This review summarizes functional long noncoding RNAs (lncRNAs), which were shown to serve as novel biomarkers for diagnosis and prognosis of digestive tract cancers (DTCs) and to act as oncogenes or tumor suppressor genes in DTC development. The potential mechanism of functional lncRNAs in DTCs is highlighted.
Keywords: Long noncoding RNAs, Digestive tract cancers, Biomarker, Epigenetic regulation
Abstract
Digestive tract cancers (DTCs) are a leading cause of cancer-related death worldwide. Current therapeutic tools for advanced stage DTCs have limitations, and patients with early stage DTCs frequently have a missed diagnosis due to shortage of efficient biomarkers. Consequently, it is necessary to develop novel biomarkers for early diagnosis and novel therapeutic targets for treatment of DTCs. In recent years, long noncoding RNAs (lncRNAs), a class of noncoding RNAs with >200 nucleotides, have been shown to be aberrantly expressed in DTCs and to have an important role in DTC development: the expression profiles of lncRNAs strongly correlated with poor survival of patients with DTCs, and lncRNAs acted as oncogenes or tumor suppressor genes in DTC progression. In this review, we summarized the functional lncRNAs and expounded on their regulatory mechanisms in DTCs.
Implications for Practice:
Digestive tract cancers (DTCs) are a leading cause of cancer-related death worldwide. It is necessary to exploit novel biomarkers for early diagnosis and novel therapeutic targets for treatment of DTCs. Long noncoding RNAs (lncRNAs), a class of noncoding RNAs with approximately 200 nucleotides to 100,000 bases, participate in the progression of a variety of diseases. This review summarizes functional lncRNAs, which were shown to serve as novel biomarkers for diagnosis and prognosis of DTCs and to act as oncogenes or tumor suppressor genes in DTC development. In addition, the potential mechanism of functional lncRNAs in DTCs is highlighted.
Abstract
摘要
消化道癌(DTC)是全球癌症相关死亡的主要原因之一。晚期 DTC 的当前治疗手段有局限性,并且早期 DTC 患者由于高效生物标志物的短缺而经常有漏诊。因此,有必要开发早期诊断的新型生物标志物以及 DTC 治疗的新治疗靶向。近年来,长链非编码核糖核酸(RNA)(lncRNA,一类超过 200 个核苷酸的非编码RNA)已被证实在 DTC 中异常表达,并且在 DTC 的发展中起到重要作用:lncRNA 的表达特性与 DTC 患者的不良生存率强烈相关,并且 lncRNA 在 DTC 进展中充当致癌基因或抑癌基因。在这次回顾中,我们总结了功能性 lncRNA,并阐述了其在 DTC 中的调节机制。 (The Oncologist) 2015;20:898–906
实践意义:消化道癌(DTC)是全球癌症相关死亡的主要原因之一。有必要开发早期诊断的新型生物标志物以及 DTC 治疗的新治疗靶向。长链非编码 RNA[一类有大约 200 个核苷酸至 100 000 个碱基对的非编码 RNA(IncRNA)]涉及各种疾病的进展。这次回顾总结了功能性 lncRNA,这些功能性 lncRNA 已被证实可用作 DTC 诊断和预后的新型生物标志物,并在 DTC 进展中充当致癌基因或抑癌基因。此外,还强调了功能性 lncRNA 在 DTC 中的潜在机制。
Introduction
Digestive tract cancers (DTCs), including multiple malignancies such as esophageal cancer (EsC), gastric cancer (GC), and colorectal cancer (CRC), are the most common tumors worldwide. In eastern Asian countries, DTCs are still the leading cause of cancer-related death [1, 2]. There were 182,410 new diagnosed cases and 77,030 deaths caused by esophageal, gastric, and colorectal cancers in the U.S. in 2013 [3]. Complete surgical resection remains the most effective treatment for early DTCs, but many patients are not diagnosed until tumors have developed to the advanced stage. The lack of effective prevention methods and treatments is a great threat to human health, and the 5-year survival rate for advanced DTCs is very low [4–7], despite multiple treatments involving surgery, radiation, and chemotherapy. Tumor metastasis and drug-resistance contribute to high mortality; therefore, identification of potential biomarkers for DTCs may help prevention and early diagnosis of such diseases, and understanding the molecular regulatory mechanism in tumor development may contribute to exploring effective treatments for advanced DTCs.
Long noncoding RNAs (lncRNAs) are a class of noncoding RNAs with approximately 200 nucleotides (nt) to 100,000 bases, without coding for proteins [8, 9]. In recent years, numerous studies reported that aberrant expressions of lncRNAs were associated with the recurrence [10], metastasis [11], and prognosis [12] of malignancies. LncRNAs were also found to play crucial roles in tumor development, including tumor cell proliferation [13], apoptosis [14], migration [15], invasion [16], and epithelial-to-mesenchymal transition [17]. The regulatory mechanism of lncRNAs in tumor development is complicated and involves chromatin modification [18, 19], transcriptional [20] and post-transcriptional regulation [21], and influence of protein stability [22]. Understanding the expression profiles of lncRNAs and their function and mechanism in DTC development will help develop novel biomarkers for early diagnosis and effective therapeutic targets. We summarized aberrantly expressed lncRNAs and expounded on their possible function and mechanism in DTCs.
Properties of lncRNAs
Numerous studies have indicated that 70%–90% of the human genome transcribes RNA products; however, less than 2% of the total genome encodes protein-coding genes [23–25], suggesting that the human transcriptome predominantly consists of abundant noncoding RNAs. Noncoding transcripts were initially thought to be “noise” generated from gene transcription, but a large number of more recent studies showed that noncoding RNAs have important roles in human diseases [26–30]. Noncoding RNAs can be divided into two classes: housekeeping noncoding RNAs and regulatory noncoding RNAs. Housekeeping noncoding RNAs contain ribosomal, transfer, small nuclear, and small nucleolar RNAs. Short regulatory noncoding RNAs include microRNAs (miRNAs), small interfering RNAs, and Piwi-associated RNAs [31]. Long regulatory noncoding RNAs were defined as lacking a significant open reading frame and having a length of more than 200 nt [32]. In 1988, Pachnis et al. reported the first lncRNA, H19, which has a very unusual structure consisting of five exons and is activated early during muscle cell differentiation [33]. Subsequently, Air [34], NRON [35], and HOTAIR [19] were gradually discovered. Recently, more than 32,000 lncRNAs have been documented, and more than 1,000 lncRNA disease entries and 475 lncRNA interaction entries were integrated in the LncRNADisease database (http://www.cuilab.cn/lncrnadisease).
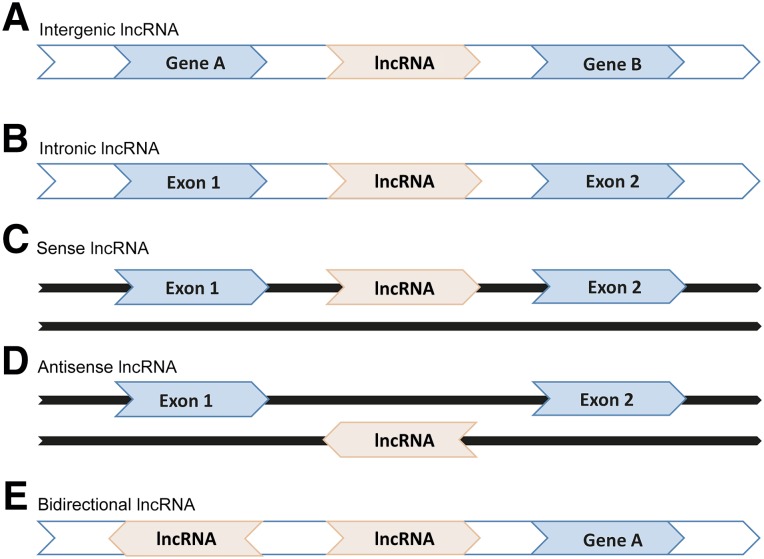
Several studies indicated that lncRNAs could be classified into five categories based on their genomic location and context. Intergenic and intronic lncRNAs are transcribed from intergenic regions and from introns of protein-coding genes, respectively. Sense lncRNAs are transcribed from the sense strand of protein-coding genes, and their sequences may overlap with parts of or entire sequences of protein-coding genes. Antisense lncRNAs are transcribed from the antisense strand of protein-coding genes, and their sequences may be complementary base pairing with exon, intron, or entire sequences of protein-coding genes. Last, bidirectional lncRNAs have transcriptional orientations opposite those of their neighboring protein-coding genes [31, 36] (Fig. 1).
Figure 1.
Genomic location of lncRNAs. Blue represents protein-coding genes and their exons, and light red represents lncRNAs. (A): Intergenic lncRNA, which is transcribed from intergenic regions. (B): Intronic lncRNA, which is transcribed from introns of protein-coding genes. (C): Sense lncRNA, which is transcribed from the sense strand of protein-coding genes. (D): Antisense lncRNA, which is transcribed from the antisense strand of protein-coding genes. (E): Bidirectional lncRNA, for which transcriptional orientation is opposite that of neighboring protein-coding genes.
Abbreviation: lncRNA, long noncoding RNA.
LncRNAs have a diverse set of functions. Perturbation experiments, such as loss of function and gain of function, showed that lncRNAs participated in epigenetic regulation. XIST, for example, is required in the process of X chromosome inactivation [37]; HOTAIR is crucial for reprograming the chromatin state in cancer metastasis [38]; and NRON represses NFAT transcription factor activity [35]. Interestingly, Guttman et al. summarized the modular regulatory principles of lncRNAs [29]. First, lncRNAs have a cis-function and affect their neighboring regions [39]. Second, lncRNAs serve as trans-regulators and modulate their neighboring genes [40]. Third, lncRNAs bind regulatory proteins and change their activity, resulting in the change in modification state and expression of the target genes [41]. Fourth, lncRNAs act as “decoys” and bind protein complexes and prevent them from binding to their proper regulatory targets [42]. Briefly, lncRNAs can interact with proteins, RNA (eg, mRNA, miRNA), and DNA to affect chromatin modification, gene expression, and the stability of proteins and mRNA. These regulatory principles may greatly enhance our understanding of the underlying role of lncRNAs in DTC development.
lncRNAs in DTC Development
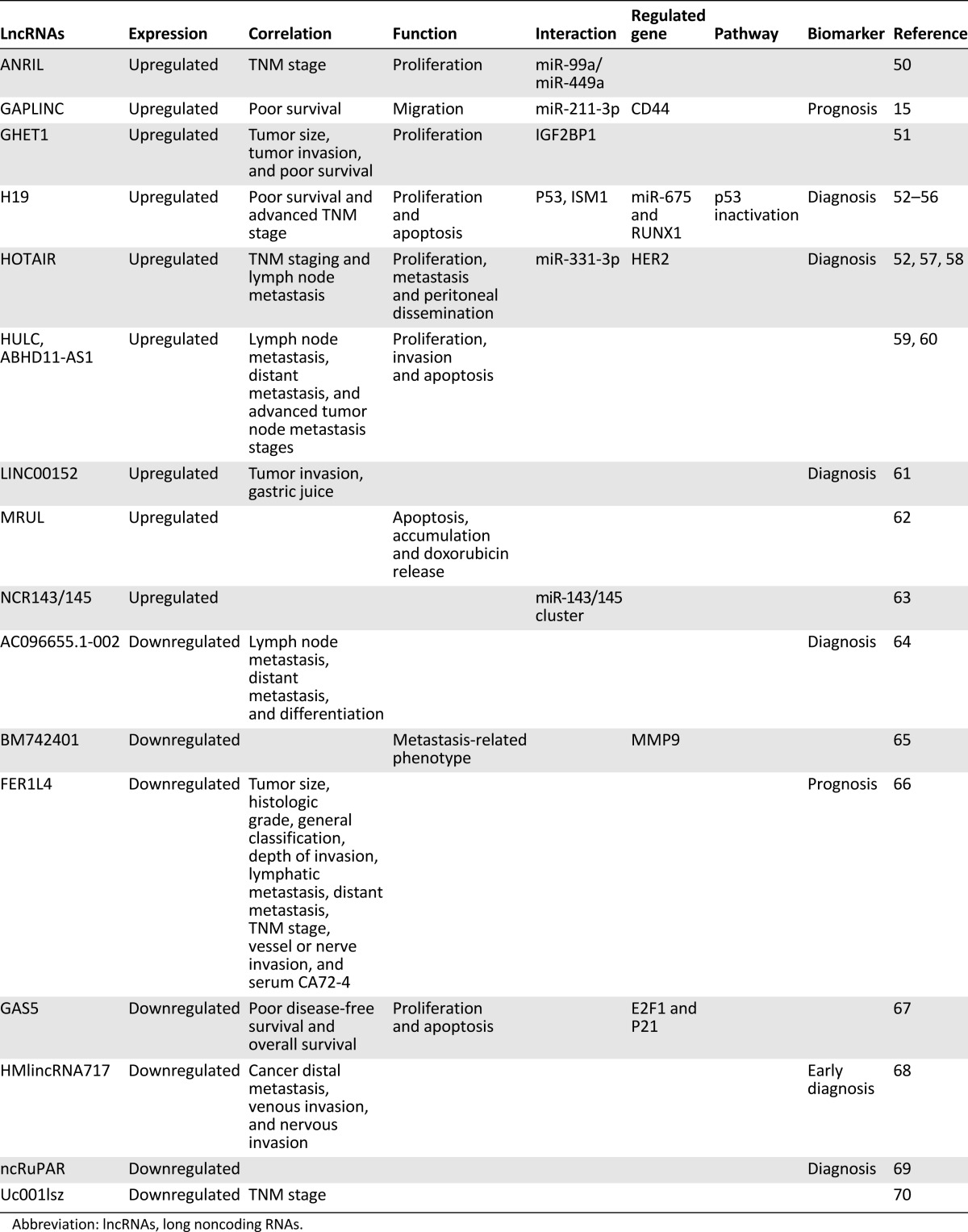
Increasing numbers of lncRNAs have been identified in DTCs in recent years, especially in EsC, GC, and CRC, in which lncRNAs participated in regulating proliferation, apoptosis, migration, and/or invasion of tumor cells in vitro and in vivo. In addition, lncRNA interacted with proteins, mRNAs, or miRNAs to modify expression of the target genes, resulting in the change in activation of some signaling pathways, such as the Wnt/β-catenin signaling pathway. The functional lncRNAs and their mechanisms are summarized in Tables 1–3. Notably, several lncRNAs, including HOTAIR and H19, were frequently reported in the three cancers.
Table 1.
The identified lncRNAs in esophageal cancer
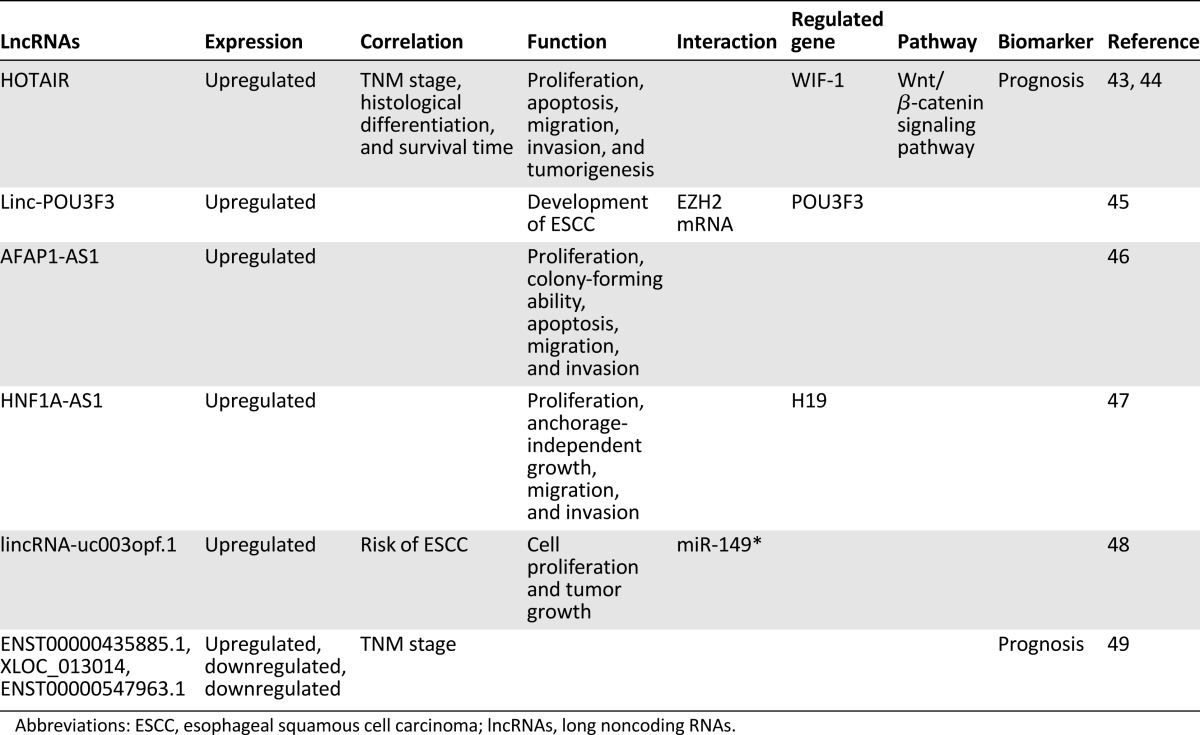
Table 3.
The function of lncRNAs in colorectal cancer
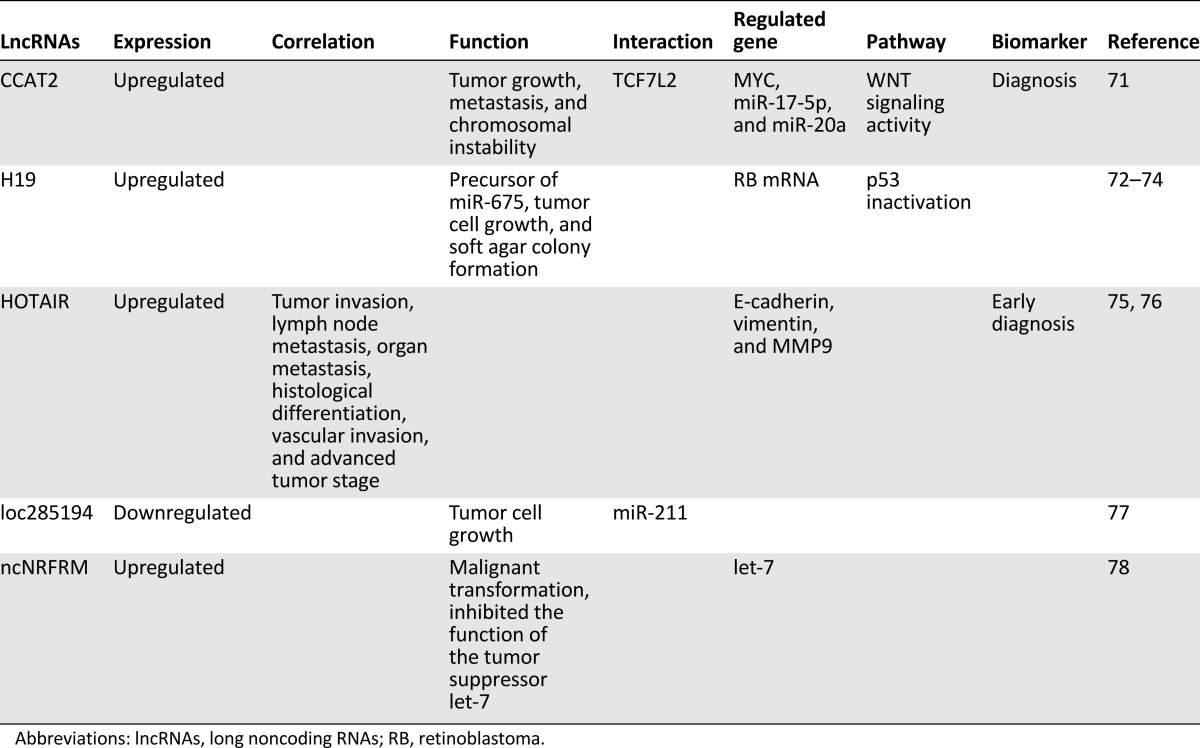
Table 2.
Dysregulation of lncRNAs in gastric cancer
HOTAIR
The long intergenic noncoding RNA (lincRNA) HOTAIR, which is transcribed from the HOXC locus, can interact with polycomb repressive complex 2 (PRC2; consisting of H3K27 methylase EZH2, SUZ12, and EED) to silence HOXD and select genes on other chromosomes [19, 38, 79]. Tsai et al. subsequently reported that a 5′ domain of HOTAIR binds PRC2, whereas a 3′ domain of HOTAIR binds LSD1 (KDM1/BHC110) [40], which is a demethylase that mediates the enzymatic demethylation of H3K4me2 [80] and a component of CoREST/REST repressor complexes [81].
In EsC, HOTAIR expression was markedly upregulated in esophageal squamous cell carcinoma (ESCC) and strongly correlated with tumor progress and poor prognosis of patients with ESCC. HOTAIR inhibition can reduce cell proliferation and migration in vitro and in vivo. Further exploration via microarray demonstrated that HOTAIR reprogrammed the gene expression profile of ESCC cells, some of which are responsible for tumorigenesis [43]. HOTAIR was also shown to promote WIF-1’s histone H3K27 methylation in the promoter region, resulting in decreased WIF expression and activation of the Wnt/β-catenin signaling pathway [44].
In GC, HOTAIR-expressing GC cells exhibited an enhanced ability of metastasis and peritoneal dissemination in a nude mouse model [57]. Liu et al. confirmed that HOTAIR was markedly increased in GC tissues compared with matched normal tissues, and its loss led to suppressed proliferation of GC cells in vitro and in vivo. The investigation of the molecular mechanism revealed that HOTAIR indirectly regulated human epithelial growth factor receptor 2 (HER2) by competitively binding to miR-331-3p [58]. In addition, the plasma levels of HOTAIR were increased in the patients with GC compared with the healthy controls [52], suggesting that HOTAIR may be a noninvasive biomarker for GC diagnosis.
In CRC, HOTAIR expression correlated with tumor invasion, lymph node metastasis, organ metastasis, histological differentiation, vascular invasion, and advanced tumor stage in colon cancer. Colon cancer patients with high HOTAIR expression had higher recurrence rates and reduced metastasis-free and overall survival compared with those with low HOTAIR expression. Further investigation revealed that HOTAIR increased the expression of E-cadherin and decreased the expression of vimentin and MMP9 [75]. Svoboda et al. demonstrated that HOTAIR expression was increased in primary tumors and blood of CRC patients, and its levels in tumors were associated with higher mortality of patients, suggesting that HOTAIR blood levels might serve as a potential surrogate prognostic marker in sporadic CRC [76].
Colon cancer patients with high HOTAIR expression had higher recurrence rates and reduced metastasis-free and overall survival compared with those with low HOTAIR expression.
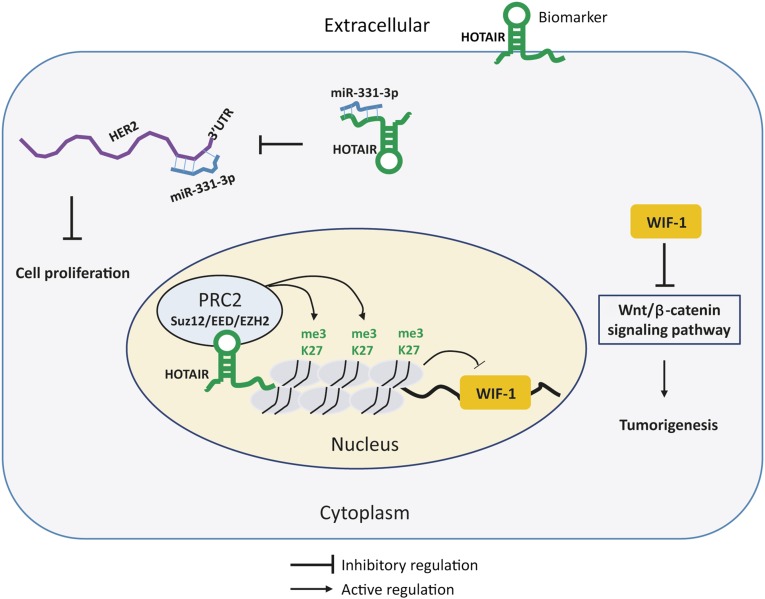
In summary, HOTAIR can interact with proteins and miRNAs to regulate expression of the target genes and activate the Wnt/β-catenin signaling pathway; HOTAIR can serve as independent and noninvasive biomarker for diagnosis of DTCs (Fig. 2).
Figure 2.
Diagrammatic sketch shows the hypothetical mechanism of HOTAIR in digestive tract cancers. HOTAIR interacts with PRC2 complex (Suz12/EED/EZH2) to methylate H3K27 and silence WIF-1 expression, further inducing activation of Wnt/β-catenin signaling pathway. HOTAIR acts as competing endogenous RNA to sponge miR-331-3p, which represses HER2 expression by binding to HER2 mRNA 3′UTR. HOTAIR can be released to blood from cells and serve as biomarker.
H19
H19, a maternally imprinted gene, is located in an imprinted region of chromosome 11p15.5 near the insulin-like growth factor 2 (IGF2) gene and contains five exons and four introns [82, 83]. In GC, Yang et al. reported that H19 was frequently increased in the GC tissues compared with the matched normal tissues and that its upregulation promoted GC cell proliferation, whereas H19 small interfering RNA led to cell apoptosis in AGS-line cells. Mechanism investigation revealed that H19 could interact with p53 and partially induce inactivation of p53 in AGS cells [53]. Thereafter, Arita et al. stated that the plasma levels of H19 were markedly increased in patients with GC compared with healthy controls, and levels were significantly decreased in postoperative samples, suggesting that lncRNAs may be novel, noninvasive biomarkers for GC diagnosis [52].
Zhang et al. showed the molecular mechanism by which c-Myc-induced upregulation of H19 can promote cell proliferation, which strongly correlates with poor survival of patients with GC [54]. Interestingly, H19-derived miR-675 could promote GC cell proliferation through the miR-675-targeting tumor suppressor gene runt-related transcription factor 1 (RUNX1) [55]. Recently, Li et al. reported that H19/miR-675 upregulation can promote the proliferation, migration, and invasion of GC cells in vitro and in vivo. RNA immunoprecipitation (RIP) and dual-luciferase reporter experiments confirmed that H19 could bind isthmin 1 (ISM1), an angiogenesis inhibitor. Further study revealed that H19 expression was positively correlated with ISM1, and H19-derived miR-675 could target calneuron 1 (CALN1); however, the function of H19/ISM1 in GC development has not been explored in detail [56].
In CRC, H19 was the first reported lncRNA. In the insulator region of IGF2/H19, loss of imprinting of the IGF2 gene strongly correlated with biallelic hypermethylation of a core of five CpG sites, and the hypermethylation created a field defect, predisposing to cancer [72]. Cui et al., however, reported that the IGF2-H19 enhancer competition model for IGF2 imprinting does not apply to the human colon [73], suggesting that lncRNAs have different functions in different types of cancer. Interestingly, Tsang et al. reported that H19, a precursor of miR-675, and miR-675 were both upregulated in human CRC tissues compared with adjacent noncancerous tissues, and the enhanced expression of miR-675 downregulated tumor suppressor retinoblastoma mRNA expression by targeting its 3′UTR, further increasing tumor cell growth and soft agar colony formation [74].
In summary, upregulation of H19 is frequently found in DTCs and promotes tumor progression. H19 can interact with p53 and induce p53 inactivation and can serve as noninvasive biomarker for GC detection.
lncRNA Interacts With Protein and miRNA
Under the regulatory principle of lncRNA, lncRNA interacting with proteins was frequently reported. In EsC, Li et al. reported that linc-POU3F3, a lincRNA encoded by a gene located next to POU3F3, could enhance proliferation and the ability to form colonies of ESCC cells and reduce the expression of POU3F3 mRNA. The ESCC cell lines with overexpressed linc-POU3F3 revealed dense hypermethylation of CpG islands in POU3F3, whereas EZH2 inhibition increased the expression of POU3F3 mRNA and reduced the binding of DNA methyltransferase (DNMT) 1, DNMT3A, and DNMT3B to POU3F3. Importantly, linc-POU3F3 can interact with EZH2 in Eca-109 and TE-1 cells, causing increased binding of DNA methyltransferase [45]. Yang et al. reported that lncRNA-GHET1 was markedly upregulated in gastric carcinoma, and its overexpression correlated with tumor size, tumor invasion, and poor survival in addition to enhanced GC cell proliferation in vitro and in vivo. Further experiments based on RNA pull-down and RIP confirmed that lncRNA-GHET1 was physically associated with insulin-like growth factor 2 mRNA binding protein 1 (IGF2BP1) and promoted a physical interaction between c-Myc mRNA and IGF2BP1 [51]. Ling et al. reported that CCAT2, a novel lncRNA encompassing the rs6983267 SNP, was increased in microsatellite-stable CRC and promoted tumor growth, metastasis, and chromosomal instability. CCAT2 could upregulate MYC, miR-17-5p, and miR-20a through TCF7L2-induced transcriptional regulation. Interestingly, CCAT2 and TCF7L2 had a physical interaction, enhancing the WNT signaling activity, which could also affect CCAT2 expression, suggesting the existence of a feedback loop [71].
Several papers reported that lncRNAs function as competing endogenous RNAs (ceRNAs) to sponge miRNAs and reduce the biological function of the miRNAs, resulting in changes in the expression of cancer-related genes. In GC, for example, Iio et al. reported that NCR143/145, the stability of which was preferentially reduced by DDX6, encompassed the miR-143/145 cluster and downregulated the expression of mature miR-143/145 in GC cells [63]. ANRIL (CDKN2B-AS1), which can recruit and bind to PRC2, was increased in human GC tissues and correlated with a higher TNM stage and tumor size. Further experiments confirmed that E2F1 could induce ANRIL-mediated growth promotion by which ANRIL acted as a ceRNA to repress the biological function of miR-99a/miR-449a, which was shown to bind to PRC2 mRNA. These findings provided support for a positive feedback loop by which ANRIL can continue to promote GC cell proliferation [50]. Liu et al. reported that lncRNA loc285194, a known lncRNA in osteosarcoma, is a p53 transcription target with two binding sites of miR-211 in its exon 4. The ectopic expression of loc285194 suppressed tumor cell growth in vitro and in vivo by repressing miR-211-induced cell growth [77]. Recently, Hu et al. reported that lincRNA GAPLINC was overexpressed in GC tissues and correlated with poor survival of patients with GC. GAPLINC suppression led to alterations in cell migration pathways in which CD44 expression was the most strongly correlated. Mechanistic investigations showed that GAPLINC acted as ceRNA to sponge miR211-3p, which can directly bind to CD44 mRNA [15].
Function of Other lncRNAs
During the past decade, a large number of functional lncRNAs had been identified and the identified lncRNAs showed multiple functions in DTC development. Several studies confirmed that lncRNAs, including lncRNA HULC [59] and ABHD11-AS1 [60], correlate with GC metastasis and differentiation and modulate the ability to perform cell proliferation, apoptosis, and invasion. Interestingly, Pang et al. reported that LINC00152, an overexpressed lncRNA in GC tissues, was increased in the gastric juice from patients with GC compared with non-GC controls [61], suggesting that GC tissue can secrete lncRNA into gastric juice, in which its expression is also stable. Some lncRNAs were shown to correlate with drug resistance in the treatment of GC. lncRNA MRUL, for instance, was markedly overexpressed in two multidrug-resistant GC cells, SGC-7901/ADR and SGC-7901/VCR, and its loss in these cells with the presence of adriamycin (ADR) or vincristine (VCR) resulted in increased rates of apoptosis, increased accumulation, and reduced doxorubicin release [62]. Sun et al. stated that GAS5 was markedly downregulated in GC tissues, and its low expression correlated with the poor disease-free survival and overall survival of patients with GC. The ectopic expression of GAS5 repressed GC cell proliferation and induced apoptosis in vitro and in vivo through regulating E2F1 and P21 expression [67]. Franklin et al. reported that ncNRFRM exhibited a malignant transformation ability when it was stably overexpressed in nontransformed and conditionally immortalized mouse colonocytes, and it inhibited the function of tumor suppressor let-7 [78].
In addition, the effects of single nucleotide polymorphisms (SNPs) of lncRNAs on ESCC susceptibility were also explored. Wu et al. scoured the exons of lincRNAs located in ESCC susceptibility loci for all probable functional SNPs and stated that in the lincRNA-uc003opf.1 exon, the functional polymorphism rs1175942A > G was considered a genetic modifier for the development of ESCC [48], suggesting that the function of lncRNAs in ESCC might be of individual significance. These findings highlight that the expression of lncRNAs was stably expressed in gastric juice; the function of some lncRNAs may be associated with multidrug resistance.
Identification of lncRNAs Using lncRNA Microarray or RNA Sequencing
Some studies used high-throughput RNA sequencing (RNA-seq) and/or lncRNA microarray to screen the dysregulated lncRNAs. Wu et al. found the CpG methylation status of 1.8 million loci distributed throughout the genome in Barrett’s esophagus (BE) tissues using a high-resolution assay with massively parallel sequencing. The authors reported that AFAP1-AS1 was hypomethylated and overexpressed in BE and esophageal adenocarcinoma (EAC) as well as in EAC cells. AFAP1-AS1 dysregulation, without changing the expression of its protein-coding counterpart AFAP1, could affect the proliferation, colony-forming ability, apoptosis, migration, and invasion of EAC cells [46].
Yang et al. used next-generation RNA-seq analysis and showed that 61 unique lncRNAs were significantly overexpressed in EACs compared with normal esophagus. Among the 61 lncRNAs, HNF1A-AS1 was strikingly overexpressed in human primary EACs and EAC cells, and its loss suppressed the proliferation, anchorage-independent growth, migration, and invasion of EAC cells in vitro. They further confirmed that HNF1A, the sense-cognate gene for HNF1A-AS1, was not an HNF1A-AS1 target. Mechanistic investigation via gene ontology enrichment analysis revealed that HNF1A-AS1 knockdown dysregulated the expression of genes involved in cell cycle regulation and affected the expression of a cancer-related lncRNA H19, suggesting that one lncRNA may influence the function of another lncRNA in EACs [47].
Park et al. used RNA-seq to screen lincRNAs in GC and normal tissues. By analyzing the RNA-seq and public microarray data, they found that 31 transcripts, including BM742401, were significantly decreased in GC tissues. The ectopic expression of BM742401 suppressed metastasis-related phenotypes and decreased the concentration of extracellular MMP9 [65].
Song et al. used lncRNA microarray and found that the expressions of 135 lncRNAs were markedly changed, including the most downregulated lncRNAs—FER1L4, uc001lsz, BG491697, AF131784, uc009ycs, BG981369, af147447, HMlincRNA1600, and AK054588—and the most upregulated ones—H19, HMlincRNA717, BM709340, BQ213083, AK054978, and DB077273. Further exploration revealed that the downregulation of uc001lsz was associated with TNM stages and suggested that uc001lsz might be a novel biomarker for the diagnosis of early GC [70].
Wang et al. used lncRNA microarray to analyze the expression levels of 21,558 lncRNAs in gastric cardia adenocarcinoma and found 2,289 upregulated lncRNAs and 1,546 downregulated lncRNAs. Among these, the most upregulated lncRNA was ASHG19A3A028863 (fold change: 169.673093), and the most downregulated lncRNA was ASHG19A3A007184 (fold change: 59.385806). Pathway analysis revealed that 18 pathways corresponded to the upregulated transcripts, and the most enriched network was “Ribosome-Homo sapiens (human).” In addition, eight pathways corresponded to the downregulated transcripts, and the most enriched network was “Mineral absorption-Homo sapiens (human)” [84].
Other data on dysregulated lncRNAs from microarray analysis revealed that 2,621 lncRNAs were differentially expressed in GC tissues compared with the matched normal tissues. KEGG pathway analysis indicated that the lncRNA-mRNA target pairs were the most significantly enriched in the p53 pathway [85]. These results suggested that many lncRNAs are dysregulated in DTCs and may have important roles in DTC development. Aberrant expression profiles of the identified lncRNAs via RNA-seq or/and lncRNA microarray need to be further confirmed, and their function and regulatory mechanism in DTC development need to be better understood.
lncRNAs as Clinical Biomarkers
Molecular markers have different types, including diagnostic markers, which aid in the diagnosis or subclassification of a particular disease state; prognostic markers, which have an association with some clinical outcomes, such as overall survival or recurrence-free survival; predictive markers, which predict the activity of a specific class or type of therapy and help determine more specific treatment; and companion diagnostic markers, which may be diagnostic, prognostic, or predictive but are used to identify a subgroup of patients for benefit therapy [86].
In recent years, several studies investigated whether lncRNAs can serve as effective diagnostic and prognostic biomarkers for DTCs. HOTAIR, for example, was shown to be an independent prognostic factor in patients with ESCC, and its high levels correlated with TNM stage, histological differentiation, and overall survival rates in ESCC patients [87, 88]. Based on the lncRNA microarray, Li et al. demonstrated the altered expressions of lncRNAs in the tumor tissues and matched normal tissues from 119 patients with ESCC, and they developed a prognostic signature from the training group (60 patients) using a random forest supervised classification algorithm and nearest shrunken centroid algorithm. In detail, the three-lncRNA signature (including the lncRNAs ENST00000435885.1, XLOC_013014, and ENST00000547963.1) had strong prognostic value and could serve as an independent prognostic factor for patients with ESCC [49]. Some lncRNAs were shown to serve as diagnostic biomarkers, such as ncRuPAR, which had sensitivity of 88.41%, specificity of 73.91%, and accuracy of 81.16% for the diagnosis of GC [69]; AC096655.1-002, which had sensitivity of 51.3%, specificity of 87.2%, and an area under the curve (AUC) of 0.731 for diagnosis of GC [64]; FER1L4, which had sensitivity of 67.2%, specificity of 80.3%, and AUC of 0.778 for the prognosis for GC [66]; and HMlincRNA717 [68]. Together, lncRNAs may serve as potential diagnostic and prognostic biomarkers for DTCs.
Conclusion
In this review, we summarized the lncRNAs that play important roles in DTCs. Some of them act as oncogenes or tumor suppressor genes, contributing to tumor invasion, metastasis, and histological differentiation as well as cell proliferation, apoptosis, and clone formation. These lncRNAs are correlated with TNM staging, tumor size, lymph node metastasis, and poor patient survival. Some lncRNAs have high sensitivity and high specificity in serving as potential biomarkers for the prognosis and diagnosis of DTCs. lncRNAs, serving as molecular biomarkers for prognosis and diagnosis of tumors may have more advantages because they have limited conservation among human organs. Consequently, lncRNA-derived biomarkers may have higher specificity. Through screening and comparison of transcripts in a variety of human organs, some lncRNAs that are only upregulated or downregulated in a single organ may be found, and specific expression profiles of these lncRNAs can be used to specifically diagnose disease without the interference of other diseases. More important, several papers reported that lncRNAs were stably detected in the plasma of patients or healthy subjects, suggesting that lncRNAs can serve as noninvasive biomarkers for the prognosis and diagnosis of DTCs.
These lncRNAs are correlated with TNM staging, tumor size, lymph node metastasis, and poor patient survival. Some lncRNAs have high sensitivity and high specificity in serving as potential biomarkers for the prognosis and diagnosis of DTCs.
There are more than 32,000 lncRNAs in the human genome, many of which have important functions in the initiation and progression of cancer. For DTCs, the identification of functional lncRNAs is the tip of iceberg, and many more functional lncRNAs must be identified and their regulatory mechanisms explored. Strikingly, several papers have shown that lncRNAs interact with proteins, mRNAs, and miRNAs in DTCs, resulting in the gain or loss of function of the corresponding elements. The molecular mechanisms by which lncRNAs contribute to DTC development seem to be more complicated, provoking researchers’ interests. As the next step, we should investigate the crucial function of lncRNAs in the initiation and progression of DTCs and find potentially effective biomarkers for prognosis and diagnosis of DTCs. More important, the molecular regulatory mechanisms of lncRNAs underlying DTC development should be explored in detail. Improving our understanding of these mechanisms will make it possible to identify an effective therapeutic target for DTCs.
Acknowledgment
This work was supported by a grant from the National Science Foundation of China (Grant 81301729 to Bo-Sheng Li).
Author Contributions
Conception/Design: Bo-Sheng Li
Provision of study material or patients: Shuo Zeng, Yu-Feng Xiao, Rei Xie
Collection and/or assembly of data: Shuo Zeng, Bo Tang
Data analysis and interpretation: Yu-Feng Xiao, Chang-Jiang Hu, Shi-Ming Yang
Manuscript writing: Shuo Zeng, Bo-Sheng Li
Final approval of manuscript: Shi-Ming Yang, Bo-Sheng Li
Disclosures
The authors indicated no financial relationships.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Leung WK, Wu MS, Kakugawa Y, et al. Screening for gastric cancer in Asia: Current evidence and practice. Lancet Oncol. 2008;9:279–287. doi: 10.1016/S1470-2045(08)70072-X. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 4.Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative folfox4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (eortc 40983): Long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208–1215. doi: 10.1016/S1470-2045(13)70447-9. [DOI] [PubMed] [Google Scholar]
- 5.Cascinu S, Labianca R, Barone C, et al. Adjuvant treatment of high-risk, radically resected gastric cancer patients with 5-fluorouracil, leucovorin, cisplatin, and epidoxorubicin in a randomized controlled trial. J Natl Cancer Inst. 2007;99:601–607. doi: 10.1093/jnci/djk131. [DOI] [PubMed] [Google Scholar]
- 6.Neuzillet C, Tijeras-Raballand A, Cros J, et al. Stromal expression of sparc in pancreatic adenocarcinoma. Cancer Metastasis Rev. 2013;32:585–602. doi: 10.1007/s10555-013-9439-3. [DOI] [PubMed] [Google Scholar]
- 7.Ciocirlan M, Lapalus MG, Hervieu V, et al. Endoscopic mucosal resection for squamous premalignant and early malignant lesions of the esophagus. Endoscopy. 2007;39:24–29. doi: 10.1055/s-2006-945182. [DOI] [PubMed] [Google Scholar]
- 8.Volders PJ, Helsens K, Wang X, et al. LNCipedia: A database for annotated human lncRNA transcript sequences and structures. Nucleic Acids Res. 2013;41:D246–D251. doi: 10.1093/nar/gks915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glover AR, Zhao JT, Ip JC, et al. Long noncoding RNA profiles of adrenocortical cancer can be used to predict recurrence. Endocr Relat Cancer. 2015;22:99–109. doi: 10.1530/ERC-14-0457. [DOI] [PubMed] [Google Scholar]
- 11.Liu B, Sun L, Liu Q, et al. A cytoplasmic NF-κB interacting long noncoding RNA blocks IκB phosphorylation and suppresses breast cancer metastasis. Cancer Cell. 2015;27:370–381. doi: 10.1016/j.ccell.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Han L, Zhang EB, Yin DD, et al. Low expression of long noncoding RNA PANDAR predicts a poor prognosis of non-small cell lung cancer and affects cell apoptosis by regulating Bcl-2. Cell Death Dis. 2015;6:e1665. doi: 10.1038/cddis.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu TP, Liu XX, Xia R, et al. SP1-induced upregulation of the long noncoding RNA TINCR regulates cell proliferation and apoptosis by affecting KLF2 mRNA stability in gastric cancer. Oncogene 2015. [Epub ahead of print]. [DOI] [PubMed]
- 14.Hirata H, Hinoda Y, Shahryari V, et al. Long noncoding RNA MALAT1 promotes aggressive renal cell carcinoma through Ezh2 and interacts with miR-205. Cancer Res. 2015;75:1322–1331. doi: 10.1158/0008-5472.CAN-14-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu Y, Wang J, Qian J, et al. Long noncoding RNA GAPLINC regulates CD44-dependent cell invasiveness and associates with poor prognosis of gastric cancer. Cancer Res. 2014;74:6890–6902. doi: 10.1158/0008-5472.CAN-14-0686. [DOI] [PubMed] [Google Scholar]
- 16.Pandey GK, Mitra S, Subhash S, et al. The risk-associated long noncoding RNA NBAT-1 controls neuroblastoma progression by regulating cell proliferation and neuronal differentiation. Cancer Cell. 2014;26:722–737. doi: 10.1016/j.ccell.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Han Y, Ye J, Wu D, et al. LEIGC long non-coding RNA acts as a tumor suppressor in gastric carcinoma by inhibiting the epithelial-to-mesenchymal transition. BMC Cancer. 2014;14:932. doi: 10.1186/1471-2407-14-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engreitz JM, Pandya-Jones A, McDonel P, et al. The Xist lncRNA exploits three-dimensional genome architecture to spread across the X chromosome. Science. 2013;341:1237973. doi: 10.1126/science.1237973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rinn JL, Kertesz M, Wang JK, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu G, Cai J, Han Y, et al. LincRNA-p21 regulates neointima formation, vascular smooth muscle cell proliferation, apoptosis, and atherosclerosis by enhancing p53 activity. Circulation. 2014;130:1452–1465. doi: 10.1161/CIRCULATIONAHA.114.011675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao C, Sun J, Zhang D, et al. The long intergenic noncoding RNA UFC1, a target of MicroRNA 34a, interacts with the mRNA stabilizing protein HuR to increase levels of β-catenin in HCC cells. Gastroenterology. 2015;148:415.e18–426.e18. doi: 10.1053/j.gastro.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 22.Hu X, Feng Y, Zhang D, et al. A functional genomic approach identifies FAL1 as an oncogenic long noncoding RNA that associates with BMI1 and represses p21 expression in cancer. Cancer Cell. 2014;26:344–357. doi: 10.1016/j.ccr.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein LD. Human genome: End of the beginning. Nature. 2004;431:915–916. doi: 10.1038/431915a. [DOI] [PubMed] [Google Scholar]
- 24.Lander ES, Linton LM, Birren B, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 25.Gutschner T, Diederichs S. The hallmarks of cancer: A long non-coding RNA point of view. RNA Biol. 2012;9:703–719. doi: 10.4161/rna.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lujambio A, Lowe SW. The microcosmos of cancer. Nature. 2012;482:347–355. doi: 10.1038/nature10888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chitwood DH, Timmermans MC. Small RNAs are on the move. Nature. 2010;467:415–419. doi: 10.1038/nature09351. [DOI] [PubMed] [Google Scholar]
- 28.Eulalio A, Mano M, Dal Ferro M, et al. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012;492:376–381. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- 29.Guttman M, Rinn JL. Modular regulatory principles of large non-coding RNAs. Nature. 2012;482:339–346. doi: 10.1038/nature10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmitt AM, Chang HY. Gene regulation: Long RNAs wire up cancer growth. Nature. 2013;500:536–537. doi: 10.1038/nature12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Perkel JM. Visiting “noncodarnia.”. Biotechniques. 2013;54:301–303–304. doi: 10.2144/000114037. [DOI] [PubMed] [Google Scholar]
- 33.Pachnis V, Brannan CI, Tilghman SM. The structure and expression of a novel gene activated in early mouse embryogenesis. EMBO J. 1988;7:673–681. doi: 10.1002/j.1460-2075.1988.tb02862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sleutels F, Zwart R, Barlow DP. The non-coding Air RNA is required for silencing autosomal imprinted genes. Nature. 2002;415:810–813. doi: 10.1038/415810a. [DOI] [PubMed] [Google Scholar]
- 35.Willingham AT, Orth AP, Batalov S, et al. A strategy for probing the function of noncoding RNAs finds a repressor of NFAT. Science. 2005;309:1570–1573. doi: 10.1126/science.1115901. [DOI] [PubMed] [Google Scholar]
- 36.Ma L, Bajic VB, Zhang Z. On the classification of long non-coding RNAs. RNA Biol. 2013;10:925–933. doi: 10.4161/rna.24604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Penny GD, Kay GF, Sheardown SA, et al. Requirement for Xist in X chromosome inactivation. Nature. 1996;379:131–137. doi: 10.1038/379131a0. [DOI] [PubMed] [Google Scholar]
- 38.Gupta RA, Shah N, Wang KC, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JT. Lessons from X-chromosome inactivation: Long ncRNA as guides and tethers to the epigenome. Genes Dev. 2009;23:1831–1842. doi: 10.1101/gad.1811209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsai MC, Manor O, Wan Y, et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–693. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X, Arai S, Song X, et al. Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Nature. 2008;454:126–130. doi: 10.1038/nature06992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hung T, Wang Y, Lin MF, et al. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat Genet. 2011;43:621–629. doi: 10.1038/ng.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X, Wu Z, Mei Q, et al. Long non-coding RNA HOTAIR, a driver of malignancy, predicts negative prognosis and exhibits oncogenic activity in oesophageal squamous cell carcinoma. Br J Cancer. 2013;109:2266–2278. doi: 10.1038/bjc.2013.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ge XS, Ma HJ, Zheng XH, et al. HOTAIR, a prognostic factor in esophageal squamous cell carcinoma, inhibits WIF-1 expression and activates Wnt pathway. Cancer Sci. 2013;104:1675–1682. doi: 10.1111/cas.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li W, Zheng J, Deng J, et al. Increased levels of the long intergenic non-protein coding rna pou3f3 promote DNA methylation in esophageal squamous cell carcinoma cells. Gastroenterology. 2014;146:1714.e5–1726.e5. doi: 10.1053/j.gastro.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Wu W, Bhagat TD, Yang X, et al. Hypomethylation of noncoding DNA regions and overexpression of the long noncoding RNA, AFAP1-AS1, in Barrett’s esophagus and esophageal adenocarcinoma. Gastroenterology. 2013;144:956.e4–966.e4. doi: 10.1053/j.gastro.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang X, Song JH, Cheng Y, et al. Long non-coding RNA HNF1A-AS1 regulates proliferation and migration in oesophageal adenocarcinoma cells. Gut. 2014;63:881–890. doi: 10.1136/gutjnl-2013-305266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu H, Zheng J, Deng J, et al. A genetic polymorphism in lincRNA-uc003opf.1 is associated with susceptibility to esophageal squamous cell carcinoma in Chinese populations. Carcinogenesis. 2013;34:2908–2917. doi: 10.1093/carcin/bgt252. [DOI] [PubMed] [Google Scholar]
- 49.Li J, Chen Z, Tian L, et al. LncRNA profile study reveals a three-lncRNA signature associated with the survival of patients with oesophageal squamous cell carcinoma. Gut. 2014;63:1700–1710. doi: 10.1136/gutjnl-2013-305806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang EB, Kong R, Yin DD, et al. Long noncoding RNA ANRIL indicates a poor prognosis of gastric cancer and promotes tumor growth by epigenetically silencing of miR-99a/miR-449a. Oncotarget. 2014;5:2276–2292. doi: 10.18632/oncotarget.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang F, Xue X, Zheng L, et al. Long non-coding RNA GHET1 promotes gastric carcinoma cell proliferation by increasing c-Myc mRNA stability. FEBS J. 2014;281:802–813. doi: 10.1111/febs.12625. [DOI] [PubMed] [Google Scholar]
- 52.Arita T, Ichikawa D, Konishi H, et al. Circulating long non-coding RNAs in plasma of patients with gastric cancer. Anticancer Res. 2013;33:3185–3193. [PubMed] [Google Scholar]
- 53.Yang F, Bi J, Xue X, et al. Up-regulated long non-coding RNA H19 contributes to proliferation of gastric cancer cells. FEBS J. 2012;279:3159–3165. doi: 10.1111/j.1742-4658.2012.08694.x. [DOI] [PubMed] [Google Scholar]
- 54.Zhang EB, Han L, Yin DD, et al. c-Myc-induced, long, noncoding H19 affects cell proliferation and predicts a poor prognosis in patients with gastric cancer. Med Oncol. 2014;31:914. doi: 10.1007/s12032-014-0914-7. [DOI] [PubMed] [Google Scholar]
- 55.Zhuang M, Gao W, Xu J, et al. The long non-coding RNA H19-derived miR-675 modulates human gastric cancer cell proliferation by targeting tumor suppressor RUNX1. Biochem Biophys Res Commun. 2014;448:315–322. doi: 10.1016/j.bbrc.2013.12.126. [DOI] [PubMed] [Google Scholar]
- 56.Li H, Yu B, Li J, et al. Overexpression of lncRNA H19 enhances carcinogenesis and metastasis of gastric cancer. Oncotarget. 2014;5:2318–2329. doi: 10.18632/oncotarget.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Endo H, Shiroki T, Nakagawa T, et al. Enhanced expression of long non-coding RNA HOTAIR is associated with the development of gastric cancer. PLoS One. 2013;8:e77070. doi: 10.1371/journal.pone.0077070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu XH, Sun M, Nie FQ, et al. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol Cancer. 2014;13:92. doi: 10.1186/1476-4598-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao Y, Guo Q, Chen J, et al. Role of long non-coding RNA HULC in cell proliferation, apoptosis and tumor metastasis of gastric cancer: A clinical and in vitro investigation. Oncol Rep. 2014;31:358–364. doi: 10.3892/or.2013.2850. [DOI] [PubMed] [Google Scholar]
- 60.Lin X, Yang M, Xia T, et al. Increased expression of long noncoding RNA ABHD11-AS1 in gastric cancer and its clinical significance. Med Oncol. 2014;31:42. doi: 10.1007/s12032-014-0042-4. [DOI] [PubMed] [Google Scholar]
- 61.Pang Q, Ge J, Shao Y, et al. Increased expression of long intergenic non-coding RNA LINC00152 in gastric cancer and its clinical significance. Tumour Biol. 2014;35:5441–5447. doi: 10.1007/s13277-014-1709-3. [DOI] [PubMed] [Google Scholar]
- 62.Wang Y, Zhang D, Wu K, et al. Long noncoding RNA MRUL promotes ABCB1 expression in multidrug-resistant gastric cancer cell sublines. Mol Cell Biol. 2014;34:3182–3193. doi: 10.1128/MCB.01580-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iio A, Takagi T, Miki K, et al. DDX6 post-transcriptionally down-regulates miR-143/145 expression through host gene NCR143/145 in cancer cells. Biochim Biophys Acta. 2013;1829:1102–1110. doi: 10.1016/j.bbagrm.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 64.Sun W, Wu Y, Yu X, et al. Decreased expression of long noncoding RNA AC096655.1-002 in gastric cancer and its clinical significance. Tumour Biol . 2013;34:2697–2701. doi: 10.1007/s13277-013-0821-0. [DOI] [PubMed] [Google Scholar]
- 65.Park SM, Park SJ, Kim HJ, et al. A known expressed sequence tag, BM742401, is a potent lincRNA inhibiting cancer metastasis. Exp Mol Med. 2013;45:e31. doi: 10.1038/emm.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shao Y, Chen H, Jiang X, et al. Low expression of lncRNA-HMlincRNA717 in human gastric cancer and its clinical significances. Tumour Biol. 2014;35:9591–9595. doi: 10.1007/s13277-014-2243-z. [DOI] [PubMed] [Google Scholar]
- 67.Sun M, Jin FY, Xia R, et al. Decreased expression of long noncoding RNA GAS5 indicates a poor prognosis and promotes cell proliferation in gastric cancer. BMC Cancer. 2014;14:319. doi: 10.1186/1471-2407-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Z, Shao Y, Tan L, et al. Clinical significance of the low expression of fer1l4 in gastric cancer patients. Tumour Biol. 2014;35:9613–9617. doi: 10.1007/s13277-014-2259-4. [DOI] [PubMed] [Google Scholar]
- 69.Liu L, Yan B, Yang Z, et al. ncRuPAR inhibits gastric cancer progression by down-regulating protease-activated receptor-1. Tumour Biol. 2014;35:7821–7829. doi: 10.1007/s13277-014-2042-6. [DOI] [PubMed] [Google Scholar]
- 70.Song H, Sun W, Ye G, et al. Long non-coding RNA expression profile in human gastric cancer and its clinical significances. J Transl Med. 2013;11:225. doi: 10.1186/1479-5876-11-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ling H, Spizzo R, Atlasi Y, et al. CCAT2, a novel noncoding RNA mapping to 8q24, underlies metastatic progression and chromosomal instability in colon cancer. Genome Res. 2013;23:1446–1461. doi: 10.1101/gr.152942.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nakagawa H, Chadwick RB, Peltomaki P, et al. Loss of imprinting of the insulin-like growth factor II gene occurs by biallelic methylation in a core region of H19-associated CTCF-binding sites in colorectal cancer. Proc Natl Acad Sci USA. 2001;98:591–596. doi: 10.1073/pnas.011528698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cui H, Onyango P, Brandenburg S, et al. Loss of imprinting in colorectal cancer linked to hypomethylation of H19 and IGF2. Cancer Res. 2002;62:6442–6446. [PubMed] [Google Scholar]
- 74.Tsang WP, Ng EK, Ng SS, et al. Oncofetal H19-derived miR-675 regulates tumor suppressor RB in human colorectal cancer. Carcinogenesis. 2010;31:350–358. doi: 10.1093/carcin/bgp181. [DOI] [PubMed] [Google Scholar]
- 75.Wu ZH, Wang XL, Tang HM, et al. Long non-coding RNA HOTAIR is a powerful predictor of metastasis and poor prognosis and is associated with epithelial-mesenchymal transition in colon cancer. Oncol Rep. 2014;32:395–402. doi: 10.3892/or.2014.3186. [DOI] [PubMed] [Google Scholar]
- 76.Svoboda M, Slyskova J, Schneiderova M, et al. HOTAIR long non-coding RNA is a negative prognostic factor not only in primary tumors, but also in the blood of colorectal cancer patients. Carcinogenesis. 2014;35:1510–1515. doi: 10.1093/carcin/bgu055. [DOI] [PubMed] [Google Scholar]
- 77.Liu Q, Huang J, Zhou N, et al. LncRNA loc285194 is a p53-regulated tumor suppressor. Nucleic Acids Res. 2013;41:4976–4987. doi: 10.1093/nar/gkt182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Franklin JL, Rankin CR, Levy S, et al. Malignant transformation of colonic epithelial cells by a colon-derived long noncoding RNA. Biochem Biophys Res Commun. 2013;440:99–104. doi: 10.1016/j.bbrc.2013.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mikkelsen TS, Ku M, Jaffe DB, et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–560. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shi Y, Lan F, Matson C, et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 81.Lunyak VV, Burgess R, Prefontaine GG, et al. Corepressor-dependent silencing of chromosomal regions encoding neuronal genes. Science. 2002;298:1747–1752. doi: 10.1126/science.1076469. [DOI] [PubMed] [Google Scholar]
- 82.Ayesh S, Matouk I, Schneider T, et al. Possible physiological role of H19 RNA. Mol Carcinog. 2002;35:63–74. doi: 10.1002/mc.10075. [DOI] [PubMed] [Google Scholar]
- 83.Thorvaldsen JL, Duran KL, Bartolomei MS. Deletion of the H19 differentially methylated domain results in loss of imprinted expression of H19 and Igf2. Genes Dev. 1998;12:3693–3702. doi: 10.1101/gad.12.23.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Y, Feng X, Jia R, et al. Microarray expression profile analysis of long non-coding RNAs of advanced stage human gastric cardia adenocarcinoma. Mol Genet Genomics. 2014;289:291–302. doi: 10.1007/s00438-013-0810-4. [DOI] [PubMed] [Google Scholar]
- 85.Lin XC, Zhu Y, Chen WB, et al. Integrated analysis of long non-coding RNAs and mRNA expression profiles reveals the potential role of lncRNAs in gastric cancer pathogenesis. Int J Oncol. 2014;45:619–628. doi: 10.3892/ijo.2014.2431. [DOI] [PubMed] [Google Scholar]
- 86.Febbo PG, Ladanyi M, Aldape KD, et al. NCCN Task Force report: Evaluating the clinical utility of tumor markers in oncology. J Natl Compr Canc Netw. 2011;9(suppl 5):S1–S32; quiz S33. doi: 10.6004/jnccn.2011.0137. [DOI] [PubMed] [Google Scholar]
- 87.Lv XB, Lian GY, Wang HR, et al. Long noncoding RNA HOTAIR is a prognostic marker for esophageal squamous cell carcinoma progression and survival. PLoS One. 2013;8:e63516. doi: 10.1371/journal.pone.0063516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen FJ, Sun M, Li SQ, et al. Upregulation of the long non-coding RNA HOTAIR promotes esophageal squamous cell carcinoma metastasis and poor prognosis. Mol Carcinog. 2013;52:908–915. doi: 10.1002/mc.21944. [DOI] [PubMed] [Google Scholar]