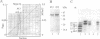Abstract
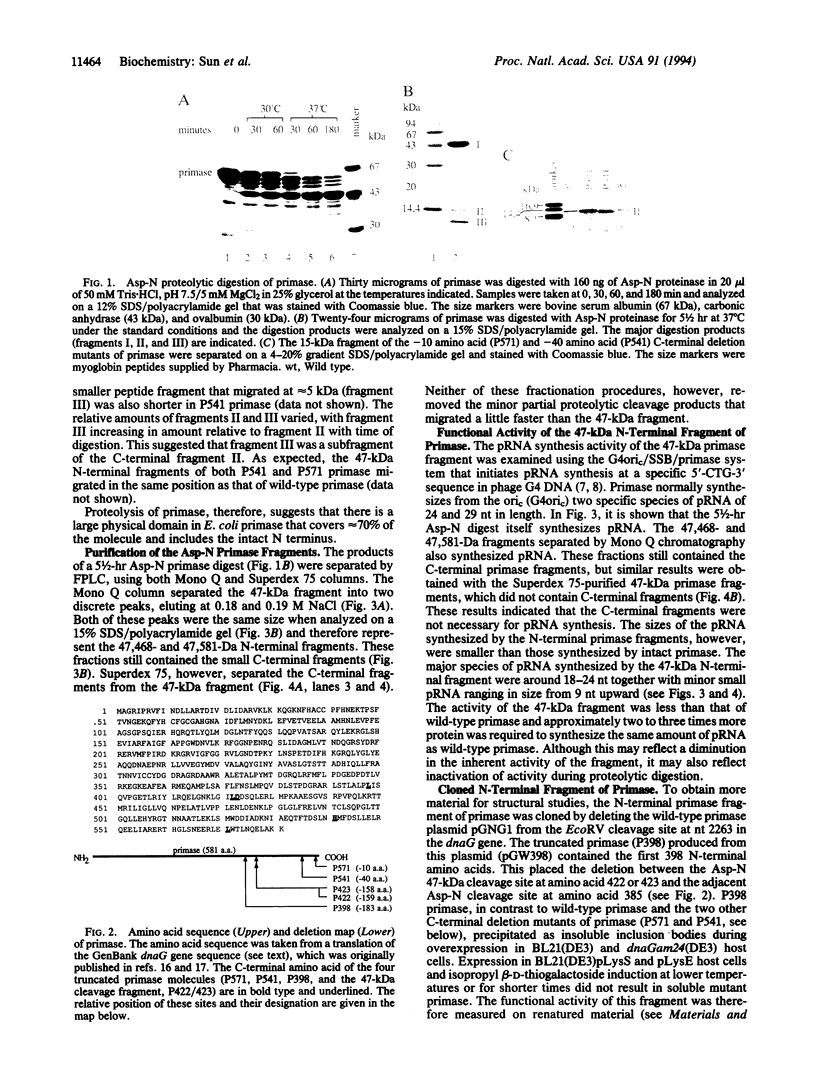
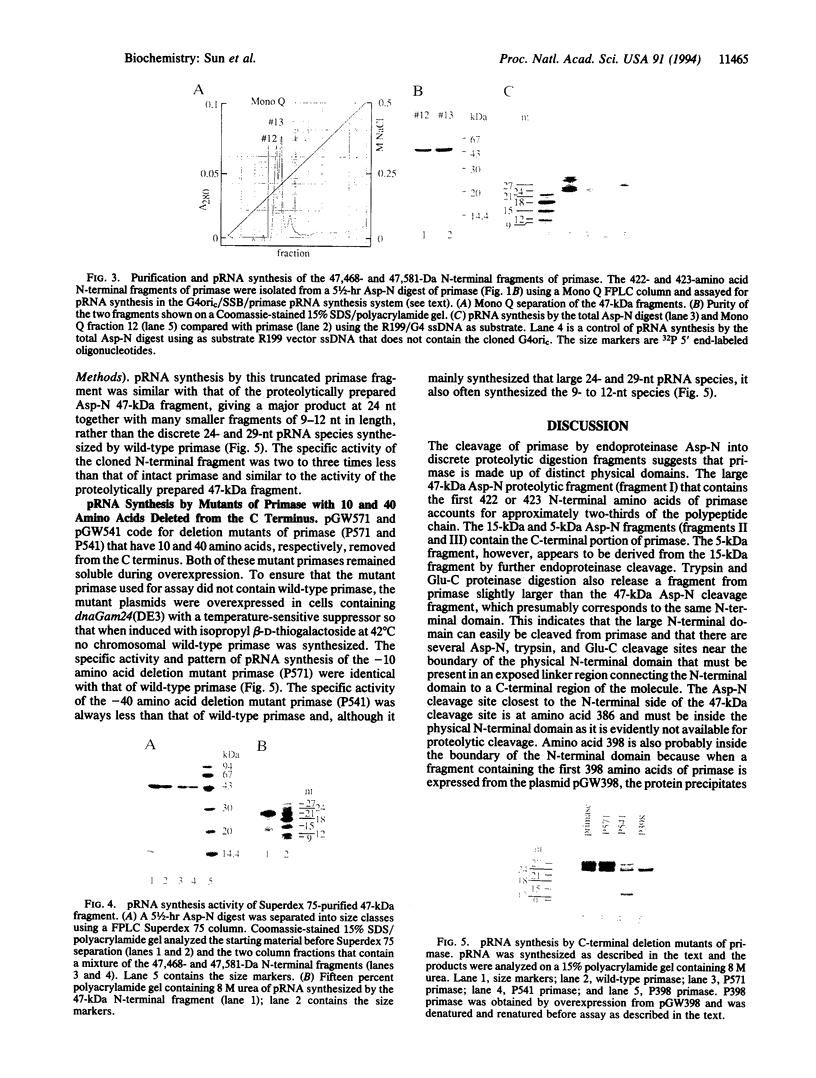
Endoproteinase Asp-N cleaves the 581-amino acid Escherichia coli primase (65,564 Da) into several major fragments. One of these, a 47-kDa fragment containing the complete N terminus and the first 422 amino acids of primase, is capable of primer RNA (pRNA) synthesis in the G4oric/single-stranded DNA binding protein/primase pRNA synthesis system. A cloned 398-amino acid N-terminal fragment of primase can also synthesize pRNA. The sizes of the pRNA synthesized by these N-terminal fragments, however, are smaller than those synthesized by intact primase, suggesting that the C-terminal region of primase plays a role in processivity or regulation of pRNA synthesis. Primase mutants with the last 10 and 40 C-terminal amino acids deleted synthesize pRNA as wild-type primase, indicating that any regulatory sequences must be internal to the C terminus of primase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K., Kornberg A. A general priming system employing only dnaB protein and primase for DNA replication. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4308–4312. doi: 10.1073/pnas.76.9.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K., Kornberg A. Mechanism of dnaB protein action. IV. General priming of DNA replication by dnaB protein and primase compared with RNA polymerase. J Biol Chem. 1981 May 25;256(10):5267–5272. [PubMed] [Google Scholar]
- Bernstein J. A., Richardson C. C. A 7-kDa region of the bacteriophage T7 gene 4 protein is required for primase but not for helicase activity. Proc Natl Acad Sci U S A. 1988 Jan;85(2):396–400. doi: 10.1073/pnas.85.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouché J. P., Zechel K., Kornberg A. dnaG gene product, a rifampicin-resistant RNA polymerase, initiates the conversion of a single-stranded coliphage DNA to its duplex replicative form. J Biol Chem. 1975 Aug 10;250(15):5995–6001. [PubMed] [Google Scholar]
- Burton Z. F., Gross C. A., Watanabe K. K., Burgess R. R. The operon that encodes the sigma subunit of RNA polymerase also encodes ribosomal protein S21 and DNA primase in E. coli K12. Cell. 1983 Feb;32(2):335–349. doi: 10.1016/0092-8674(83)90453-1. [DOI] [PubMed] [Google Scholar]
- Drapeau G. R. Substrate specificity of a proteolytic enzyme isolated from a mutant of Pseudomonas fragi. J Biol Chem. 1980 Feb 10;255(3):839–840. [PubMed] [Google Scholar]
- Godson G. N. An over-expression plasmid for Escherichia coli primase. Gene. 1991 Apr;100:59–64. doi: 10.1016/0378-1119(91)90350-k. [DOI] [PubMed] [Google Scholar]
- Grompe M., Versalovic J., Koeuth T., Lupski J. R. Mutations in the Escherichia coli dnaG gene suggest coupling between DNA replication and chromosome partitioning. J Bacteriol. 1991 Feb;173(3):1268–1278. doi: 10.1128/jb.173.3.1268-1278.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiasa H., Sakai H., Tanaka K., Honda Y., Komano T., Godson G. N. Mutational analysis of the primer RNA template region in the replication origin (oric) of bacteriophage G4: priming signal recognition by Escherichia coli primase. Gene. 1989 Dec 7;84(1):9–16. doi: 10.1016/0378-1119(89)90133-9. [DOI] [PubMed] [Google Scholar]
- Ilyina T. V., Gorbalenya A. E., Koonin E. V. Organization and evolution of bacterial and bacteriophage primase-helicase systems. J Mol Evol. 1992 Apr;34(4):351–357. doi: 10.1007/BF00160243. [DOI] [PubMed] [Google Scholar]
- Kornberg A. DNA replication. J Biol Chem. 1988 Jan 5;263(1):1–4. [PubMed] [Google Scholar]
- Lupski J. R., Smiley B. L., Blattner F. R., Godson G. N. Cloning and characterization of the Escherichia coli chromosomal region surrounding the dnaG Gene, with a correlated physical and genetic map of dnaG generated via transposon Tn5 mutagenesis. Mol Gen Genet. 1982;185(1):120–128. doi: 10.1007/BF00333800. [DOI] [PubMed] [Google Scholar]
- Marston F. A., Hartley D. L. Solubilization of protein aggregates. Methods Enzymol. 1990;182:264–276. doi: 10.1016/0076-6879(90)82022-t. [DOI] [PubMed] [Google Scholar]
- Masai H., Arai K. Leading strand synthesis of R1 plasmid replication in vitro is primed by primase alone at a specific site downstream of oriR. J Biol Chem. 1989 May 15;264(14):8082–8090. [PubMed] [Google Scholar]
- McMacken R., Kornberg A. A multienzyme system for priming the replication of phiX174 viral DNA. J Biol Chem. 1978 May 10;253(9):3313–3319. [PubMed] [Google Scholar]
- Mok M., Marians K. J. The Escherichia coli preprimosome and DNA B helicase can form replication forks that move at the same rate. J Biol Chem. 1987 Dec 5;262(34):16644–16654. [PubMed] [Google Scholar]
- Nakamura Y. Amber dnaG mutation exerting a polar effect on the synthesis of RNA polymerase sigma factor in Escherichia coli. Mol Gen Genet. 1984;196(1):179–182. doi: 10.1007/BF00334114. [DOI] [PubMed] [Google Scholar]
- Rowen L., Kornberg A. A ribo-deoxyribonucleotide primer synthesized by primase. J Biol Chem. 1978 Feb 10;253(3):770–774. [PubMed] [Google Scholar]
- Rowen L., Kornberg A. Primase, the dnaG protein of Escherichia coli. An enzyme which starts DNA chains. J Biol Chem. 1978 Feb 10;253(3):758–764. [PubMed] [Google Scholar]
- Sims J., Benz E. W., Jr Initiation of DNA replication by the Escherichia coli dnaG protein: evidence that tertiary structure is involved. Proc Natl Acad Sci U S A. 1980 Feb;77(2):900–904. doi: 10.1073/pnas.77.2.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley B. L., Lupski J. R., Svec P. S., McMacken R., Godson G. N. Sequences of the Escherichia coli dnaG primase gene and regulation of its expression. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4550–4554. doi: 10.1073/pnas.79.15.4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamford N. P., Lilley P. E., Dixon N. E. Enriched sources of Escherichia coli replication proteins. The dnaG primase is a zinc metalloprotein. Biochim Biophys Acta. 1992 Aug 17;1132(1):17–25. doi: 10.1016/0167-4781(92)90047-4. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Sun W., Godson G. N. Binding and phasing of Escherichia coli single-stranded DNA-binding protein by the secondary structure of phage G4 origin of complementary DNA strand synthesis (G4oric). J Biol Chem. 1993 Apr 15;268(11):8026–8039. [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Tougu K., Peng H., Marians K. J. Identification of a domain of Escherichia coli primase required for functional interaction with the DnaB helicase at the replication fork. J Biol Chem. 1994 Feb 11;269(6):4675–4682. [PubMed] [Google Scholar]
- Versalovic J., Lupski J. R. The Haemophilus influenzae dnaG sequence and conserved bacterial primase motifs. Gene. 1993 Dec 22;136(1-2):281–286. doi: 10.1016/0378-1119(93)90480-q. [DOI] [PubMed] [Google Scholar]
- Wickner S. DNA or RNA priming of bacteriophage G4 DNA synthesis by Escherichia coli dnaG protein. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2815–2819. doi: 10.1073/pnas.74.7.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoda K., Okazaki T. Specificity of recognition sequence for Escherichia coli primase. Mol Gen Genet. 1991 May;227(1):1–8. doi: 10.1007/BF00260698. [DOI] [PubMed] [Google Scholar]
- Zipursky S. L., Marians K. J. Identification of two Escherichia coli factor Y effector sites near the origins of replication of the plasmids (ColE1 and pBR322. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6521–6525. doi: 10.1073/pnas.77.11.6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ende A., Baker T. A., Ogawa T., Kornberg A. Initiation of enzymatic replication at the origin of the Escherichia coli chromosome: primase as the sole priming enzyme. Proc Natl Acad Sci U S A. 1985 Jun;82(12):3954–3958. doi: 10.1073/pnas.82.12.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]