Abstract
Vertebrate gene members of the HoxD complex are essential for proper development of the appendicular skeletons. Inactivation of these genes induces severe alterations in the size and number of bony elements. Evx-2, a gene related to the Drosophila even-skipped (eve) gene, is located close to Hoxd-13 and is expressed in limbs like the neighbouring Hoxd genes. To investigate whether this tight linkage reflects a functional similarity, we produced a null allele of Evx-2. Furthermore, and because Hoxd-13 function is prevalent over that of nearby Hoxd genes, we generated two different double mutant loci wherein both Evx-2 and Hoxd-13 were inactivated in cis. The analysis of these various genetic configurations revealed the important function of Evx-2 during the development of the autopod as well as its genetic interaction with Hoxd-13. These results show that, in limbs, Evx-2 functions like a Hoxd gene. A potential evolutionary scenario is discussed, in which Evx-2 was recruited by the HoxD complex in conjunction with the emergence of digits in an ancestral tetrapod.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahringer J. Posterior patterning by the Caenorhabditis elegans even-skipped homolog vab-7. Genes Dev. 1996 May 1;10(9):1120–1130. doi: 10.1101/gad.10.9.1120. [DOI] [PubMed] [Google Scholar]
- Bastian H., Gruss P. A murine even-skipped homologue, Evx 1, is expressed during early embryogenesis and neurogenesis in a biphasic manner. EMBO J. 1990 Jun;9(6):1839–1852. doi: 10.1002/j.1460-2075.1990.tb08309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian H., Gruss P., Duboule D., Izpisúa-Belmonte J. C. The murine even-skipped-like gene Evx-2 is closely linked to the Hox-4 complex, but is transcribed in the opposite direction. Mamm Genome. 1992;3(4):241–243. doi: 10.1007/BF00355726. [DOI] [PubMed] [Google Scholar]
- Cohn M. J., Izpisúa-Belmonte J. C., Abud H., Heath J. K., Tickle C. Fibroblast growth factors induce additional limb development from the flank of chick embryos. Cell. 1995 Mar 10;80(5):739–746. doi: 10.1016/0092-8674(95)90352-6. [DOI] [PubMed] [Google Scholar]
- Crossley P. H., Minowada G., MacArthur C. A., Martin G. R. Roles for FGF8 in the induction, initiation, and maintenance of chick limb development. Cell. 1996 Jan 12;84(1):127–136. doi: 10.1016/s0092-8674(00)80999-x. [DOI] [PubMed] [Google Scholar]
- D'Esposito M., Morelli F., Acampora D., Migliaccio E., Simeone A., Boncinelli E. EVX2, a human homeobox gene homologous to the even-skipped segmentation gene, is localized at the 5' end of HOX4 locus on chromosome 2. Genomics. 1991 May;10(1):43–50. doi: 10.1016/0888-7543(91)90482-t. [DOI] [PubMed] [Google Scholar]
- Davis A. P., Capecchi M. R. A mutational analysis of the 5' HoxD genes: dissection of genetic interactions during limb development in the mouse. Development. 1996 Apr;122(4):1175–1185. doi: 10.1242/dev.122.4.1175. [DOI] [PubMed] [Google Scholar]
- Davis A. P., Capecchi M. R. Axial homeosis and appendicular skeleton defects in mice with a targeted disruption of hoxd-11. Development. 1994 Aug;120(8):2187–2198. doi: 10.1242/dev.120.8.2187. [DOI] [PubMed] [Google Scholar]
- Davis A. P., Witte D. P., Hsieh-Li H. M., Potter S. S., Capecchi M. R. Absence of radius and ulna in mice lacking hoxa-11 and hoxd-11. Nature. 1995 Jun 29;375(6534):791–795. doi: 10.1038/375791a0. [DOI] [PubMed] [Google Scholar]
- Doetschman T. C., Eistetter H., Katz M., Schmidt W., Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985 Jun;87:27–45. [PubMed] [Google Scholar]
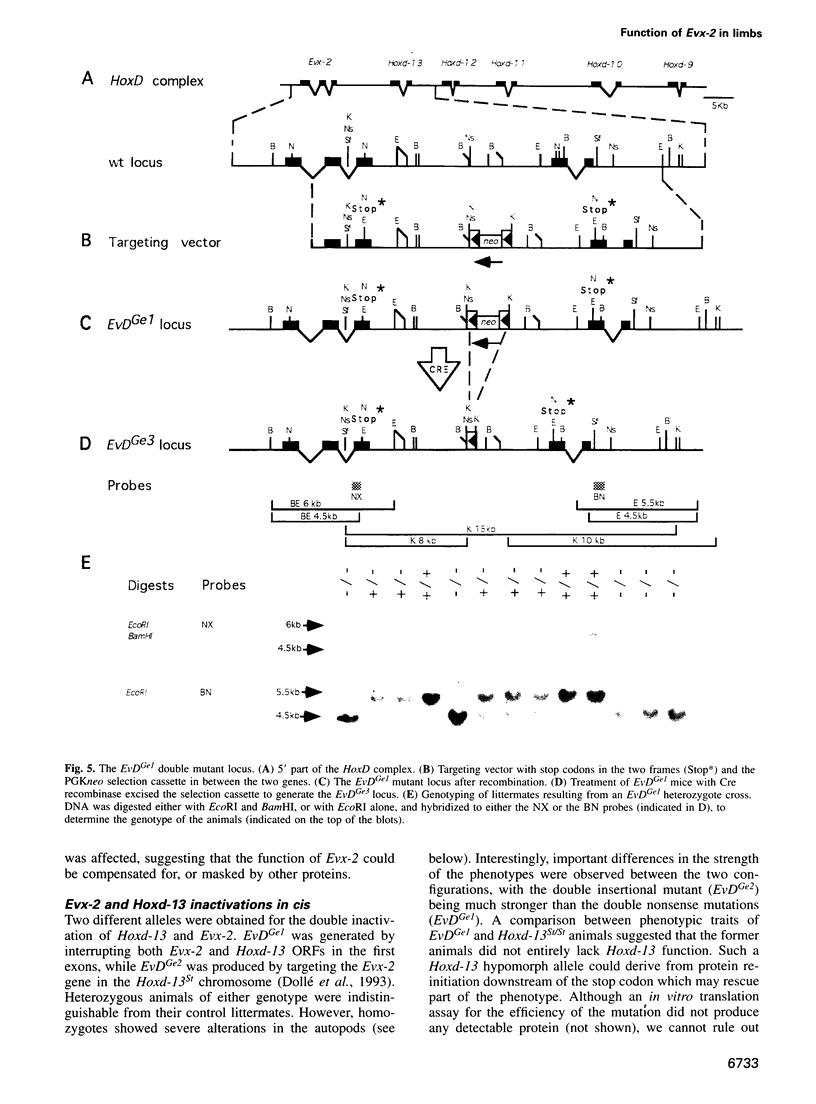
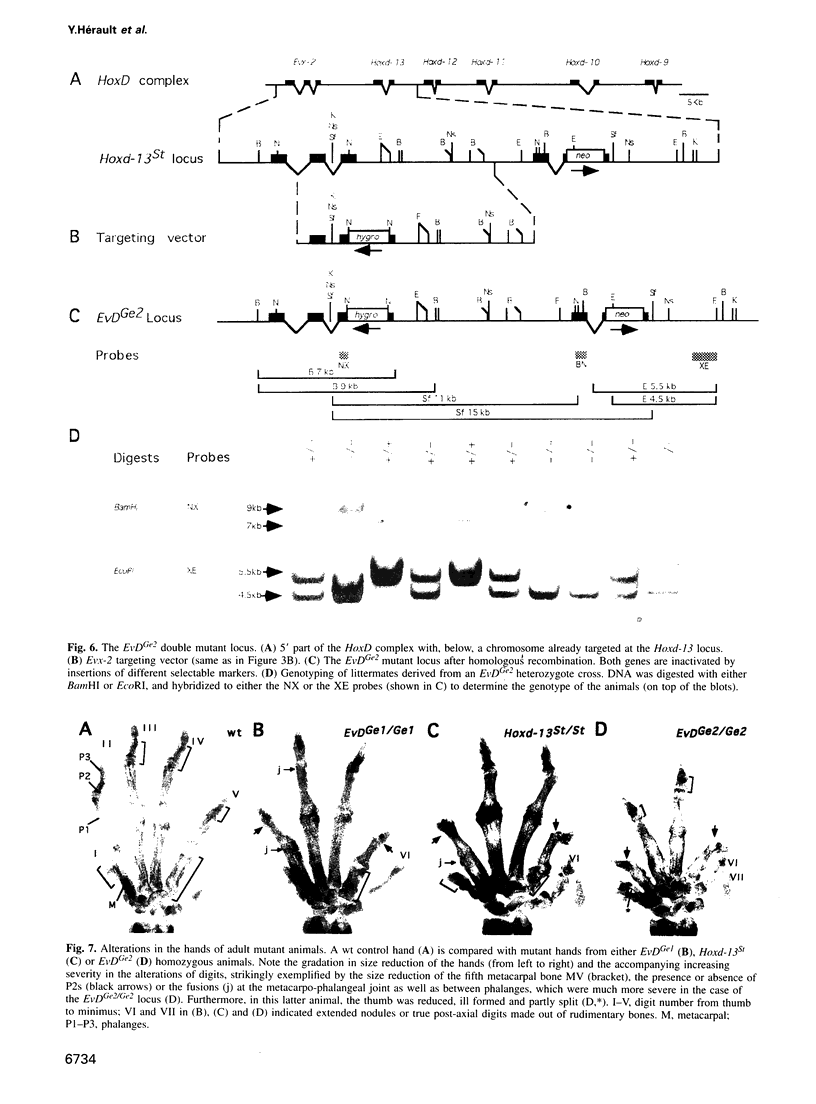
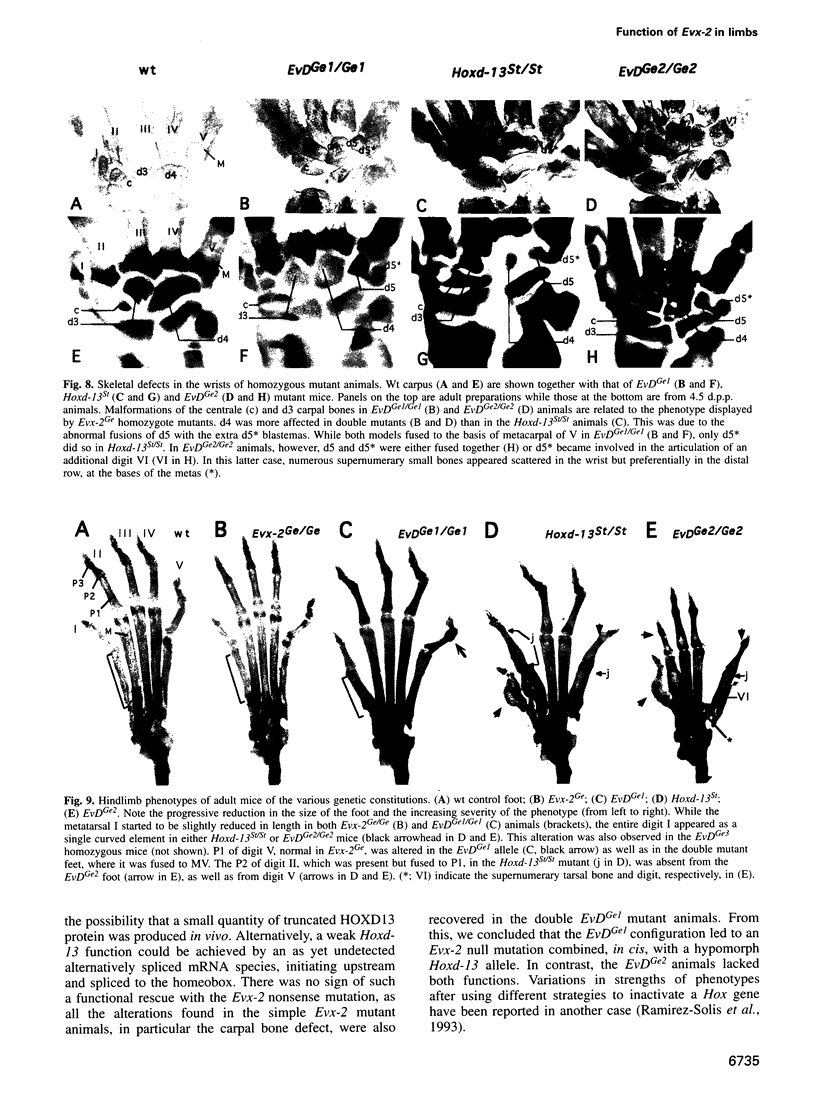
- Dollé P., Dierich A., LeMeur M., Schimmang T., Schuhbaur B., Chambon P., Duboule D. Disruption of the Hoxd-13 gene induces localized heterochrony leading to mice with neotenic limbs. Cell. 1993 Nov 5;75(3):431–441. doi: 10.1016/0092-8674(93)90378-4. [DOI] [PubMed] [Google Scholar]
- Dollé P., Fraulob V., Duboule D. Developmental expression of the mouse Evx-2 gene: relationship with the evolution of the HOM/Hox complex. Dev Suppl. 1994:143–153. [PubMed] [Google Scholar]
- Dollé P., Izpisúa-Belmonte J. C., Boncinelli E., Duboule D. The Hox-4.8 gene is localized at the 5' extremity of the Hox-4 complex and is expressed in the most posterior parts of the body during development. Mech Dev. 1991 Dec;36(1-2):3–13. doi: 10.1016/0925-4773(91)90067-g. [DOI] [PubMed] [Google Scholar]
- Dollé P., Izpisúa-Belmonte J. C., Falkenstein H., Renucci A., Duboule D. Coordinate expression of the murine Hox-5 complex homoeobox-containing genes during limb pattern formation. Nature. 1989 Dec 14;342(6251):767–772. doi: 10.1038/342767a0. [DOI] [PubMed] [Google Scholar]
- Dush M. K., Martin G. R. Analysis of mouse Evx genes: Evx-1 displays graded expression in the primitive streak. Dev Biol. 1992 May;151(1):273–287. doi: 10.1016/0012-1606(92)90232-6. [DOI] [PubMed] [Google Scholar]
- Faiella A., D'Esposito M., Rambaldi M., Acampora D., Balsofiore S., Stornaiuolo A., Mallamaci A., Migliaccio E., Gulisano M., Simeone A. Isolation and mapping of EVX1, a human homeobox gene homologous to even-skipped, localized at the 5' end of HOX1 locus on chromosome 7. Nucleic Acids Res. 1991 Dec 11;19(23):6541–6545. doi: 10.1093/nar/19.23.6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favier B., Le Meur M., Chambon P., Dollé P. Axial skeleton homeosis and forelimb malformations in Hoxd-11 mutant mice. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):310–314. doi: 10.1073/pnas.92.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favier B., Rijli F. M., Fromental-Ramain C., Fraulob V., Chambon P., Dollé P. Functional cooperation between the non-paralogous genes Hoxa-10 and Hoxd-11 in the developing forelimb and axial skeleton. Development. 1996 Feb;122(2):449–460. doi: 10.1242/dev.122.2.449. [DOI] [PubMed] [Google Scholar]
- Fromental-Ramain C., Warot X., Lakkaraju S., Favier B., Haack H., Birling C., Dierich A., Doll e P., Chambon P. Specific and redundant functions of the paralogous Hoxa-9 and Hoxd-9 genes in forelimb and axial skeleton patterning. Development. 1996 Feb;122(2):461–472. doi: 10.1242/dev.122.2.461. [DOI] [PubMed] [Google Scholar]
- Garcia-Fernández J., Holland P. W. Archetypal organization of the amphioxus Hox gene cluster. Nature. 1994 Aug 18;370(6490):563–566. doi: 10.1038/370563a0. [DOI] [PubMed] [Google Scholar]
- Gaunt S. J., Krumlauf R., Duboule D. Mouse homeo-genes within a subfamily, Hox-1.4, -2.6 and -5.1, display similar anteroposterior domains of expression in the embryo, but show stage- and tissue-dependent differences in their regulation. Development. 1989 Sep;107(1):131–141. doi: 10.1242/dev.107.1.131. [DOI] [PubMed] [Google Scholar]
- Graham A., Papalopulu N., Krumlauf R. The murine and Drosophila homeobox gene complexes have common features of organization and expression. Cell. 1989 May 5;57(3):367–378. doi: 10.1016/0092-8674(89)90912-4. [DOI] [PubMed] [Google Scholar]
- Hsieh-Li H. M., Witte D. P., Weinstein M., Branford W., Li H., Small K., Potter S. S. Hoxa 11 structure, extensive antisense transcription, and function in male and female fertility. Development. 1995 May;121(5):1373–1385. doi: 10.1242/dev.121.5.1373. [DOI] [PubMed] [Google Scholar]
- Izpisúa-Belmonte J. C., Falkenstein H., Dollé P., Renucci A., Duboule D. Murine genes related to the Drosophila AbdB homeotic genes are sequentially expressed during development of the posterior part of the body. EMBO J. 1991 Aug;10(8):2279–2289. doi: 10.1002/j.1460-2075.1991.tb07764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly J. S., Joly C., Schulte-Merker S., Boulekbache H., Condamine H. The ventral and posterior expression of the zebrafish homeobox gene eve1 is perturbed in dorsalized and mutant embryos. Development. 1993 Dec;119(4):1261–1275. doi: 10.1242/dev.119.4.1261. [DOI] [PubMed] [Google Scholar]
- Kondo T., Dollé P., Zákány J., Duboule D. Function of posterior HoxD genes in the morphogenesis of the anal sphincter. Development. 1996 Sep;122(9):2651–2659. doi: 10.1242/dev.122.9.2651. [DOI] [PubMed] [Google Scholar]
- Miles A., Miller D. J. Genomes of diploblastic organisms contain homeoboxes: sequence of eveC, an even-skipped homologue from the cnidarian Acropora formosa. Proc Biol Sci. 1992 May 22;248(1322):159–161. doi: 10.1098/rspb.1992.0057. [DOI] [PubMed] [Google Scholar]
- Morgan B. A., Tabin C. Hox genes and growth: early and late roles in limb bud morphogenesis. Dev Suppl. 1994:181–186. [PubMed] [Google Scholar]
- Olson E. N., Arnold H. H., Rigby P. W., Wold B. J. Know your neighbors: three phenotypes in null mutants of the myogenic bHLH gene MRF4. Cell. 1996 Apr 5;85(1):1–4. doi: 10.1016/s0092-8674(00)81073-9. [DOI] [PubMed] [Google Scholar]
- Parr B. A., McMahon A. P. Dorsalizing signal Wnt-7a required for normal polarity of D-V and A-P axes of mouse limb. Nature. 1995 Mar 23;374(6520):350–353. doi: 10.1038/374350a0. [DOI] [PubMed] [Google Scholar]
- Ramírez-Solis R., Zheng H., Whiting J., Krumlauf R., Bradley A. Hoxb-4 (Hox-2.6) mutant mice show homeotic transformation of a cervical vertebra and defects in the closure of the sternal rudiments. Cell. 1993 Apr 23;73(2):279–294. doi: 10.1016/0092-8674(93)90229-j. [DOI] [PubMed] [Google Scholar]
- Rijli F. M., Matyas R., Pellegrini M., Dierich A., Gruss P., Dollé P., Chambon P. Cryptorchidism and homeotic transformations of spinal nerves and vertebrae in Hoxa-10 mutant mice. Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8185–8189. doi: 10.1073/pnas.92.18.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satokata I., Benson G., Maas R. Sexually dimorphic sterility phenotypes in Hoxa10-deficient mice. Nature. 1995 Mar 30;374(6521):460–463. doi: 10.1038/374460a0. [DOI] [PubMed] [Google Scholar]
- Small K. M., Potter S. S. Homeotic transformations and limb defects in Hox A11 mutant mice. Genes Dev. 1993 Dec;7(12A):2318–2328. doi: 10.1101/gad.7.12a.2318. [DOI] [PubMed] [Google Scholar]
- Spyropoulos D. D., Capecchi M. R. Targeted disruption of the even-skipped gene, evx1, causes early postimplantation lethality of the mouse conceptus. Genes Dev. 1994 Aug 15;8(16):1949–1961. doi: 10.1101/gad.8.16.1949. [DOI] [PubMed] [Google Scholar]
- Tabin C. The initiation of the limb bud: growth factors, Hox genes, and retinoids. Cell. 1995 Mar 10;80(5):671–674. doi: 10.1016/0092-8674(95)90343-7. [DOI] [PubMed] [Google Scholar]
- Tickle C. Vertebrate limb development. Curr Opin Genet Dev. 1995 Aug;5(4):478–484. doi: 10.1016/0959-437x(95)90052-i. [DOI] [PubMed] [Google Scholar]
- Yokouchi Y., Nakazato S., Yamamoto M., Goto Y., Kameda T., Iba H., Kuroiwa A. Misexpression of Hoxa-13 induces cartilage homeotic transformation and changes cell adhesiveness in chick limb buds. Genes Dev. 1995 Oct 15;9(20):2509–2522. doi: 10.1101/gad.9.20.2509. [DOI] [PubMed] [Google Scholar]
- van der Hoeven F., Sordino P., Fraudeau N., Izpisúa-Belmonte J. C., Duboule D. Teleost HoxD and HoxA genes: comparison with tetrapods and functional evolution of the HOXD complex. Mech Dev. 1996 Jan;54(1):9–21. doi: 10.1016/0925-4773(95)00455-6. [DOI] [PubMed] [Google Scholar]
- van der Hoeven F., Zákány J., Duboule D. Gene transpositions in the HoxD complex reveal a hierarchy of regulatory controls. Cell. 1996 Jun 28;85(7):1025–1035. doi: 10.1016/s0092-8674(00)81303-3. [DOI] [PubMed] [Google Scholar]










