Abstract
Acute Helicobacter pylori infection of gastric epithelial cells and human gastric biopsies represses H,K-ATPase α subunit (HKα) gene expression and inhibits acid secretion, causing transient hypochlorhydria and supporting gastric H. pylori colonization. Infection by H. pylori strains deficient in the cag pathogenicity island (cag PAI) genes cagL, cagE, or cagM, which do not transfer CagA into host cells or induce interleukin-8 secretion, does not inhibit HKα expression, nor does a cagA-deficient strain that induces IL-8. To test the hypothesis that virulence factors other than those mediating CagA translocation or IL-8 induction participate in HKα repression by activating NF-κB, AGS cells transfected with HKα promoter-Luc reporter constructs containing an intact or mutated NF-κB binding site were infected with wild-type H. pylori strain 7.13, isogenic mutants lacking cag PAI genes responsible for CagA translocation and/or IL-8 induction (cagA, cagζ, cagε, cagZ, and cagβ), or deficient in genes encoding two peptidoglycan hydrolases (slt and cagγ). H. pylori-induced AGS cell HKα promoter activities, translocated CagA, and IL-8 secretion were measured by luminometry, immunoblotting, and ELISA, respectively. Human gastric biopsy acid secretion was measured by microphysiometry. Taken together, the data showed that HKα repression is independent of IL-8 expression, and that CagA translocation together with H. pylori transglycosylases encoded by slt and cagγ participate in NF-κB-dependent HKα repression and acid inhibition. The findings are significant because H. pylori factors other than CagA and IL-8 secretion are now implicated in transient hypochlorhydria which facilitates gastric colonization and potential triggering of epithelial progression to neoplasia.
Keywords: acid secretion, Helicobacter pylori, proton pump, type 4 secretory system, virulence factors
human gastric colonization by the Gram-negative bacterium Helicobacter pylori causes chronic active gastritis that may progress to peptic ulcer disease, gastric mucosa-associated lymphoid tissue lymphoma, and gastric adenocarcinoma (40). A consistent feature of acute H. pylori infection in humans is transient hypochlorhydria that arises within days of infection and lasts for several weeks (18, 19, 28, 34, 41, 49). Antral and corpus mucosal inflammation follows the same time course. Subsequently, both gastric pH and corpus inflammation decrease, while antral inflammation persists throughout infection. Given that transient H. pylori-induced hypochlorhydria, which facilitates gastric colonization (31) and corpus gastritis, initiate a pathological progression that may culminate in gastric cancer, understanding their mechanistic basis clarifies disease etiology and illustrates a novel and important bacterial adaptation to a hostile ecological niche.
A major virulence determinant of H. pylori is the cytotoxin-associated gene (cag) pathogenicity island (PAI), a 40-kb genetic locus encoding ∼28 protein components of type IV secretory system (T4SS) forming pili that project from the bacterial outer membrane. H. pylori strains expressing T4SS genes induce secretion of the proinflammatory cytokine IL-8 in vitro (3, 24), and in vivo lead to increased mucosal IL-8 expression, mucosal inflammation, ulceration, and increased risk of gastric cancer (1, 5, 12, 37, 38). However, the relevance of cag PAI or other H. pylori genes and induction of IL-8 secretion to acid inhibition is still not fully understood. T4SS pili mediate H. pylori adherence to cell membranes, enabling delivery of virulence factor(s) into the host cell. CagE subunits with ATPase activity in the cytoplasmic part of the T4SS energize such transfer. The extracellular shaft of the T4SS incorporates the structural protein CagM and also expresses surface-bound CagL, which interacts specifically with α5β1 and other integrins on gastric epithelial cells, contributing to bacterial adherence and facilitating T4SS function (26, 55). CagA, a secreted protein encoded by cagA, the 3′-terminal gene of the cag PAI, has been extensively studied as a mediator of H. pylori pathogenesis and is the first reported bacterial oncoprotein that acts in mammals (2, 36). CagA transferred into gastric epithelial cells through the T4SS mimics adapter proteins by interacting with host cell proteins through tyrosine phosphorylation-dependent and -independent mechanisms (20).
Gastric acid secretion is mediated by the parietal cell H,K-ATPase proton pump (48). Our studies of infected gastric epithelial AGS cells have shown that the cag PAI-encoded T4SS proteins CagL, CagM, and CagE, which encode structural T4SS components, and secreted CagA participate in repression of transfected H,K-ATPase α subunit (HKα) promoter constructs (15–17, 43–46) and that such repression is mediated by NF-κB p50 homodimer interaction with an HKα promoter NF-κB binding site (46). Notably, HKα promoter activity was also repressed, but to a lesser extent, by an H. pylori cagA deletion mutant (43), suggesting that bacterial factors other than Cag A may play a role in HKα repression. Recently, we reported that in vitro H. pylori infection of AGS cells and human gastric biopsies upregulates a microRNA (miR-1289) that represses translation of HKα mRNA in a CagA-dependent manner (57). miR-1289 upregulation was also dependent on another H. pylori virulence factor, a product of bacterial peptidoglycan (PGN) cleavage by soluble lytic transglycosylase (SLT) (7) encoded by the non-cag PAI gene slt (HP0645 homolog). H. pylori also expresses putative PGN hydrolase activity (Cagγ) encoded by the cag PAI gene cagγ (HP0523 homolog) (58). The present study sought to determine the role of H. pylori-induced IL-8 secretion and the H. pylori virulence genes slt and cagγ on acid inhibition. Infection of AGS cells with a series of isogenic H. pylori knockout mutants and measurement of transfected HKα promoter activity indicated that bacterial factors other than those mediating CagA transfer or IL-8 secretion participate in HKα gene repression.
MATERIALS AND METHODS
Cells, bacteria, and reagents.
Human gastric epithelial AGS cells (ATCC, Manassas, VA) were maintained in Ham's F12 medium containing 10% FBS as described (46). The carcinogenic H. pylori wild-type strain 7.13 and isogenic mutants (Table 1) were cultured on Brucella-agar plates at 37°C under microaerobic conditions (5% O2, 7% CO2, 88% N2). Bacterial multiplicities of infection (MOI) were calculated as described (46). All reagents were of molecular biology grade.
Table 1.
Genetic characterization of H. pylori strains used in this study
| H. pylori Strains | Inactivated Gene | Proposed Gene Function | References |
|---|---|---|---|
| Wild-type 7.13 | 11 | ||
| ΔcagA | HP0547 | CagA oncoprotein | 20 |
| Δcagζ | HP0520 | Unknown | 10 |
| Δcagϵ | HP0521 | Unknown | 10 |
| Δcagβ | HP0524 | Nucleoside triphosphatase coupling protein | 23 |
| ΔcagZ | HP0526 | cag-specific translocation factor | 23 |
| Δcagγ | HP0523 | Peptidoglycan hydrolase | 56, 58 |
| Δslt | HP0645 | Soluble lytic transglycosylase | 7 |
HKα promoter-luc reporter plasmid transfection.
An HKα promoter-Luc reporter construct (HKα206) was formed by integrating 206 bp of proximal human gastric HKα 5′-flanking sequence into the luciferase reporter plasmid pGL2-Basic Vector as described (16). Point mutations at −159 bp (A>C, forward strand; T>G, reverse strand) and at −161 bp (G>A, forward strand; C>T, reverse strand) were introduced into the NF-κB1 site of the HKα construct using QuikChange Lightning Site-Directed Mutagenesis Kit (Stratagene) (46), and mutagenesis was verified by dideoxy sequencing. AGS cells were cotransfected with wild-type or mutated promoter-Luc reporter construct (HKαΔ206) and pMaxGFP for transfection efficiency control and normalization, and promoter-reporter activities were measured and normalized as described (44).
Generation of isogenic H. pylori strain 7.13 mutants.
Five wild-type H. pylori strain 26695 DNA plasmids (gift of Dr. Rainer Haas) containing a chloramphenicol acetyltransferase (cat gene) cassette in place of the cag PAI genes cagζ, cagε, cagβ, cagZ, and cagγ, respectively, were used to naturally transform wild-type H. pylori strain 7.13 (11). The replacement of specific cag PAI genes with cat cassettes utilized TnMax5 or TNMax5gfp transposon insertion mutagenesis, yielding isogenic mutant plasmids with no polar effects on expression of downstream genes (10). Briefly, H. pylori strain 7.13 were grown for 2–3 days on Brucella-agar with 10% serum and diluted to an A550 ∼0.1 in 1 ml BHI broth with 10% serum in a 24-well culture plate. After 2–3 days culture at 37°C with 5% CO2, when the culture density increased to A550 ∼0.2, supercoiled DNA plasmid (1–5 μg) was added and culture continued for 12 h (37°C, 5% CO2). Culture aliquots (100 μl) were spread on Brucella-agar plates with 10% serum and chloramphenicol (8 μg/ml). Chloramphenicol-resistant transformants were isolated after 3–6 days incubation under microaerophilic conditions (5% O2, 7% CO2, 88% N2,) at 37°C and were checked for correct insertion of the cassette into the cag PAI by PCR. Insertion of a kanamycin resistance cassette into the BamH1 site of a cloned PCR product of the full-length slt gene (HP0645 homolog), followed by transformation into H. pylori and kanamycin selection, yielded a Δslt mutant, confirmed to be nonpolar by equivalent expression of the downstream gene HP0646 in both wild-type 7.13 H. pylori and the Δslt mutant. The resulting isogenic mutants are shown in Table 1.
Human gastric biopsies.
Gastric endoscopic biopsies were acquired from patients who provided written informed consent (21–60 years old) to undergo esophagogastroduodenoscopy or endoscopic ultrasound at the MUSC Digestive Disease Center (IRB-approved protocol HR16941). Exclusion criteria included patients with positive urea breath and CLO tests. Four endoscopic biopsies (6–42 mg each) per patient were obtained from normal-appearing corpus mucosa on the greater curvature of the stomach. Corpus mucosa, unlike antral mucosa, is responsible for physiological acid secretion by virtue of parietal cell expression of H,K-ATPase, the focus of this study. Single biopsies were incubated in individual wells of 96 well culture plates with F12 culture medium (100 μl, 1 h, 37°C), infected for varying periods of time with 24 h cultures of H. pylori (1–2 × 105 bacteria/mg wet weight biopsy), and then rinsed 3 × with F12 medium. Same-patient biopsies incubated with F12 medium alone served as mock-infection controls.
Biopsy acid secretion.
Gastric biopsies were infected in vitro with H. pylori (1–2 × 105 bacteria/mg wet weight biopsy, 24 h), and then treated for 30 min with 5-(N-ethyl-n-isopropyl) amiloride (EIPA; 150 μM) to block Na+/H+ exchanger activity. Biopsies were divided into four parts along the mucosal-serosal axis, distributed into wells of a 24-well XF24 culture plate thermostatted to 37°C, and overlaid with pyruvate- and bicarbonate-free DMEM (600 μl; Mediatech, Manassas VA). The wells had been pretreated overnight with Cell-Tak (1 μl; BD Biosciences, Bedford, MA) to facilitate adhesion of biopsies. After 15 min acclimatization, measurement of gastric biopsy medium acidification was initiated using an XF24 Extracellular Flux Analyzer (Seahorse Biosciences, Boston, MA). Automated placement of fiber-optic pH-sensitive fluorescent hydrogel probes within ∼300 μm of the biopsies created a “virtual chamber” enclosing ∼28 μl of medium immediately overlying a biopsy. Measurements of [H+] in this limited diffusion region were carried out in six 10-min cycles, each cycle involving probe retraction and vibration for 4 min to mix and reequilibrate formerly enclosed medium with bulk medium, a 3 min pause, and [H+]-dependent fluorescence measurement for 3 min. The rate of medium acidification was calculated from the slope of change in [H+]. pH measurements were made without significant depression of oxygen tension or medium acidification, achieving microphysiometer-like sensitivity. Constitutive H+ secretion was measured for ∼30 min before programmed remote injection of histamine (1 mM final concentration).
IL-8 secretion.
IL-8 protein levels in AGS cell/H. pylori-conditioned media were measured by ELISA (R&D Systems, Minneapolis-St. Paul, MN) according to the manufacturer's protocol.
Immunoblotting.
AGS cells (80% confluent, serum-deprived for 15–20 h) were infected (MOI = 50, 1 h) with WT and isogenic mutant H. pylori strain 7.13, washed and harvested in ice-cold PBS, and centrifuged (520 g, 5 min). Cell pellets were dissolved in SDS-PAGE sample buffer and analyzed by immunoblotting as described (14) using pan-phosphotyrosine specific antibody PY99 (Santa Cruz Biotechnology, CA) (5 μg antibody/mg lysate protein). Uniformity of sample loading onto gels was confirmed by sequential probing of PVDF gel replicas with PY99 and GAPDH or β-actin specific antibodies. Immunoblots shown are representative of three experimental replications.
Statistical analysis.
Two-way analysis of variance (ANOVA) followed by Bonferroni posttest were used to analyze the effect of H. pylori infection on the transcriptional activity of HKα promoter-reporter constructs. Mann-Whitney tests were used for analysis of H. pylori effects on IL-8 secretion. Analyses were done with GraphPad Prism 4.0 or Microsoft Excel 2007. P values < 0.05 were considered significant.
RESULTS
cag PAI gene dependency of HKα repression.
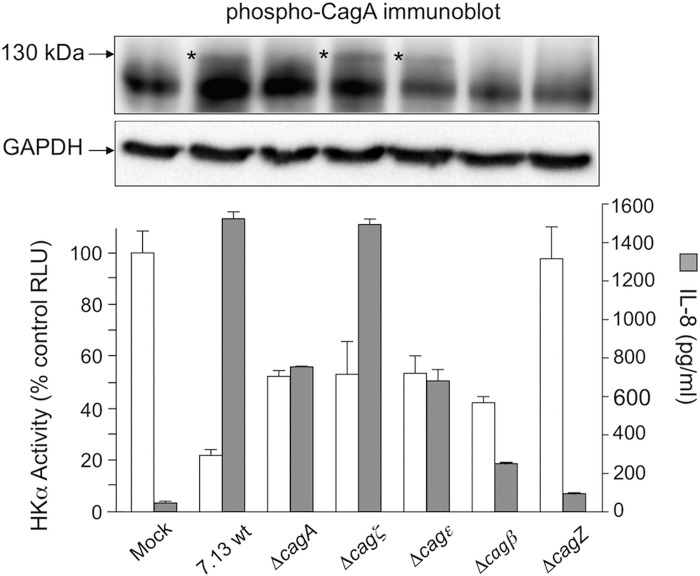
Having previously shown that H. pylori-induced repression of HKα transcription was only partially relieved when cells were infected with a cagA-deficient isogenic mutant (43), we reasoned that other bacterial factors may also exert HKα-repressive effects. Thus we examined the effects on HKα transcription of gastric epithelial cell infection with H. pylori isogenic mutants deficient in selected cag PAI genes but still competent in terms of CagA translocation. A previous seminal study utilizing systematic mutagenesis of 27 individual genes in the cag PAI of strain 26695 H. pylori and subsequent infection of gastric epithelial cells in vitro had revealed that 12 cag PAI genes were required for induction of IL-8 secretion, and 9 of these genes were also required for CagA transfer into host cells (10). We selected four strain 26695 cag PAI gene knockout strains that retained both CagA phosphorylation and IL-8 secretion competence to generate corresponding H. pylori mutants in the 7.13 strain background (Δcagζ, Δcagε, Δcagβ, and ΔcagZ; Table 1). Figure 1 shows constitutive HKα promoter activity (corresponding to ∼5500 RLU) and IL-8 secretion (∼200 pg/ml) in mock-infected cells and corresponding data for H. pylori-infected cells. Wild-type infection inhibited transfected HKα promoter activity by ∼80%, but Δcagζ and Δcagε infections inhibited HKα by only 50%, comparable to HKα inhibition exerted by ΔcagA. Δcagε infection induced ∼50% less IL-8 secretion than wild-type H. pylori or Δcagζ. HKα promoter activity and IL-8 secretion were unaffected by ΔcagZ mutant infection. Phospho-CagA was detected only in cells infected with wild-type H. pylori and Δcagζ and Δcagε. Two mutants that did not induce phospho-CagA (ΔcagA and Δcagβ) nevertheless inhibited HKα promoter activity by ∼50%, and induced 14-fold and 5-fold induction of IL-8 secretion, respectively. These data suggest that neither CagA- nor IL-8-mediated pathways, alone or in concert, fully account for the observed impact of H. pylori on gastric acid secretion.
Fig. 1.
Effect of Helicobacter pylori infection of AGS cells on transfected HKα promoter activity and on IL-8 secretion. AGS cells were transfected with a 206-bp HKα-promoter-Luc reporter construct, and independently infected 24 h later (MOI = 50, 6 h) with wild-type H. pylori or ΔcagA, Δcagζ, Δcagε, Δcagβ, or ΔcagZ isogenic mutants. CagA delivery into the cells was assessed by phospho-CagA immunoblotting, IL-8 secretion was measured by ELISA, and the transcriptional activity of the HKα promoter construct was measured by luminometry. Data are shown as means ± SD, n = 3. Presence of phospho-CagA in corresponding AGS cell lysate immunoblots is indicated as *, and β-actin served as a gel loading control.
NF-κB dependency of H. pylori-induced HKα repression.
To determine whether intracellular CagA alone and its phosphorylation are sufficient to repress HKα and whether NF-κB signaling plays a role in repression, AGS cells expressing wild-type HKα (HKα206) or mutated HKα promoter (HKαΔ206) were transfected with wild-type or phosphorylation-resistant (PR) CagA expression plasmids. HKαΔ206 is a Luc reporter construct in which the NF-κB binding site on the HKα promoter is rendered nonfunctional by mutation of two nucleotides (46), and the PR CagA expression plasmid contains a tyrosine > alanine mutation in the COOH-terminal EPIYA motif. Intracellular expression of CagA was confirmed by immunoblotting (Fig. 2, inset), and was associated with significant repression of wild-type HKα promoter activity (Fig. 2). Mutation of the HKα promoter NF-κB binding site partially reduced CagA-mediated HKα promoter repression, indicating that CagA activates mobilization and nuclear targeting of NF-κB. Interestingly, wild-type CagA expression repressed HKα promoter activity significantly more than PR CagA expression; both repressive effects were sensitive to NF-κB binding site mutation. These data suggest that although phosphorylated and nonphosphorylated CagA both signal to NF-κB, the latter CagA does so more efficiently than the former.
Fig. 2.
Repression of HKα promoter activity by phosphorylated and nonphosphorylated intracellular CagA is NF-κB-dependent. AGS cells were cotransfected with HKα206 or HKαΔ206 promoter-Luc reporter constructs and CagA or phosphorylation resistant (PR)-CagA expression vectors, and HKα promoter activity was measured by luminometry after 24 h to ensure maximal expression of the CagA vectors. Data are shown as means ± SD, n = 3. Inset shows CagA immunoblots of the corresponding lysates of CagA-transfected AGS cells, and β-actin served as a gel loading control.
To determine whether H. pylori-induced HKα repression was mediated by NF-κB, whether or not cagA was delivered into host cells, AGS cells expressing wild-type HKα206 or HKαΔ206 Luc reporter constructs were infected (6 h, MOI = 50) with wild-type or Δcagζ, Δcagε, or Δcagβ H. pylori mutants, and HKα promoter activity was measured as before. As shown in Fig. 3, HKα repression induced by infection of transfected cells with 7.13 wild-type or Δcagζ, Δcagε, or Δcagβ isogenic mutants was significantly or completely abrogated by the presence of a nonfunctional NF-κB binding site on the HKα promoter. Two of the isogenic mutants, Δcagζ and Δcagε, mediated CagA phosphorylation in host cells, while Δcagβ did not. These data suggest that H. pylori virulence factor(s) other than CagA infiltrate the cell to activate NF-κB and cause HKα repression.
Fig. 3.
Repression of HKα promoter activity by wild-type H. pylori or Δcagζ, Δcagε, or Δcagβ isogenic mutants is NF-κB-dependent. AGS cells were transfected with HKα206 or HKαΔ206-Luc reporter constructs, infected with wild-type or H. pylori isogenic mutants as indicated, and HKα promoter activity was measured by luminometry. Data are shown as means ± SD, n = 3. Presence of phospho-CagA in corresponding AGS cell lysate immunoblots is indicated as *.
Role of slt and cagγ genes in HKα repression.
Bacterial soluble lytic transglycosylase (SLT) activity was previously shown to signal to host intracellular nucleotide-oligomerization domain (NOD)1 receptor with subsequent NF-κB activation (54). Additionally, the H. pylori cag PAI gene cagγ (HP0523) has been reported to express PGN hydrolase activity, generating GM-tripeptide (58). To determine whether slt and/or cagγ gene products repress HKα gene transcription, AGS cells expressing HKα206 or HKαΔ206 were infected (6 h, MOI = 50) with wild-type H. pylori, Δslt, Δcagγ, or Δslt/Δcagγ H. pylori isogenic mutants. As shown in Fig. 4A, Δcagγ and Δslt isogenic mutants repressed HKα promoter activity by ∼75%, the same degree of repression exerted by wild-type H. pylori. In contrast, in AGS cells infected with the double mutant Δslt/Δcagγ, HKα promoter activity was repressed by only 35%, suggesting some degree of participation in HKα repression by cooperative SLT and Cagγ secretory activity. IL-8 secretion into culture medium was markedly stimulated by wild-type H. pylori infection of AGS cells, and was equally stimulated by Δcagγ, Δslt, and Δslt/Δcagγ isogenic mutants (Fig. 4A), indicating that H. pylori-induced IL-8 secretion is not dependent on bacterial Cagγ or SLT expression. As shown by phospho-CagA immunoblotting of infected AGS cells (Fig. 4B), Δslt, Δcagγ, and Δslt/Δcagγ isogenic mutants remained capable of CagA transfer into the cells. When transfected AGS cells were separated from bacteria by Transwell filters, HKα promoter activity was unaffected by infection with Δslt, Δcagγ, or Δslt/Δcagγ isogenic mutants (data not shown). As shown in Fig. 4C, AGS cells expressing HKαΔ206 infected with Δslt or Δcagγ isogenic mutants showed significantly less repression of HKα promoter activity than observed with cells expressing wild-type HKα promoter.
Fig. 4.
A: effect of H. pylori infection of AGS cells on transfected HKα promoter activity and on IL-8 secretion. AGS cells were transfected with a 206 bp HKα-promoter-Luc reporter construct, and independently infected 24 h later (MOI = 50, 6 h) with wild-type H. pylori or Δslt, Δcagγ, or Δslt/Δcagγ H. pylori isogenic mutants. IL-8 secretion was measured by ELISA, and the transcriptional activity of the HKα promoter construct was measured by luminometry. B: CagA transfer into the cells was assessed in two separate phospho-CagA immunoblotting experiments, and β-actin served as a gel loading control. C: repression of HKα promoter activity by Δslt or Δcagγ H. pylori isogenic mutants is NF-κB-dependent. AGS cells were transfected with HKα206 or HKαΔ206 promoter-Luc reporter constructs, and independently mock-infected or infected 24 h later (MOI = 50, 6 h) with Δslt or Δcagγ H. pylori isogenic mutants. Data are shown as means ± SD, n = 3. ** P < 0.05.
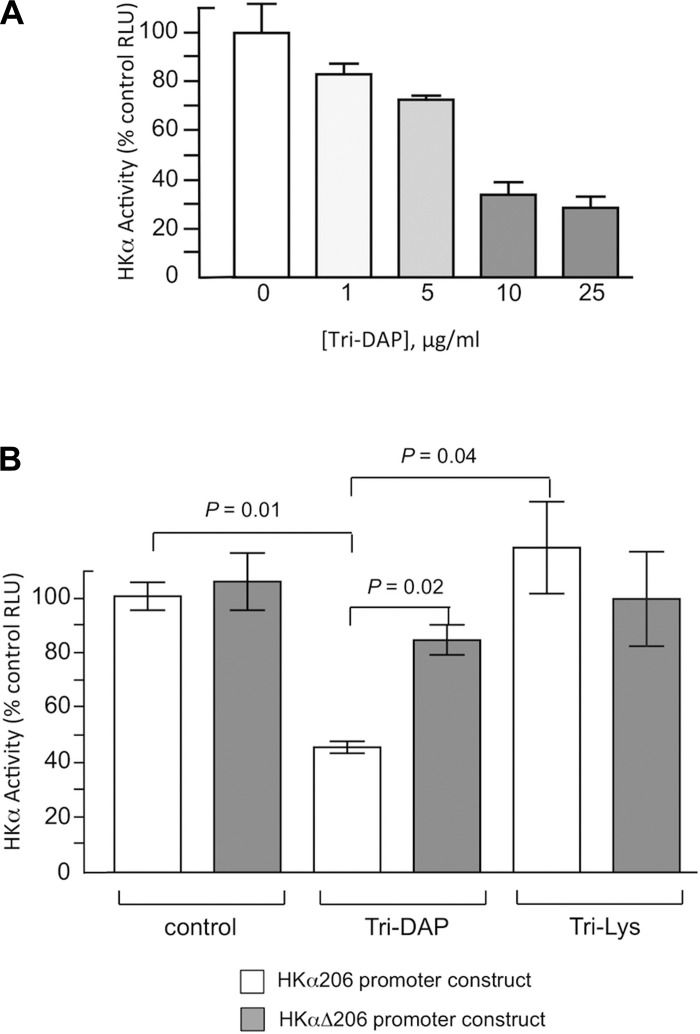
Since both SLT and Cagγ give rise to N-acetyl-glucosamine-N-acetyl-muramic acid-l-alanine-d-glutamate-mesodiaminopimelic acid (GM-3), we investigated the potential role of this PGN in modulating HKα transcription. HKα206 or HKαΔ206-transfected AGS cells were treated with the GM-3 mimetic Tri-DAP (MurNAc-l-Ala-γ-d-Glu-mDAP), a diaminopimelic acid-containing muramyl tripeptide NOD1 receptor agonist. Tri-Lys, a peptide found in the PGN of Gram-positive bacteria that is not recognized by NOD1 receptor, was used as a negative control for Tri-DAP. Tri-DAP dose-dependently repressed HKα206 promoter-Luc reporter activity (Fig. 5A), and HKα promoter activity in Tri-DAP-treated cells (10 μg/ml, 6 h) was repressed by ∼50% compared with control; Tri-Lys had no effect on HKα promoter activity (Fig. 5B). The repressive effect of Tri-DAP was significantly reduced (P = 0.02) by mutation of the HKα NF-κB site (Fig. 5B). Taken together, these data indicate that cooperative secretory activity of both SLT and Cagγ is involved in cell contact-dependent, NOD1-mediated, and NF-κB-dependent repression of HKα promoter activity.
Fig. 5.
Repression of HKα promoter activity by the GM-3 analog Tri-DAP is NF-κB-dependent. AGS cells were transfected with HKα206 or HKαΔ206 promoter-Luc reporter constructs, treated with Tri-DAP or Tri-Lys, and HKα promoter activity was measured by luminometry. A: HKα206 promoter activity as a function of Tri-DAP concentration. B: HKα206 and HKαΔ206 promoter activities in the presence or absence of Tri-DAP or Tri-Lys. Data are shown as means ± SD, n = 3.
Role of slt and cagγ genes in physiological acid secretion.
To assess the contribution of H. pylori slt and cagγ genes to functional regulation of H,K-ATPase in terms of physiological acid secretion, human gastric corpus biopsies were infected (MOI ∼ 50, 24 h) with wild-type 7.13 H. pylori, Δslt, or Δcagγ isogenic mutant strains. The biopsies were then treated with EIPA (150 μM, 30 min), and biopsy medium [H+] was recorded continuously for 3 min at intervals of 7 min. After 15 min, histamine (1 mM final) or vehicle alone was added to some biopsies. The slopes of the initial rates of change of extracellular pH were transformed for buffer capacity to yield the proton production rate (PPR; pmol H+/min) (43). Urease-induced ammonia production factors into the measured PPR, as does CO2 production during substrate oxidation (33). Both processes contribute to measured PPR in wild-type and mutant H. pylori infections alike, and so changes in PPR following infection can be ascribed to changes in parietal cell proton secretion (43). As shown in Fig. 6A, mock-infected biopsies (●) maintained a stable constitutive PPR of ∼180 pmol H+/min for 15 min. Histamine addition to mock-infected biopsies (●) increased PPR to ∼220 pmol H+/min within 10 min, and this rate of acid secretion was maintained over the next 30 min. Pretreatment of biopsies with 50 μM SCH28080 for 30 min abrogated histamine-stimulated medium acidification (not shown), confirming mechanistic involvement of biopsy H,K-ATPase activity in this acidification. In contrast, wild-type H. pylori-infected biopsies (▲) showed significantly attenuated PPR of ∼75 pmol H+/min, and histamine addition caused minimal change in PPR. Biopsies infected with the Δslt isogenic mutant (■) exhibited an intermediate relatively stable PPR of ∼140 pmol H+/min throughout the experimental time-course with no sensitivity to added histamine. Since wild-type H. pylori and Δslt isogenic mutant both express CagA, these data are consistent with SLT acting synergistically with CagA in parietal cells to suppress acid secretion. In contrast, as shown in Fig. 6B, the acid secretory response in biopsies infected with the Δcagγ isogenic mutant was more attenuated than that observed in biopsies infected with wild-type H. pylori, although both wild type-infected and Δcagγ-infected biopsies had reduced constitutive PPR compared with mock-infected controls, and histamine addition failed to stimulate extracellular acidification. Since wild-type H. pylori and Δcagγ isogenic mutant both express CagA, the data are consistent with products of the putative PGN hydrolase activity expressed by the cagPAI gene cagγ countering to some extent CagA-driven inhibition of acid secretion. Overall, microphysiometric data confirm our previous report of inhibition of parietal cell H,K-ATPase-mediated acid secretion as a physiological consequence of H. pylori infection (43), and additionally implicate H. pylori soluble lytic transglycosylase activity expressed by the slt gene in the form of GM-3 tripeptide as a mechanistic intermediary in the inhibitory pathway.
Fig. 6.
Acid secretion by human gastric mucosal biopsies is inhibited by wild-type H. pylori and by Δslt and Δcagγ isogenic mutants. Gastric biopsies were infected with wild-type H. pylori strain 7.13 or Δslt or Δcagγ isogenic mutants (1–2 × 105 bacteria/mg wet wt biopsy, 24 h) or Brucella broth alone, and then incubated with 150 μM EIPA for 30 min. The [H+] of the medium overlying the biopsies was measured continuously for 3 min at 7 min intervals. Histamine (or vehicle) was added to some biopsies to a 1 mM final concentration (arrows). The slopes of the initial rates of change of medium [H+] were calculated from buffer capacity and volume to yield the biopsy proton production rate (PPR; pmoles H+/min). A: ●, mock infection; ▲, infection with wild-type H. pylori; ■, infection with Δslt mutant. B: ●, mock infection; ▲, infection with wild-type H. pylori; ■, infection with Δcagγ mutant. Data points in A and B are the mean PPR ± SD, n = 3 biopsies.
cagA and slt genes work in concert to repress HKα transcription.
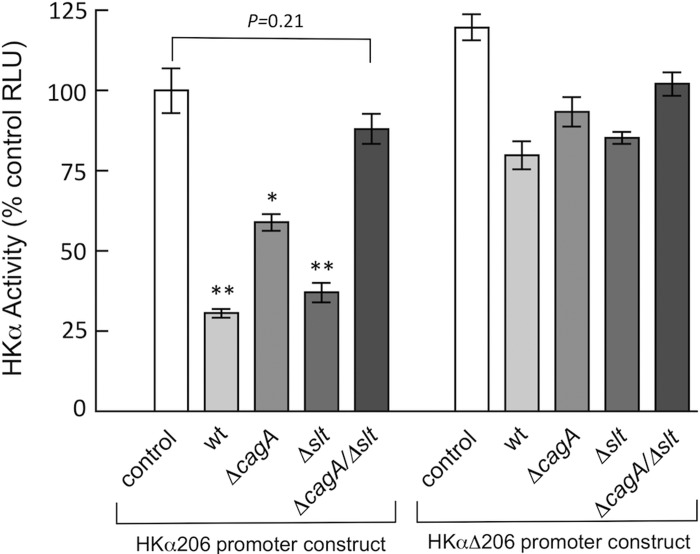
Given our findings that CagA and GM-3 independently promote repression of HKα transcription, we reasoned that concurrent deletion of both the cagA and the slt genes would deprive H. pylori of its acid-suppressive capabilities. Accordingly, AGS cells transfected with the HKα206 promoter-Luc reporter construct were infected (6 h, MOI = 50) with an H. pylori double isogenic mutant deficient in both genes. As shown in Fig. 7, HKα promoter activity was not significantly attenuated by infection of transfected AGS cells with the double mutant, although infection with the single mutants markedly repressed HKα promoter activity. These data indicate that expression of both the cagA and the slt genes contributes to repression of HKα transcription, and together with our foregoing findings of NF-κB participation in this repression, strongly suggest that CagA and GM-3 tripeptide transported into host gastric epithelial cells are the principal H. pylori effectors of NF-κB-mediated suppression of H,K-ATPase activity. Table 2 summarizes the outcomes of H. pylori infection of AGS cells in this study in terms of CagA translocation, IL-8 secretion, and HKα repression.
Fig. 7.
Repression of HKα promoter activity by wild-type H. pylori, ΔcagA, or Δslt H. pylori isogenic mutants is NF-κB-dependent. AGS cells were transfected with HKα206 or HKαΔ206 promoter-Luc reporter constructs, and independently mock-infected or infected 24 h later (MOI = 50, 6 h) with Δslt or Δcagγ H. pylori isogenic mutants. Data are shown as means ± SD, n = 3. *P < 0.05; **P < 0.01.
Table 2.
Experimental end points of H. pylori infection of AGS cells
| Outcome of Infection* |
|||
|---|---|---|---|
| H. pylori Strain | CagA translocation | IL-8 secretion | HKα promoter repression |
| wild-type 7.13 | + | 3 | 3 |
| ΔcagA | − | 2 | 2 |
| Δcagζ | + | 3 | 2 |
| Δcagϵ | + | 2 | 2 |
| Δcagβ | − | 1 | 2 |
| ΔcagZ | − | 1 | 1 |
| Δcagγ | + | 3 | 3 |
| Δslt | + | 3 | 3 |
DISCUSSION
H. pylori has successfully colonized the human gastric mucosa for at least 60,000 years (32), evolving adaptations to an ostensibly corrosive environment of low pH that most bacteria cannot survive. The fact that most H. pylori infections are asymptomatic and clinically benign, together with putative inverse relationships between H. pylori infection and Barrett's esophagus (51), childhood asthma (8) and gastroenteritis (6), suggest that H. pylori, in exchange for a noncompetitive, nutritive niche, may confer benefits to its Homo sapiens hosts. One such benefit may be modulation of the composition of the gastrointestinal microbiome by H. pylori-induced hypochlorhydria (21, 27, 30, 47). An extensive literature documents inhibition of acid secretion secondary to gastric infection by H. pylori (9, 18, 19, 29, 34, 41, 49, 50). We have shown that activity of HKα promoter-Luc reporter constructs transfected into AGS cells is repressed by infection with wild-type H. pylori, is partially repressed (∼50%) by CagA-deficient H. pylori, and is unaffected by ΔcagE, ΔcagM, and ΔcagL H. pylori mutants (16, 43). These data demonstrate a role for CagA and a requirement for T4SS integrity in H. pylori-induced HKα repression. We have also shown that H. pylori stimulates AGS cell ADAM 17 activity and represses transfected HKα promoter activity. Mechanistic data in that study established that acute H. pylori infection causes CagL to dissociate ADAM 17 from α1β5 integrin, activating ADAM 17-dependent, NF-κB-mediated repression of HKα promoter (42). The mechanisms underlying H. pylori-induced hypochlorhydria include inhibition of H,K-ATPase α-subunit (HKα) gene expression by ERK 1/2-mediated NF-κB p50 subunit homodimer binding to HKα promoter (46). We recently reviewed the mechanistic evidence for H. pylori-induced acid secretory inhibition, including evidence that acute H. pylori-induced acid inhibition is not caused by parietal cell ablation, neutrophil IL-1β secretion, or H. pylori vacuolating toxin (VacA) (48). More recently, we reported that H. pylori infection upregulates gastric epithelial cell microRNA (miR-1289), that CagA and bacterial soluble lytic transglycosylase (SLT) (54) are implicated in HKα-specific miR-1289 upregulation, and that miR-1289 with a highly-conserved HKα distal UTR binding site plays a role in repressing HKα mRNA translation (57).
The present study investigated the role of selected cag and non-cag PAI genes in H. pylori-induced IL-8 secretion and transfected HKα promoter activity, with a view to establishing their participation in H. pylori-induced hypochlorhydria. Cag PAI+ H. pylori strains have been characterized as high (>2,000 pg/ml) or low (<500 pg/ml) IL-8 inducers, depending on specific COOH-terminal sequence variations in CagA (3). Our finding that isogenic ΔcagA mutant induced significantly less IL-8 than wild-type infection suggests that H. pylori strain 7.13 is a high IL-8 inducer strain and confirms CagA participation in IL-8 secretion. High IL-8 inducer H. pylori strains induce IL-8 secretion by activation of a Ras > Raf > MEK > ERK > NF-κB signaling pathway (3), and neither Shp-2 tyrosine phosphatase, a binding partner of CagA (22), nor c-Met, nor MAP kinases p38 and c-Jun, nor PKC are required for signaling to IL-8 secretion (3). We have shown that NF-κB-mediated transcriptional regulation of HKα promoter activity is dependent on a functional ERK1/2 signaling pathway, and that neither p39 nor the JNK signaling pathways are involved in NF-κB-mediated inhibition of HKα promoter activity (46). Our findings here that the highest levels of H. pylori-induced IL-8 secretion coexist with maximal, intermediate or minimal stimulation of HKα promoter activity, depending on specific cag PAI or non-cag PAI gene knockouts, and that both high (1,500 pg/ml) and intermediate (700 pg/ml) levels of IL-8 secretion induced by Δcagζ and Δcagε mutants, respectively, were accompanied by comparable HKα promoter activity, not significantly different from that induced by ΔcagA mutant infection, indicate that the IL-8 pathway per se plays no role in HKα transcription. The data confirm a previous report that cagζ and cagε gene products are unnecessary for IL-8 induction and that the cag PAI does not encode a secreted IL-8 inducing effector protein (10).
Notably, AGS cell infection with Δcagβ mutant, which did not translocate CagA, nonetheless repressed constitutive HKα promoter activity by ∼60%, comparable to the repression exerted by the ΔcagA mutant. Yeast two-hybrid and immunoprecipitation pull-down assays have shown that Cagβ interacts with the Cag-specific translocation factor CagZ (4) and with CagA, forming a putative T4SS substrate-translocation factor complex (23). Thus partial repression of HKα promoter activity by a Δcagβ mutant deficient in T4SS function suggests involvement of H. pylori virulence factor(s) that by-pass the T4SS to infiltrate host cells. Partial abrogation of wild-type H. pylori or isogenic mutant-induced HKα repression by mutation of the HKα promoter NF-κB binding site indicated that nuclear localized NF-κB was a primary effector of acid inhibition, whether CagA or other T4SS-dependent or -independent H. pylori factors are responsible for triggering NF-κB nuclear translocation. The potential involvement in HKα repression of another H. pylori-induced signaling pathway, e.g., AKT targeting by phosphotidylinositol 3-phosphate kinase (35), remains to be investigated.
Our data relating to the effects of Δslt and Δcagγ mutant infections on HKα promoter activity in AGS cells and on acid secretion by human gastric biopsies are consistent with SLT activity, and to a lesser extent Cagγ activity, playing a role in HKα gene repression and acid inhibition. SLT cleaves the unique diaminopimelidate-containing tripeptide GM-3 from the nonreducing ends of glycan strands constituting the cell wall PGN heteropolymer encapsulating H. pylori. GM-3 interacts with the intracellular NOD1 receptor, which in turn induces NF-κB activation in HEK 293 cells (13) and AGS cells (54), raising the possibility that secreted GM-3 may modulate HKα promoter activity through a NOD1 receptor pathway. H. pylori PGN export leads to decreased apoptosis and increased cell proliferation and migration (35), and inactivation of the H. pylori deacetylase PgdA decreases NOD1-dependent NF-κB activation and autophagy (52), conferring on GM-3 and NOD1 important roles in gastric carcinogenesis. The putative PGN hydrolase activity (Cagγ) encoded by the cag PAI gene cagγ (HP0523) has also been reported to digest H. pylori glycan strands resulting in the formation of a 1,6-anhydro bond in the MurNAc residue and degraded PGN fragments including GM-3 (56, 58). Cagγ expressed by H. pylori strain 26695 was reported to be essential for both CagA translocation and IL-8 secretion (10), consistent with the proposed role of PAI-encoded lytic transglycosylases in facilitating PGN penetration by the macromolecular complexes constituting the T4SS (25).
In the present study, however, human gastric mucosal biopsies infected in vitro with Δslt or Δcagγ H. pylori mutants failed to sustain the levels of histamine-induced acid secretion shown by mock-infected biopsies, indicating persistence of a functional T4SS. This finding recapitulates the wild-type H. pylori-induced acid inhibition that we reported previously (43), which was attributed to CagL and CagA signaling to NF-κB through ERK 1/2 pathways (42, 46). Infection with independent or joint slt and cagγ preserved CagA phosphorylation and H. pylori-induced IL-8 secretion, consistent with H. pylori strain 7.13 being a CagA-dependent, high IL-8 inducer, and suggesting that structural and functional assembly of the T4SS in the 7.13 strain depends on lytic transglycosylases other than SLT or Cagγ. However, the partial abrogation of HKα promoter repression induced by the joint slt/cagγ deletion, or by mutation of the HKα promoter NF-κB binding site, argues for cumulative SLT and Cagγ-dependent GM-3 activation of intracellular NOD1 receptor as a significant contributor to NF-κB mobilization and nuclear localization, and thus HKα gene repression. This conclusion is further supported by our data demonstrating that a cell permeable, exogenous GM-3 NOD1 receptor analog (Tri-DAP), but not a Gram-positive-specific GM-3 analog (Tri-Lys), dose-dependently represses HKα promoter activity, but not in the presence of a mutated NF-κB site on the promoter. Complementary determination of wild-type or mutated HKα promoter activity following AGS cell infection with a ΔcagA/Δslt double mutant also pointed to synergistic activation of NF-κB by CagA and SLT activity; independent deletions of cagA or slt significantly repressed HKα promoter activity, while double mutant infections did not, and all repression was abolished by a dysfunctional HKα NF-κB binding site.
Involvement of the pro-carcinogenic cag PAI in acid secretory inhibition confers clinical relevance to mechanisms of H. pylori-induced hypochlorhydria. This study complements earlier findings that an intact T4SS is required for inhibition by presenting several lines of evidence that induction of host cell IL-8 secretion does not per se repress HKα gene expression, and that the translocated oncoprotein CagA, together with secreted PGN, are the primary bacterial effectors of acid inhibition caused by acute H. pylori infection. The physiological relevance of such inhibition may be manifold. First, amelioration of an otherwise hostile acidic environment clearly facilitates gastric colonization by H. pylori. Second, while antral or antral-corpus transitional zone colonization is more typical, corpus and cardia may also be colonized (53). Infection of the acid-secreting corpus induces transient gastric hypochlorhydria which may predispose mucosal susceptibility to atrophic gastritis. The resulting parietal cell attrition reinforces hypochlorhydria, with consequent mucosal progression to intestinal metaplasia, dysplasia, and gastric cancer. Last, transient gastric H. pylori acid inhibition may contribute to gastrointestinal homeostasis by modulating gastrointestinal microbial composition. H. pylori-induced microbiota changes have been reported in gerbil large intestine (21), mouse stomach (27) and human stomach (30, 47). Since gut dysbiosis is associated with many diseases (39), dissection of the molecular mechanisms underlying transient H. pylori-induced hypochlorhydria may augment understanding of both the deleterious and potentially beneficial effects of gastric colonization by this microorganism.
GRANTS
This study was supported by National Institutes of Health Grants DK-064371 to A. J. Smolka; DK-58587, CA-77955, and CA-116087 to R. M. Peek, Jr; and the German Science Foundation CRC-796 (Project B10) to S. Backert.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.E.H., G.S., S.B., and A.J.S. performed experiments; C.E.H., C.B., and A.J.S. analyzed data; C.E.H., C.B., G.S., R.M.P.J., S.B., and A.J.S. edited and revised manuscript; C.E.H., C.B., G.S., R.M.P.J., S.B., and A.J.S. approved final version of manuscript; C.B., G.S., R.M.P.J., S.B., and A.J.S. interpreted results of experiments; S.B. and A.J.S. prepared figures; A.J.S. conception and design of research; A.J.S. drafted manuscript.
ACKNOWLEDGMENTS
We thank Dr. R. Haas (Max von Pettenkofer Institut, Munich, Germany) for a panel of H. pylori strain 26695 DNA plasmids with individual cag PAI gene knockouts, Dr. G. Shull (Univ. of Cincinnati) for genomic DNA incorporating a portion of the human gastric H,K-ATPase α subunit 5′-flanking sequence, and Dr. B. Hoffman [Division of Gastroenterology and Hepatology, Medical University of South Carolina (MUSC)] and A. Wood (Clinical Coordinator, Digestive Disease Center, MUSC) for provision of human gastric biopsies. We thank also G. Beeson (Dept. of Drug Discovery and Biomedical Sciences, MUSC) for expertise and guidance in measuring biopsy extracellular acidification.
REFERENCES
- 1.Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, Stemmermann GN, Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res 55: 2111–2115, 1995. [PubMed] [Google Scholar]
- 2.Bourzac KM, Guillemin K. Helicobacter pylori-host cell interactions mediated by type IV secretion. Cell Microbiol 7: 911–919, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Brandt S, Kwok T, Hartig R, Konig W, Backert S. NF-kappaB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc Natl Acad Sci USA 102: 9300–9305, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cendron L, Seydel A, Angelini A, Battistutta R, Zanotti G. Crystal structure of CagZ, a protein from the Helicobacter pylori pathogenicity island that encodes for a type IV secretion system. J Mol Biol 340: 881–889, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Censini S, Lange C, Xiang Z, Crabtree JE, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA 93: 14648–14653, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang AH, Haggerty TD, de Martel C, Leung CW, Parsonnet J. Effect of Helicobacter pylori infection on symptoms of gastroenteritis due to enteropathogenic Escherichia coli in adults. Dig Dis Sci 56: 457–464, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaput C, Labigne A, Boneca IG. Characterization of Helicobacter pylori Lytic transglycosylases Slt and MltD. J Bacteriol 189: 422–429, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Blaser MJ. Helicobacter pylori colonization is inversely associated with childhood asthma. J Infect Dis 198: 553–560, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Omar EM, Oien K, El-Nujumi A, Gillen D, Wirz A, Dahill S, Williams C, Ardill JE, McColl KE. Helicobacter pylori infection and chronic gastric acid hyposecretion. Gastroenterology 113: 15–24, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Fischer W, Puls J, Buhrdorf R, Gebert B, Odenbreit S, Haas R. Systematic mutagenesis of the Helicobacter pylori cag pathogenicity island: essential genes for CagA translocation in host cells and induction of interleukin-8. Mol Microbiol 42: 1337–1348, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Franco AT, Israel DA, Washington MK, Krishna U, Fox JG, Rogers AB, Neish AS, Collier-Hyams L, Perez-Perez GI, Hatakeyama M, Whitehead R, Gaus K, O'Brien DP, Romero-Gallo J, Peek RM Jr. Activation of b-catenin by carcinogenic Helicobacter pylori. Proc Natl Acad Sci USA 102: 10646–10651, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franco AT, Johnston E, Krishna U, Yamaoka Y, Israel DA, Nagy TA, Wroblewski LE, Piazuelo MB, Correa P, Peek RM Jr. Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer Res 68: 379–387, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girardin SE, Boneca IG, Carneiro LA, Antignac A, Jehanno M, Viala J, Tedin K, Taha MK, Labigne A, Zahringer U, Coyle AJ, DiStefano PS, Bertin J, Sansonetti PJ, Philpott DJ. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science 300: 1584–1587, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Gooz M, Gooz P, Luttrell LM, Raymond JR. 5-HT2A receptor induces ERK phosphorylation and proliferation through ADAM-17 tumor necrosis factor-alpha-converting enzyme (TACE) activation and heparin-bound epidermal growth factor-like growth factor (HB-EGF) shedding in mesangial cells. J Biol Chem 281: 21004–21012, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Gooz M, Gooz P, Smolka AJ. Epithelial and bacterial metalloproteinases and their inhibitors in H. pylori infection of human gastric cells. Am J Physiol Gastrointest Liver Physiol 281: G823–G832, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Gooz M, Hammond CE, Larsen K, Mukhin YV, Smolka AJ. Inhibition of human gastric H+-K+-ATPase alpha-subunit gene expression by Helicobacter pylori. Am J Physiol Gastrointest Liver Physiol 278: G981–G991, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Gooz M, Shaker M, Gooz P, Smolka AJ. Interleukin 1beta induces gastric epithelial cell matrix metalloproteinase secretion and activation during Helicobacter pylori infection. Gut 52: 1250–1256, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham DY, Alpert LC, Smith JL, Yoshimura HH. Iatrogenic Campylobacter pylori infection is a cause of epidemic achlorhydria. Am J Gastroenterol 83: 974–980, 1988. [PubMed] [Google Scholar]
- 19.Harford WV, Barnett C, Lee E, Perez-Perez G, Blaser MJ, Peterson WL. Acute gastritis with hypochlorhydria: report of 35 cases with long term follow up. Gut 47: 467–472, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatakeyama M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat Rev Cancer 4: 688–694, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Heimesaat MM, Fischer A, Plickert R, Wiedemann T, Loddenkemper C, Gobel UB, Bereswill S, Rieder G. Helicobacter pylori induced gastric immunopathology is associated with distinct microbiota changes in the large intestines of long-term infected mongolian gerbils. PLoS One 9: e100362, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higashi H, Tsutsumi R, Fujita A, Yamazaki S, Asaka M, Azuma T, Hatakeyama M. Biological activity of the Helicobacter pylori virulence factor CagA is determined by variation in the tyrosine phosphorylation sites. Proc Natl Acad Sci USA 99: 14428–14433, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jurik A, Hausser E, Kutter S, Pattis I, Prassl S, Weiss E, Fischer W. The coupling protein Cagbeta and its interaction partner CagZ are required for type IV secretion of the Helicobacter pylori CagA protein. Infect Immun 78: 5244–5251, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keates S, Hitti YS, Upton M, Kelly CP. Helicobacter pylori infection activates NF-kappa B in gastric epithelial cells. Gastroenterology 113: 1099–1109, 1997. [DOI] [PubMed] [Google Scholar]
- 25.Koraimann G. Lytic transglycosylases in macromolecular transport systems of Gram-negative bacteria. Cell Mol Life Sci 60: 2371–2388, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwok T, Zabler D, Urman S, Rohde M, Hartig R, Wessler S, Misselwitz R, Berger J, Sewald N, Konig W, Backert S. Helicobacter exploits integrin for type IV secretion and kinase activation. Nature 449: 862–866, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Lofgren JL, Whary MT, Ge Z, Muthupalani S, Taylor NS, Mobley M, Potter A, Varro A, Eibach D, Suerbaum S, Wang TC, Fox JG. Lack of commensal flora in Helicobacter pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia. Gastroenterology 140: 210–220, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall BJ. Helicobacter pylori in peptic ulcer: have Koch's postulates been fulfilled? Ann Med 27: 565–568, 1995. [DOI] [PubMed] [Google Scholar]
- 29.Marshall BJ, Armstrong JA, McGechie DB, Glancy RJ. Attempt to fulfil Koch's postulates for pyloric Campylobacter. Med J Austral 142: 436–439, 1985. [DOI] [PubMed] [Google Scholar]
- 30.Mattarelli P, Brandi G, Calabrese C, Fornari F, Prati GM, Biavati B, Sgorbati B. Occurrence of Bifidobacteriaceae in human hypochlorhydria stomach. Microb Ecol Health Dis 25, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer-Rosberg K, Scott DR, Rex D, Melchers K, Sachs G. The effect of environmental pH on the proton motive force of Helicobacter pylori. Gastroenterology 111: 886–900, 1996. [DOI] [PubMed] [Google Scholar]
- 32.Moodley Y, Linz B, Bond RP, Nieuwoudt M, Soodyall H, Schlebusch CM, Bernhoft S, Hale J, Suerbaum S, Mugisha L, van der Merwe SW, Achtman M. Age of the association between Helicobacter pylori and man. PLoS Pathog 8: e1002693, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mookerjee SA, Goncalves RL, Gerencser AA, Nicholls DG, Brand MD. The contributions of respiration and glycolysis to extracellular acid production. Biochim Biophys Acta 1847: 171–181, 2015. [DOI] [PubMed] [Google Scholar]
- 34.Morris A, Nicholson G. Ingestion of Campylobacter pyloridis causes gastritis and raised fasting gastric pH. Am J Gastroenterol 82: 192–199, 1987. [PubMed] [Google Scholar]
- 35.Nagy TA, Frey MR, Yan F, Israel DA, Polk DB, Peek RM Jr. Helicobacter pylori regulates cellular migration and apoptosis by activation of phosphatidylinositol 3-kinase signaling. J Infect Dis 199: 641–651, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohnishi N, Yuasa H, Tanaka S, Sawa H, Miura M, Matsui A, Higashi H, Musashi M, Iwabuchi K, Suzuki M, Yamada G, Azuma T, Hatakeyama M. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc Natl Acad Sci USA 105: 1003–1008, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peek RM Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer 2: 28–37, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Peek RM Jr, Miller GG, Tham KT, Perez-Perez GI, Zhao X, Atherton JC, Blaser MJ. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab Invest 73: 760–770, 1995. [PubMed] [Google Scholar]
- 39.Pflughoeft KJ, Versalovic J. Human microbiome in health and disease. Annu Rev Pathol 7: 99–122, 2012. [DOI] [PubMed] [Google Scholar]
- 40.Polk DB, Peek RM. Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer 10: 403–414, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramsey EJ, Carey KV, Peterson WL, Jackson JJ, Murphy FK, Read NW, Taylor KB, Trier JS, Fordtran JS. Epidemic gastritis with hypochlorhydria. Gastroenterology 76: 1449–1457, 1979. [PubMed] [Google Scholar]
- 42.Saha A, Backert S, Hammond CE, Gooz M, Smolka AJ. Helicobacter pylori CagL activates ADAM17 to induce repression of the gastric H, K-ATPase alpha subunit. Gastroenterology 139: 239–248, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saha A, Hammond CE, Beeson C, Peek RM, Smolka AJ. Helicobacter pylori represses proton pump expression and inhibits acid secretion in human gastric mucosa. Gut 59: 874–881, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saha A, Hammond CE, Gooz M, Smolka AJ. IL-1beta modulation of H,K-ATPase-alpha-subunit gene transcription in Helicobacter pylori infection. Am J Physiol Gastrointest Liver Physiol 292: G1055–G1061, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Saha A, Hammond CE, Gooz M, Smolka AJ. The role of Sp1 in IL-1beta and H. pylori-mediated regulation of H,K-ATPase gene transcription. Am J Physiol Gastrointest Liver Physiol 295: G977–G986, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saha A, Hammond CE, Trojanowska M, Smolka AJ. Helicobacter pylori-induced H,K-ATPase-alpha-subunit gene repression is mediated by NF-kappaB p50 homodimer promoter binding. Am J Physiol Gastrointest Liver Physiol 294: G795–G807, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Sheh A, Fox JG. The role of the gastrointestinal microbiome in Helicobacter pylori pathogenesis. Gut Microbes 4: 505–531, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smolka AJ, Backert S. How Helicobacter pylori infection controls gastric acid secretion. J Gastroenterol 47: 609–618, 2012. [DOI] [PubMed] [Google Scholar]
- 49.Sobala GM, Crabtree JE, Dixon MF, Schorah CJ, Taylor JD, Rathbone BJ, Heatley RV, Axon AT. Acute Helicobacter pylori infection: clinical features, local and systemic immune response, gastric mucosal histology, and gastric juice ascorbic acid concentrations. Gut 32: 1415–1418, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sonnenberg A, Bartmess J, Kern L, Siebenmann RE, Joris F, Blum AL. Hypochlorhydrie bei akuter Gastritis. Deutsche Medizin Wochenschr 104: 1814–1816, 1979. [DOI] [PubMed] [Google Scholar]
- 51.Sonnenberg A, Lash RH, Genta RM. A national study of Helicobactor pylori infection in gastric biopsy specimens. Gastroenterology 139: 1894–1901, e1892, 2010. [DOI] [PubMed] [Google Scholar]
- 52.Suarez G, Romero-Gallo J, Piazuelo MB, Wang G, Maier R, Forsberg LS, Azadi P, Gomez MA, Correa P, Peek RM Jr. Modification of Helicobacter pylori peptidoglycan enhances NOD1 activation and promotes cancer of the stomach. Cancer Res 75: 749–759, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Zanten SJ, Dixon MF, Lee A. The gastric transitional zones: neglected links between gastroduodenal pathology and helicobacter ecology. Gastroenterology 116: 1217–1229, 1999. [DOI] [PubMed] [Google Scholar]
- 54.Viala J, Chaput C, Boneca IG, Cardona A, Girardin SE, Moran AP, Athman R, Memet S, Huerre MR, Coyle AJ, DiStefano PS, Sansonetti PJ, Labigne A, Bertin J, Philpott DJ, Ferrero RL. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol 5: 1166–1174, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Wiedemann T, Hofbaur S, Tegtmeyer N, Huber S, Sewald N, Wessler S, Backert S, Rieder G. Helicobacter pylori CagL dependent induction of gastrin expression via a novel alphavbeta5-integrin-integrin linked kinase signalling complex. Gut 61: 986–996, 2012. [DOI] [PubMed] [Google Scholar]
- 56.Zahrl D, Wagner M, Bischof K, Bayer M, Zavecz B, Beranek A, Ruckenstuhl C, Zarfel GE, Koraimann G. Peptidoglycan degradation by specialized lytic transglycosylases associated with type III and type IV secretion systems. Microbiology 151: 3455–3467, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Zhang YM, Hammond CE, Barth JL, Argraves WS, Backert S, Peek RM, Smolka AJ. Helicobacter pylori-induced post-transcriptional regulation of H,K-ATPase alpha subunit gene by miRNA. Am J Physiol Gastrointest Liver Physiol 306: G606–G613, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhong Q, Shao S, Mu R, Wang H, Huang S, Han J, Huang H, Tian S. Characterization of peptidoglycan hydrolase in Cag pathogenicity island of Helicobacter pylori. Mol Biol Rep 38: 503–509, 2011. [DOI] [PubMed] [Google Scholar]