Abstract
Context:
IGF-2 is highly expressed in the granulosa cells of human dominant ovarian follicles; however, little is known about the regulation of the IGF-2 gene or the interaction of IGF-2 and FSH during follicle development.
Objective:
To examine the mechanisms involved in the regulation of the IGF-2 gene by FSH and the interplay between FSH and IGF-2 during granulosa cell differentiation.
Design, Setting, Patients, and Interventions:
Cumulus granulosa cells were separated from cumulus-oocyte complexes isolated from the follicular aspirates of in vitro fertilization patients and cultured for in vitro studies.
Main Outcome:
Protein and mRNA levels of IGF-2 and CYP19A1 (aromatase) were quantified using Western blot and quantitative real-time PCR. IGF-2 promoter-specific activation was determined by the amplification of alternative exons by PCR. Cell proliferation was assessed after treatment with FSH and/or IGF-2.
Results:
FSH significantly enhanced IGF-2 expression after 8 hours of treatment and at low doses (1 ng/mL). Reciprocally, IGF-2 synergized with FSH to increase cell proliferation and the expression of CYP19A1. When IGF-2 activity was blocked, FSH was no longer able to stimulate CYP19A1 expression. Determination of IGF-2 promoter usage in human cumulus cells showed that the IGF-2 gene is driven by promoters P3 and P4. However, FSH exclusively increased P3 promoter-derived transcripts. Moreover, the FSH-induced stimulation of P3-driven IGF-2 transcripts was blocked by cotreatment with inhibitors of AKT or IGF-1 receptor (IGF-1R). The inhibitory effect of the IGF-1R inhibitor on FSH-induced IGF-2 mRNA accumulation was reversed by overexpression of a constitutively active AKT construct.
Conclusions:
FSH is a potent enhancer of IGF-2 expression in human granulosa cells. In return, IGF-2 activation of the IGF-1R and AKT is required for FSH to stimulate CYP19A1 expression and proliferation of granulosa cells. These findings suggest a positive loop interaction between FSH and IGF-2 that is critical for human granulosa cell proliferation and differentiation.
In humans, the yearlong process of ovarian follicle recruitment, growth, and maturation comes to its climax with the release of a mature oocyte at ovulation. The last step of this process is relatively fast, lasting approximately 15 days; it is controlled by FSH, and to some degree by LH (1, 2). During this time, FSH stimulates follicle progression from the preantral to the preovulatory stage. In particular, FSH promotes granulosa cell proliferation and differentiation. The differentiation of the granulosa cells from the preantral to the preovulatory stage is essential, not only for ovulation but also for the maintenance of early pregnancy. During the preantral to preovulatory transition, granulosa cells acquire the capacity to produce high quantities of estradiol by expressing aromatase (CYP19A1) and the capacity to respond to LH by expression of the LH receptor, which is required for ovulation and the formation and maintenance of the corpus luteum.
Although FSH is required for preovulatory follicle formation and granulosa cell differentiation, FSH alone is not enough, and other factors are required. For example, in rodents that lack either FSH or the FSH receptor, the progression of follicles beyond the preantral stage is blocked (3, 4); however, a block on preovulatory follicle formation is also observed in IGF-1 knockout mice (5), demonstrating that an active IGF system is required for follicle maturation to the preovulatory stage. In rodents and humans, IGF-1 receptor (IGF-1R) activity is necessary for FSH to stimulate CYP19A1 expression (6, 7). Therefore, FSH and IGF signaling are crucial for the transition of follicles from the preantral to the preovulatory stage. However, the mechanisms by which FSH and IGFs regulate these processes are not fully understood, particularly in humans.
Significant differences in the ovarian IGF system exist between rodents and humans. In rodents, granulosa cells produce mostly IGF-1 (8). In contrast, IGF-2 mRNA levels are high in human granulosa cells, and IGF-1 mRNA is not detectable (7, 9, 10). Consequently, the follicular fluid of human dominant follicles contains up to 10-fold more IGF-2 than IGF-1 (11, 12). The high concentration of intrafollicular IGF-2 provides an explanation as to why a 1.6-fold increase in intrafollicular IGF-1 has no effect on follicle maturation in primates (13). A central role of IGF-2 in regulating follicle growth in humans is further supported by the positive association between intrafollicular IGF-2 levels, follicle maturation, and oocyte maturation (12, 14, 15). Together, these observations suggest that IGF-2 is the main player of the IGF system in human granulosa cells.
In support of this idea, IGF-2 has been shown to stimulate proliferation (16) and the production of estradiol and progesterone in human luteinized granulosa cells (17–20). In primates, IGF-2 increases steroidogenesis and promotes vascular endothelial growth factor production in preovulatory follicles (17, 21–23). IGF-2 stimulates estradiol and progesterone accumulation in granulosa cells of small (2 to 7 mm) follicles and preovulatory follicles, but it seems to have no effect on proliferation (22). Finally, the preferential gene expression and secretion of IGF-2 by the granulosa cells of the human dominant follicle strongly suggest that IGF-2 plays a critical role in follicular maturation by the way of autocrine actions (9).
Despite this evidence, the interaction between FSH and IGF-2 during human granulosa cell differentiation has not been examined. We hypothesize that granulosa cell-produced IGF-2 acts in concert with FSH to regulate granulosa cell differentiation and follicle growth in humans. The aim of this study was to examine the mechanisms involved in the regulation of the IGF-2 gene by FSH in human granulosa cells and the interplay between FSH and IGF-2 during granulosa cell differentiation. To accomplish this aim, we used primary cultures of human cumulus granulosa cells for in vitro studies. We previously observed that cumulus cells express low levels of the preovulatory differentiation markers including aromatase (CYP19A1), P450 side chain cleavage (CYP11A1), LH/choriogonadotropin receptor, and steroidogenic acute regulator. In addition, we demonstrated that FSH stimulates the expression of these genes and estradiol synthesis (7). In this report, we show that IGF-2 and FSH synergize to promote the proliferation and the expression of differentiation markers in human cumulus cells and that IGF-2 is required for the stimulation of these cells by FSH. In addition, we show that FSH exclusively activates promoter-3 of the IGF-2 gene. Finally, we demonstrate that AKT activity stimulated by endogenous IGF-2 is critical for the FSH-induced IGF-2 expression in human granulosa cells.
Materials and Methods
Human cumulus cell culture
Human cumulus cells were collected from patients undergoing in vitro fertilization (IVF) at the University of Illinois Hospital under an institutional review board-exempt protocol. No patient information was collected for reporting. After controlled ovarian stimulation, mature follicles were aspirated from women undergoing IVF. The cumulus-oocyte complexes were then removed from the follicular aspirates, and the cumulus cells were manually separated from the oocyte. The cumulus cells from all follicles for a single patient were then pooled, centrifuged at 1000 × g for 5 minutes, resuspended in phenol-red free DMEM F/12 (Sigma-Aldrich), and broken up to a single-cell suspension by gentle pipetting. Cells were then cultured on plates precoated with BD Matrigel (BD Biosciences) at a density of 1.0 × 105/mL in phenol-red free DMEM/F12 supplemented with penicillin (50 IU/mL), streptomycin (50 μg/mL), sodium bicarbonate (1.2 g/L; Sigma-Aldrich), and BSA (0.25 w/v; Sigma-Aldrich). Cells were cultured for 24 hours, followed by the treatments indicated below in the legends of Figures 1–6. Treatments include human recombinant FSH (Serono), human recombinant IGF-2 (Sigma-Aldrich), human recombinant IGF binding protein 4 (IGFBP-4; Sigma-Aldrich), NVP-AEW451 (AEW, a specific inhibitor of IGF-1R; Cayman Chemical Co), MK2206 (an inhibitor of AKT; Selleck Chemicals), U0126 (an inhibitor of ERK1/2 activation; Millipore), or dimethyl sulfoxide (DMSO; vehicle) control.
Figure 1.
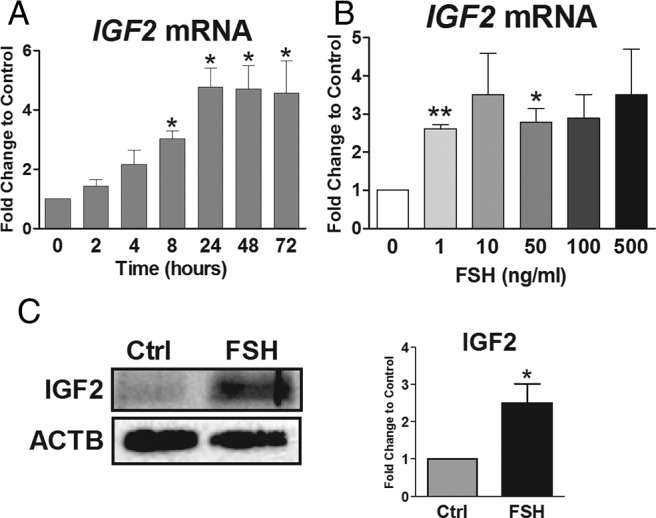
FSH up-regulates IGF-2 expression. A and B, Cumulus cells were cultured in serum-free and phenol red-free media for 24 hours, followed by treatment with (A) FSH (50 ng/mL) for increasing amounts of time (2 to 72 h) or (B) increasing doses of FSH (1–500 ng/mL) for 48 hours. RNA was isolated and reverse-transcribed, and IGF-2 transcript abundance was quantified by qPCR. For A and B, columns represent the mean ± SEM for three independent experiments. *, P < .05; **, P < .01, relative to control (one-way ANOVA). C, Cumulus cells cultured in serum-free and phenol red-free media for 24 hours were treated with FSH (50 ng/mL) or left untreated (Ctrl) for 48 hours. IGF-2 and ACTB expression (loading control) was assessed by Western blot. The IGF-2 band intensities were adjusted to band intensity of ACTB on the same blot, and the ratio of FSH-treated relative to untreated cells is reported on the right graph. Columns represent the mean ± SEM for three independent experiments. *, P < .05; Mann-Whitney U test.
Figure 2.
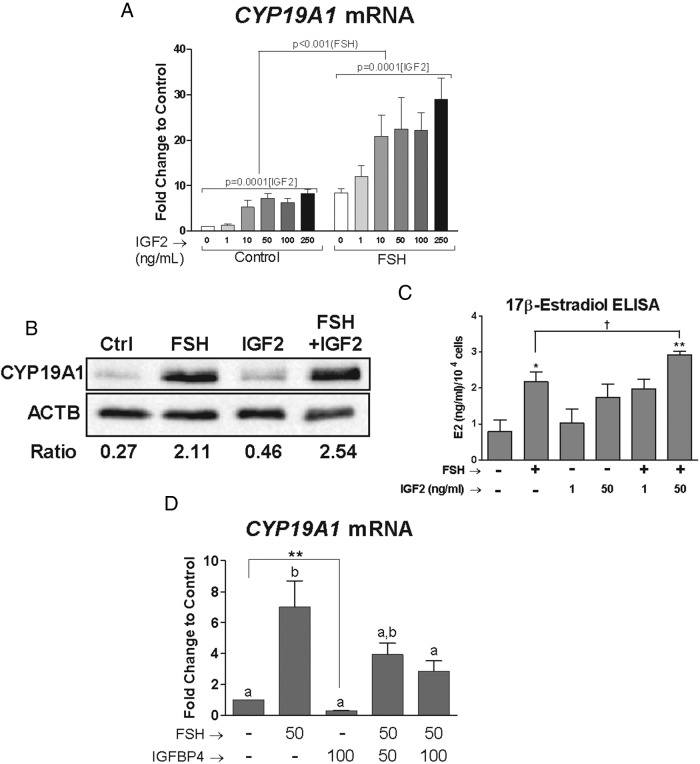
IGF-2 is essential for FSH-induced cumulus cell differentiation. A, Cumulus cells were cultured for 24 hours in serum-free and phenol red-free media, then treated with FSH (50 ng/mL), IGF-2 (1–250 ng/mL), or both for 48 hours. Two-way ANOVA analysis of these results indicated that IGF-2 treatment has a significant effect on the stimulation of CYP19A1 by FSH (P = .0001) and that FSH significantly affects the IGF-2 stimulation of CYP19A1 (P < .001). B, Cumulus cells from one patient were cultured in serum-free and phenol red-free media for 24 hours and treated with FSH (50 ng/mL), IGF-2 (50 ng/mL), or their combination for 48 hours. Total protein was isolated from the cells, and the expression of CYP19A1 and ACTB (loading control) was assessed by Western blot. CYP19A1 band intensities were adjusted to band intensity of ACTB for the same sample, and the ratio is indicated below the Western blot images. C, Cumulus cells from three patients were cultured in serum-free and phenol red-free media for 24 hours and treated with FSH (50 ng/mL), IGF-2 (1 or 50 ng/mL), or their combination for 48 hours. Media was collected from the cells, and 17β-estradiol (E2) levels were measured by ELISA (intra-assay coefficient of variability = 3.5%). The E2 levels are reported per 10 000 cells. *, P < .05; **, P < .01 compared to control, one-way ANOVA; †, P < .05, t test. D, Cumulus cells were cultured for 24 hours in serum- and phenol red-free media, then treated with FSH (50 ng/mL), IGFBP-4 (50–100 ng/mL), or both for 48 hours. RNA was isolated from the cumulus cells, and CYP19A1 mRNA levels were measured by qPCR. Columns represent the mean ± SEM of at least three independent experiments. Different letters denote significant difference of at least P < .05 (one-way ANOVA) between treatment groups. Control and IGFBP-4-treated groups are significantly different when compared using a t test. **, P < .01.
Figure 3.
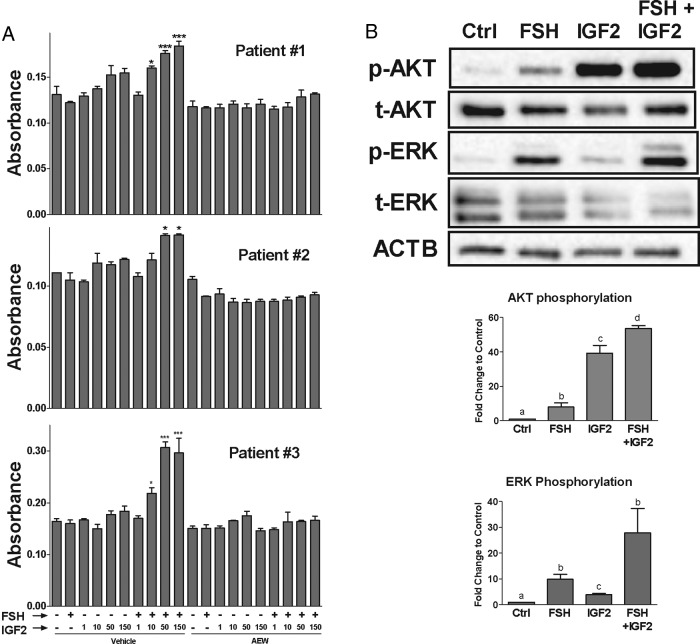
FSH and IGF-2 synergize to stimulate cumulus cell proliferation. A, Cumulus cells were cultured in serum-free and phenol red-free media for 24 hours. Cells were treated with AEW (0.5 μm) or vehicle (DMSO) for 1 hour, followed by treatment with FSH (50 ng/mL), IGF-2 (1–150 ng/mL), or both for 72 hours in triplicate wells. Cell proliferation was measured by MTT assay. For each patient graph, the columns represent the mean absorbance of triplicate wells ± SEM for each treatment in cumulus cells of that patient. *, P < .05; ***, P < .001, relative to control; one-way ANOVA. B, Cumulus cells from three patients were cultured in serum-free and phenol red-free media for 24 hours and treated with FSH (50 ng/mL), IGF-2 (50 ng/mL), or their combination for 1 hour. Total protein was isolated from the cells and phosphorylated, and total AKT and ERK were assessed by Western blot. Band intensities were normalized to ACTB (loading control), and the ratio of phosphorylated to total AKT and ERK is reported. Different letters denote significant difference of at least P < .05 (one-way ANOVA) between treatment groups.
Figure 4.
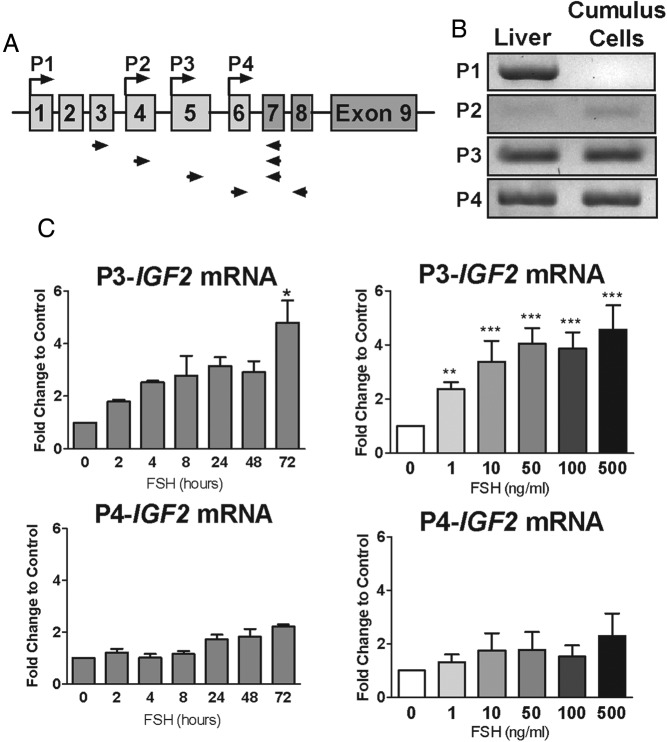
FSH-induced up-regulation of IGF-2 expression is driven by the P3 promoter. A, Scheme of IGF-2 gene region indicating the promoter that drives the expression of specific exons. Arrows represent locations of primer pairs designed to detect promoter-specific IGF-2 transcripts. B, RNA was isolated from cumulus cells after culture in serum-free and phenol red-free media for 24 hours. Cumulus cell RNA and RNA from human liver was reverse-transcribed, and PCR with promoter-specific primers (indicated in panel A) was used to detect promoter-specific (P1–P4) IGF-2 transcripts. PCR products were visualized on an agarose gel. C, Expression of P3 and P4 promoter-specific IGF-2 transcripts was measured in cumulus cells treated with increasing doses of FSH (1–500 ng/mL) for 48 hours or FSH (50 ng/mL) for increasing amounts of time (2 to 72 h) as in Figure 1. Columns represent the mean ± SEM for three independent experiments. *, P < .05; **, P < .01; ***, P < .001, relative to control; one-way ANOVA.
Figure 5.
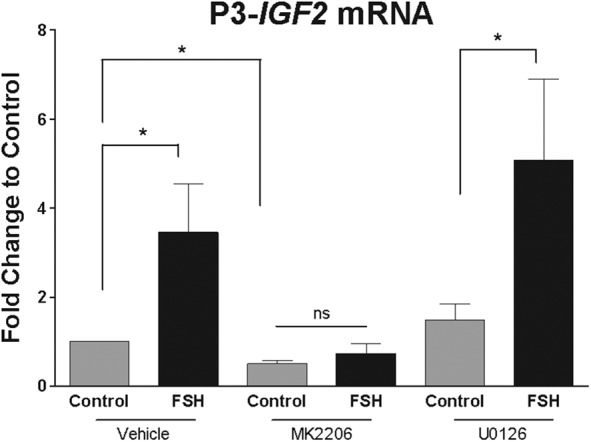
FSH regulates P3 promoter-driven IGF-2 expression in an AKT-dependent manner. Cumulus granulosa cells were cultured in serum-free and phenol red-free media for 24 hours, followed by treatment with MK2206 (1 μm), U0126 (5 μm), or vehicle (DMSO) for 1 hour. Cells were then treated with FSH (50 ng/mL) for 48 hours and RNA was isolated and reverse-transcribed. P3 promoter-driven IGF-2 mRNA expression was measured by qPCR. Columns represent the mean ± SEM for at least three independent experiments. *, P < .05; t test. ns, not significant.
Figure 6.
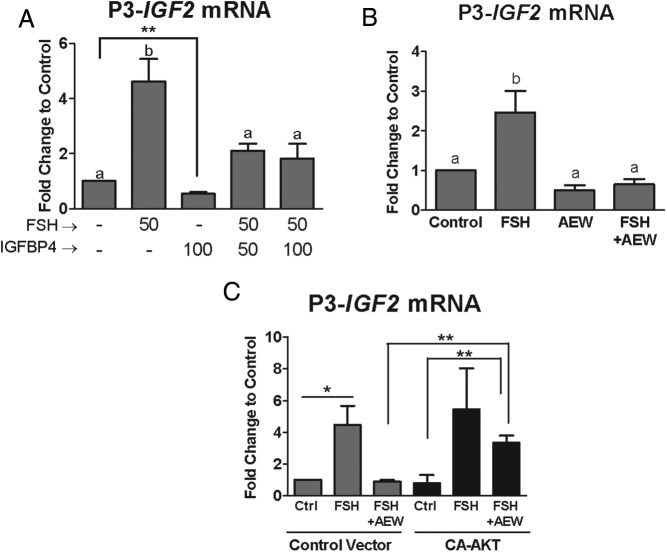
Activation of the IGF-1R is required for P3 promoter-driven IGF-2 expression. Cumulus cells were cultured for 24 hours in serum-free and phenol red free media. A, Cumulus cells were treated with FSH (50 ng/mL), IGFBP-4 (50–100 ng/mL), or both for 48 hours. B, Cumulus cells were treated with AEW (0.5 μm) or vehicle (DMSO) for 1 hour followed by treatment with FSH (50 ng/mL) for 48 hours. C, Cumulus cells were infected with a lentivirus expressing CA-AKT or an empty virus (control vector). These cells were then treated with AEW (0.5 μm) or vehicle (DMSO) for 1 hour, followed by treatment with FSH (50 ng/mL) for 48 hours. RNA was isolated from cumulus cells at the end of each experiment and reverse-transcribed. P3 promoter-driven IGF-2 mRNA expression was measured by qPCR. Columns represent the mean ± SEM for at least four independent experiments in panels A and B and three independent experiments in panel C. Different letters denote significant difference of at least P < .05 (one-way ANOVA) between treatment groups. In panel A, control and IGFBP-4-treated groups are significantly different when compared using a t test. **, P < .01. In panel C, indicated treatments are significantly different from each other. *, P < .05; **, P < .01, by one-way ANOVA.
Polymerase chain reaction
Total RNA was isolated using TRIzol Reagent (Invitrogen) as recommended in the manufacturer's protocol. Total RNA (1 μg) was reverse-transcribed using anchored oligo-dT primers (Integrated DNA Technologies) and Moloney murine leukemia virus reverse transcriptase (Invitrogen) at 42°C for 1 hour. The resulting cDNA was diluted to a final concentration of 10 ng/μL. Quantitative real-time PCR (qPCR) was performed using intron-spanning primers specific for the detection of ribosomal protein L19 (RPL19), CYP19A1, total IGF-2, and promoter-specific IGF-2 transcripts. Primer sequences are available upon request. Target gene expression was adjusted to the expression of RPL19, an internal control, for each sample. In Figure 2B, the final qPCR product was visualized using 3% agarose gel electrophoresis.
Western blotting
Cultured cumulus cells were harvested in ice-cold radioimmunoprecipitation assay lysis buffer, and whole cell lysates were used in Western blotting as previously described (6, 7). The primary antibodies used were IGF-2 (1:200; Santa Cruz Biotechnology), CYP19A1 (1:1000; Epitomics), and β-actin (ACTB) (1:1000; Proteintech) as a loading control. Image Lab software (Bio-Rad Laboratories) was used to quantify band intensities, which were adjusted relative to ACTB.
17β-Estradiol measurement
Cultured cumulus cells were treated with FSH (50 ng/mL), IGF-2 (1 ng/mL or 50 ng/mL), or their combination for 48 hours, and androstenedione (50 nm) was added to the cell culture media 3 hours before the experimental endpoint. Cell culture medium was then collected and diluted 1:250, and 17β-estradiol levels were determined using the estradiol ELISA kit (DRG Instruments GmbH) following the manufacturer's protocol and reported per 10 000 cells. The intra-assay coefficient of variability was 3.5%.
Proliferation assay
Cumulus granulosa cells were cultured in 96-well plates at a density of 8 × 103 cells per well. After culture for 24 hours, triplicate wells were treated with FSH, IGF-2, or their combination in the presence or absence of AEW for 96 hours. At 96 hours, 10 μL of MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] 5 mg/mL stock solution was added to each well. The plates were incubated at 37°C for 2.5 hours. Wells were carefully aspirated, and Formosan crystals were dissolved in 100 μL DMSO. The absorbance of each well was measured at 562 nm.
Lentivirus transduction
A lentivirus construct containing a constitutively activated AKT (CA-AKT) was kindly provided by Michael Robinson, Children's Hospital of Philadelphia, Philadelphia, Pennsylvania (24). The construct was subcloned into the pTY-CMV lentivirus transfer plasmid, and the virus was generated in HEK (human embryonic kidney)-293 cells. For experiments, CA-AKT or green fluorescent protein-expressing (control) lentivirus was added to human cumulus granulosa cells 2 hours after plating. After 36 hours, cells were treated for 1 hour with AEW or DMSO vehicle followed by 48-hour treatment with FSH.
Statistical analysis
Each experiment was conducted at least three times, each one using cells from different patients. Data in each graph represent the mean value ± SEM from data obtained from at least three different patients. For Figure 3, proliferation is presented for three individual patients as the mean value ± SEM of triplicate determinations. Statistical comparisons were carried out as noted in the figure legends, using t test when comparing two groups and one-way or two-way ANOVA when comparing more than two groups. Differences between groups was considered to be statistically significant at P < .05.
Results
FSH stimulates IGF-2 expression in human cumulus cells
To examine the mechanisms involved in the regulation of IGF-2 gene expression in human granulosa cells, we first explored the time- and concentration-dependent effects of FSH. Using 50 ng/mL FSH, a concentration that maximally stimulates aromatase expression in human granulosa cells (7), we quantified IGF-2 mRNA transcript abundance from 2 to 72 hours after initiation of treatment (Figure 1A). After 2 and 4 hours of treatment, a nonstatistically significant increase in the relative amount of IGF-2 mRNA was observed. At 8 hours, FSH significantly increased IGF-2 transcript levels to nearly 3-fold when compared to nontreated cells. IGF-2 mRNA abundance reached a maximum of 4-fold stimulation with respect to nontreated cells at 24 hours and remained at this level at 48 and 72 hours. Based on this result, dose-response experiments were conducted using 48-hour treatment. The average IGF-2 transcript abundance was increased more than 2.5-fold at all FSH concentrations, even at 1 ng/mL, the lowest concentration tested (Figure 1B). However, due to wide confidence intervals related to patient-to-patient variability, statistical significance was only reached at 1 and 50 ng/mL concentrations of FSH (P < .01 vs 0 ng/mL one-way ANOVA). Treatment of cumulus cells with FSH (50 ng/mL) for 48 hours also stimulated an increase in the expression of IGF-2 protein (Figure 1C). These results suggest that FSH is a potent stimulator of IGF-2 expression in human granulosa cells.
IGF-2 is essential for FSH-induced stimulation of CYP19A1 and cell proliferation
To determine the interplay between IGF-2 and FSH on the regulation of granulosa cell differentiation, we quantified CYP19A1 mRNA levels in cells treated with increasing concentrations of IGF-2 in the absence or presence of FSH. When cells were treated with 1 to 250 ng/mL of IGF-2, a concentration-dependent increase in CYP19A1 mRNA transcripts was observed in which maximal stimulation was reached at 50 ng/mL and remained at this level at higher concentrations of IGF-2 (Figure 2A). Cotreatment with increasing concentrations of IGF-2 and FSH (50 ng/mL) synergistically increased CYP19A1 transcript abundance. For example, IGF-2 at 1 ng/mL did not stimulate CYP19A1 expression alone, but it synergized with FSH to enhance CYP19A1 expression above levels induced by FSH alone. Two-way ANOVA of these results indicated that IGF-2 treatment has a significant effect on the stimulation of CYP19A1 by FSH (P = .0001). Reciprocally, this analysis demonstrated that FSH significantly affects the IGF-2 stimulation of CYP19A1 (P < .001). Similarly, FSH and IGF-2 synergized in stimulating the expression of CYP19A1 protein (Figure 2B) and estradiol accumulation (Figure 2C). These results suggest that IGF-2, even at low concentrations, has a major impact on FSH-induced granulosa cell differentiation.
Because human granulosa cells produce IGF-2 (Figure 1) and IGF-2 synergizes with FSH (Figure 2A), we next investigated the extent to which FSH action in granulosa cells depends on locally produced IGF-2. For this purpose, we treated cells with IGFBP-4. IGFBP-4 binds IGFs and, consequently, blocks IGF-1R activation. Cotreatment with IGFBP-4 blunted the increase in CYP19A1 induced by FSH (Figure 2D). The addition of IGFBP-4 (100 ng/mL) not only significantly decreased FSH-induced stimulation of CYP19A1 but also diminished basal CYP19A1 transcript levels by approximately 70%. The effect of IGFBP-4 on basal CYP19A1 mRNA abundance was statistically significant (P < .01) when control and IGFBP-4-treated groups were compared using a t test. These results suggest that IGF-2 actions are required for FSH-induced granulosa cell differentiation and for basal CYP19A1 expression and are in agreement with our previous findings demonstrating that IGF-1R activity is required for FSH-induced granulosa cell differentiation (7). In addition, because human granulosa cells produce only IGF-2, the results demonstrate that IGF-2 is a physiological activator of the IGF-1R in these cells.
Next, we evaluated the impact of IGF-2 on granulosa cell proliferation, a crucial event in follicle progression toward the preovulatory state. For this purpose, cells were treated with FSH, increasing doses of IGF-2, or both factors combined. Treatment with FSH or IGF-2 alone had no effect on granulosa cell proliferation. However, when cells were cotreated with FSH and IGF-2, a significant increase in cell proliferation was observed (Figure 3A, left). The effect of IGF-2 dosage varied from patient to patient; therefore, proliferation is presented for each individual patient as the mean of triplicate determination. Thus, as shown in Figure 3, a clear concentration-dependent synergism was observed between IGF-2 and FSH in patients no. 1 and 3; whereas, for patient no. 2, only the highest concentrations of IGF-2 used were able to stimulate cell proliferation in the presence of FSH.
To explore possible mechanisms by which FSH and IGF-2 synergize to promote granulosa cell proliferation, we examined the AKT and ERK signaling pathways, which are known to regulate proliferation and are targets of FSH and IGF-2. When cumulus cells were treated with FSH or IGF-2, an increase in AKT and ERK phosphorylation was observed (Figure 3B). However, the results suggest that FSH stimulates ERK1/2 more strongly than IGF-2, whereas IGF-2 stimulates AKT more strongly than FSH. More importantly, the combination of FSH and IGF-2 further stimulated the phosphorylation of both AKT and ERK above the level of either factor alone. Because the main effect of IGF-2 is the stimulation of AKT, we next examined its contribution to cell proliferation. For this purpose, cumulus cells were treated with AEW, an inhibitor of IGF-1R activity that inhibits AKT phosphorylation but has no effect on ERK signaling. When cumulus cells were treated with AEW, the stimulation of granulosa cell proliferation by the combination of IGF-2 and FSH was abolished in all patients (Figure 3A, right). This demonstrates that IGF-1R activity is critical for granulosa cell proliferation.
Together, these results demonstrate that FSH-induced differentiation and proliferation of human granulosa cells depends on IGF-2 and its activation of the IGF-1R. This demonstrates an interdependent relationship where FSH stimulates IGF-2 expression, and IGF-2 is required and synergizes with FSH to stimulate granulosa cell proliferation and differentiation. Therefore, it is critical to understand how FSH regulates IGF-2 expression in human granulosa cells.
FSH regulates IGF-2 expression via activation of the P3 promoter
IGF-2 gene transcription is driven by four different promoters (25) (Figure 4A). Activity of each promoter generates IGF-2 mRNA with promoter-specific untranslated exons upstream of the protein-coding exons 7 to 9 that are common to all transcripts. To determine the activation status of IGF-2 alternative promoters in human granulosa cells, exon analysis of RNAs was performed using 5′-untranslated region-specific forward primers and reverse primers located in exon 7 or 8 (Figure 4A). The results demonstrated that P1-driven IGF-2 transcripts were absent in human cumulus cells, and P2-driven transcripts were barely detectable after 40 cycles, even in human liver cDNA. This is consistent with previous reports of low P2-driven IGF-2 expression in liver (26, 27). In contrast, P3- and P4-driven IGF-2 transcripts were abundant (Figure 4B).
To determine whether the P3 or the P4 promoter was regulated by FSH, the mRNA abundance of the promoter-specific transcripts was quantified in cells treated with FSH (50 ng/mL) for up to 72 hours (Figure 4C). Over this period, P3-specific transcript levels increased in a manner that resembles the changes observed for the IGF-2 coding region described in Figure 1. In contrast, P4-driven IGF-2 transcripts did not increase significantly over time. When cumulus cells were treated with increasing concentrations of FSH for 48 hours, P3-driven IGF-2 mRNA transcripts significantly increased in a concentration-dependent manner, whereas P4-specific IGF-2 mRNA abundance was not affected by any of the concentrations of FSH used. Taken together, these results suggest that the P3 and P4 promoters of the IGF-2 gene are active in human granulosa cells, and of these promoters, only P3 is activated by FSH.
FSH regulation of IGF-2 expression is AKT-dependent
In cultured human cumulus cells, FSH activates both the AKT and ERK pathways (7). To investigate whether FSH regulates IGF-2 expression in an AKT- or ERK-dependent manner, we treated cells with MK2206, an inhibitor of AKT, and U0126, an inhibitor of ERK activation, and measured the effect of FSH treatment on IGF-2 mRNA. In the presence of MK2206, FSH was no longer able to increase IGF-2 mRNA transcript abundance (Figure 5). In contrast, inhibition of ERK activation did not alter the ability of FSH to simulate IGF-2 (Figure 5), suggesting that FSH-induced IGF-2 expression depends on AKT, but not on ERK. Moreover, in the absence of FSH, MK2206 treatment significantly decreased IGF-2 transcript levels when compared with vehicle-treated cells. These results suggest that AKT activity is crucial for both basal and FSH-stimulated IGF-2 expression in human granulosa cells.
Endogenous IGF-2 is essential for basal and FSH-stimulated IGF-2 expression
Because IGF-2 activates the IGF-1R, which results in downstream AKT activation, we examined whether endogenous IGF-2 contributes to IGF-2 expression by incubating cells with IGFBP-4 as described for Figure 2. Treatment of granulosa cells with IGFBP-4 alone reduced promoter P3-driven IGF-2 transcripts by 50% (Figure 6A). This decrease was statistically significant when control and IGFBP-4 groups were compared using t test (P < .01). In addition, cotreatment with IGFBP-4 caused a 60% decrease in FSH-induced stimulation of IGF-2 mRNA accumulation, suggesting a positive autoregulation of IGF-2 in human granulosa cells.
To further support this idea, we examined whether IGF-1R activity is required for basal and FSH-induced IGF-2 expression. For this purpose, cells were treated with AEW, a specific inhibitor of IGF-1R activity (28, 29). As observed for IGFBP-4 treatment, in the presence of the IGF-1R inhibitor, basal IGF-2 mRNA transcript abundance was reduced nearly 50%, and FSH was unable to increase IGF-2 transcript levels (Figure 6B). These data suggest that activation of the IGF-1R by IGF-2 is required for both basal and FSH-stimulated IGF-2 expression in human granulosa cells.
Inhibition of AKT activity prevented the stimulation of IGF-2 expression by FSH (Figure 5), and because previous reports demonstrated that AKT is downstream of the IGF-1R in granulosa cells (6, 7), we next explored whether AKT mediates IGF-2 autostimulation. For this purpose, cells were treated with AEW, to eliminate basal AKT activation (7), and infected with lentivirus carrying expression constructs for green fluorescent protein or CA-AKT. In the presence of AEW and CA-AKT, FSH treatment overcame the inhibitory effect of AEW and was able to stimulate IGF-2 mRNA transcript accumulation to the levels observed in cells treated with FSH alone (Figure 6C). These results suggest that AKT acts downstream of the IGF-1R in the stimulation of IGF-2 expression. In addition, this finding demonstrates that in human granulosa cells, AKT activity is sufficient for FSH to stimulate IGF-2 expression.
Discussion
Herein, we contribute to the understanding of the interplay between FSH and IGF-2 during the process of human granulosa cell differentiation. We illustrate that IGF-2 synergizes with FSH to stimulate granulosa cell proliferation and differentiation and, furthermore, that IGF-2 action is required for FSH to stimulate granulosa cell differentiation. We also provide the first evidence of the mechanisms of IGF-2 regulation in human granulosa cells. We demonstrate that FSH enhances IGF-2 expression in human granulosa cells by stimulating P3 promoter-driven IGF-2 transcription in an AKT-dependent manner. Furthermore, basal activation of AKT, possibly by endogenous IGF-2, is necessary for FSH to stimulate IGF-2 expression in human granulosa cells. We carried out these studies in cultured human cumulus cells, which we have previously demonstrated share characteristics with undifferentiated, preantral granulosa cells and respond to FSH treatment within 24 hours of culture (7). Therefore, we believe our findings are indicative of FSH and IGF-2 actions during the process of human granulosa cell differentiation and follicle maturation in the ovary.
The IGF system in human and rodent granulosa cells differs in many respects. In addition to the differences in IGF expression in humans and rodents (IGF-2 and IGF-1, respectively), the regulation of IGF expression by FSH is also significantly different. In rodents, FSH decreases IGF-1 levels in granulosa cells (6). In contrast, here, we demonstrate a rapid (within 8 h) and potent (at 1 ng/mL concentration) stimulation of IGF-2 expression by FSH. On the other hand, there are striking similarities in FSH and IGF action observed in human and rodent granulosa cells. IGF-1 can synergize with FSH to enhance proliferation and differentiation of rodent granulosa cells (30–33). Here we demonstrate that the same is true for IGF-2 in humans, where IGF-2 synergizes with FSH to stimulate human granulosa cell proliferation and CYP19A1 expression in a dose-dependent manner. Additionally, activity of the IGF-1R, the receptor that mediates both IGF-2 and IGF-1 action in granulosa cells, is required for FSH to stimulate granulosa cell differentiation in both humans and rodents (6, 7).
In addition, we demonstrate that when IGF-2 action is blunted by the addition of IGFBP-4, FSH no longer fully stimulates CYP19A1 expression in human granulosa cells. These findings agree with early evidence showing that IGF-2 has a significant role in human ovarian follicle maturation and follicle selection (34). A high level of IGF-2 in the follicular fluid of women undergoing IVF correlates with good prognosis for oocyte maturation and early embryo development (15). IGF-2 has also been shown to prevent follicle loss in ovarian tissue cultured in vitro (35). We also demonstrate that IGF-2, by activating the IGF-1R, synergizes with FSH to stimulate granulosa cell proliferation, suggesting a positive effect of IGF-2/IGF-1R on follicle growth. In agreement with the positive role of IGF-2 in follicle maturation, a study of follicular cells from IVF patients with diminished ovarian reserve found a significant decrease in components of the IGF system, including IGF-2, in comparison to patients with normal ovarian reserve (36). Additionally, the follicular fluid of women with polycystic ovary syndrome has been shown to contain higher levels of IGFBPs, which can bind and sequester IGFs and blunt their normal activity in the follicle (37, 38). This suggests that insufficient IGF activity in ovarian follicles could contribute to the decreased reproductive capacity of women with diminished ovarian reserve, polycystic ovary syndrome, and other etiologies of impaired fertility.
Because IGF-2 is critical during follicle maturation and in particular for FSH-induced granulosa cell proliferation and differentiation, we explored the mechanisms by which IGF-2 expression is regulated in human cells. In the liver, GH regulates IGF-2 expression, but this is not the case in granulosa cells (26, 39, 40). This suggests that mechanisms of IGF-2 expression are cell type-specific, and a closer inspection of these mechanisms specifically in human granulosa cells is necessary to understand IGF-2 regulation during follicle development. Here, we demonstrate that the P3 promoter drives FSH-stimulated IGF-2 expression. This promoter-specific increase in IGF-2 expression is dependent on FSH-stimulated AKT activation. These findings, together with our previous report that FSH-induced CYP19A1 expression requires AKT activation by FSH (7), adds to the increasing evidence that FSH-induced AKT activation is crucial for human granulosa cell differentiation; however, the molecular mechanisms involved in FSH stimulation of AKT and IGF-2 P3 promoter activity are unknown, and further research is warranted.
In summary, we set forth a mechanism for IGF-2 expression in human granulosa cells, where FSH enhances IGF-2 expression by stimulating P3 promoter-driven IGF-2 transcription in an AKT-dependent manner. Basal activation of AKT by endogenous IGF-2 is critical for FSH-induced IGF-2 expression, which provides evidence of IGF-2 autoregulation in human granulosa cells. In addition, we show that locally produced IGF-2 is required for and synergizes with FSH to drive the proliferation and differentiation of human granulosa cells. Thus, our findings suggest a unique relationship between FSH and IGF-2 during human follicle development in which only follicles capable of producing an adequate amount of IGF-2 will respond to the low and decreasing levels of FSH found in the latter part of the follicular phase. We speculate that this interdependent relationship between FSH and IGF-2 could be a driving force in dominant follicle selection in humans.
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grant R01HD057110 (to C.S.). S.C.B. and S.M.C. are supported by NIH Training Grant T32HL07692.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACTB
- β-actin
- CA-AKT
- constitutively activated AKT
- DMSO
- dimethyl sulfoxide
- IGF-1R
- IGF-1 receptor
- IGFBP-4
- IGF binding protein 4
- IVF
- in vitro fertilization
- qPCR
- quantitative real-time PCR.
References
- 1. Filicori M, Cognigni GE, Taraborrelli S, et al. Luteinizing hormone activity supplementation enhances follicle-stimulating hormone efficacy and improves ovulation induction outcome. J Clin Endocrinol Metab. 1999;84:2659–2663. [DOI] [PubMed] [Google Scholar]
- 2. Jeppesen JV, Kristensen SG, Nielsen ME, et al. LH-receptor gene expression in human granulosa and cumulus cells from antral and preovulatory follicles. J Clin Endocrinol Metab. 2012;97:E1524–E1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–204. [DOI] [PubMed] [Google Scholar]
- 4. Dierich A, Sairam MR, Monaco L, et al. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci USA. 1998;95:13612–13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baker J, Hardy MP, Zhou J, et al. Effects of an Igf1 gene null mutation on mouse reproduction. Mol Endocrinol. 1996;10:903–918. [DOI] [PubMed] [Google Scholar]
- 6. Zhou P, Baumgarten SC, Wu Y, et al. IGF-I signaling is essential for FSH stimulation of AKT and steroidogenic genes in granulosa cells. Mol Endocrinol. 2013;27:511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baumgarten SC, Convissar SM, Fierro MA, Winston NJ, Scoccia B, Stocco C. IGF-1R signaling is necessary for FSH-induced activation of AKT and differentiation of human cumulus granulosa cells. J Clin Endocrinol Metab. 2014;99:2995–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hernandez ER, Roberts CT, Jr, LeRoith D, Adashi EY. Rat ovarian insulin-like growth factor I (IGF-I) gene expression is granulosa cell-selective: 5′-untranslated mRNA variant representation and hormonal regulation. Endocrinology. 1989;125:572–574. [DOI] [PubMed] [Google Scholar]
- 9. el-Roeiy A, Chen X, Roberts VJ, LeRoith D, Roberts CT, Jr, Yen SS. Expression of insulin-like growth factor-I (IGF-I) and IGF-II and the IGF-I, IGF-II, and insulin receptor genes and localization of the gene products in the human ovary. J Clin Endocrinol Metab. 1993;77:1411–1418. [DOI] [PubMed] [Google Scholar]
- 10. Qu J, Godin PA, Nisolle M, Donnez J. Expression of receptors for insulin-like growth factor-I and transforming growth factor-β in human follicles. Mol Hum Reprod. 2000;6:137–145. [DOI] [PubMed] [Google Scholar]
- 11. Klein NA, Battaglia DE, Woodruff TK, et al. Ovarian follicular concentrations of activin, follistatin, inhibin, insulin-like growth factor I (IGF-I), IGF-II, IGF-binding protein-2 (IGFBP-2), IGFBP-3, and vascular endothelial growth factor in spontaneous menstrual cycles of normal women of advanced reproductive age. J Clin Endocrinol Metab. 2000;85:4520–4525. [DOI] [PubMed] [Google Scholar]
- 12. Seifer DB, Giudice LC, Dsupin BA, Haning RV, Jr, Frishman GN, Burger HG. Follicular fluid insulin-like growth factor-I and insulin-like growth factor-II concentrations vary as a function of day 3 serum follicle stimulating hormone. Hum Reprod. 1995;10:804–806. [DOI] [PubMed] [Google Scholar]
- 13. Zeleznik AJ, Little-Ihrig L, Ramasawamy S. Administration of insulin-like growth factor I to rhesus monkeys does not augment gonadotropin-stimulated ovarian steroidogenesis. J Clin Endocrinol Metab. 2002;87:5722–5729. [DOI] [PubMed] [Google Scholar]
- 14. Thierry van Dessel HJ, Chandrasekher Y, Yap OW, et al. Serum and follicular fluid levels of insulin-like growth factor I (IGF-I), IGF-II, and IGF-binding protein-1 and -3 during the normal menstrual cycle. J Clin Endocrinol Metab. 1996;81:1224–1231. [DOI] [PubMed] [Google Scholar]
- 15. Wang TH, Chang CL, Wu HM, Chiu YM, Chen CK, Wang HS. Insulin-like growth factor-II (IGF-II), IGF-binding protein-3 (IGFBP-3), and IGFBP-4 in follicular fluid are associated with oocyte maturation and embryo development. Fertil Steril. 2006;86:1392–1401. [DOI] [PubMed] [Google Scholar]
- 16. Di Blasio AM, Viganó P, Ferrari A. Insulin-like growth factor-II stimulates human granulosa-luteal cell proliferation in vitro. Fertil Steril. 1994;61:483–487. [DOI] [PubMed] [Google Scholar]
- 17. Devoto L, Christenson LK, McAllister JM, Makrigiannakis A, Strauss JF., 3rd Insulin and insulin-like growth factor-I and -II modulate human granulosa-lutein cell steroidogenesis: enhancement of steroidogenic acute regulatory protein (StAR) expression. Mol Hum Reprod. 1999;5:1003–1010. [DOI] [PubMed] [Google Scholar]
- 18. Kamada S, Kubota T, Taguchi M, Ho WR, Sakamoto S, Aso T. Effects of insulin-like growth factor-II on proliferation and differentiation of ovarian granulosa cells. Horm Res. 1992;37:141–149. [DOI] [PubMed] [Google Scholar]
- 19. Mason HD, Willis DS, Holly JM, Franks S. Insulin preincubation enhances insulin-like growth factor-II (IGF-II) action on steroidogenesis in human granulosa cells. J Clin Endocrinol Metab. 1994;78:1265–1267. [DOI] [PubMed] [Google Scholar]
- 20. Kubota T, Kamada S, Ohara M, et al. Insulin-like growth factor II in follicular fluid of the patients with in vitro fertilization and embryo transfer. Fertil Steril. 1993;59:844–849. [DOI] [PubMed] [Google Scholar]
- 21. Poretsky L, Chun B, Liu HC, Rosenwaks Z. Insulin-like growth factor II (IGF-II) inhibits insulin-like growth factor binding protein I (IGFBP-1) production in luteinized human granulosa cells with a potency similar to insulin-like growth factor I (IGF-I) and higher than insulin. J Clin Endocrinol Metab. 1996;81:3412–3414. [DOI] [PubMed] [Google Scholar]
- 22. Willis DS, Mason HD, Watson H, Franks S. Developmentally regulated responses of human granulosa cells to insulin-like growth factors (IGFs): IGF-I and IGF-II action mediated via the type-I IGF receptor. J Clin Endocrinol Metab. 1998;83:1256–1259. [DOI] [PubMed] [Google Scholar]
- 23. Martinez-Chequer JC, Stouffer RL, Hazzard TM, Patton PE, Molskness TA. Insulin-like growth factors-1 and -2, but not hypoxia, synergize with gonadotropin hormone to promote vascular endothelial growth factor-A secretion by monkey granulosa cells from preovulatory follicles. Biol Reprod. 2003;68:1112–1118. [DOI] [PubMed] [Google Scholar]
- 24. Li LB, Toan SV, Zelenaia O, et al. Regulation of astrocytic glutamate transporter expression by Akt: evidence for a selective transcriptional effect on the GLT-1/EAAT2 subtype. J Neurochem. 2006;97:759–771. [DOI] [PubMed] [Google Scholar]
- 25. Sussenbach JS, Steenbergh PH, Jansen E, et al. Structural and regulatory aspects of the human genes encoding IGF-I and -II. Adv Exp Med Biol. 1991;293:1–14. [DOI] [PubMed] [Google Scholar]
- 26. von Horn H, Ekström C, Ellis E, et al. GH is a regulator of IGF2 promoter-specific transcription in human liver. J Endocrinol. 2002;172:457–465. [DOI] [PubMed] [Google Scholar]
- 27. Uchida K, Kondo M, Takeda S, et al. Altered transcriptional regulation of the insulin-like growth factor 2 gene in human hepatocellular carcinoma. Mol Carcinog. 1997;18:193–198. [PubMed] [Google Scholar]
- 28. Riedemann J, Macaulay VM. IGF1R signalling and its inhibition. Endocr Relat Cancer. 2006;13(suppl 1):S33–S43. [DOI] [PubMed] [Google Scholar]
- 29. García-Echeverría C, Pearson MA, Marti A, et al. In vivo antitumor activity of NVP-AEW541-A novel, potent, and selective inhibitor of the IGF-IR kinase. Cancer Cell. 2004;5:231–239. [DOI] [PubMed] [Google Scholar]
- 30. Adashi EY, Resnick CE, Brodie AM, Svoboda ME, Van Wyk JJ. Somatomedin-C-mediated potentiation of follicle-stimulating hormone-induced aromatase activity of cultured rat granulosa cells. Endocrinology. 1985;117:2313–2320. [DOI] [PubMed] [Google Scholar]
- 31. Adashi EY, Resnick CE, Svoboda ME, Van Wyk JJ. Somatomedin-C synergizes with follicle-stimulating hormone in the acquisition of progestin biosynthetic capacity by cultured rat granulosa cells. Endocrinology. 1985;116:2135–2142. [DOI] [PubMed] [Google Scholar]
- 32. Adashi EY, Resnick CE, Svoboda ME, Van Wyk JJ. Somatomedin-C enhances induction of luteinizing hormone receptors by follicle-stimulating hormone in cultured rat granulosa cells. Endocrinology. 1985;116:2369–2375. [DOI] [PubMed] [Google Scholar]
- 33. Zhou J, Refuerzo J, Bondy C. Granulosa cell DNA synthesis is strictly correlated with the presence of insulin-like growth factor I and absence of c-fos/c-jun expression. Mol Endocrinol. 1995;9:924–931. [DOI] [PubMed] [Google Scholar]
- 34. Yuan W, Giudice LC. Insulin-like growth factor-II mediates the steroidogenic and growth promoting actions of follicle stimulating hormone on human ovarian pre-antral follicles cultured in vitro. J Clin Endocrinol Metab. 1999;84:1479–1482. [DOI] [PubMed] [Google Scholar]
- 35. Louhio H, Hovatta O, Sjöberg J, Tuuri T. The effects of insulin, and insulin-like growth factors I and II on human ovarian follicles in long-term culture. Mol Hum Reprod. 2000;6:694–698. [DOI] [PubMed] [Google Scholar]
- 36. Greenseid K, Jindal S, Hurwitz J, Santoro N, Pal L. Differential granulosa cell gene expression in young women with diminished ovarian reserve. Reprod Sci. 2011;18:892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cataldo NA, Giudice LC. Follicular fluid insulin-like growth factor binding protein profiles in polycystic ovary syndrome. J Clin Endocrinol Metab. 1992;74:695–697. [DOI] [PubMed] [Google Scholar]
- 38. Cataldo NA, Giudice LC. Insulin-like growth factor binding protein profiles in human ovarian follicular fluid correlate with follicular functional status. J Clin Endocrinol Metab. 1992;74:821–829. [DOI] [PubMed] [Google Scholar]
- 39. Peñarrubia J, Balasch J, García-Bermúdez M, Casamitjana R, Vanrell JA, Hernandez ER. Growth hormone does not increase the expression of insulin-like growth factors and their receptor genes in the pre-menopausal human ovary. Hum Reprod. 2000;15:1241–1246. [DOI] [PubMed] [Google Scholar]
- 40. Voutilainen R, Miller WL. Coordinate tropic hormone regulation of mRNAs for insulin-like growth factor II and the cholesterol side-chain-cleavage enzyme, P450scc [corrected], in human steroidogenic tissues. Proc Natl Acad Sci USA. 1987;84:1590–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]