Abstract
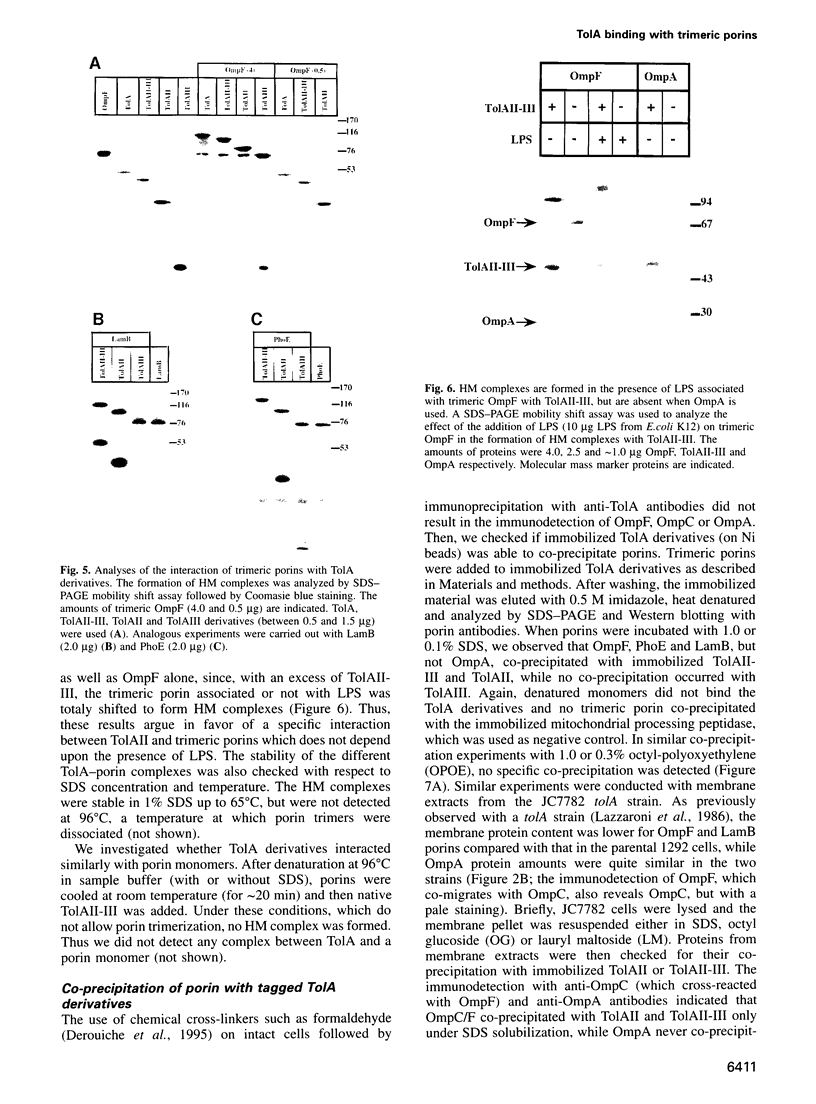
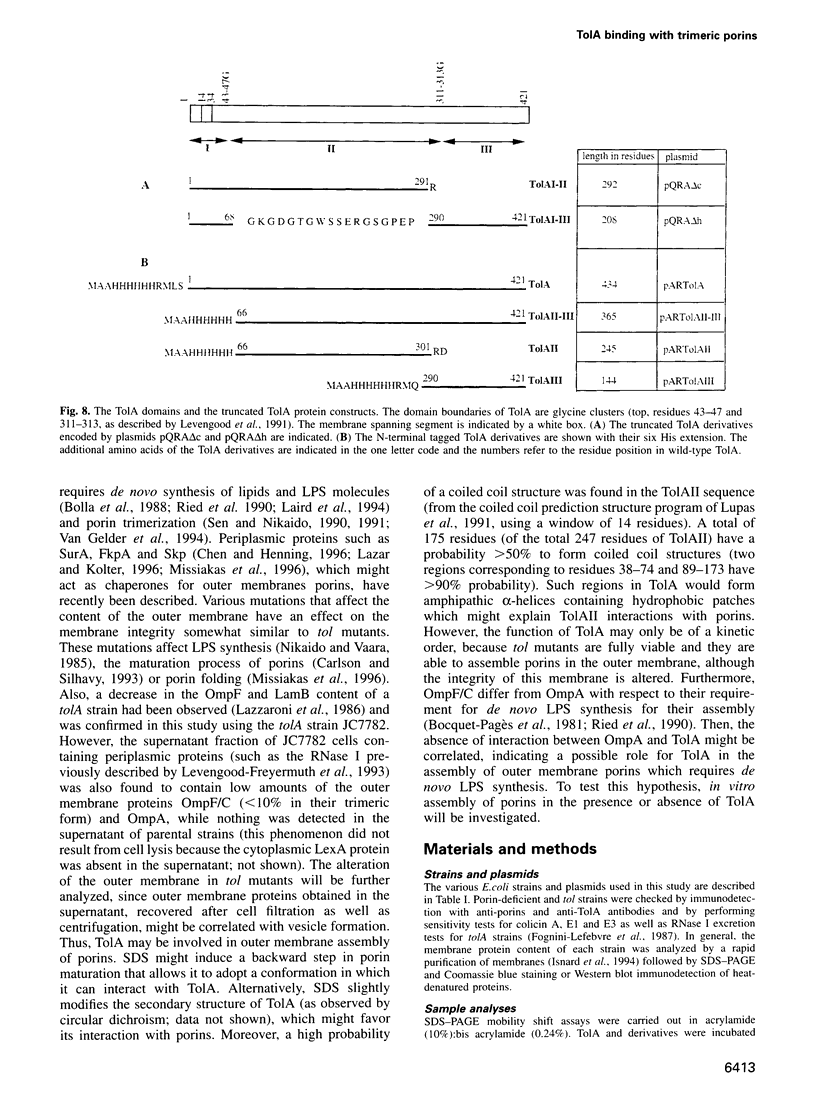
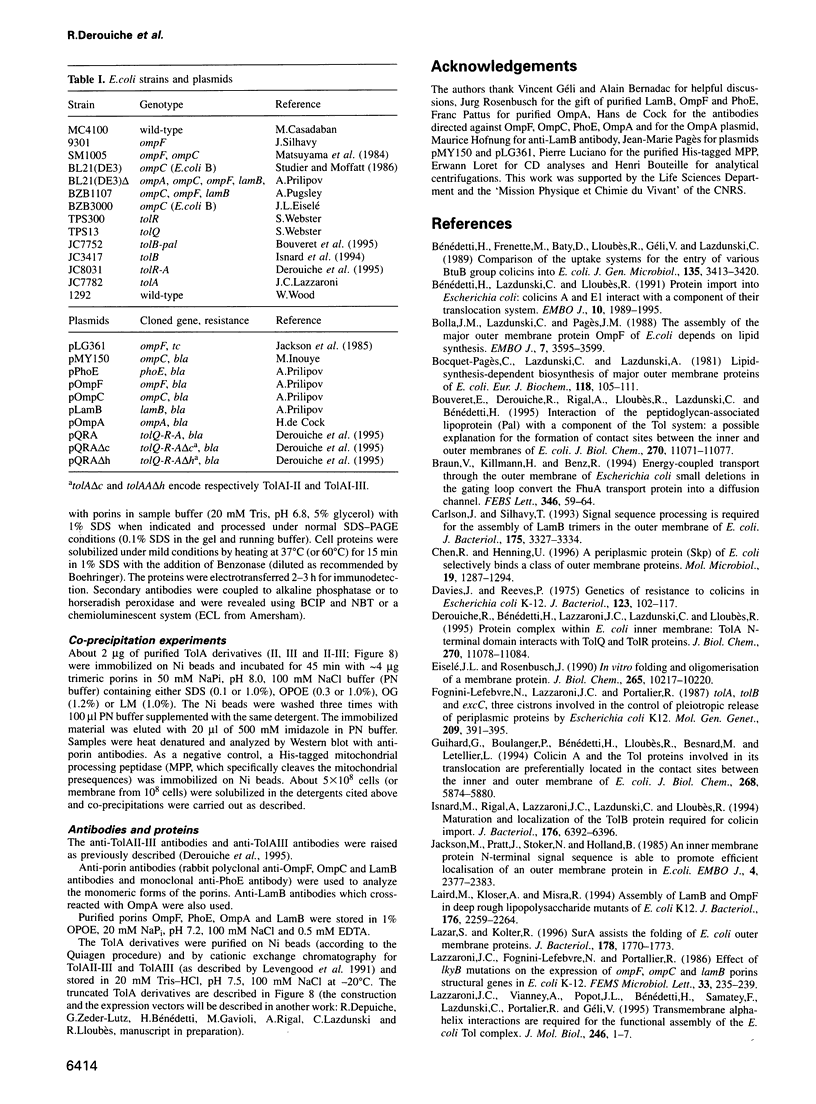
TolA is an inner membrane protein with three domains: a transmembrane N-terminus and periplasmic central and C-terminal domains. The interaction of TolA with outer membrane porins of Escherichia coli was investigated. Western blot analyses of cell extracts with anti-TolA antibodies indicated that TolA forms high molecular weight complexes specifically with trimeric OmpF, OmpC, PhoE and LamB, but not with OmpA. The interaction of purified TolA domains with purified porins was also studied. TolA interacted with OmpF, PhoE and LamB porins via its central domain, but not with either their denatured monomeric forms or OmpA. Moreover, the presence or absence of lipopolysaccharides associated with trimeric porins did not modify the interactions. These results suggest that the specific interaction of TolA with outer membrane porins might be relevant to the function of Tol proteins.
Full text
PDF
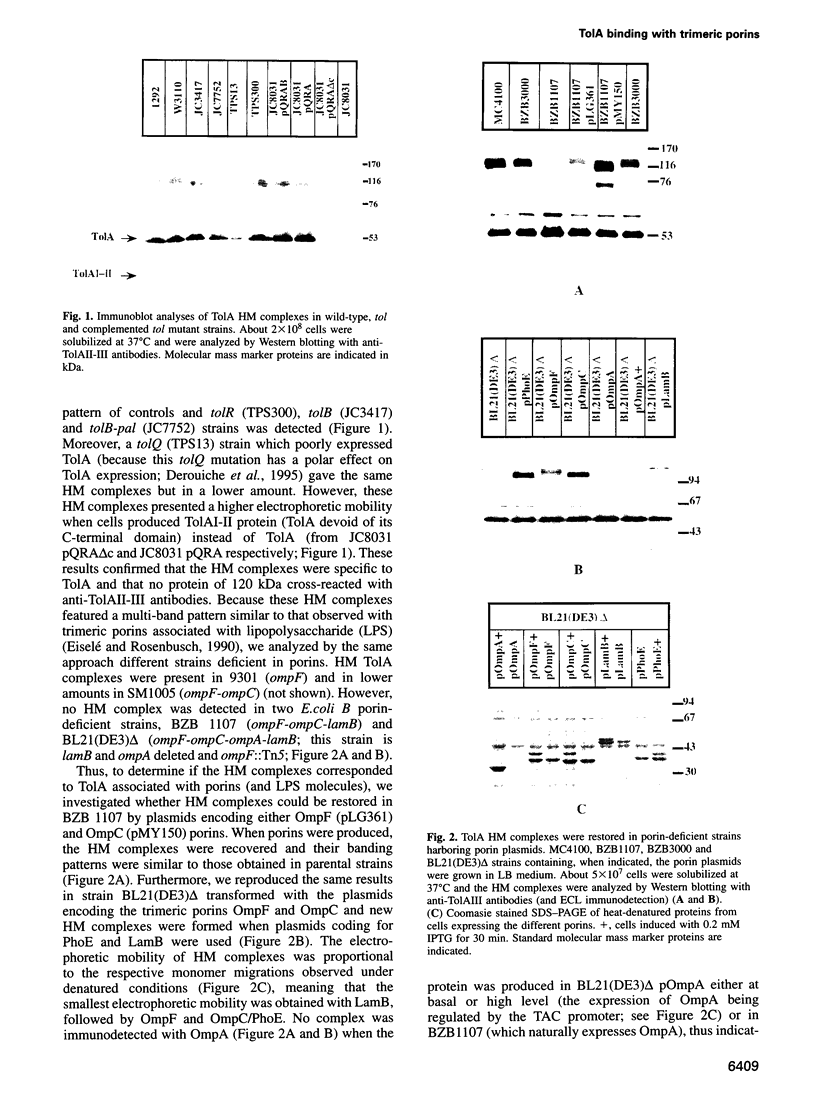
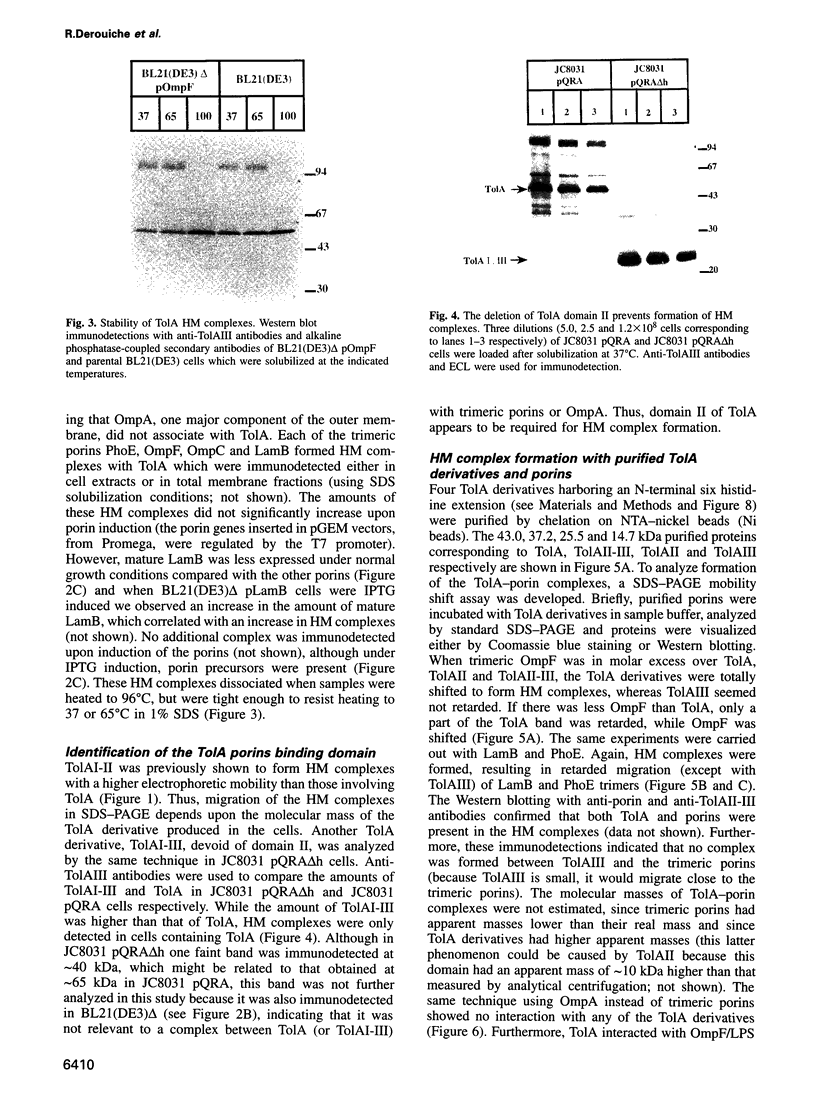


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedetti H., Frenette M., Baty D., Lloubès R., Geli V., Lazdunski C. Comparison of the uptake systems for the entry of various BtuB group colicins into Escherichia coli. J Gen Microbiol. 1989 Dec;135(12):3413–3420. doi: 10.1099/00221287-135-12-3413. [DOI] [PubMed] [Google Scholar]
- Benedetti H., Lazdunski C., Lloubès R. Protein import into Escherichia coli: colicins A and E1 interact with a component of their translocation system. EMBO J. 1991 Aug;10(8):1989–1995. doi: 10.1002/j.1460-2075.1991.tb07728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocquet-Pages C., Lazdunski C., Lazdunski A. Lipid-synthesis-dependent biosynthesis (or assembly) of major outer-membrane proteins of Escherichia coli. Eur J Biochem. 1981 Aug;118(1):105–111. doi: 10.1111/j.1432-1033.1981.tb05491.x. [DOI] [PubMed] [Google Scholar]
- Bolla J. M., Lazdunski C., Pagès J. M. The assembly of the major outer membrane protein OmpF of Escherichia coli depends on lipid synthesis. EMBO J. 1988 Nov;7(11):3595–3599. doi: 10.1002/j.1460-2075.1988.tb03237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouveret E., Derouiche R., Rigal A., Lloubès R., Lazdunski C., Bénédetti H. Peptidoglycan-associated lipoprotein-TolB interaction. A possible key to explaining the formation of contact sites between the inner and outer membranes of Escherichia coli. J Biol Chem. 1995 May 12;270(19):11071–11077. doi: 10.1074/jbc.270.19.11071. [DOI] [PubMed] [Google Scholar]
- Braun V., Killmann H., Benz R. Energy-coupled transport through the outer membrane of Escherichia coli small deletions in the gating loop convert the FhuA transport protein into a diffusion channel. FEBS Lett. 1994 Jun 6;346(1):59–64. doi: 10.1016/0014-5793(94)00431-5. [DOI] [PubMed] [Google Scholar]
- Carlson J. H., Silhavy T. J. Signal sequence processing is required for the assembly of LamB trimers in the outer membrane of Escherichia coli. J Bacteriol. 1993 Jun;175(11):3327–3334. doi: 10.1128/jb.175.11.3327-3334.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Henning U. A periplasmic protein (Skp) of Escherichia coli selectively binds a class of outer membrane proteins. Mol Microbiol. 1996 Mar;19(6):1287–1294. doi: 10.1111/j.1365-2958.1996.tb02473.x. [DOI] [PubMed] [Google Scholar]
- Davies J. K., Reeves P. Genetics of resistance to colicins in Escherichia coli K-12: cross-resistance among colicins of group A. J Bacteriol. 1975 Jul;123(1):102–117. doi: 10.1128/jb.123.1.102-117.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derouiche R., Bénédetti H., Lazzaroni J. C., Lazdunski C., Lloubès R. Protein complex within Escherichia coli inner membrane. TolA N-terminal domain interacts with TolQ and TolR proteins. J Biol Chem. 1995 May 12;270(19):11078–11084. doi: 10.1074/jbc.270.19.11078. [DOI] [PubMed] [Google Scholar]
- Eisele J. L., Rosenbusch J. P. In vitro folding and oligomerization of a membrane protein. Transition of bacterial porin from random coil to native conformation. J Biol Chem. 1990 Jun 25;265(18):10217–10220. [PubMed] [Google Scholar]
- Fognini-Lefebvre N., Lazzaroni J. C., Portalier R. tolA, tolB and excC, three cistrons involved in the control of pleiotropic release of periplasmic proteins by Escherichia coli K12. Mol Gen Genet. 1987 Sep;209(2):391–395. doi: 10.1007/BF00329670. [DOI] [PubMed] [Google Scholar]
- Guihard G., Boulanger P., Bénédetti H., Lloubés R., Besnard M., Letellier L. Colicin A and the Tol proteins involved in its translocation are preferentially located in the contact sites between the inner and outer membranes of Escherichia coli cells. J Biol Chem. 1994 Feb 25;269(8):5874–5880. [PubMed] [Google Scholar]
- Isnard M., Rigal A., Lazzaroni J. C., Lazdunski C., Lloubes R. Maturation and localization of the TolB protein required for colicin import. J Bacteriol. 1994 Oct;176(20):6392–6396. doi: 10.1128/jb.176.20.6392-6396.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. E., Pratt J. M., Stoker N. G., Holland I. B. An inner membrane protein N-terminal signal sequence is able to promote efficient localisation of an outer membrane protein in Escherichia coli. EMBO J. 1985 Sep;4(9):2377–2383. doi: 10.1002/j.1460-2075.1985.tb03942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird M. W., Kloser A. W., Misra R. Assembly of LamB and OmpF in deep rough lipopolysaccharide mutants of Escherichia coli K-12. J Bacteriol. 1994 Apr;176(8):2259–2264. doi: 10.1128/jb.176.8.2259-2264.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar S. W., Kolter R. SurA assists the folding of Escherichia coli outer membrane proteins. J Bacteriol. 1996 Mar;178(6):1770–1773. doi: 10.1128/jb.178.6.1770-1773.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzaroni J. C., Vianney A., Popot J. L., Bénédetti H., Samatey F., Lazdunski C., Portalier R., Géli V. Transmembrane alpha-helix interactions are required for the functional assembly of the Escherichia coli Tol complex. J Mol Biol. 1995 Feb 10;246(1):1–7. doi: 10.1006/jmbi.1994.0058. [DOI] [PubMed] [Google Scholar]
- Levengood-Freyermuth S. K., Click E. M., Webster R. E. Role of the carboxyl-terminal domain of TolA in protein import and integrity of the outer membrane. J Bacteriol. 1993 Jan;175(1):222–228. doi: 10.1128/jb.175.1.222-228.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levengood S. K., Beyer W. F., Jr, Webster R. E. TolA: a membrane protein involved in colicin uptake contains an extended helical region. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):5939–5943. doi: 10.1073/pnas.88.14.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A., Van Dyke M., Stock J. Predicting coiled coils from protein sequences. Science. 1991 May 24;252(5009):1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- Matsuyama S., Inokuchi K., Mizushima S. Promoter exchange between ompF and ompC, genes for osmoregulated major outer membrane proteins of Escherichia coli K-12. J Bacteriol. 1984 Jun;158(3):1041–1047. doi: 10.1128/jb.158.3.1041-1047.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missiakas D., Betton J. M., Raina S. New components of protein folding in extracytoplasmic compartments of Escherichia coli SurA, FkpA and Skp/OmpH. Mol Microbiol. 1996 Aug;21(4):871–884. doi: 10.1046/j.1365-2958.1996.561412.x. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Transport across the bacterial outer membrane. J Bioenerg Biomembr. 1993 Dec;25(6):581–589. doi: 10.1007/BF00770245. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle K. TonB protein and energy transduction between membranes. J Bioenerg Biomembr. 1993 Dec;25(6):591–601. doi: 10.1007/BF00770246. [DOI] [PubMed] [Google Scholar]
- Ried G., Hindennach I., Henning U. Role of lipopolysaccharide in assembly of Escherichia coli outer membrane proteins OmpA, OmpC, and OmpF. J Bacteriol. 1990 Oct;172(10):6048–6053. doi: 10.1128/jb.172.10.6048-6053.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Sen K., Nikaido H. In vitro trimerization of OmpF porin secreted by spheroplasts of Escherichia coli. Proc Natl Acad Sci U S A. 1990 Jan;87(2):743–747. doi: 10.1073/pnas.87.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen K., Nikaido H. Trimerization of an in vitro synthesized OmpF porin of Escherichia coli outer membrane. J Biol Chem. 1991 Jun 15;266(17):11295–11300. [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Van Gelder P., De Cock H., Tommassen J. Detergent-induced folding of the outer-membrane protein PhoE, a pore protein induced by phosphate limitation. Eur J Biochem. 1994 Dec 15;226(3):783–787. doi: 10.1111/j.1432-1033.1994.00783.x. [DOI] [PubMed] [Google Scholar]
- Webster R. E. The tol gene products and the import of macromolecules into Escherichia coli. Mol Microbiol. 1991 May;5(5):1005–1011. doi: 10.1111/j.1365-2958.1991.tb01873.x. [DOI] [PubMed] [Google Scholar]