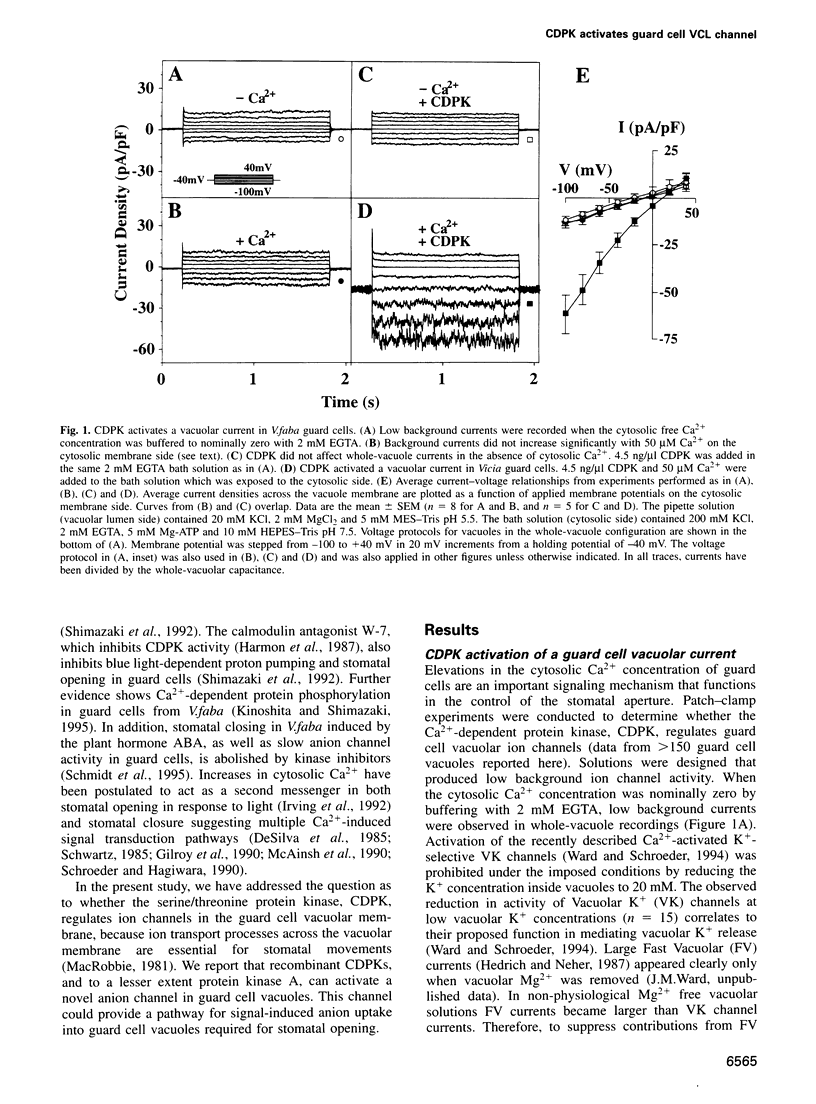
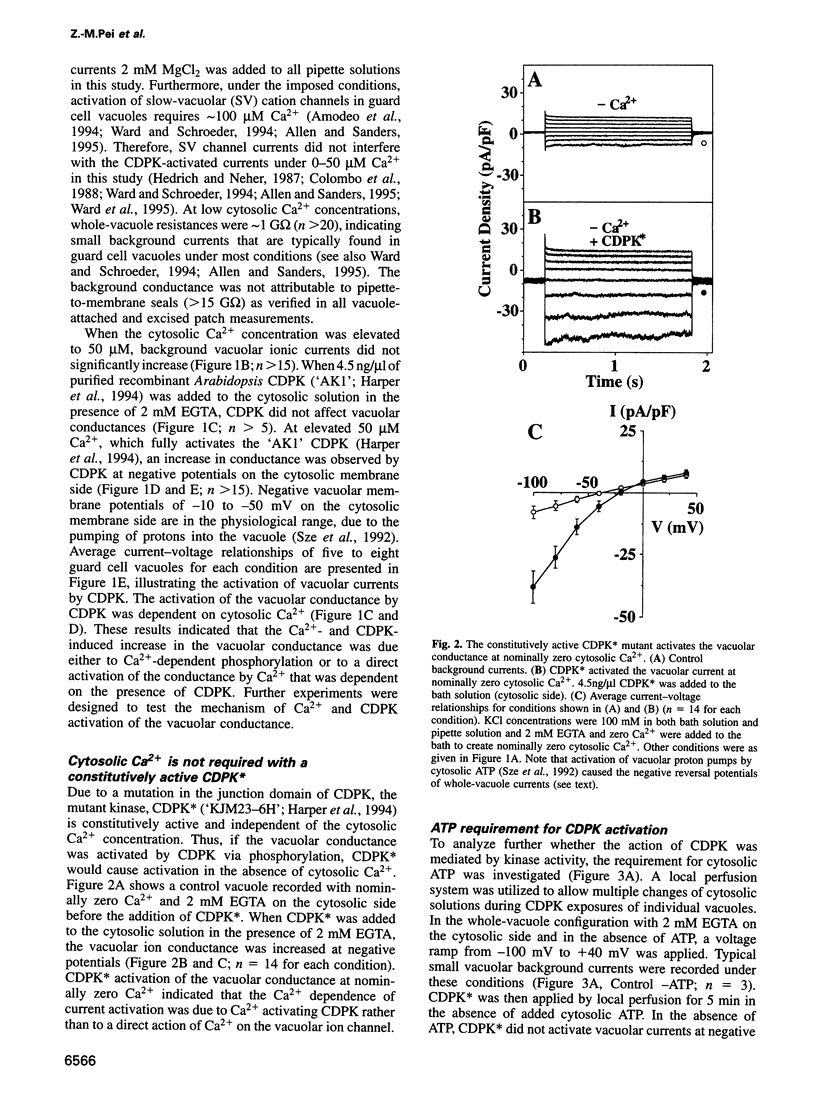
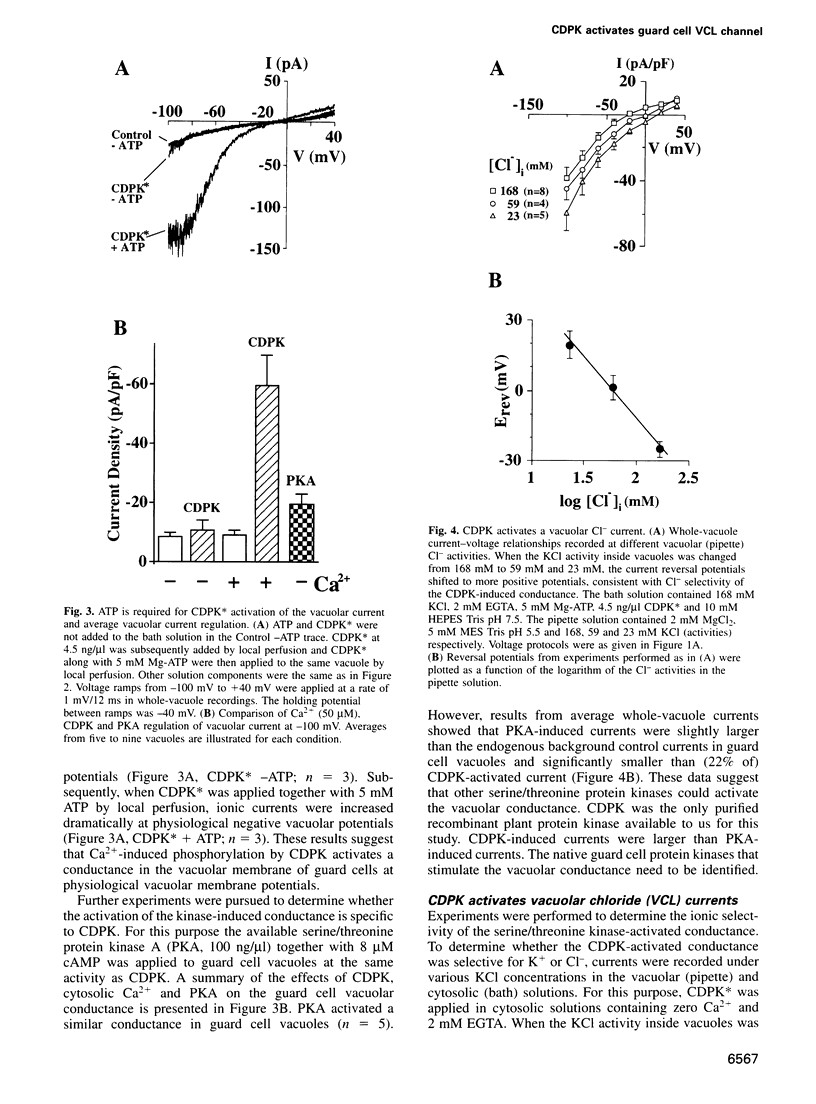
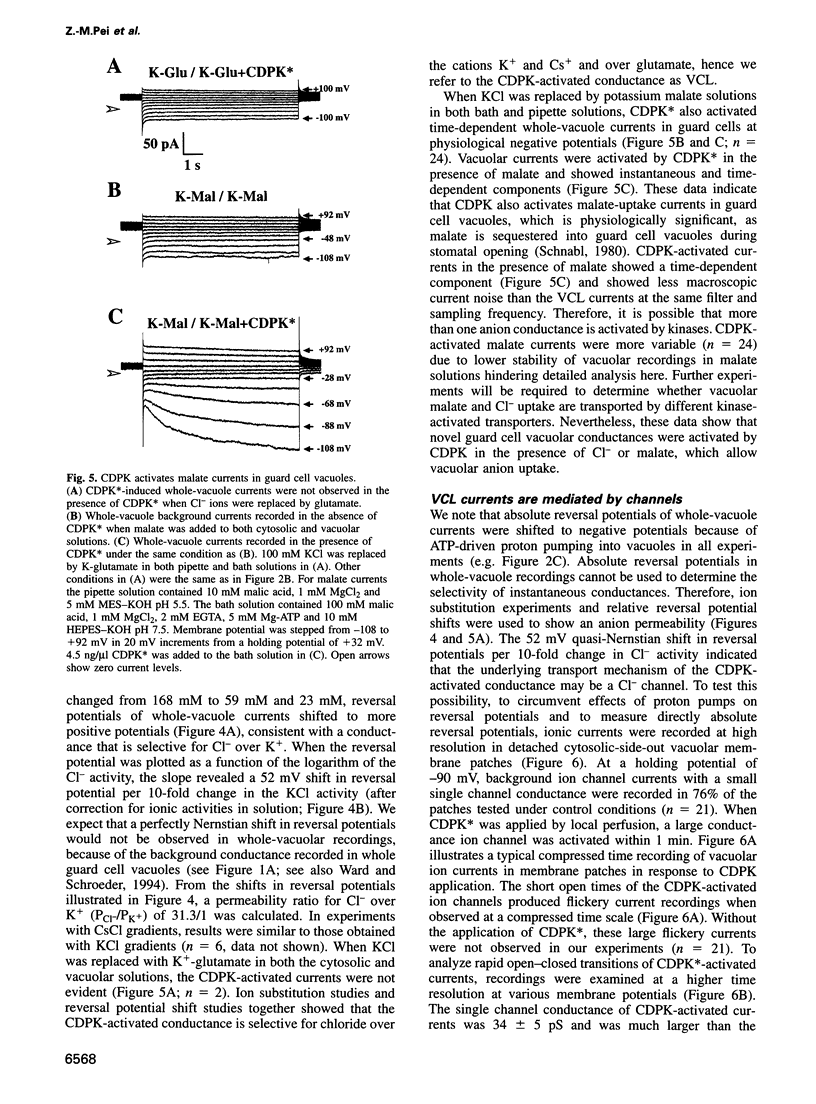
Abstract
Calcium-Dependent Protein Kinases (CDPKs) in higher plants contain a C-terminal calmodulin-like regulatory domain. Little is known regarding physiological CDPK targets. Both kinase activity and multiple Ca2+-dependent signaling pathways have been implicated in the control of stomatal guard cell movements. To determine whether CDPK or other protein kinases could have a role in guard cell signaling, purified and recombinant kinases were applied to Vicia faba guard cell vacuoles during patch-clamp experiments. CDPK activated novel vacuolar chloride (VCL) and malate conductances in guard cells. Activation was dependent on both Ca2+ and ATP. Furthermore, VCL activation occurred in the absence of Ca2+ using a Ca2+-independent, constitutively active, CDPK* mutant. Protein kinase A showed weaker activation (22% as compared with CDPK). Current reversals in whole vacuole recordings shifted with the Nernst potential for Cl-and vanished in glutamate. Single channel recordings showed a CDPK-activated 34 +/- 5 pS Cl- channel. VCL channels were activated at physiological potentials enabling Cl- uptake into vacuoles. VCL channels may provide a previously unidentified, but necessary, pathway for anion uptake into vacuoles required for stomatal opening. CDPK-activated VCL currents were also observed in red beet vacuoles suggesting that these channels may provide a more general mechanism for kinase-dependent anion uptake.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan A. C., Fricker M. D., Ward J. L., Beale M. H., Trewavas A. J. Two Transduction Pathways Mediate Rapid Effects of Abscisic Acid in Commelina Guard Cells. Plant Cell. 1994 Sep;6(9):1319–1328. doi: 10.1105/tpc.6.9.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G. J., Sanders D. Calcineurin, a Type 2B Protein Phosphatase, Modulates the Ca2+-Permeable Slow Vacuolar Ion Channel of Stomatal Guard Cells. Plant Cell. 1995 Sep;7(9):1473–1483. doi: 10.1105/tpc.7.9.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodeo G., Escobar A., Zeiger E. A Cationic Channel in the Guard Cell Tonoplast of Allium cepa. Plant Physiol. 1994 Jul;105(3):999–1006. doi: 10.1104/pp.105.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann M., McMichael R. W., Jr, Huber J. L., Kaiser W. M., Huber S. C. Partial Purification and Characterization of a Calcium-Dependent Protein Kinase and an Inhibitor Protein Required for Inactivation of Spinach Leaf Nitrate Reductase. Plant Physiol. 1995 Jul;108(3):1083–1091. doi: 10.1104/pp.108.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H. A., Travis S. M., Welsh M. J. Regulation of the cystic fibrosis transmembrane conductance regulator Cl- channel by specific protein kinases and protein phosphatases. J Biol Chem. 1993 Jan 25;268(3):2037–2047. [PubMed] [Google Scholar]
- Binder B. M., Harper J. F., Sussman M. R. Characterization of an Arabidopsis calmodulin-like domain protein kinase purified from Escherichia coli using an affinity sandwich technique. Biochemistry. 1994 Mar 1;33(8):2033–2041. doi: 10.1021/bi00174a008. [DOI] [PubMed] [Google Scholar]
- Chen L., Huang L. Y. Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature. 1992 Apr 9;356(6369):521–523. doi: 10.1038/356521a0. [DOI] [PubMed] [Google Scholar]
- Faux M. C., Scott J. D. Molecular glue: kinase anchoring and scaffold proteins. Cell. 1996 Apr 5;85(1):9–12. doi: 10.1016/s0092-8674(00)81075-2. [DOI] [PubMed] [Google Scholar]
- Gadsby D. C., Nagel G., Hwang T. C. The CFTR chloride channel of mammalian heart. Annu Rev Physiol. 1995;57:387–416. doi: 10.1146/annurev.ph.57.030195.002131. [DOI] [PubMed] [Google Scholar]
- Gilroy S., Read N. D., Trewavas A. J. Elevation of cytoplasmic calcium by caged calcium or caged inositol triphosphate initiates stomatal closure. Nature. 1990 Aug 23;346(6286):769–771. doi: 10.1038/346769a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harmon A. C., Putnam-Evans C., Cormier M. J. A calcium-dependent but calmodulin-independent protein kinase from soybean. Plant Physiol. 1987 Apr;83(4):830–837. doi: 10.1104/pp.83.4.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon A. C., Yoo B. C., McCaffery C. Pseudosubstrate inhibition of CDPK, a protein kinase with a calmodulin-like domain. Biochemistry. 1994 Jun 14;33(23):7278–7287. doi: 10.1021/bi00189a032. [DOI] [PubMed] [Google Scholar]
- Harper J. F., Binder B. M., Sussman M. R. Calcium and lipid regulation of an Arabidopsis protein kinase expressed in Escherichia coli. Biochemistry. 1993 Apr 6;32(13):3282–3290. doi: 10.1021/bi00064a010. [DOI] [PubMed] [Google Scholar]
- Harper J. F., Huang J. F., Lloyd S. J. Genetic identification of an autoinhibitor in CDPK, a protein kinase with a calmodulin-like domain. Biochemistry. 1994 Jun 14;33(23):7267–7277. doi: 10.1021/bi00189a031. [DOI] [PubMed] [Google Scholar]
- Harper J. F., Sussman M. R., Schaller G. E., Putnam-Evans C., Charbonneau H., Harmon A. C. A calcium-dependent protein kinase with a regulatory domain similar to calmodulin. Science. 1991 May 17;252(5008):951–954. doi: 10.1126/science.1852075. [DOI] [PubMed] [Google Scholar]
- Hille B., Schwarz W. Potassium channels as multi-ion single-file pores. J Gen Physiol. 1978 Oct;72(4):409–442. doi: 10.1085/jgp.72.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabak E. M., Dickmann L. J., Satterlee J. S., Sussman M. R. Characterization of eight new members of the calmodulin-like domain protein kinase gene family from Arabidopsis thaliana. Plant Mol Biol. 1996 May;31(2):405–412. doi: 10.1007/BF00021802. [DOI] [PubMed] [Google Scholar]
- Humble G. D., Raschke K. Stomatal opening quantitatively related to potassium transport: evidence from electron probe analysis. Plant Physiol. 1971 Oct;48(4):447–453. doi: 10.1104/pp.48.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving H. R., Gehring C. A., Parish R. W. Changes in cytosolic pH and calcium of guard cells precede stomatal movements. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1790–1794. doi: 10.1073/pnas.89.5.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. D., Chrispeels M. J. Tonoplast-bound protein kinase phosphorylates tonoplast intrinsic protein. Plant Physiol. 1992 Dec;100(4):1787–1795. doi: 10.1104/pp.100.4.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestner K. H., Sze H. Potential-dependent anion transport in tonoplast vesicles from oat roots. Plant Physiol. 1987 Mar;83(3):483–489. doi: 10.1104/pp.83.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T., Hayashida N., Baba T., Shinozaki K., Shimada H. The gene encoding a calcium-dependent protein kinase located near the sbe1 gene encoding starch branching enzyme I is specifically expressed in developing rice seeds. Gene. 1993 Jul 30;129(2):183–189. doi: 10.1016/0378-1119(93)90267-7. [DOI] [PubMed] [Google Scholar]
- Lee J. W., Zhang Y., Weaver C. D., Shomer N. H., Louis C. F., Roberts D. M. Phosphorylation of nodulin 26 on serine 262 affects its voltage-sensitive channel activity in planar lipid bilayers. J Biol Chem. 1995 Nov 10;270(45):27051–27057. doi: 10.1074/jbc.270.45.27051. [DOI] [PubMed] [Google Scholar]
- McAinsh M. R., Webb AAR., Taylor J. E., Hetherington A. M. Stimulus-Induced Oscillations in Guard Cell Cytosolic Free Calcium. Plant Cell. 1995 Aug;7(8):1207–1219. doi: 10.1105/tpc.7.8.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G., Hwang T. C., Nastiuk K. L., Nairn A. C., Gadsby D. C. The protein kinase A-regulated cardiac Cl- channel resembles the cystic fibrosis transmembrane conductance regulator. Nature. 1992 Nov 5;360(6399):81–84. doi: 10.1038/360081a0. [DOI] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- Pantoja O., Gelli A., Blumwald E. Characterization of Vacuolar Malate and K Channels under Physiological Conditions. Plant Physiol. 1992 Nov;100(3):1137–1141. doi: 10.1104/pp.100.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant P. J., Gelli A., Blumwald E. Vacuolar chloride regulation of an anion-selective tonoplast channel. J Membr Biol. 1994 May;140(1):1–12. doi: 10.1007/BF00234481. [DOI] [PubMed] [Google Scholar]
- Poovaiah B. W., Reddy A. S. Calcium and signal transduction in plants. CRC Crit Rev Plant Sci. 1993;12(3):185–211. doi: 10.1080/07352689309701901. [DOI] [PubMed] [Google Scholar]
- Schmidt C., Schelle I., Liao Y. J., Schroeder J. I. Strong regulation of slow anion channels and abscisic acid signaling in guard cells by phosphorylation and dephosphorylation events. Proc Natl Acad Sci U S A. 1995 Oct 10;92(21):9535–9539. doi: 10.1073/pnas.92.21.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. I., Fang H. H. Inward-rectifying K+ channels in guard cells provide a mechanism for low-affinity K+ uptake. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11583–11587. doi: 10.1073/pnas.88.24.11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. I., Hagiwara S. Repetitive increases in cytosolic Ca2+ of guard cells by abscisic acid activation of nonselective Ca2+ permeable channels. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9305–9309. doi: 10.1073/pnas.87.23.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A. Role of Ca and EGTA on Stomatal Movements in Commelina communis L. Plant Physiol. 1985 Dec;79(4):1003–1005. doi: 10.1104/pp.79.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki K., Kinoshita T., Nishimura M. Involvement of Calmodulin and Calmodulin-Dependent Myosin Light Chain Kinase in Blue Light-Dependent H Pumping by Guard Cell Protoplasts from Vicia faba L. Plant Physiol. 1992 Aug;99(4):1416–1421. doi: 10.1104/pp.99.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J. M., Walker J. C. Plant protein kinase families and signal transduction. Plant Physiol. 1995 Jun;108(2):451–457. doi: 10.1104/pp.108.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen K. L., Choi J. H. Isolation and sequence analysis of a cDNA clone for a carrot calcium-dependent protein kinase: homology to calcium/calmodulin-dependent protein kinases and to calmodulin. Plant Mol Biol. 1991 Oct;17(4):581–590. doi: 10.1007/BF00037045. [DOI] [PubMed] [Google Scholar]
- Sze H., Ward J. M., Lai S. Vacuolar H(+)-translocating ATPases from plants: structure, function, and isoforms. J Bioenerg Biomembr. 1992 Aug;24(4):371–381. doi: 10.1007/BF00762530. [DOI] [PubMed] [Google Scholar]
- Wang Y. T., Salter M. W. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature. 1994 May 19;369(6477):233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- Ward J. M., Pei Z. M., Schroeder J. I. Roles of Ion Channels in Initiation of Signal Transduction in Higher Plants. Plant Cell. 1995 Jul;7(7):833–844. doi: 10.1105/tpc.7.7.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. M., Schroeder J. I. Calcium-Activated K+ Channels and Calcium-Induced Calcium Release by Slow Vacuolar Ion Channels in Guard Cell Vacuoles Implicated in the Control of Stomatal Closure. Plant Cell. 1994 May;6(5):669–683. doi: 10.1105/tpc.6.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver C. D., Crombie B., Stacey G., Roberts D. M. Calcium-dependent phosphorylation of symbiosome membrane proteins from nitrogen-fixing soybean nodules : evidence for phosphorylation of nodulin-26. Plant Physiol. 1991 Jan;95(1):222–227. doi: 10.1104/pp.95.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Kappes B., Franklin R. M. Gene structure and expression of an unusual protein kinase from Plasmodium falciparum homologous at its carboxyl terminus with the EF hand calcium-binding proteins. J Biol Chem. 1993 Feb 25;268(6):4347–4354. [PubMed] [Google Scholar]
- van der Hoeven P. C., Siderius M., Korthout H. A., Drabkin A. V., de Boer A. H. A calcium and free fatty acid-modulated protein kinase as putative effector of the fusicoccin 14-3-3 receptor. Plant Physiol. 1996 Jul;111(3):857–865. doi: 10.1104/pp.111.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]


