in DA neurons in the SN. Using a ROSA26 reporter mouse, the pattern of CamKII expression is identified by expression of β-galactosidase (blue cells). These are colocalized with (A) TH+ positive cells in the SN (arrows) but not in (B) GFAP+ astrocytes (arrowhead).
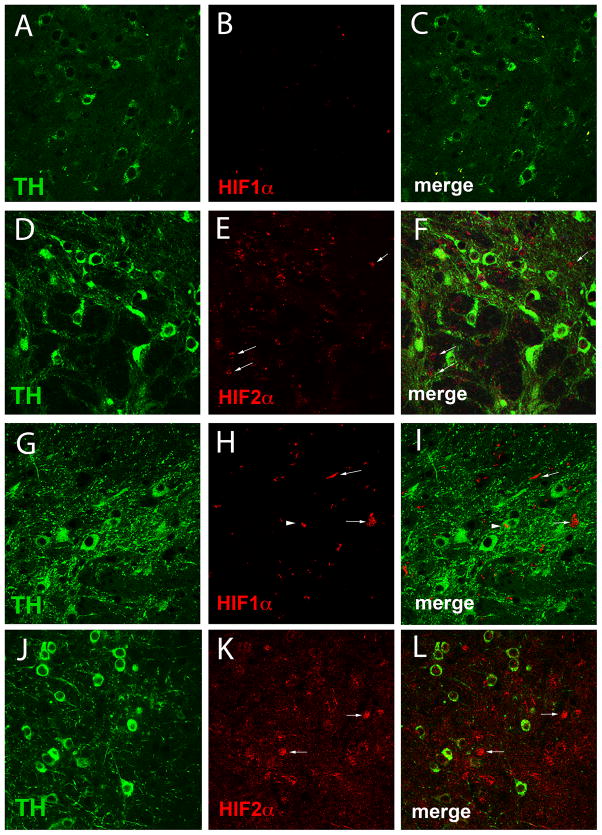
Although we did not expect to see HIF1a immunopositive SNpc DA neurons in the hif1α-CKO from SH (Fig. 8A–C), the veracity of the deletion is shown by our observations of no HIF1α immunopositive SNpc DA neurons following treatment with MPTP, exercise, or exercise followed by MPTP treatment (compare Fig. 8G, H, I exercise plus MPTP with Fig. 1G–I). Loss of hif1α does not appear to affect HIF2a staining as DA neurons are labeled with HIF2α in both SH (Fig. 8D–F) and exercise plus MPTP conditions (Fig. 8J–L).
Fig. 8.
HIF in the SNpc of Hif1α-CKO mice. SNpc sections from Hif1α-CKO mice in SH condition (A–F) or 3 months of exercise followed by MPTP administration (G–L) were labeled with antibodies to TH, HIF1α, and HIF2α. HIF1α (B, C, H, I) is not expressed in SNpc TH+ DA neurons (A, C, G, I), while HIF2α (E, F, K, L) is expressed in SNpc TH+ DA neurons (D, F, J, L). Arrows mark TH+ cells.
We also examined HIF1α and HIF2α immunostaining in the SN of epas1-CKO mice. Sections through the SN from epas1-CKO mice labeled with antibodies to HIF1α revealed little HIF1α in the SH condition (Fig. 9A–C) and slightly more HIF1α labeling in the exercise plus MPTP condition (Fig. 9G–I). HIF2α labeling in the SN from epas1-CKO mice shows robust staining of cells other than TH-positive neurons in both SH (Fig. 9D–F, Fig. 10A–C) and exercise plus MPTP conditions (Fig. 9J–L).
Fig. 9.
HIF in the SNpc of Epas1-CKO mice. Co-expression of TH, (A, C, D, F, G, I, J, L) HIF1α (B, C, H, I) and HIF2α (E, F, K, L) in Epas1-CKO SNpc in SH (A–F) and after 3 months of exercise + MPTP (G–L). Merged images are shown in C, F, I, L. While HIF1α is absent in SH conditions (B, C), it appears more abundant with MPTP administration after 3 months of exercise (H, I). HIF2α is present in cells other than DA neurons in SH (E,F) and also with MPTP after 3 months of exercise (K, L). Arrows point to HIF1α or HIF2α labeled cells. Arrowhead identifies HIF1α-TH+ neuron (H, I).
Fig. 10.
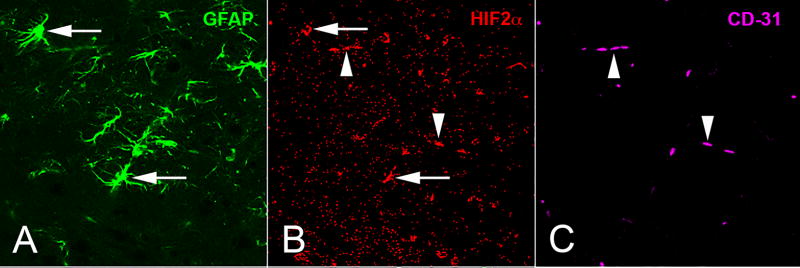
Localization of HIF2α in the SN of Epas1-CKO mice. While absent from neurons, Hif2α (B) is found in (A) GFAP+ astrocytes (arrows) and (C) CD31+ endothelial cells in the SN. Arrowheads mark HIF2α and CD-31 regions of co-localization.
3.5 Loss of HIF alters TH cell number, sensitivity to MPTP, and neuroprotection from oxidative stress
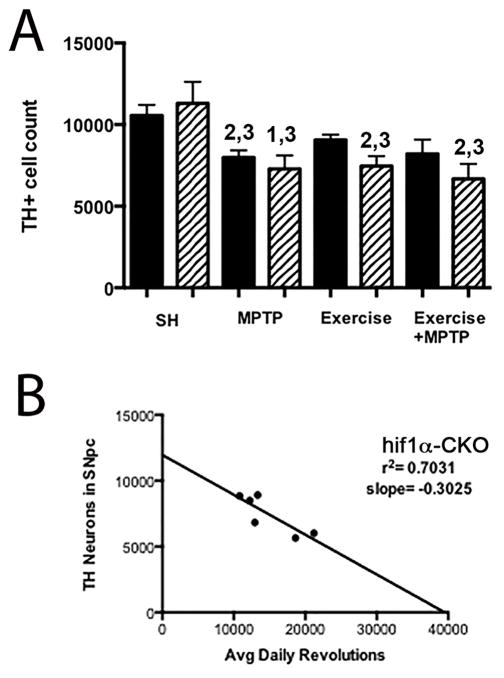
There is no difference in the number of DA neurons in the SNpc of adult WT and hif1α-CKO mice in SH conditions (Fig. 11A). Following administration of acute dosing of MPTP, the number of DA neurons is significantly reduced in the WT strain (25 % cell loss, p≤0.0001) and in hif1α-CKO mice as well (p≤0.05).
Fig. 11.
Reduced TH+ cell number in the SNpc of Hif1α-CKO mice with oxidative stress. TH+ neuron number is not different in the SNpc of WT and Hif1α-CKO mice in SH (A). However, Hif1α-CKO results in significantly reduced TH+ cell number with MPTP, exercise, or exercise + MPTP. TH+ cell number is also reduced in WT SNpc after MPTP administration. 1=p <0.05 vs. WT SH, 2= p <0.01 vs. WT SH, 3= p <0.01 vs. Hif1α-CKO SH. Error bars = ± SEM (B) SNpc TH+ cell number is inversely related to daily exercise in Hif1α-CKO mice. Solid bars are WT mice, Hatched bars are Hif1a-CKO mice.
To determine if loss of hif1α affected exercise-induced neuroprotection, we examined these mice after 3 months of exercise and after 3 months of exercise followed by MPTP treatment. Previously we have shown that the number of SNpc DA neurons in WT mice is not affected by 3 months of running wheel exercise and that 3 months of exercise protects SNpc DA neurons from MPTP toxicity (Gerecke et al., 2010, Gerecke et al., 2012). These findings in WT mice are replicated here (Fig. 11A). Unlike WT mice, hif1α-CKO allowed 3 months of exercise demonstrate a decrease in DA neuron number compared to both WT and hif1α-CKO in SH conditions (p<0.01, Fig. 11A). Hif1α-CKO mice exercised for 3 months and administered MPTP also show a loss of DA neurons compared to WT SH and hif1α-CKO SH conditions (p<0.01) (Fig 11A).
Wheel running was monitored for individual mice in exercise and exercise followed by MPTP treatment conditions for these WT, hif1α-CKO, and epas1-CKO cohorts. We have previously determined that 3 months of unrestricted wheel running (12–18k average daily revolutions) is necessary for exercise-induced neuroprotection, while 2 months of unrestricted running provides partial protection (Gerecke et al., 2010). Daily wheel running varies between (Lerman et al., 2002, Lightfoot et al., 2004) and within (Coletti et al., 2013) mouse strains, so to determine whether running is protective for DA neurons, only mice running an average of 16k or greater revolutions per day for 3 months were used in this analysis. Average daily wheel revolutions (range = 16,196 to 33,766 revolutions per day) were calculated and correlated to the number of TH+ neurons in the SNpc after 3 months of exercise. A negative correlation between daily wheel running and TH+ cell number was found for hif1a-CKO mice (r2=0.70, p≤0.03, Fig. 11B) in the exercise only condition. This is in agreement with the finding that after 3 months of exercise, hif1a-CKO mice have a reduced number of DA neurons, a decrease similar to WT and hif1α-CKO mice in SH conditions that are treated with MPTP. No linear relationship for average daily wheel revolutions and TH cell number was observed for WT and Epas1-CKO mice (not shown).
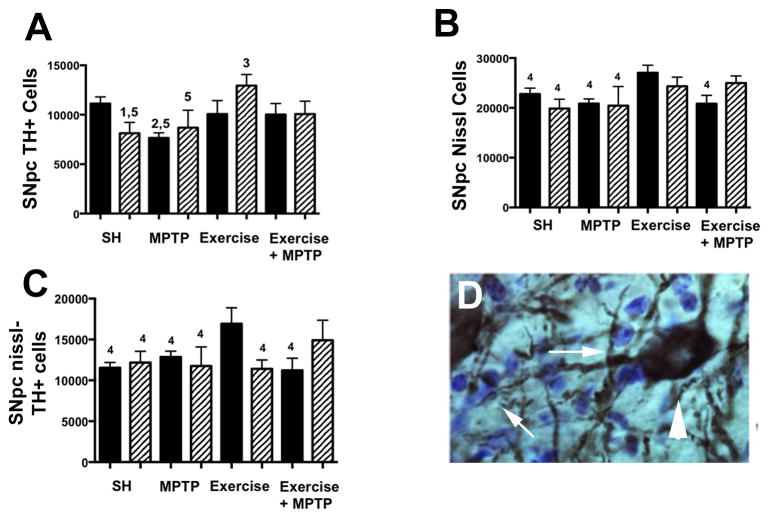
In contrast to hif1α-CKO mice, baseline DA neuron number in the SNpc of epas1-CKO mice is significantly lower than WT mice (p≤0.02) (Fig. 12A, Table 1). Another disparity between hif1α-CKO and epas1-CKO mice is the lack of a significant TH+ cell loss with MPTP treatment in epas1-CKO SNpc (Fig. 12A). Epas1-CKO mice in exercise conditions have TH+ cell number similar to, and slightly higher than WT mice in both SH and exercise conditions, and a significantly higher number of TH+ cells compared to either epas1-CKO mice in SH (p≤0.005) or WT mice in MPTP treatment conditions (p≤0.0004)(Fig. 12A). Exercise is protective for both WT and epas1-CKO mice in exercise followed by MPTP treatment conditions, as each have TH+ cell numbers similar to WT mice in SH conditions.
Fig. 12.
TH and Nissl cell count reveals DA neuron loss in the SNpc of Epas1-CKO mice. (A) TH+ cells are reduced in Epas1-CKO SH (p≤0.05) and WT SH+MPTP (p≤0.01) conditions compared to WT SH. TH+ cell number is rescued in the Epas1-CKO exercise condition, and is reduced in the Epas1-CKO SH + MPTP (p≤0.05) condition when compared to the Epas1-CKO exercise condition. (B) Nissl cell count is significantly lower in the WT and Epas1-CKO SH and SH+MPTP conditions as well as the WT exercise + MPTP condition when compared to the WT Exercise condition (p≤0.05). (C) SNpc Nissl minus TH cell count reveals significantly less cells in the WT and Epas1-CKO SH and SH + MPTP conditions, Epas1-CKO exercise, and WT exercise + MPTP conditions compared to the WT exercise condition (p≤0.05). (D) Appearance of Nissl cells (arrows) and TH+ cell (arrowhead) in the SNpc. Statistics: 1=p≤0.05 compared to WT SH, 2=p≤0.01 compared to WT SH, 3=p≤0.01 compared to Epas1-CKO SH, 4=p≤0.05 compared to WT Exercise, 5=p≤0.05 compared to Epas1-CKO Exercise. Error bars = ± SEM. Solid bars are WT mice, Hatched bars are epas1-CKO mice.
Table 1.
WT and Epas1-CKO SNpc Nissl Cell and TH+ Cell Numbers
| Mean total Nissl cell number | SEM | Statistics | Mean TH+ cell number | SEM | Statistics | Mean Nissl- TH+ cell number | n | Statistics | |
|---|---|---|---|---|---|---|---|---|---|
| WT SH | 22693 | 1100 | 4 | 11294 | 755 | - | 11399 | 15 | 4 |
| WT SH+MPTP | 20865 | 921 | 4 | 7999 | 707 | 1,5 | 12866 | 16 | 4 |
| WT Exercise | 27037 | 1523 | - | 10117 | 1342 | - | 16920 | 5 | - |
| WT Exercise +MPTP | 20831 | 1680 | 4 | 9607 | 774 | - | 11224 | 7 | 4 |
| Epas1 CKO SH | 19854 | 1877 | 4 | 7681 | 1285 | 1,5 | 12173 | 4 | 4 |
| Epas1 CKO SH +MPTP | 20449 | 3845 | 4 | 8697 | 1758 | 5 | 11753 | 3 | 4 |
| Epas1 CKO Exercise | 24341 | 1819 | - | 12937 | 1134 | - | 11405 | 4 | 4 |
| Epas1 CKO Exercise +MPTP | 24979 | 1422 | - | 10068 | 1301 | - | 14911 | 4 | - |
Statistics:
1=p•0.05 compared to WT SH; 2=p•0.01 compared to WT SH; 3=p•0.01 compared to CKO SH; 4=p•0.05 compared to WT Exercise; 5=p•0.05 compared to Epas1-CKO Exercise
It is possible that the loss of TH+ SNpc DA neurons in the epas1-CKO is not a true cell loss but results from a loss of its TH phenotype since epas1 (Brown et al., 2009) but not hif1α (Gammella et al., 2010) has been shown to contribute to the expression of tyrosine hydroxylase in catecholamine synthesis. To examine this possibility, we counterstained the sections used to determine TH+ number with a Nissl stain, cresyl violet (fig 12B, D). If the reduction in TH+ cell number in the epas1-CKO is due to the physical loss of these cells, then the Nissl cell number will be reduced compared to WT by an amount equal to that seen in TH+ counts (Nissl counts – TH counts will be equal) (Table 1). If the reduction in TH+ is due to a loss of phenotype, then the Nissl cell counts will be identical (Nissl counts – TH counts will be unequal). As previously noted, the number of SNpc TH+ DA neurons in the epas1-CKO mouse was reduced by 32% (11294 compared to 7681, a difference of 3613 neurons, Table 1). Counts of Nissl stained cells between WT and epas1-CKO mice showed a similar cell reduction and there were no differences in Nissl – TH+ cell numbers between these groups of mice (Fig 12C, Table 1). This suggests that the reduction in TH+ DA neurons in the SNpc is a true cell loss and is not due to a loss of TH cell phenotype.
We also noted that WT mice allowed exercise had a significant increase in the total cell number compared to WT SH, WT SH + MPTP, epas1-CKO SH, epas1 CKO SH + MPTP, and WT Exercise + MPTP (p<0.05) (Fig 12B, C). This increase is likely due to proliferation of astrocytes in the exercised mice (Li et al., 2005, Ferreira et al., 2011) since we (Gerecke et al., 2010) and other labs (Smith et al., 2011, Wu et al., 2011) have reported no evidence of neuron increases in the SNpc after exercise in the brain.
4.0 Discussion
It is likely that the interaction of numerous factors contribute to the neuroprotection seen in the complex environment of the CNS. Here, we have investigated two transcription factors, Hif1α and Hif2α that are induced with an exercise regime intense enough to produce hypoxia in neurons. We show that neuronal HIF1α, but not HIF2α, is necessary for exercise-induced neuroprotection.
Hif1α and hif2α, two related but unique transcription factors, function to activate a number of genes that enable cells to survive under a variety of unfavorable circumstances (Semenza, 2000, Scortegagna et al., 2003). For example, conditions that increase cellular oxidative stress or alter ROS homeostasis activate hif1α (Brunelle et al., 2005), while antioxidants and reducing agents reduce hif1α activity (Tajima et al., 2009). Hif1α has been shown to induce genes in a number of pathways involved in protecting cells from oxidative stress. These include proteins in the glycolytic pathway (Semenza et al., 1994) the production of erythropoietin (Wang and Semenza, 1993), VEGF (Levy et al., 1995) and VEGF receptor (Okuyama et al., 2006); each of which has been shown to have neuroprotective effects (Yasuhara et al., 2004). Hif2α (epas1) is necessary for mitochondrial homeostasis, control of glycemic and lactic acid levels, regulation of ROS, redox signaling, expression of antioxidant enzymes (Lando et al., 2000, Scortegagna et al., 2003, Peng et al., 2011), control of TH expression (Schnell et al., 2003) and vascular remodeling (Peng et al., 2000).
Previous reports in the literature suggest that HIF1α is expressed in virtually all cells, while HIF2α has a more limited expression (Chavez et al., 2006, Vangeison et al., 2008). Our studies show HIF1α is expressed in SNpc DA neurons and endothelial cells, while HIF2α is expressed in DA and glutamatergic neurons, astrocytes, and endothelial cells of the SN. The identification of the cells that express HIF1α or HIF2α is important to interpret the studies that use conditional gene deletion based on expression of CaMKII. CaMKII-cre is expressed in postnatal neurons in the SN. Thus, any effects of hif1α or hif2α deletion would be due to effects on this specific population, without direct effects from non-neuronal (astroctyes, microglia, vascular) cells. Given that the neurons in the SN account for approximately half of the total number of SN cells (Table 1 and (Smeyne et al., 2005), it is noteworthy that we observe any significant changes in overall HIF1α and HIF2α protein and mRNA levels in the SN. In addition, although the reduction of HIF1α in the SN of the hif1α-CKO is less than 15%, this reduction is critical to the survival of the DA neurons during the hypoxia induced by exercise or generation of oxidative stress by administration of MPTP.
A major finding in this study is that there is differential loss of SNpc DA neurons in hif1α and epas1 conditional knockouts, with and without MPTP; and this suggests that these genes serve different biological processes in neurons of the SN. Loss of hif1α from postnatal SN neurons in standard conditions appears to have little effect, a not unexpected finding since its expression in the brain with normal O2 levels is low. However, when mice exercise at a level that can induce neuroprotection (Gerecke et al., 2010), these same neurons, as shown by visualization of EF-5, become hypoxic. In non-transgenic mice, this induction of hypoxia has no effect on cell survival. However, in exercised hif1α–CKO mice, we observe a reduction in TH+ SNpc DAergic neurons; at a level similar to mice treated with the Complex I inhibitor, MPTP. We also find no additional loss when these two treatments are combined. Both hypoxia (Chandel and Budinger, 2007) and MPTP (Jackson-Lewis and Smeyne, 2005) have been shown to induce ROS formation. The lack of a combinatorial loss of SNpc DA neurons may be explained by the observation that different tiers of the SNpc DA neurons are particularly sensitive to oxidative stress (German et al., 1996); and that exercise-induced hypoxic effects and MPTP act upon the same population of cells. The importance of HIF1α in exercise-induced SNpc DA neuroprotection is supported by our findings that TH+ cell number in the hif1α-CKO mouse is inversely related to the total amount of exercise performed.
The loss of DA neurons in the SNpc of epas1-CKO mice housed in standard conditions suggests that HIF2α plays a different role than HIF1α in regard to development and maintenance of the SN. In standard conditions, adult epas1-CKO mice have lower SNpc TH+ DA cell numbers; however this reduction is rescued by 3 months of exercise. Additionally, a significant loss of TH+ neurons following the administration of MPTP in epas1-CKO mice is not seen. The low TH+ neuron number in standard conditions has two potential explanations. First, in the absence of postnatal neuronal hif2α, there is an increase in the naturally occurring cell death that takes place during the early postnatal period. This hypothesis is supported by studies showing that loss of hif2α, but not hif1α, leads to increases in cell death based on downregulation of members of the IAP family (Dong et al., 2001, Ko et al., 2011). The second explanation for the lower number of TH+ neurons, is that we are observing only a loss of the DA neuron phenotype without true neuronal loss. Loss of hif2α leads to a deficiency in catecholamine production (Tian et al., 1998), likely via indirect control of transcription of the von Hippel–Lindau tumor suppressor protein (Schnell et al., 2003), whose expression has been shown to be inversely related to TH production (Czyzyk-Krzeska et al., 2003). The reduction in total Nissl stained cells in SNpc from epas1-CKO mice in SH (compared to WT SH), which vanishes when the TH+ population is subtracted from the Nissl stained number, supports the first explanation for reduced TH+ cell number, that is true loss of DA neurons when epas1 is absent. In regard to the exercise-induced rescue of TH+ neurons, we suggest two possibilities: first, exercise upregulates hif2α in non-neuronal cells and is subsequently protective to neurons, perhaps by increased antioxidant or trophic factor activity. Second, it is also known that exercise (Tajiri et al., 2010, Lau et al., 2011), and specifically astrocytes, provide trophic (e.g. GDNF, BDNF, VEGF) and antioxidant (glutathione and its constitutive amino acids) (Smeyne and Smeyne, 2013) factors. The increased Nissl cell number in the SNpc of exercised WT mice likely reflects an increase in astrocyte population. Thus, epas1 may directly (Takeda et al., 2004) or indirectly function as a modulator for the protection of DA neurons in exercised epas1-CKO mice that may bestow protection before the inhibition of Complex I by MPTP.
Transient sublethal hypoxia has been shown to induce a phenomenon called preconditioning; and this is a well-recognized cellular process that is protective against later insult in a number of physiological systems (Chavez et al., 2000, Semenza, 2011, Dornbos et al., 2013). In this study, we suggest that regular and sustained exercise produces a recurring and intermittent hypoxia that induces members of the HIF family. This family of transcription factors that are responsible for the induction of numerous adaptive pathways involved in cell metabolism and survival may activate processes that have long lasting effects. Recurrent oxidative stress produces more efficient energy use (Mason et al., 2007, Semenza, 2011, Dornbos et al., 2013). Endurance training increases the oxidative capacity in skeletal muscle, a phenomenon that is reproduced in Hif1α null muscle without training (Mason et al., 2007). Low tissue oxygenation also initiates angiogenesis through HIF and VEGF-A induction, factors that initially rise then return to prehypoxic levels, while vascular changes remain for several weeks (LaManna et al., 2004). The intensity and period of exercise necessary for neuroprotection in the SN may reflect the initial induction of hif and the subsequent expression of adaptive genes leading to long term changes such as increased vascularity and oxidative capacity that are advantageous in the event of ischemic or xenobiotic trauma. Hif1α loss in postnatal DA neurons of the SNpc eliminates exercise-induced neuroprotection and provides clues to the pathways whose activation is critical to initiate this process. Future investigation to determine if hif1α or epas1 can compensate for one another, the role that exercise and Hif induction play in the generation of or adaptation to ROS in the SN, and to discover which downstream signaling pathways are affected by the reduction of neuronal HIFs may further our understanding of how exercise benefits neuron vitality.
Bullet Points.
Exercise produces hypoxia in SNpc dopaminergic (DA) neurons.
Hif1α and Hif2α are modulated by exercise in the SNpc.
Loss of Hif1α in postnatal neurons results in lower DA numbers following exercise.
Hif2α loss in postnatal neurons lowers SNpc DA neuron number in standard conditions.
Exercise rescues the DA neuron loss mediated by Hif2α reduction.
Acknowledgments
The authors thank Jarad Hopper, Yannan Ouyang and Rolanda Peterson for technical assistance. This work is supported by RO1-NS070825 and ALSAC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agani FH, Pichiule P, Chavez JC, LaManna JC. The role of mitochondria in the regulation of hypoxia-inducible factor 1 expression during hypoxia. J Biol Chem. 2000;275:35863–35867. doi: 10.1074/jbc.M005643200. [DOI] [PubMed] [Google Scholar]
- Agani FH, Puchowicz M, Chavez JC, Pichiule P, LaManna J. Role of nitric oxide in the regulation of HIF-1alpha expression during hypoxia. Am J Physiol Cell Physiol. 2002;283:C178–186. doi: 10.1152/ajpcell.00381.2001. [DOI] [PubMed] [Google Scholar]
- Baquet ZC, Williams D, Brody J, Smeyne RJ. A comparison of model-based (2D) and design-based (3D) stereological methods for estimating cell number in the substantia nigra pars compacta (SNpc) of the C57BL/6J Mouse. Neuroscience. 2009;161:1082–1090. doi: 10.1016/j.neuroscience.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranova O, Miranda LF, Pichiule P, Dragatsis I, Johnson RS, Chavez JC. Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci. 2007;27:6320–6332. doi: 10.1523/JNEUROSCI.0449-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron M, Evans SM, Sharp FR, Koch CJ, Lord EM, Ferriero DM. Detection of hypoxic cells with the 2-nitroimidazole, EF5, correlates with early redox changes in rat brain after perinatal hypoxia-ischemia. Neuroscience. 1999;89:1357–1366. doi: 10.1016/s0306-4522(98)00377-7. [DOI] [PubMed] [Google Scholar]
- Boyd JD, Jang H, Shepherd KR, Faherty C, Slack S, Jiao Y, Smeyne RJ. Response to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) differs in mouse strains and reveals a divergence in JNK signaling and COX-2 induction prior to loss of neurons in the substantia nigra pars compacta. Brain Res. 2007;1175:107–116. doi: 10.1016/j.brainres.2007.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones TL. Environment, physical activity, and neurogenesis: implications for prevention and treatment of Alzhemier’s disease. Curr Alzheimer Res. 2006;3:49–54. doi: 10.2174/156720506775697197. [DOI] [PubMed] [Google Scholar]
- Brown ST, Kelly KF, Daniel JM, Nurse CA. Hypoxia inducible factor (HIF)-2 alpha is required for the development of the catecholaminergic phenotype of sympathoadrenal cells. J Neurochem. 2009;110:622–630. doi: 10.1111/j.1471-4159.2009.06153.x. [DOI] [PubMed] [Google Scholar]
- Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC, Chandel NS. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 2005;1:409–414. doi: 10.1016/j.cmet.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshert E. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- Chandel NS, Budinger GR. The cellular basis for diverse responses to oxygen. Free Radic Biol Med. 2007;42:165–174. doi: 10.1016/j.freeradbiomed.2006.10.048. [DOI] [PubMed] [Google Scholar]
- Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez JC, Agani F, Pichiule P, LaManna JC. Expression of hypoxia-inducible factor-1alpha in the brain of rats during chronic hypoxia. J Appl Physiol. 2000;89:1937–1942. doi: 10.1152/jappl.2000.89.5.1937. [DOI] [PubMed] [Google Scholar]
- Chavez JC, Baranova O, Lin J, Pichiule P. The transcriptional activator hypoxia inducible factor 2 (HIF-2/EPAS-1) regulates the oxygen-dependent expression of erythropoietin in cortical astrocytes. J Neurosci. 2006;26:9471–9481. doi: 10.1523/JNEUROSCI.2838-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez JC, LaManna JC. Activation of hypoxia-inducible factor-1 in the rat cerebral cortex after transient global ischemia: potential role of insulin-like growth factor-1. J Neurosci. 2002;22:8922–8931. doi: 10.1523/JNEUROSCI.22-20-08922.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coletti D, Berardi E, Aulino P, Rossi E, Moresi V, Li Z, Adamo S. Substrains of inbred mice differ in their physical activity as a behavior. ScientificWorldJournal. 2013;2013:237260. doi: 10.1155/2013/237260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Curry A, Guo M, Patel R, Liebelt B, Sprague S, Lai Q, Zwagerman N, Cao FX, Jimenez D, Ding Y. Exercise pre-conditioning reduces brain inflammation in stroke via tumor necrosis factor-alpha, extracellular signal-regulated kinase 1/2 and matrix metalloproteinase-9 activity. Neurol Res. 2010;32:756–762. doi: 10.1179/174313209X459101. [DOI] [PubMed] [Google Scholar]
- Czyzyk-Krzeska MF, Schnell PO, Bauer AL, Streit JB, Nash JA, Kuznetsova AV, Hui AS. Regulation of Tyrosine Hydroxylase Gene Expression by Hypoxia in Neuroendocrine Cells. New York: Marcel Dekker, Inc; 2003. [Google Scholar]
- Dong Z, Venkatachalam MA, Wang J, Patel Y, Saikumar P, Semenza GL, Force T, Nishiyama J. Up-regulation of apoptosis inhibitory protein IAP-2 by hypoxia. Hif-1-independent mechanisms. J Biol Chem. 2001;276:18702–18709. doi: 10.1074/jbc.M011774200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornbos D, 3rd, Zwagerman N, Guo M, Ding JY, Peng C, Esmail F, Sikharam C, Geng X, Guthikonda M, Ding Y. Preischemic exercise reduces brain damage by ameliorating metabolic disorder in ischemia/reperfusion injury. J Neurosci Res. 2013;91:818–827. doi: 10.1002/jnr.23203. [DOI] [PubMed] [Google Scholar]
- Dragatsis I, Zeitlin S. CaMKIIalpha-Cre transgene expression and recombination patterns in the mouse brain. Genesis. 2000;26:133–135. doi: 10.1002/(sici)1526-968x(200002)26:2<133::aid-gene10>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Evans SM, Joiner B, Jenkins WT, Laughlin KM, Lord EM, Koch CJ. Identification of hypoxia in cells and tissues of epigastric 9L rat glioma using EF5 [2-(2-nitro-1H-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoropropyl) acetamide] Br J Cancer. 1995;72:875–882. doi: 10.1038/bjc.1995.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira AF, Real CC, Rodrigues AC, Alves AS, Britto LR. Short-term, moderate exercise is capable of inducing structural, BDNF-independent hippocampal plasticity. Brain Res. 2011;1425:111–122. doi: 10.1016/j.brainres.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Gammella E, Cairo G, Tacchini L. Adenosine A(2)A receptor but not HIF-1 mediates Tyrosine hydroxylase induction in hypoxic PC12 cells. J Neurosci Res. 2010;88:2007–2016. doi: 10.1002/jnr.22366. [DOI] [PubMed] [Google Scholar]
- Gerecke KM, Jiao Y, Pagala V, Smeyne RJ. Exercise does not protect against MPTP-induced neurotoxicity in BDNF haploinsufficient mice. PLoS One. 2012;7:e43250. doi: 10.1371/journal.pone.0043250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerecke KM, Jiao Y, Pani A, Pagala V, Smeyne RJ. Exercise protects against MPTP-induced neurotoxicity in mice. Brain Res. 2010 doi: 10.1016/j.brainres.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German DC, Nelson EL, Liang CL, Speciale SG, Sinton CM, Sonsalla PK. The neurotoxin MPTP causes degeneration of specific nucleus A8, A9 and A10 dopaminergic neurons in the mouse. Neurodegeneration. 1996;5:299–312. doi: 10.1006/neur.1996.0041. [DOI] [PubMed] [Google Scholar]
- Hamre K, Tharp R, Poon K, Xiong X, Smeyne RJ. Differential strain susceptibility following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration acts in an autosomal dominant fashion: quantitative analysis in seven strains of Mus musculus. Brain Res. 1999;828:91–103. doi: 10.1016/s0006-8993(99)01273-1. [DOI] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Smeyne RJ. MPTP and SNpc DA neuronal vulnerability: role of dopamine, superoxide and nitric oxide in neurotoxicity. Minireview. Neurotox Res. 2005;7:193–202. doi: 10.1007/BF03036449. [DOI] [PubMed] [Google Scholar]
- Jain S, Maltepe E, Lu MM, Simon C, Bradfield CA. Expression of ARNT, ARNT2, HIF1 alpha, HIF2 alpha and Ah receptor mRNAs in the developing mouse. Mech Dev. 1998;73:117–123. doi: 10.1016/s0925-4773(98)00038-0. [DOI] [PubMed] [Google Scholar]
- Jang H, Boltz D, McClaren J, Pani AK, Smeyne M, Korff A, Webster R, Smeyne RJ. Inflammatory effects of highly pathogenic H5N1 influenza virus infection in the CNS of mice. J Neurosci. 2012;32:1545–1559. doi: 10.1523/JNEUROSCI.5123-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko CY, Tsai MY, Tseng WF, Cheng CH, Huang CR, Wu JS, Chung HY, Hsieh CS, Sun CK, Hwang SP, Yuh CH, Huang CJ, Pai TW, Tzou WS, Hu CH. Integration of CNS survival and differentiation by HIF2alpha. Cell Death Differ. 2011;18:1757–1770. doi: 10.1038/cdd.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch CJ, Evans SM, Lord EM. Oxygen dependence of cellular uptake of EF5 [2-(2-nitro-1H-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoropropyl)a cet amide] : analysis of drug adducts by fluorescent antibodies vs bound radioactivity. Br J Cancer. 1995;72:869–874. doi: 10.1038/bjc.1995.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaManna JC, Chavez JC, Pichiule P. Structural and functional adaptation to hypoxia in the rat brain. J Exp Biol. 2004;207:3163–3169. doi: 10.1242/jeb.00976. [DOI] [PubMed] [Google Scholar]
- Lando D, Pongratz I, Poellinger L, Whitelaw ML. A redox mechanism controls differential DNA binding activities of hypoxia-inducible factor (HIF) 1alpha and the HIF-like factor. J Biol Chem. 2000;275:4618–4627. doi: 10.1074/jbc.275.7.4618. [DOI] [PubMed] [Google Scholar]
- Lau YS, Patki G, Das-Panja K, Le WD, Ahmad SO. Neuroprotective effects and mechanisms of exercise in a chronic mouse model of Parkinson’s disease with moderate neurodegeneration. Eur J Neurosci. 2011;33:1264–1274. doi: 10.1111/j.1460-9568.2011.07626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman I, Harrison BC, Freeman K, Hewett TE, Allen DL, Robbins J, Leinwand LA. Genetic variability in forced and voluntary endurance exercise performance in seven inbred mouse strains. J Appl Physiol. 2002;92:2245–2255. doi: 10.1152/japplphysiol.01045.2001. [DOI] [PubMed] [Google Scholar]
- Levy AP, Levy NS, Wegner S, Goldberg MA. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem. 1995;270:13333–13340. doi: 10.1074/jbc.270.22.13333. [DOI] [PubMed] [Google Scholar]
- Li J, Ding YH, Rafols JA, Lai Q, McAllister JP, 2nd, Ding Y. Increased astrocyte proliferation in rats after running exercise. Neurosci Lett. 2005;386:160–164. doi: 10.1016/j.neulet.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Liebelt B, Papapetrou P, Ali A, Guo M, Ji X, Peng C, Rogers R, Curry A, Jimenez D, Ding Y. Exercise preconditioning reduces neuronal apoptosis in stroke by up-regulating heat shock protein-70 (heat shock protein-72) and extracellular-signal-regulated-kinase 1/2. Neuroscience. 2010;166:1091–1100. doi: 10.1016/j.neuroscience.2009.12.067. [DOI] [PubMed] [Google Scholar]
- Lightfoot JT, Turner MJ, Daves M, Vordermark A, Kleeberger SR. Genetic influence on daily wheel running activity level. Physiol Genomics. 2004;19:270–276. doi: 10.1152/physiolgenomics.00125.2004. [DOI] [PubMed] [Google Scholar]
- Marques de Olivera M, Bonatto D, Pegas Henriques JA. Nitroreductases: Enzymes with Environmental, Biotechnological and Clinical Importance. In: Mendez-Vilas A, editor. Current Research Technology and Education Topics in Applied Microbiology and Microbial Biotechnology. Vol. 2. Formatex Research Center; Spain: 2010. pp. 1008–1019. [Google Scholar]
- Mason SD, Rundqvist H, Papandreou I, Duh R, McNulty WJ, Howlett RA, Olfert IM, Sundberg CJ, Denko NC, Poellinger L, Johnson RS. HIF-1alpha in endurance training: suppression of oxidative metabolism. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2059–2069. doi: 10.1152/ajpregu.00335.2007. [DOI] [PubMed] [Google Scholar]
- Murray DK, Sacheli MA, Eng JJ, Stoessl AJ. The effects of exercise on cognition in Parkinson’s disease: a systematic review. Transl Neurodegener. 2014;3:5. doi: 10.1186/2047-9158-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdick J, Wallace JD, Lewin A, Smeyne RJ. Transgenic expression to monitor dynamic organization of neuronal development: use of the E. coli lacZ gene product, beta-galactosidase. Neuroprotocols. 1994;5:54–62. [Google Scholar]
- Okuyama H, Krishnamachary B, Zhou YF, Nagasawa H, Bosch-Marce M, Semenza GL. Expression of vascular endothelial growth factor receptor 1 in bone marrow-derived mesenchymal cells is dependent on hypoxia-inducible factor 1. J Biol Chem. 2006;281:15554–15563. doi: 10.1074/jbc.M602003200. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 2001. [Google Scholar]
- Peng J, Zhang L, Drysdale L, Fong GH. The transcription factor EPAS-1/hypoxia-inducible factor 2alpha plays an important role in vascular remodeling. Proc Natl Acad Sci U S A. 2000;97:8386–8391. doi: 10.1073/pnas.140087397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Nanduri J, Khan SA, Yuan G, Wang N, Kinsman B, Vaddi DR, Kumar GK, Garcia JA, Semenza GL, Prabhakar NR. Hypoxia-inducible factor 2alpha (HIF-2alpha) heterozygous-null mice exhibit exaggerated carotid body sensitivity to hypoxia, breathing instability, and hypertension. Proc Natl Acad Sci U S A. 2011;108:3065–3070. doi: 10.1073/pnas.1100064108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzinger GM, Fisher BE, McEwen S, Beeler JA, Walsh JP, Jakowec MW. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson’s disease. Lancet Neurol. 2013;12:716–726. doi: 10.1016/S1474-4422(13)70123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radak Z, Chung HY, Koltai E, Taylor AW, Goto S. Exercise, oxidative stress and hormesis. Ageing Res Rev. 2008;7:34–42. doi: 10.1016/j.arr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Radak Z, Zhao Z, Koltai E, Ohno H, Atalay M. Oxygen consumption and usage during physical exercise: the balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid Redox Signal. 2013;18:1208–1246. doi: 10.1089/ars.2011.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell PO, Ignacak ML, Bauer AL, Striet JB, Paulding WR, Czyzyk-Krzeska MF. Regulation of tyrosine hydroxylase promoter activity by the von Hippel-Lindau tumor suppressor protein and hypoxia-inducible transcription factors. J Neurochem. 2003;85:483–491. doi: 10.1046/j.1471-4159.2003.01696.x. [DOI] [PubMed] [Google Scholar]
- Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, Yan LJ, Marck BT, Matsumoto AM, Shelton JM, Richardson JA, Bennett MJ, Garcia JA. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1-/- mice. Nat Genet. 2003;35:331–340. doi: 10.1038/ng1266. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol (1985) 2000;88:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1: regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochim Biophys Acta. 2011;1813:1263–1268. doi: 10.1016/j.bbamcr.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeyne M, Boyd J, Raviie Shepherd K, Jiao Y, Pond BB, Hatler M, Wolf R, Henderson C, Smeyne RJ. GSTpi expression mediates dopaminergic neuron sensitivity in experimental parkinsonism. Proc Natl Acad Sci U S A. 2007;104:1977–1982. doi: 10.1073/pnas.0610978104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeyne M, Jiao Y, Shepherd KR, Smeyne RJ. Glia cell number modulates sensitivity to MPTP in mice. Glia. 2005;52:144–152. doi: 10.1002/glia.20233. [DOI] [PubMed] [Google Scholar]
- Smeyne M, Smeyne RJ. Glutathione metabolism and Parkinson’s disease. Free Radic Biol Med. 2013;62:13–25. doi: 10.1016/j.freeradbiomed.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeyne RJ, Jackson-Lewis V. The MPTP model of Parkinson’s disease. Mol Brain Res. 2005;134:57–66. doi: 10.1016/j.molbrainres.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Smith BA, Goldberg NR, Meshul CK. Effects of treadmill exercise on behavioral recovery and neural changes in the substantia nigra and striatum of the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse. Brain Res. 2011;1386:70–80. doi: 10.1016/j.brainres.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Martin B, Maudsley S. Anti-inflammatory effects of physical activity in relationship to improved cognitive status in humans and mouse models of Alzheimer’s disease. Curr Alzheimer Res. 2012;9:86–92. doi: 10.2174/156720512799015019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima M, Kurashima Y, Sugiyama K, Ogura T, Sakagami H. The redox state of glutathione regulates the hypoxic induction of HIF-1. Eur J Pharmacol. 2009;606:45–49. doi: 10.1016/j.ejphar.2009.01.026. [DOI] [PubMed] [Google Scholar]
- Tajiri N, Yasuhara T, Shingo T, Kondo A, Yuan W, Kadota T, Wang F, Baba T, Tayra JT, Morimoto T, Jing M, Kikuchi Y, Kuramoto S, Agari T, Miyoshi Y, Fujino H, Obata F, Takeda I, Furuta T, Date I. Exercise exerts neuroprotective effects on Parkinson’s disease model of rats. Brain Res. 2010;1310:200–207. doi: 10.1016/j.brainres.2009.10.075. [DOI] [PubMed] [Google Scholar]
- Takeda N, Maemura K, Imai Y, Harada T, Kawanami D, Nojiri T, Manabe I, Nagai R. Endothelial PAS domain protein 1 gene promotes angiogenesis through the transactivation of both vascular endothelial growth factor and its receptor, Flt-1. Circ Res. 2004;95:146–153. doi: 10.1161/01.RES.0000134920.10128.b4. [DOI] [PubMed] [Google Scholar]
- Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL. The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev. 1998;12:3320–3324. doi: 10.1101/gad.12.21.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- Vangeison G, Carr D, Federoff HJ, Rempe DA. The good, the bad, and the cell type-specific roles of hypoxia inducible factor-1 alpha in neurons and astrocytes. J Neurosci. 2008;28:1988–1993. doi: 10.1523/JNEUROSCI.5323-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci U S A. 1993;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- Wu SY, Wang TF, Yu L, Jen CJ, Chuang JI, Wu FS, Wu CW, Kuo YM. Running exercise protects the substantia nigra dopaminergic neurons against inflammation-induced degeneration via the activation of BDNF signaling pathway. Brain Behav Immun. 2011;25:135–146. doi: 10.1016/j.bbi.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Yasuhara T, Shingo T, Kobayashi K, Takeuchi A, Yano A, Muraoka K, Matsui T, Miyoshi Y, Hamada H, Date I. Neuroprotective effects of vascular endothelial growth factor (VEGF) upon dopaminergic neurons in a rat model of Parkinson’s disease. Eur J Neurosci. 2004;19:1494–1504. doi: 10.1111/j.1460-9568.2004.03254.x. [DOI] [PubMed] [Google Scholar]
- Yuan G, Peng YJ, Reddy VD, Makarenko VV, Nanduri J, Khan SA, Garcia JA, Kumar GK, Semenza GL, Prabhakar NR. Mutual antagonism between hypoxia-inducible factors 1alpha and 2alpha regulates oxygen sensing and cardio-respiratory homeostasis. Proc Natl Acad Sci U S A. 2013;110:E1788–1796. doi: 10.1073/pnas.1305961110. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zigmond MJ, Smeyne RJ. Exercise: is it a neuroprotective and if so, how does it work? Parkinsonism Relat Disord. 2014;20(Suppl 1):S123–127. doi: 10.1016/S1353-8020(13)70030-0. [DOI] [PubMed] [Google Scholar]