Abstract
Context:
Most studies show sleep homeostasis and continuity remain stable across the menstrual cycle in young women. The influence of the menstrual cycle on physiological sleep in midlife women is unknown.
Objective:
The objective of the study was to assess the impact of menstrual cycle phase on the polysomnogram and electroencephalographic (EEG) features of sleep in midlife women, accounting for the presence of an insomnia disorder.
Design and Participants:
This was a laboratory study of 20 women in the early menopausal transition (48.8 ± 2.9 y), 11 with a Diagnostic and Statistical Manual of Mental Disorders, fourth edition, diagnosis of insomnia, studied on one night each in the follicular and luteal menstrual cycle phases.
Main Outcome Measures:
Polysomnographic and sleep EEG indices were measured.
Results:
Both groups of women had more awakenings (P = .003) and arousals (P = .025) per hour of sleep and less percentage slow wave sleep (P = .024) when progesterone was raised (≥3 ng/mL−1) during the luteal compared with the follicular phase. Both groups had greater spindle density (P = .007), longer spindles (P = .037), and increased 14–17 Hz EEG activity in the luteal phase (P < .05), although for the 15- to 16-Hz bin, this effect was significant only in women without insomnia (P < .001). Women with insomnia had a shorter sleep duration (P = .012), more wakefulness after sleep onset (P = .031), and a lower sleep efficiency (P = .034) than women without insomnia, regardless of menstrual cycle phase.
Conclusion:
Sleep is more disrupted in the luteal phase compared with the follicular phase in midlife women, whether or not they have an insomnia disorder. There is a prominent increase in sleep spindles and spindle frequency activity in the luteal phase, likely an effect of progesterone and/or its neuroactive metabolites acting on sleep regulatory systems.
As women transition menopause, reproductive hormone levels change, with an overall rise in FSH and a decline in estradiol relative to premenopause (1). Many women approaching menopause experience bothersome symptoms, such as hot flashes, sleep disruption, and mood disturbances that have been linked with changes in their hormone levels (2).
Superimposed on these menopausal hormone changes are menstrual cycle-related fluctuations in hormones within cycles that are ovulatory in women who are in the early menopausal transition, as defined by Stages of Reproductive Aging Workshop criteria (3). Estradiol is the dominant reproductive hormone in the preovulatory follicular phase, whereas progesterone dominates in the postovulatory luteal phase. Several studies have investigated the impact of the menstrual cycle on sleep in young women, with most finding that sleep homeostasis, as reflected by sleep onset latency, slow wave (N3) sleep, and the time course of slow-wave activity (SWA) within nonrapid eye movement (NREM) sleep across the night, remains constant across menstrual phases (4–6). Most studies also show that sleep efficiency, wakefulness after sleep onset (WASO), and number of awakenings are similar across the menstrual cycle in young women. The most prominent effect of the menstrual cycle in young women is a marked increase in σ-electroencephalographic (EEG) activity (12–15 Hz), presumed to reflect an increase in sleep spindles (brief bursts of ∼12–15 Hz synchronous activity), in the luteal compared with the follicular phase (7–9), although only one small study has assessed menstrual cycle modulation of sleep spindle characteristics (10).
Reproductive aging may modulate the influence of the menstrual cycle on sleep architecture, but few studies have investigated sleep in midlife women at different menstrual cycle phases. The Study of Women Across the Nation (SWAN) showed that trouble sleeping is more common in perimenopausal than premenopausal women (11). In addition, more women, both pre- and early perimenopausal, reported trouble sleeping in the late luteal and early follicular phases compared with other phases of the menstrual cycle (11). Recently additional SWAN data showed that actigraphy-derived sleep measures varied with menstrual phase in midlife women, with total sleep time and sleep efficiency declining gradually across the menstrual cycle, being lowest in the premenstrual (late-luteal) phase (12). It remains unknown how the polysomnogram (PSG) and sleep EEG are impacted by menstrual cycle phase in perimenopausal women.
Studies of younger women typically focus on good sleepers with regular menstrual cycles and have considered the impact of menstrual-associated disorders such as premenstrual syndrome (PMS) or dysmenorrhea on sleep (7, 8, 13, 14). To our knowledge, studies have not investigated whether menstrual cycle variation in sleep architecture is affected by the presence of an insomnia disorder in young or midlife women. Insomniacs have a high night-to-night variability in their sleep patterns (15) and a heightened level of arousal that prevents them from obtaining a restorative sleep (16). During the menopausal transition, some women who have previously been good sleepers develop an insomnia disorder, suggesting altered sensitivity to the corresponding hormonal and/or other midlife changes. We previously found evidence of differences in hormone-sleep relationships between women with and without insomnia in the context of the menopausal transition (17). It therefore is possible that insomniacs may respond differently to the hormonal changes of the menstrual cycle.
Here we compared PSG and sleep EEG measures in the follicular and luteal phases of ovulatory menstrual cycles in women with and without clinical insomnia in the early menopausal transition. We hypothesized that women would have a more disturbed sleep in the luteal compared with the follicular phase. In addition, we hypothesized that they would show increased sleep spindles in the luteal phase relative to the follicular phase.
Materials and Methods
Participants
The 20 participants included in this analysis were drawn from a study investigating sleep in women in the menopausal transition; details are described by Sassoon et al (18). Participants were recruited from the San Francisco Bay Area community area through flyers, advertisements, or word of mouth. The study was approved by SRI International's Institutional Review Board. All participants gave written informed consent. Main exclusion criteria were having a body mass index (BMI) of 33 kg · m2 or greater, taking hormone therapy or hormonal contraception (during the previous 3 mo), having a hysterectomy or bilateral oophorectomy, having amenorrhea for 12 months or longer, having current severe medical and/or psychiatric conditions (eg, major depression, hypertension), having an apnea-hypopnea index greater than 5 and/or periodic leg movement index greater than 10 (based on a laboratory clinical PSG assessment), and current use of medication known to affect sleep (eg, hypnotics, antidepressants).
Women were selected for the current analysis if they had one PSG recording in each of the follicular and luteal phases of the menstrual cycle (see below); all were in the early menopause transition, as defined by Stages of Reproductive Aging Workshop criteria (3). Participants completed the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, fourth edition, (DSM-IV), Text Revision axis I disorders (19) including a customized module evaluating DSM-IV criteria for primary insomnia (20). Eleven women presented with a prominent self-described disturbance in sleep characterized by difficulty falling asleep, maintaining sleep, or early morning awakening, evident at least three times per week for at least a month and that caused clinically significant distress or impairment, qualifying them for a diagnosis of insomnia. The onset of sleep disturbance was coincident with the menopausal transition, and none of the women had a prior history of DSM-IV insomnia. The remaining nine women reported absent or minimal sleep difficulties. Sleep quality over the past month was assessed with the Pittsburgh Sleep Quality Index (PSQI) (21) with global scores greater than 5, reflecting poor sleep.
Experimental design
After initial screening, all participants had a clinical PSG to confirm the absence of a breathing-related or periodic limb movement sleep disorder and to adapt to the laboratory. Women then were scheduled for two overnight recordings, one in the follicular phase (mean ± SD 5.8 ± 2.6 d after period onset) and one in the luteal phase (mean ± SD 5.2 ± 3.3 d before their period). Nine women had their follicular phase recording first, and in 11 women (seven controls and four insomniacs), data from follicular and luteal phases were collected within the same menstrual cycle.
All recordings took place in sound-attenuated and temperature-controlled bedrooms at the sleep laboratory at SRI International. All women were asked to refrain from drinking beverages containing alcohol or caffeine after 3:00 pm of each recording day. Women had to register 0 on the breathalyzer at the start of each recording. Lights-out and lights-on were self-selected by participants.
Mood and hormone measures
At each PSG recording, current mood was assessed in the evening using the Profile of Mood States (22). A constant of 50 was added to the total score to avoid negative values.
Blood samples were collected either in the evening or morning of the recording based on the availability of the phlebotomist and participant, with most samples being collected in the evening. Samples were centrifuged and frozen at −70°C until analysis for FSH, progesterone, and estradiol using standard immunoassay kits. The intraassay and interassay coefficients of variations were 2.6% and 5.5%, respectively, for FSH (Siemens Healthcare Diagnostics), 4.0% and 5.7%, respectively, for progesterone (Siemens Medical Solutions Diagnostics), and 6.7% and 7.6%, respectively, for estradiol (Beckman Coulter Inc). One insomniac did not have a blood draw but gave a saliva sample at each visit, the analysis of which confirmed a higher level of progesterone during the luteal phase relative to the follicular phase.
Sleep assessment
EEG (F3, F4, C3, C4, Cz, O1, and O2 referenced to the mastoids; sampled at 256 Hz and 0.3–35 Hz filtered), bilateral electrooculographic, and submental electromyographic signals were recorded using Compumedics amplifiers and Profusion software (Compumedics) according to American Academy of Sleep Medicine criteria (23). Sleep stages and brief arousals (≥3 sec, <15 sec) were scored according to American Academy of Sleep Medicine criteria (23). Time in bed (TIB; minutes) was calculated as the time from lights-out to lights-on, total sleep time (TST; minutes) as the time spent asleep minus the total time spent awake, sleep efficiency (percentage) as TST/TIB100, sleep-onset latency (minutes) as the time from lights-out to the first epoch of any stage of sleep, REM-onset latency (minutes) as the time between sleep onset and the first epoch of REM sleep. The percentage of TIB spent awake after sleep onset (WASO, percentage), and in each stage of sleep (N1, N2, N3, and REM; percentage), the arousal index (as number of arousals multiplied by 60 and divided by TST), and the awakening index (number of awakenings multiplied by 60 and divided by TST) were also calculated.
Quantitative EEG analysis of artifact-free NREM (N2+N3) sleep was performed using EEGLAB (24). Epochs containing arousals were discharged from analysis (4.4% ± 1.9%). In addition, epochs that exceeded the mean ± 3 SD for each frequency band were excluded (maximum percentage excluded: 1.9% ± 0.7%). All EEG analyses are based on channel Cz. One insomniac was excluded from EEG analysis due to recording problems. EEG was rereferenced to the average mastoid and filtered at 0.3–30 Hz using an eighth-order Butterworth filter. Fast Fourier Transform analysis was conducted on each 30-sec epoch using a 4-sec sliding Hanning window to calculate power density values with 0.125 Hz resolution. Power density (square microvolts per Hertz) values were then averaged across individual hertz bins (from 1 to 29 Hz; bands are identified by the lower boundary).
A more refined analysis was conducted to characterize sleep spindles. Semiautomated spindle detection and quantification were conducted using an adaptation of methods used elsewhere (25, 26). Peak spindle frequency, between 9 and 16 Hz, was identified within the power spectrum for all artifact-free NREM sleep epochs. Data were then bandpass filtered to a 1.5-Hz band around the peak frequency (ie, peak frequency ± 0.75 Hz) for each participant. Thus, if the peak frequency was identified as 15 Hz, the band pass would have been set for 14.25–15.75 Hz. The Hilbert transform was then applied to the filtered data to calculate the instantaneous amplitude envelope of the spindle frequency band. As reported by Andrillon et al (26), a threshold of mean +3SD of the amplitude envelope was set for spindle detection and a threshold of mean +1SD for the start and end of the spindle. Spindles between 500 and 3000 msec were included in subsequent analyses. Mean spindle length (milliseconds), peak spindle amplitude (microvolts), and spindle density (number of spindles per minute) were calculated for each participant.
Hot flash measurement
All-night skin conductance was recorded from the sternum with the BioDerm Skin Conductance Meter (model 2701; UFI), and hot flashes were identified according to standard criteria (27). Skin conductance was missing due to technical problems for one insomniac's luteal phase PSG recording.
Statistical analysis
Demographic and self-report measures were compared between women with and without insomnia using independent t tests by group for continuous variables and χ2 tests for categorical variables. Reproductive hormone levels, mood scores, PSG sleep parameters, single-Hertz EEG power spectral measures, and EEG-derived spindle measures were analyzed using repeated-measure ANOVAs with phase (follicular and luteal) as a within factor and group (control vs insomnia) as a between factor. Bonferroni post hoc tests were used when interactions were significant. PSQI scores, hormone levels, and single-Hertz EEG power spectral measures were log transformed to normalize their distribution before analysis. The α-level was set at .05 for all analyses.
Results
Demographic and self-report measures
There were no differences in age (P = .13), BMI (P = .91), or education level (P = .73) between women with and without insomnia. Insomniacs had elevated PSQI scores (P = .001) (Table 1).
Table 1.
Demographic and Self-Report Measures for Women in the Early Menopausal Transition With and Without Insomnia Assessed at the Initial Visit
| Women With Insomnia Mean ± SD | Women Without Insomnia Mean ± SD | t or χ2 | P Value | |
|---|---|---|---|---|
| Sample size | 11 | 9 | ||
| Age, y | 49.6 ± 3.2 | 47.8 ± 2.1 | 1.58 | .13 |
| BMI, kg/m−2 | 24.0 ± 4.2 | 24.2 ± 3.8 | −0.12 | .91 |
| Education, y | 16.3 ± 2.1 | 16.6 ± 1.3 | −0.36 | .73 |
| Caucasian, n | 10 | 7 | 0.67 | .41 |
| Being in a relationship, n | 9 | 7 | 0.05 | .82 |
| Employed, n | 10 | 7 | 0.67 | .41 |
| Have children, n | 10 | 7 | 0.67 | .41 |
| Past history of DSM-IV major depression or alcohol/substance abuse/dependence, n | 3 | 1 | 0.81 | .37 |
| PSQI total scoresa | 8.4 ± 3.4 | 3.7 ± 1.4 | 4.38 | <.001 |
Analysis based on log-transformed data.
Hormones, mood, and hot flashes
There was a significant phase effect for progesterone and FSH, with both groups of women having higher progesterone and lower FSH levels in the luteal compared with the follicular phase (P < .001) (Table 2). Estradiol levels did not differ between phases or according to group. All women had progesterone levels of 3 ng/mL−1 or greater in the luteal phase, indicative of an ovulatory cycle (28). There were no significant phase, group, or interaction effects for perceived evening mood (Table 2). Hot flashes were detected in only three women. Presence of hot flashes was therefore not considered in the analysis.
Table 2.
Reproductive Hormone Levels, Evening Mood, and Statistical Comparisons for Women in the Early Menopausal Transition With and Without Insomnia in the Follicular and Luteal Phases of the Menstrual Cycle
| Follicular Phase Mean ± SD | Luteal Phase Mean ± SD | ANOVA | |
|---|---|---|---|
| FSH, IU/L−1a | |||
| Insomnia | 25.0 (19.4) | 5.4 (3.6) | Phase: F1,17 = 37.2, P < .001 |
| No insomnia | 19.2 (15.2) | 5.6 (2.4) | Group: F1,17 = 0.00, P = .98 |
| Group × phase: F1,17 = 2.05, P = .17 | |||
| Estradiol, pg/mL−1a | |||
| Insomnia | 43.5 (25.9) | 77.9 (40.2) | Phase: F1,17 = 1.32, P = .27 |
| No insomnia | 76.3 (53.5) | 52.6 (23.0) | Group: F1,17 = 0.06, P = .81 |
| Group × phase: F1,17 = 2.16, P = .16 | |||
| Progesterone, ng/mL−1a | |||
| Insomnia | 0.66 (0.39) | 8.04 (5.13) | Phase: F1,17 = 112.3, P < .001 |
| No insomnia | 0.75 (0.66) | 9.59 (5.91) | Group: F1,17 = 0.10, P = .75 |
| Group × phase: F1,17 = 0.25, P = .62 | |||
| POMS total scores | |||
| Insomnia | 52.4 (9.6) | 54.4 (13.0) | Phase: F1,18 = 0.19, P = .67 |
| No insomnia | 53.8 (17.5) | 49.2 (16.9) | Group: F1,18 = 0.11, P = .75 |
| Group × phase: F1,18 = 1.24, P = .28 | |||
Abbreviation: POMS, Profile of Mood States.
Analysis based on log-transformed data. One insomniac was excluded from the analyses because we did not obtain a blood draw for both menstrual phases (phases were confirmed based on salivary hormones).
Polysomnographic sleep
Women had a lower percentage of N3 sleep (P = .024) and more awakenings (P = .003) and arousals (P = .025) per hour of sleep during the luteal compared with the follicular phase (Table 3). Women with insomnia had a shorter total sleep time (P = .012), a poorer sleep efficiency (P = .034), and a greater amount of WASO (P = .031) compared with women without insomnia, regardless of menstrual cycle phase (Table 3). There were no significant group by menstrual phase interaction effects for any variables.
Table 3.
Polysomnographic Variables and Statistical Comparison for Women in the Early Menopausal Transition With and Without Insomnia in the Follicular and Luteal Phases of the Menstrual Cycle
| Follicular Phase Mean ± SD | Luteal Phase Mean ± SD | ANOVA | ||
|---|---|---|---|---|
| TIB, min | ||||
| Insomnia | 433.3 ± 46.7 | 437.7 ± 39.4 | Phase: F1,18 = 0.29, P = .60 | |
| No insomnia | 452.2 ± 34.1 | 457.6 ± 38.2 | Group: F1,18 = 1.54, P = .23 | |
| Group × phase: F1,18 = 0.00, P = .96 | ||||
| TST, min | ||||
| Insomnia | 375.6 ± 46.6 | 368.2 ± 31.8 | Phase: F1,18 = 0.84, P = .37 | |
| No insomnia | 416.6 ± 35.4 | 404.6 ± 39.7 | ▶ | Group: F1,18 = 7.76, P = .012 |
| Group × phase: F1,18 = 0.05, P = .83 | ||||
| SE, % | ||||
| Insomnia | 86.8 ± 6.5 | 84.4 ± 6.8 | Phase: F1,18 = 2.83, P = .11 | |
| No insomnia | 92.2 ± 4.4 | 88.5 ± 5.9 | ▶ | Group: F1,18 = 5.29, P = .034 |
| Group × phase: F1,18 = 0.13, P = .72 | ||||
| SOL, min | ||||
| Insomnia | 13.2 ± 9.5 | 10.5 ± 10.1 | Phase: F1,18 = 0.03, P = .87 | |
| No insomnia | 7.8 ± 7.6 | 11.4 ± 9.7 | Group: F1,18 = 0.45, P = .51 | |
| Group × phase: F1,18 = 1.47, P = .24 | ||||
| REML, min | ||||
| Insomnia | 88.1 ± 37.1 | 88.4 ± 62.6 | Phase: F1,18 = 0.00, P = .99 | |
| No insomnia | 75.0 ± 20.2 | 74.8 ± 18.1 | Group: F1,18 = 0.79, P = .39 | |
| Group × phase: F1,18 = 0.00, P = .99 | ||||
| Arousal index | ||||
| Insomnia | 8.2 ± 3.1 | 9.1 ± 3.0 | ▶ | Phase: F1,18 = 5.97, P = .025 |
| No insomnia | 7.1 ± 2.8 | 9.7 ± 3.1 | Group: F1,18 = 0.05, P = .83 | |
| Group × phase: F1,18 = 1.32, P = .27 | ||||
| Awakening index | ||||
| Insomnia | 3.1 ± 1.2 | 3.9 ± 1.1 | ▶ | Phase: F1,18 = 11.80, P = .003 |
| No insomnia | 2.5 ± 1.2 | 3.3 ± 0.9 | Group: F1,18 = 1.53, P = .23 | |
| Group × phase: F1,18 = 0.00, P = .99 | ||||
| WASO, %a | ||||
| Insomnia | 10.2 ± 6.1 | 13.3 ± 6.2 | Phase: F1,18 = 3.28, P = .087 | |
| No insomnia | 6.1 ± 3.8 | 9.1 ± 4.5 | ▶ | Group: F1,18 = 5.48, P = .031 |
| Group × phase: F1,18 = 0.00, P = .98 | ||||
| REM, %a | ||||
| Insomnia | 20.7 ± 4.8 | 19.3 ± 3.3 | Phase: F1,18 = 2.90, P = .11 | |
| No insomnia | 22.4 ± 3.3 | 19.7 ± 4.0 | Group: F1,18 = 0.63, P = .44 | |
| Group × phase: F1,18 = 0.33, P = .57 | ||||
| N1, %a | ||||
| Insomnia | 7.2 ± 2.7 | 7.2 ± 2.0 | Phase: F1,18 = 2.08, P = .17 | |
| No insomnia | 6.3 ± 2.6 | 8.3 ± 2.4 | Group: F1,18 = 0.01, P = .91 | |
| Group × phase: F1,18 = 2.12, P = .16 | ||||
| N2, %a | ||||
| Insomnia | 46.5 ± 6.1 | 47.4 ± 8.3 | Phase: F1,18 = 0.04, P = .85 | |
| No insomnia | 50.8 ± 5.8 | 50.5 ± 6.6 | Group: F1,18 = 1.82, P = .19 | |
| Group × phase: F1,18 = 0.18, P = .67 | ||||
| N3, %a | ||||
| Insomnia | 12.4 ± 6.8 | 10.5 ± 5.4 | ▶ | Phase: F1,18 = 6.08, P = .024 |
| No insomnia | 12.6 ± 3.6 | 10.0 ± 6.6 | Group: F1,18 = 0.00, P = .96 | |
| Group × phase: F1,18 = 0.18, P = .68 | ||||
Abbreviations: REM, rapid eye movement; REML, REM latency; SE, sleep efficiency; SOL, sleep onset latency.
▶, P < .05.
Calculated as percentage of time in bed.
EEG spectral analysis
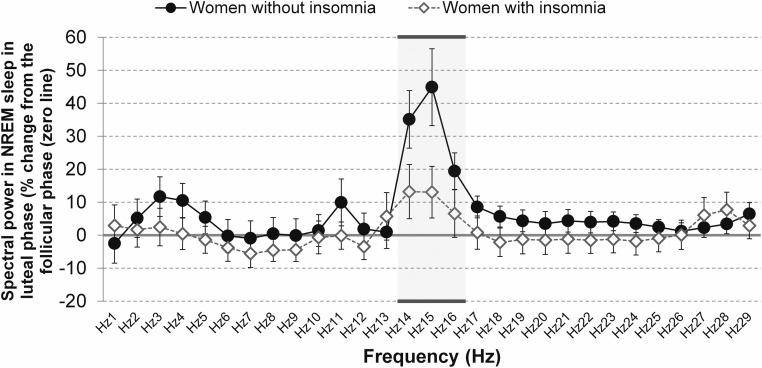
Changes in NREM sleep spectral power in the luteal phase relative to the follicular phase are shown in Figure 1, separately for women with and without insomnia.
Figure 1.
Spectral power for each single Hertz of the EEG spectrum (from 1 to < 30 Hz) analyzed in NREM sleep in the luteal phase as a percentage change from the follicular phase for women in the early menopausal transition with and without insomnia. The frequency bins in which ANOVA showed significant phase and/or phase by group effects are underlined. Bands are identified by their lower boundary.
ANOVAs revealed elevated spectral power in the luteal relative to follicular phase for activity at 14 to less than 15 Hz (F1,17 = 16.51, P < .001), 15 to less than 16 Hz (F1,17 = 20.85, P < .001), and 16 to less than 17 Hz (F1,17 = 7.05, P = .017). There was also a group by menstrual phase interaction for spectral power in the 15 to less than 16 Hz band (F1,17 = 6.27, P = .023), with post hoc analysis showing that spectral power was higher in the luteal compared with the follicular phase only in women without insomnia (P < .001). No significant menstrual phase, group or group by menstrual phase interaction effects were found for other frequency bands, including slow wave frequency bands (between 0.3 and 4 Hz) and frequencies in the highest range of the spectrum (>17 Hz).
Sleep spindle characteristics
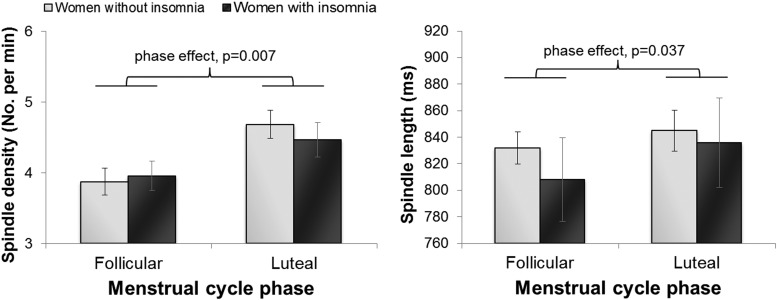
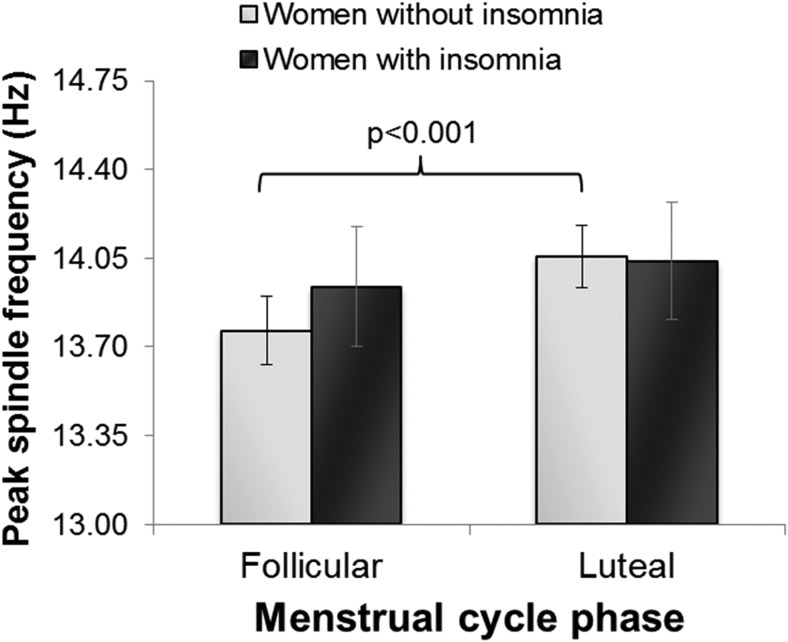
A more refined characterization of sleep spindles showed main effects of menstrual cycle phase for spindle density (F1,17 = 4.13, P = .007; Figure 2) and average length of spindles (phase: F1,17 = 5.11, P = .037; Figure 2), with spindle density being greater and spindles longer in the luteal phase. Peak spindle frequency was faster in the luteal phase (F1,17 = 28.64, P < .001). Post hoc analysis of the significant group by menstrual phase interaction effect (F1,17 = 6.86, P = .018; Figure 3) revealed that faster spindle frequency in the luteal phase only occurred in women without insomnia (P < .001). No phase, group or group by menstrual phase interactions were found for peak spindle amplitude (mean ± SD, follicular phase: insomnia: 18.0 ± 4.5 μV, no insomnia: 15.1 ± 3.7 μV; luteal phase: insomnia: 18.1 ± 4.5 μV, no insomnia: 15.1 ± 3.8 μV).
Figure 2.
Spindle density (number of spindles per minute) and average spindle length (milliseconds) in NREM sleep in the follicular and luteal phases of the menstrual cycle in women in the early menopausal transition with and without insomnia.
Figure 3.
Peak spindle frequency (Hertz) identified between 9 and 16 Hz in NREM sleep in the follicular and luteal phases of the menstrual cycle in women in the early menopausal transition with and without insomnia.
Discussion
Menstrual cycle phase impacted sleep PSG and EEG in women who still had ovulatory cycles in the early menopausal transition, with the luteal phase being characterized by more awakenings and arousals and less percentage N3 sleep. The luteal phase was also associated with elevated EEG activity in the range of 14 to less than 17 Hz, which encompasses the upper frequency range of spindles, as well as greater spindle density compared with the follicular phase. These menstrual cycle phase effects were evident in women with and without an insomnia disorder in the context of the menopausal transition.
Our findings of increased sleep disruption in the luteal phase based on PSG-derived measures of sleep complement findings from two SWAN studies showing an increase in reported trouble sleeping (11) and a decline in actigraphy-measured sleep efficiency (12) during the late-luteal phase in women approaching menopause. Whereas studies of younger women have also shown an increase in reported poor quality sleep in the late-luteal phase (29), most but not all (8, 30),PSG studies in regularly cycling women (typically investigating women younger than 40 y of age) have found that sleep efficiency, WASO, and sleep-onset latency remain stable at different phases of the menstrual cycle (4–6, 9). Rate of change rather than absolute hormone levels may influence sleep disturbance; Sharkey and colleagues (31) found an association between a steeper rise in progesterone from the follicular to the early to midluteal phase and percentage WASO in the luteal phase.
Most studies in younger women found no difference in N3 sleep and/or SWA at different phases of the menstrual cycle (4–6, 9), although an analysis across sleep cycles showed higher SWA in the first NREM sleep episode and lower SWA in the second NREM sleep episode in the midluteal relative to the midfollicular phase (4). In contrast, we found an overall reduction in percentage of N3 sleep (although SWA within NREM sleep was unchanged) in the luteal relative to the follicular phase. Taken together, our findings suggest that sleep in midlife women may be more vulnerable to the physiological changes associated with the luteal phase, although studies in larger samples are needed to directly compare the influence of menstrual cycle phase on the PSG and sleep EEG in young and midlife women.
Our finding of increased activity in the upper spindle frequency range and adjacent higher frequency bands (14 to <17 Hz) in the luteal phase supports previous findings, although the band with maximal increase in activity was 1 Hz higher than that in younger women (14.25–15.0 Hz) (7–9). There is an age-related increase in sigma frequency (32), which may interact with menstrual cycle-related effects on spindle frequency activity. Peak spindle frequency increased from the follicular to the luteal phase, significantly in the women without insomnia, which supports findings of Ishizuka et al (10). They did not find a significant variation in spindle density, amplitude, or duration across the menstrual cycle in their small sample of five young women. We found that spindle density and duration were increased in the absence of a change in spindle amplitude in the luteal phase. Spindles are generated by a network involving γ-aminobutyric acid (GABA) thalamic reticular, thalamocortical, and corticothalamic neurons (33). It is hypothesized that progesterone metabolites acting at GABA-A receptors are responsible for the luteal phase increase in spindle frequency EEG activity (9), which is partly supported by findings that progesterone administration is associated with enhanced EEG activity above 10 Hz in rats and above 15 Hz in men (34) and that women taking synthetic progestins have higher sleep spindle density and maximal spindle amplitude compared with women not taking progestins (35). However, we and others (9) have not found a corresponding suppression of SWA in the luteal phase, in contrast to the effects on sleep of administration of modulators of GABA-A receptors, including progesterone, allopregnanolone, and benzodiazepine and nonbenzodiazepine hypnotics (34). It has also been argued that increased spindle frequency activity is a consequence of the progesterone-mediated rise in body temperature in the luteal phase (36). The precise mechanism underlying the luteal phase increase in sleep spindles remains to be elucidated.
It has been hypothesized that sleep spindles serve a sleep-protective function by blocking the transfer of information to the cortex (37) and that increased sleep spindles may be a mechanism through which sleep quality is maintained in the presence of hormonal and physiological changes in the luteal phase (6), supported by findings from most studies in younger women that menstrual cycle phase has minimal impact on sleep continuity. However, the women in our study had a more disrupted sleep in the luteal phase despite having increased spindles, raising the possibility that midlife women may be more vulnerable to the hormonal changes of the menstrual cycle or that spindles are less effective at protecting sleep than in younger women. Although we focused on the impact of menstrual cycle-related variation on sleep in the early menopausal transition, SWAN data indicate that there is also a menopause status-related variation in σ- and β-EEG activity in NREM sleep, being higher in late peri- and postmenopausal women, suggesting a higher arousal level, than in premenopausal and early menopausal women recorded in the follicular phase (38). Possibly the vulnerability to arousal we detected in the luteal phase is a precursor to a change in arousal levels as women transition menopause and experience further hormone changes and the emergence of hot flashes.
Previous PSG studies have focused on menstrual cycle variation in young, good sleepers, and there are no data, to our knowledge, in younger or midlife women with insomnia with which to compare our results. Overall, insomniacs had more WASO and shorter TST than controls, and, like controls, they showed an increase in number of awakenings and arousals in the luteal phase compared with the follicular phase. The menstrual cycle therefore appears to influence sleep macroarchitecture similarly in women with and without insomnia in the context of the menopausal transition. Analysis of sleep microarchitecture revealed less consistency in the sleep spindle response to the luteal phase in insomniacs, with a blunted rise in spindle frequency EEG activity and the absence of an increase in peak spindle frequency found in noninsomniacs; possibly the influence of the menstrual cycle hormone changes on sleep EEG is weaker in the presence of an already disturbed sleep that characterized the insomniacs in our study. Because we focused on women who developed insomnia in the context of the menopausal transition, it would be interesting for future studies to investigate the influence of the menstrual cycle on sleep in younger women with insomnia.
Our findings need to be considered in context of the study limitations. We recorded sleep on only one night in the follicular phase and one night in the luteal phase. As such, we were unable to investigate the progression of sleep changes across the menstrual cycle. Most women were recorded in the last week of the luteal phase, and our results therefore may be more reflective of the late-luteal phase, when progesterone and estradiol levels are declining, as opposed to the midluteal phase, when hormone levels peak. Also, we specifically focused on women in the menopausal transition with confirmed ovulatory cycles. There are other hormonal changes occurring across the menopausal transition, with a rise in FSH and decrease in estradiol and consequently more irregular menstrual cycle and more annovulatory cycles. As shown elsewhere (11, 17, 38, 39), this overall changing hormone environment affects sleep over and above the menstrual cycle-related changes in sleep shown here. As such, changes in sleep related to the menstrual cycle are one piece in the overall picture of how sleep is affected in midlife women.
Our study also has several strengths. We confirmed that none of the women had a breathing- or movement-related sleep disorder. We also confirmed with blood samples that women had ovulatory cycles, which is critical, especially in this population, who have frequent annovulatory cycles. We evaluated sleep spindle characteristics in addition to EEG activity and could show clear increases in spindle density and spindle duration in the luteal phase. We also measured hot flashes because hot flashes are known to disturb sleep (40, 41). In this sample of early-transition women, few women had hot flashes; these events are likely to disrupt sleep more in late transition and early postmenopausal women. Finally, we considered for the first time the potential moderating effect of insomnia in the context of the menopausal transition on menstrual cycle-related effects on sleep.
In conclusion, we show that women with ovulatory cycles in the early menopausal transition have more awakenings and arousals in the luteal phase, whether or not they have an insomnia disorder. Menstrual cycle-related variation in hormones may contribute to cyclical sleep disturbances in women in the menopausal transition.
Acknowledgments
We thank our research assistants Justin Greco, David Sugarbaker, David Dresser, Stephanie Claudatos, Sarah Inkelis, and Lena Kardos for their effort in collecting data for this project.
This study was supported by National Institutes of Health Grant HL103688 (to F.C.B.). The hormone analysis was conducted by The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core, which is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health (National Centers for Translational Research in Reproduction and Infertility) Grant P50-HD28934.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders, fourth edition
- EEG
- electroencephalographic
- GABA
- γ-aminobutyric acid
- N3
- slow wave
- NREM
- nonrapid eye movement
- PMS
- premenstrual syndrome
- PSG
- polysomnogram
- PSQI
- Pittsburgh Sleep Quality Index
- SWA
- slow-wave activity
- SWAN
- Study of Women Across the Nation
- TIB
- Time in bed
- TST
- total sleep time
- WASO
- wakefulness after sleep onset.
References
- 1. Santoro N. The menopausal transition. Am J Med. 2005;118(suppl 12B):18–23. [DOI] [PubMed] [Google Scholar]
- 2. Joffe H, Massler A, Sharkey K. Evaluation and management of sleep disturbance during the menopause transition. Semin Reprod Med. 2010;28:404–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Soules M, Sherman S, Parrott E, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW). Climacteric. 2001;4:267–272. [PubMed] [Google Scholar]
- 4. Driver HS, Werth E, Dijk D-J, Borbély AA. The menstrual cycle effects on sleep. Sleep Med Clin. 2008;3:1–11. [Google Scholar]
- 5. Baker F, Driver H. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 2007;8:613–622. [DOI] [PubMed] [Google Scholar]
- 6. Shechter A, Boivin DB. Sleep, hormones, and circadian rhythms throughout the menstrual cycle in healthy women and women with premenstrual dysphoric disorder. Int J Endocrinol. 2010;2010:259345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baker F, Sassoon S, Kahan T, et al. Perceived poor sleep quality in the absence of polysomnographic sleep disturbance in women with severe premenstrual syndrome. J Sleep Res. 2012;21:535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baker FC, Kahan TL, Trinder J, Colrain IM. Sleep quality and the sleep electroencephalogram in women with severe premenstrual syndrome. Sleep. 2007;30:1283–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Driver H, Dijk D, Werth E, Biedermann K, Borbély A. Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women. J Clin Endocrinol Metab. 1996;81:728–735. [DOI] [PubMed] [Google Scholar]
- 10. Ishizuka Y, Pollak C, Shirakawa S, et al. Sleep spindle frequency changes during the menstrual cycle. J Sleep Res. 1994;3:26–29. [DOI] [PubMed] [Google Scholar]
- 11. Kravitz H, Janssen I, Santoro N, et al. Relationship of day-to-day reproductive hormone levels to sleep in midlife women. Arch Intern Med. 2005;165:2370–2376. [DOI] [PubMed] [Google Scholar]
- 12. Zheng H, Harlow S, Kravitz H, et al. Actigraphy-defined measures of sleep and movement across the menstrual cycle in midlife menstruating women: Study of Women's Health Across the Nation Sleep Study. Menopause. 2015;22:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parry B, Martínez L, Maurer E, López A, Sorenson D, Meliska C. Sleep, rhythms and women's mood. Part I. Menstrual cycle, pregnancy and postpartum. Sleep Med Rev. 2006;10:129–144. [DOI] [PubMed] [Google Scholar]
- 14. Shechter A, Lespérance P, Ng YKN, Boivin D. Nocturnal polysomnographic sleep across the menstrual cycle in premenstrual dysphoric disorder. Sleep Med. 2012;13:1071–1078. [DOI] [PubMed] [Google Scholar]
- 15. Vallières A, Ivers H, Bastien C, Beaulieu-Bonneau S, Morin C. Variability and predictability in sleep patterns of chronic insomniacs. J Sleep Res. 2005;14:447–453. [DOI] [PubMed] [Google Scholar]
- 16. Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14:19–31. [DOI] [PubMed] [Google Scholar]
- 17. de Zambotti M, Colrain IM, Baker FC. Interaction between reproductive hormones and physiological sleep in women. J Clin Endocrinol Metab. 2015;100(4):1426–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sassoon S, de Zambotti M, Colrain I, Baker F. Association between personality traits and DSM-IV diagnosis of insomnia in peri-and postmenopausal women. Menopause. 2014;21:602–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID). Version 2.0 New York: Biometrics Research Department, New York State Psychiatric Institute; 1998. [Google Scholar]
- 20. Morin CM, Espie CA. Insomnia: A Clinical Guide to Assessment and Treatment. New York: Springer; 2003. [Google Scholar]
- 21. Buysse D, Reynolds C, III, Monk T, Berman S, Kupfer D. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 22. McNair D, Lorr M, Droppleman L. Profile of Mood State Manual. San Diego: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 23. Iber C. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Darien, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 24. Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. [DOI] [PubMed] [Google Scholar]
- 25. Bódizs R, Körmendi J, Rigó P, Lázár A. The individual adjustment method of sleep spindle analysis: methodological improvements and roots in the fingerprint paradigm. J Neurosci Methods. 2009;178:205–213. [DOI] [PubMed] [Google Scholar]
- 26. Andrillon T, Nir Y, Staba RJ, et al. Sleep spindles in humans: insights from intracranial EEG and unit recordings. J Neurosci. 2011;31:17821–17834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Freedman R. Laboratory and ambulatory monitoring of menopausal hot flashes. Psychophysiology. 1989;26:573–579. [DOI] [PubMed] [Google Scholar]
- 28. Israel R, Mishell D, Jr, Stone S, Thorneycroft I, Moyer D. Single luteal phase serum progesterone assay as an indicator of ovulation. Am J Obstet Gynecol. 1972;112:1043–1046. [DOI] [PubMed] [Google Scholar]
- 29. Baker F, Driver H. Self-reported sleep across the menstrual cycle in young, healthy women. J Psychosom Res. 2004;56:239–243. [DOI] [PubMed] [Google Scholar]
- 30. Parry B, Mendelson W, Duncan W, Sack D, Wehr T. Longitudinal sleep EEG, temperature, and activity measurements across the menstrual cycle in patients with premenstrual depression and in age-matched controls. Psychiatry Res. 1989;30:285–303. [DOI] [PubMed] [Google Scholar]
- 31. Sharkey K, Crawford S, Kim S, Joffe H. Objective sleep interruption and reproductive hormone dynamics in the menstrual cycle. Sleep Med. 2014;15:688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nicolas A, Petit D, Rompré S, Montplaisir J. Sleep spindle characteristics in healthy subjects of different age groups. Clin Neurophysiol. 2001;112:521–527. [DOI] [PubMed] [Google Scholar]
- 33. Steriade M. Corticothalamic resonance, states of vigilance and mentation. Neuroscience. 2000;101:243–276. [DOI] [PubMed] [Google Scholar]
- 34. Lancel M. Role of GABAA receptors in the regulation of sleep: initial sleep responses to peripherally administered modulators and agonists. Sleep. 1999;22:33–42. [DOI] [PubMed] [Google Scholar]
- 35. Plante DT, Goldstein MR. Medroxyprogesterone acetate is associated with increased sleep spindles during non-rapid eye movement sleep in women referred for polysomnography. Psychoneuroendocrinology. 2013; 38(12):3160–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Deboer T. Brain temperature dependent changes in the electroencephalogram power spectrum of humans and animals. J Sleep Res. 1998;7:254–262. [DOI] [PubMed] [Google Scholar]
- 37. Steriade M, McCormick D, Sejnowski T. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. [DOI] [PubMed] [Google Scholar]
- 38. Campbell I, Bromberger J, Buysse D, et al. Evaluation of the association of menopausal status with delta and beta EEG activity during sleep. Sleep. 2011;34:1561–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sowers MF, Zheng H, Kravitz HM, et al. Sex steroid hormone profiles are related to sleep measures from polysomnography and the Pittsburgh Sleep Quality Index. Sleep. 2008;31:1339–1349. [PMC free article] [PubMed] [Google Scholar]
- 40. de Zambotti M, Colrain IM, Javitz HS, Baker FC. Magnitude of the impact of hot flashes on sleep in perimenopausal women. Fertil Steril. 2014;102:1708–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Woods N, Smith-Dijulio K, Percival D, Tao E, Taylor H, Mitchell E. Symptoms during the menopausal transition and early postmenopause and their relation to endocrine levels over time: observations from the Seattle Midlife Women's Health Study. J Womens Health (Larchmt). 2007;16:667–677. [DOI] [PubMed] [Google Scholar]