Abstract
Second-line therapies for non—small-cell lung cancer (NSCLC) provide modest disease control. Weekly topotecan in combination with bevacizumab was evaluated in advanced, refractory NSCLC. Median progression-free survival was 5.1 months and overall survival was 11.5 months. Based on its favorable disease control rate and tolerable side effect profile, this combination should be further evaluated in refractory NSCLC.
Background
This clinical trial evaluated whether topotecan in combination with bevacizumab improved progression-free survival (PFS) in patients with advanced, refractory non—small-cell lung cancer in a second-line setting.
Patient and Methods
Patients aged 18 years old and older received topotecan (4.0 mg/m2) on days 1, 8, and 15, and bevacizumab (10 mg/kg) on days 1 and 15 as intravenous infusions on a 28-day treatment cycle. Available tumor specimens were analyzed for ISG15 gene expression as a biomarker of response to topotecan.
Results
Forty-two patients were enrolled in the study, with a median age of 62.5 years and a median of 3 (range, 1-7) prior treatment regimens. Almost half (n = 18, 42.9%) of the patients received prior bevacizumab therapy. PFS was 5.1 months (95% CI, 3.7-7.8 months), and overall survival was 11.5 months (95% CI, 6.8-15.5 months). Response rates were as follows: 14.3% partial response, 54.8% stable disease, and 28.6% progressive disease. Hematologic toxicities included grade 3 thrombocytopenia (n = 7, 16.7%), neutropenia (n = 4, 9.5%), and anemia (n = 2, 4.8%). One toxic death occurred due to pulmonary hemorrhage, and one patient experienced a grade 4 pulmonary embolism. Grade 3 nonhematologic adverse events were uncommon (< 8%). There was a trend for improved median PFS, 3.5 months vs. 1.8 months (P = 26), in patients with high ISG15 expression.
Conclusion
Bevacizumab in combination with topotecan as a salvage therapy for metastatic non—small-cell lung cancer is well tolerated and is worthy of further investigation.
Keywords: Bevacizumab, ISG15 expression, Non-small-cell lung cancer, Refractory, Second-line therapy, Topotecan
Introduction
Non—small-cell lung cancer (NSCLC) remains the leading cause of cancer-related deaths in the United States.1 Second-line docetaxel, pemetrexed, and erlotinib for recurrent or refractory metastatic NSCLC improves progression-free survival (PFS) by a median of only 2 to 3 months.2-5 New therapies for refractory NSCLC could be effective by targeting increased tumor vascularization and elevated levels of angiogenic factors both of which are associated with increased risk for metastases and worsened survival.6, 7 Regulation of vascular endothelial growth factor and its receptors have been implicated in the angiogenesis pathway. Inhibition of this pathway is being rigorously evaluated in a variety of malignancies. Bevacizumab, an antibody against vascular endothelial growth factor, has clinical activity in a number of malignancies, including renal cell carcinoma,8 colorectal cancer,9 NSCLC10 and glioblastoma.11 When combined with standard chemotherapy, bevacizumab correlates with improved survival in several of these malignancies. Bevacizumab is currently approved for use with carboplatin and paclitaxel in locally advanced and metastatic nonsquamous NSCLC in a first-line setting.10 Current approved second-line options for NSCLC only provide modest responses, in the approximately 10%. Whereas analysis of some data suggests that adding bevacizumab with these approved agents in recurrent and/or refractory NSCLC has improved responses, its role as a second-line therapy in this disease is still being investigated.12 Novel combinations, that include bevacizumab, may provide better responses and could potentially improve survival in the second-line setting.
Topotecan is a topoisomerase-I inhibitor with activity in numerous tumor types, including NSCLC.13 In patients with previously treated NSCLC, topotecan given intravenously (I.V.) at a daily dose of 1.5-2.0 mg/m2 on days 1-5 of a 21-day cycle achieved a median overall survival (OS) that ranged from 32 to 38 weeks.14 When topotecan was compared with docetaxel in a phase III trial, the median OS times and time to progression were similar, which suggests that topotecan may be a reasonable alternative to docetaxel in patients previously treated with platinum-based chemotherapy. Because cytopenias are a major dose-limiting toxicity of topotecan, attempts to modify the administration schedule of this drug have been evaluated. In ovarian cancer, topotecan was administered on a weekly schedule at a dose of 4 mg/m2 given on days 1, 8, and 15 of a 28-day cycle; this schedule reduced the incidence of neutropenia without limiting efficacy compared with the standard dosing schedule on days 1-5.15
On the basis of these data, we explored a weekly dosing schedule of topotecan administered at 4 mg/m2 I.V. on days 1, 8, and 15 given in combination with bevacizumab on days 1 and 15 of a 28-day cycle. The purpose of this study was to determine the efficacy and safety of combining topotecan and bevacizumab in patients with previously treated NSCLC, as measured by the primary outcome of PFS. In addition, an exploratory analysis of clinical tumor samples was performed to investigate ubiquitin-like protein, interferon stimulated gene 15 (ISG15) expression as a predictive biomarker for topotecan response in NSCLC on the basis of preclinical reports.16, 17
Patients and Methods
Patient Selection
Eligible patients were aged 18 years and older, with histologically or cytologically confirmed nonsquamous NSCLC that was locally advanced or metastatic (ie, stage IIIB or IV). Additional eligibility criteria included failure of at least one or more prior therapies, an ECOG PS (Eastern Cooperative Oncology Group performance status) of 0 or 1, measurable disease as determined by RECIST (Response Evaluation Criteria In Solid Tumors), adequate bone marrow reserve (absolute neutrophil count ≥ 1.5 × 109/L, platelets > 100 × 109/L, hemoglobin level > 9 g/dL), adequate liver function (total bilirubin level < 1.5 times the upper limit of normal [ref. range 0.2-1.3 mg/dL]; alkaline phosphatase [ref. range 40-150 U/L], aspartate transaminase [ref. range 0-55 U/L], and alanine transaminase levels < 3.0 [ref. range 0-50 U/L] × the upper limit of normal or < 5.0 × the upper limit of normal if the liver was involved with the tumor), adequate coagulation parameters (international normalized ratio < 1.5, and partial thromboplastin time [ref. range 22-37 seconds] less than the upper limits of normal), adequate renal function (serum creatinine concentration < 2.0, urine dipstick for proteinuria < 2+ or for patients discovered to have ≥ 2+ proteinuria on dipstick urinalysis at baseline a 24-hour urine collection, which demonstrated ≤ 1 g of protein in 24 hours), and no radiation or systemic therapy for at least 14 days before study enrollment and at least 30 days for investigational agents. All the patients provided written informed consent. This study was approved by the institutional review board at the University of Minnesota Medical School and Park Nicollet Institute, and it was registered at clinicaltrials.gov (NCT00365547).
Patients were excluded for any of the following reasons: pregnant or breast feeding; known hypersensitivity to any component of bevacizumab; inadequately controlled hypertension as defined by systolic blood pressure > 150 mm Hg or diastolic blood pressure > 100 mm Hg; a history of hypertensive crisis or encephalopathy; New York Heart Association grade II or greater congestive heart failure; a history of myocardial infarction, unstable angina, stroke, or transient ischemic attack within 6 months of starting in the study; untreated brain metastases (treated brain metastases are defined as having no evidence of progression or hemorrhage after treatment and no ongoing requirement for dexamethasone, as ascertained by clinical examination and brain imaging during the screening period); a history of hemoptysis (≥ 2.5 mL of bright red blood per episode) within 1 month before enrollment; active malignancy other than NSCLC; major surgical procedure, open biopsy, or significant trauma within 28 days before enrollment into study; a history of abdominal fistula, gastrointestinal perforation, or intra-abdominal abscess within 6 months before enrollment in the study; or serious nonhealing wounds, ulcer, or fracture.
Treatment Schedule
Topotecan was administered weekly at a dose of 4 mg/m2 as a 30-minute I.V. infusion on days 1, 8, and 15. Topotecan was not given on day 22 of the 28-day treatment cycle. Bevacizumab was given at a dose of 10 mg/kg I.V. on days 1 and 15. The initial dose of bevacizumab was delivered over 90 minutes as a continuous I.V. infusion. If the first infusion was tolerated without infusion-related adverse events (fever or chills), then the second dose was given over 60 minutes. If the 60-minute infusion was well tolerated, then all subsequent infusions were administered over 30 minutes, unless adverse effects occurred. Treatment cycles were repeated every 28 days until disease progression or intolerance of the study drugs.
Assessments
A baseline clinical evaluation, including a history and physical examination, baseline laboratory data (complete blood cell count with differential, electrolytes, creatinine level, transaminase values, urine dipstick, and coagulation tests), and imaging, was performed before entry into the study. On day 1 of each treatment cycle, a history and physical examination was performed and repeated laboratory data were reviewed. A complete blood cell count with differential was performed and reviewed on days 1, 8, 15, and 22. Radiologic studies (computed tomography, magnetic resonance imaging) were performed at baseline and every 8 weeks (2 cycles) to assess disease response. Criteria for discontinuation of the study included the following: progression of disease, unwillingness or inability to comply with study requirements, withdrawal of consent, grade 4 hypertension (or grade 3 hypertension not controlled with medication), nephrotic syndrome, grade ≥ 2 pulmonary or central nervous system hemorrhage, any grade 4 hemorrhage, venous thromboembolic event that required full-dose warfarin or equivalent, arterial thromboembolic event (any grade), grade 4 congestive heart failure, gastrointestinal perforation, tracheoesophageal fistula (any grade), or grade 4 fistula, grade ≥ 2 bowel obstruction not fully recovered after medical or surgical intervention, wound dehiscence that required medical or surgical intervention, more than a 4-week delay due to toxicity from either drug, and any event in which the investigator had concerns about the patient continuing on therapy.
All the patients were seen for approximately 30 days (days 28-42) after the last dose of study drug for safety assessment. Patients who had an ongoing treatment-related grade 4 or serious adverse event at the time of study discontinuation were followed up as medically indicated until resolution or until the event was considered irreversible. All the patients, including those who discontinued therapy early, were followed up for response until progression or for survival for 1 year from the date of registration.
ISG15 Gene Expression
Available formalin-fixed paraffin-embedded (FFPE) tissues obtained at the time of initial diagnosis were evaluated for ISG15 gene expression. Total RNA was isolated from the FFPE tissues by using the RNeasy FFPE kit for microdissected tissue (QIAGEN, Valencia, CA) and from lung cancer cell lines: Calu3, A549, H358, and H1975, as described.18 RNA was reverse transcribed by using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) and quantitative real-time polymerase chain reaction was carried out with the ABI PRISM 7900HT by using inventoried TaqMan assays for ISG15 and the endogenous control beta-actin (Applied Biosystems). NSCLC cell lines with defined levels of ISG15 expression and topotecan sensitivity, Calu3 and A549 (low expression and sensitivity), and H358 and H1975 (high expression and sensitivity) were included in each experiment as calibrator samples.17 Experiments were run twice in duplicates, and the data generated were normalized to the endogenous control and analyzed with respect to the calibrator samples by using the 2−ΔΔCt method19. A scoring system developed in our previous study, based on ISG15 expression in NSCLC cell lines, normal human bronchial epithelial cells, and tumor-normal pairs from patients with lung cancer, was used to score the tumor samples as high (> 25-fold higher than the average expression in Calu3 and A549), borderline (20- to 25-fold), and low (< 20-fold).
Statistical Analysis
This primary objective of this single-arm, phase II clinical study was to determine the PFS of weekly topotecan and biweekly (every 2 weeks) bevacizumab in patients with NSCLC. In this study, all the patients were followed up for 1 year from the date of registration. To achieve 80% power by using a 1-sided, 1-sample test on exponential means at a 05 significance level, a sample size of 42 patients was needed to detect a difference between the null hypothesis that the median PFS was 8 weeks and the alternative hypothesis that the median PFS was 12 weeks. The study was terminated after each patient had been followed up for 26 weeks.
PFS was defined as the time from the start of treatment until the first documented sign of disease progression or death due to progressive disease. For subjects who did not progress, time to progression was censored at the time of last follow-up. For subjects who died without progression, time to progression was censored at the time of death. Kaplan-Meier estimates were used to summarize PFS and the median time to progression from the estimated survival curve. The Brookmeyer and Crowley method was to be used to estimate a 95% confidence interval for median PFS.
Response rate (RR) was defined as the percentage of subjects who achieved a complete response (CR) or partial response (PR) as determined by RECIST (version 1.0), and the clinical benefit rate was defined as the percentage of subjects who achieved a CR, PR, or stable disease (SD). A 95% confidence interval for RR was constructed by using a hypergeometric distribution. The time to response was defined as the time from the start of treatment until the first documented evidence of tumor response. For subjects who did not show a tumor response, the time was censored at the time of the last contact. The times to response were summarized by a Kaplan-Meier survival curve. For subjects who showed a response, the duration of response was defined as the time from first documented evidence of response until the first documented sign of disease progression or death due to progressive disease. For subjects who did not progress or die, the duration of response was censored at the time of the last contact. The duration of response was summarized by using medians and quartiles. OS was defined as the time from the start of treatment until death due to whatever cause. For subjects alive at study completion, the time to death was censored at the time of the last contact. OS was summarized by a Kaplan-Meier survival curve. Toxicities were recorded and classified according to National Cancer Institute Common Terminology Criteria for Adverse Events V 3.0. Exploratory analysis of the degree of ISG15 gene expression was correlated with RRs. Median PFS was compared between groups, with high vs. borderline and/or low ISG15 expression by using the t test.
Results
Patient Characteristics
A total of 42 evaluable patients were enrolled in this study between August 2006 and September 2010 (Table 1). The patient population was predominantly Caucasian, with a median age of 62.5 years (range, 36-80 years) and 71.4% (n = 30) having an ECOG PS of 0. The patients in our study were heavily pretreated, with the majority having received the study regimen as at least third-line therapy, and almost half of the patients having received prior bevacizumab as part of past treatments. Almost all of the patients had stage IV disease (n = 39, 92.9%) and adenocarcinoma tumor histology (n = 40, 95.2%).
Table 1. Baseline characterstics.
| Median (range) age, years | 62.5 (36-80) |
| Sex, no. (%) | |
| Men | 18 (42.9) |
| Women | 24 (57.1) |
| ECOG PS, no. (%) | |
| 0 | 30 (71.4) |
| 1 | 12 (28.6) |
| Ethnicity, no. (%) | |
| White | 39 (92.8) |
| Asian | 2 (4.8) |
| Other | 1 (2.4) |
| Stage of disease, no. (%) | |
| IIIB | 3 (7.1) |
| IV | 39 (92.8) |
| Prior chemotherapy regimens | |
| Average no. (range) | 2.8 (1-7) |
| Prior bevacizumab, no. (%) | 18 (42.9) |
| Histology, no. (%) | |
| Adenocarcinoma | 40 (95.2) |
| Large cell | 1 (2.4) |
| Undifferentiated | 1 (2.4) |
Abbreviation: ECOG PS = Eastern Cooperative Oncology Group performance status.
Clinical Outcome
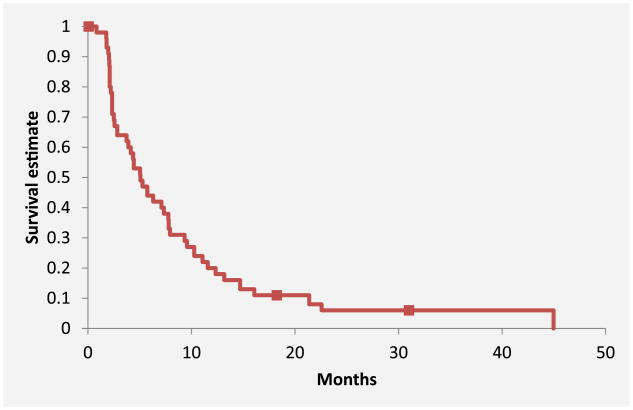
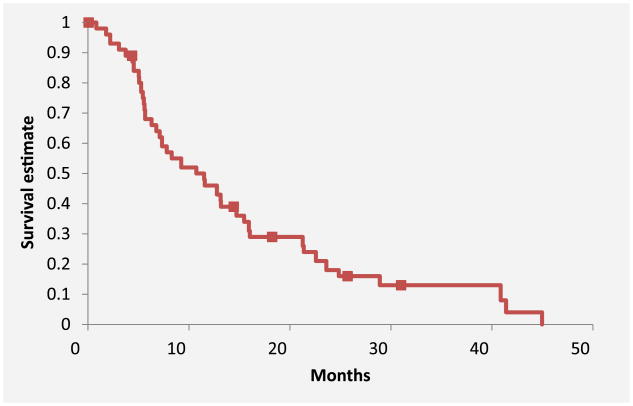
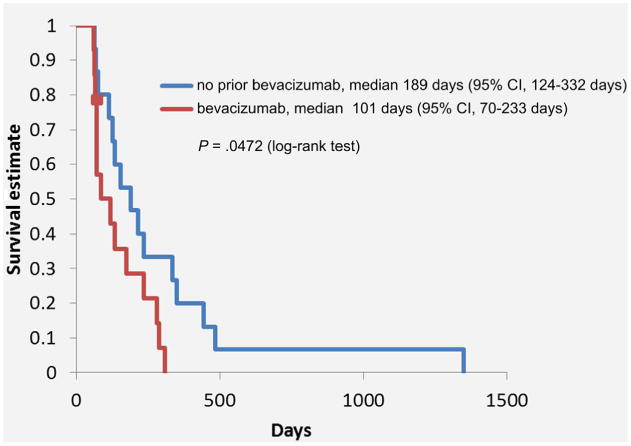
Forty patients died by the time of the final analysis. The median number of cycles received per patient was 3 (range, 1-19), with a total of 219 cycles. The primary outcome of median PFS was 5.1 months (95% CI, 3.7-7.8 months) (Figure 1). For PFS analysis, there were 3 (6.5%) censored cases with a median duration of follow-up of 547 days. The median OS was 11.5 months (95% CI, 6.8-15.5 months) (Figure 2). For OS analysis, there were 6 (13%) censored cases with a median duration of follow-up of 490 days. For all 42 patients, the clinical benefit rate was 69.1% and included 6 patients who achieved a PR and 23 patients with SD (Table 2). There was no difference in median OS between patients without vs. with prior bevacizumab therapy (11.6 months vs. 9.9 months; P = 74), although PFS was statistically different among these groups (6.3 months vs. 3.4 months; P = 047) (Figure 3).
Figure 1. Progression-Free survival.
Figure 2. Median overall survival.
Table 2. Overall Response Rates.
| No. (%) | |
|---|---|
| Complete response | 0 (0) |
| Partial response | 6 (14.3) |
| Stable disease | 23 (54.8) |
| Progressive disease | 12 (28.6) |
| Clinical benefit rate (response rate + stable disease) | 29 (69.1) |
Figure 3. Progression-Free survival in patients with and without prior use of Bevacizumab.
Toxicity
Eight (19%) patients withdrew from the study because of toxicity, whereas the majority of patients withdrew because of disease progression (Table 3). A total of 11 (31%) patients experienced a grade 3 hematologic toxicity (Table 4), with the most common being thrombocytopenia, which was seen in 7 (16.7%) patients. There were no grade 4 or 5 hematologic toxicities. There was one (2.4%) grade 5 toxicity due to pulmonary hemorrhage. Grade 3 and 4 nonhematologic toxicities were seen in 13 patients (Table 5), the most common of which was grade 3 musculoskeletal pain.
Table 3. Patient Disposition.
| Reason for Stopping | No. (%) |
|---|---|
| Progression | 28 (66.7) |
| Toxicity | 8 (19) |
| Patient request | 4 (9.5) |
| Death | 1 (2.4) |
| Ongoing | 1 (2.4) |
Table 4. Grade 3 and 4 Hematology toxicities.
| Hematologic Events | Grade 3, no. (%) | Grade 4, no. (%) | All Grades, no. (%) |
|---|---|---|---|
| Neutropenia | 4 (9.5) | 0 (0) | 5 (11.9) |
| Thrombocytopenia | 7 (16.7) | 0 (0) | 17 (40.5) |
| Anemia | 2 (4.8) | 0 (0) | 11 (26.2) |
Table 5. Series (grade 3 and 4) and common Nonhematologic toxicities.
| Nonhematologic Events | Grade 3, no. (%) | Grade 4, no. (%) | All Grades, no. (%) |
|---|---|---|---|
| Musculoskeletal pain | 3 (7.1) | 0 (0) | 19 (45.2) |
| Nausea and vomiting | 1 (2.4) | 0 (0) | 17 (40.5) |
| Fatigue | 0 (0) | 0 (0) | 14 (33.3) |
| Cough | 1 (2.4) | 0 (0) | 10 (23.8) |
| Headache | 0 (0) | 0 (0) | 9 (21.4) |
| Infection | 0 (0) | 0 (0) | 7 (16.7) |
| Shortness of breath | 2 (4.8) | 0 (0) | 7 (16.7) |
| Diarrhea | 1 (2.4) | 0 (0) | 6 (14.3) |
| Pain, other | 1 (2.4) | 1 (2.4) | 6 (14.3) |
| Mucositis | 0 (0) | 0 (0) | 4 (9.5) |
| Abdominal pain | 1 (2.4) | 0 (0) | 4 (9.5) |
| Loss of appetite | 0 (0) | 0 (0) | 3 (7.1) |
| Malaise | 0 (0) | 0 (0) | 3 (7.1) |
| Fever, non-neutropenic | 0 (0) | 0 (0) | 3 (7.1) |
| Congestion | 0 (0) | 0 (0) | 3 (7.1) |
| Vision loss | 1 (2.4) | 0 (0) | 1 (2.4) |
| Bowel obstruction | 1 (2.4) | 0 (0) | 1 (2.4) |
| Deep venous thrombosis | 1 (2.4) | 0 (0) | 1 (2.4) |
| Pseudogout | 1 (2.4) | 0 (0) | 1 (2.4) |
| Superior vena cava thrombosis | 1 (2.4) | 0 (0) | 1 (2.4) |
| Increased creatinine concentration | 1 (2.4) | 0 (0) | 1 (2.4) |
| Pulmonary embolism | 0 (0) | 1 (2.4) | 1 (2.4) |
All grade 3 and 4 adverse events are included; all-grade events that occurred in > 5% of cases are included in the table.
ISG15 Gene Expression and Response
A total of 10 patient biopsy specimens were available and adequate for ISG15 gene expression analysis. Of these, 5 had high expression, 2 had borderline expression, and 3 had low expression. In the cohort, there was 1 patient who achieved a PR with high expression. There were no responses seen in those with borderline or low expression. Comparison of patients with high ISG15 expression vs. low and borderline expression revealed a median PFS of 3.5 months vs. 1.8 months, respectively (P = 26).
Discussion
Despite our heavily pretreated patient population (on average, 2.8 prior therapy regimens), topotecan combined with bevacizumab resulted in a median PFS of 5.1 months, which exceeded our expectations, especially given the refractory nature of the disease. Furthermore, this finding is encouraging when compared with other second-line NSCLC therapies. Second-line docetaxel has a median PFS of 2 months,2 pemetrexed has a median PFS of 2.9 months,3 and erlotinib has a median PFS of 2.2 months.4 The median OS of 11.5 months also exceeded our expectations and is substantially higher than what is historically seen in patients with refractory advanced NSCLC. This duration of survival can be attributed to the relatively high number of patients with overall disease control (PR plus SD).
In addition to being heavily pretreated, almost half of our patients had received prior bevacizumab, which indicates that reintroduction of bevacizumab may still contribute to a response to therapy (3.4 months of PFS in bevacizumab pretreated group), perhaps by improving delivery of chemotherapy to tumor tissue.20 In comparison, topotecan used as a single agent in patients with previously pretreated NSCLC produced PFS of 2.6 months in a randomized phase III study.13
The combination of topotecan and bevacizumab was well tolerated despite our heavily pretreated population. Neutropenia remained a dose-limiting toxicity when administering the usual schedule of topotecan at a dose of 1.5-2.0 mg/m2 on days 1-5 of a 21-day cycle. Dose-limiting neutropenia was fairly low in our patient cohort, with only 4 (9.5%) patients experiencing grade 3 or higher neutropenia. This may, in part, be due to our inclusion criteria requirement of a baseline absolute neutrophil count being greater than 1.5. As a result, we may have screened for a patient population with a more robust bone marrow reserve. Thrombocytopenia was the most common hematologic toxicity in our population, with grade 3 events occurring in 7 (16.7%) patients. Regardless of this, significant myelosuppressive events were surprisingly low for this combination. Aside from hematologic toxicities, the regimen was fairly well tolerated, with grade 3 or higher toxicities occurring in 14 (33.3%) patients. Grade 3 or higher bevacizumab-associated bleeding events were relatively low and seen in only 4 patients. There was one toxic death from pulmonary hemorrhage, which was likely due to bevacizumab. Our single event of pulmonary hemorrhage falls in the range of the fatal hemorrhage rate secondary to bevacizumab (1.3% [95% CI, 0.6%-2.9%]) seen in a recent meta-analysis.21 This toxicity is similar to that of patients with refractory ovarian carcinoma treated with the same regimen.22 Overall, the combination of topotecan and bevacizumab was well tolerated, and bevacizumab-related toxicity was at or below expected levels.
Our exploratory analysis of high ISG15 gene expression as a predictive biomarker for topotecan response showed a trend for improved PFS; however, the overall difference was not statistically significant. Of the 10 specimens, only one response was seen, and this response was in a patient with high ISG15 expression. This analysis was limited by the number of specimens available. To our knowledge, there is no other reported clinical data that evaluated ISG15 expression as a predictor of topotecan response. Further investigation in NSCLC and other tumors treated with topotecan is necessary to determine its role as a predictive biomarker.
The strength of our conclusions is limited by the relatively homogenous patient sample and the single-arm study design with no control group for comparison. Many of our patients, although treated with this regimen as a third-line or greater therapy, had good performance status (71.4% had ECOG PS of 0).
Conclusion
Overall, results of our study suggest that the combination of topotecan and bevacizumab is a promising salvage therapy for advanced, nonsquamous NSCLC. By administering topotecan on a weekly schedule, we were able to avoid significant dose-limiting neutropenia. In addition, due to its tolerable nonhematologic adverse effects, it could be considered in older, more heavily pretreated patients. Bevacizumab-related hemorrhagic and thromboembolic events remain a concern, but these adverse effects were no more frequent than those observed in other similar studies. We think that further evaluation of this combination is warranted in a larger, randomized study.
Clinical Practice Points.
Salvage therapies for refractory NSCLC provide only modest benefit at the cost of toxicity.
Targeting of the VEGF pathway is an emerging as an approach to improve efficacy of available therapies.
This study showed that weekly topotecan in combination with biweekly (every 2 weeks) bevacizumab provided promising disease control in heavily pretreated patients with non-small cell lung cancer.
Hematologic toxicities of this regimen were manageable.
Further evaluation of this regimen compared to standard second-line therapies for refractory NSCLC is warranted.
Acknowledgments
We thank University of Minnesota Cancer Experimental Therapeutics Initiative for support of this study. We thank Michael J. Franklin for editorial support.
This study was partially funded by Glaxo SmithKline and Genentech Inc.
Footnotes
Disclosure: The authors have stated that they have no conflicts of interest.
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. 2000;18:2354–62. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- 3.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–97. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 4.Shepherd FA, Pereira JR, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;252:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 5.Azzoli CG, Baker S, Temin S, et al. American Society of Clinical Oncology Clinical Practice Guideline Update on Chemotherapy for Stage IV Non-Small-Cell Lung Cancer. J Clin Oncol. 2009;27:6251–66. doi: 10.1200/JCO.2009.23.5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrara N. The role of vascular endothelial growth factor in pathological angiogenesis. Breast Cancer Res Treat. 1995;36:127–37. doi: 10.1007/BF00666035. [DOI] [PubMed] [Google Scholar]
- 7.Fontanini G, Lucchi M, Vignati S, et al. Angiogenesis as a prognostic indicator of survival in non-small-cell lung carcinoma: a prospective study. J Natl Cancer Inst. 1997;89:881–6. doi: 10.1093/jnci/89.12.881. [DOI] [PubMed] [Google Scholar]
- 8.Escudier B, Bellmunt J, Négrier S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol. 2010;28:2144–50. doi: 10.1200/JCO.2009.26.7849. [DOI] [PubMed] [Google Scholar]
- 9.Cassidy J, Clarke S, Díaz-Rubio E, et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol. 2008;26:2006–12. doi: 10.1200/JCO.2007.14.9898. [DOI] [PubMed] [Google Scholar]
- 10.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. New Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 11.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–40. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 12.Herbst RS, O'Neill VJ, Fehrenbacher L, et al. Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non small-cell lung cancer. J Clin Oncol. 2007;25:4743–50. doi: 10.1200/JCO.2007.12.3026. [DOI] [PubMed] [Google Scholar]
- 13.Ramlau R, Gervais R, Krzakowski M, et al. Phase III study comparing oral topotecan to intravenous docetaxel in patients with pretreated advanced non-small-cell lung cancer. J Clin Oncol. 2006;24:2800–7. doi: 10.1200/JCO.2005.03.6491. [DOI] [PubMed] [Google Scholar]
- 14.Lynch T, Kalish L, Strasuss G, et al. Phase II study of topotecan in metastatic non-small-cell lung cancer. J Clin Oncol. 1994;12:347–52. doi: 10.1200/JCO.1994.12.2.347. [DOI] [PubMed] [Google Scholar]
- 15.Levy T, Inbar M, Menczer J, et al. Phase II study of weekly topotecan in patients with recurrent or persistent epithelial ovarian cancer. Gynecol Oncol. 2004;95:686–90. doi: 10.1016/j.ygyno.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Desai SD, Wood LM, Tsai YC, et al. ISG15 as a novel tumor biomarker for drug sensitivity. Mol Cancer Ther. 2008;7:1430–9. doi: 10.1158/1535-7163.MCT-07-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tessema M, Yingling CM, Thomas CL, et al. SULF2 methylation is prognostic for lung cancer survival and increases sensitivity to topoisomerase-I inhibitors via induction of ISG15. Oncogene. 2012;31:4107–16. doi: 10.1038/onc.2011.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tessema M, Willink R, Do K, et al. Promoter methylation of genes in and around the candidate lung cancer susceptibility locus 6q23-25. Cancer Res. 2008;68:1707–14. doi: 10.1158/0008-5472.CAN-07-6325. [DOI] [PubMed] [Google Scholar]
- 19.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 20.Willett CG, Duda DG, di Tomaso E, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. J Clin Oncol. 2009;27:3020–6. doi: 10.1200/JCO.2008.21.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ranpura V, Hapani S, Wu S. Treatment-related mortality with bevacizumab in cancer patients: a meta-analysis. JAMA. 2011;305:487–94. doi: 10.1001/jama.2011.51. [DOI] [PubMed] [Google Scholar]
- 22.McGonigle KF, Muntz HG, Vuky J, et al. Combined weekly topotecan and biweekly bevacizumab in women with platinum-resistant ovarian, peritoneal, or fallopian tube cancer: results of a phase 2 study. Cancer. 2011;117:3731–40. doi: 10.1002/cncr.25967. [DOI] [PubMed] [Google Scholar]