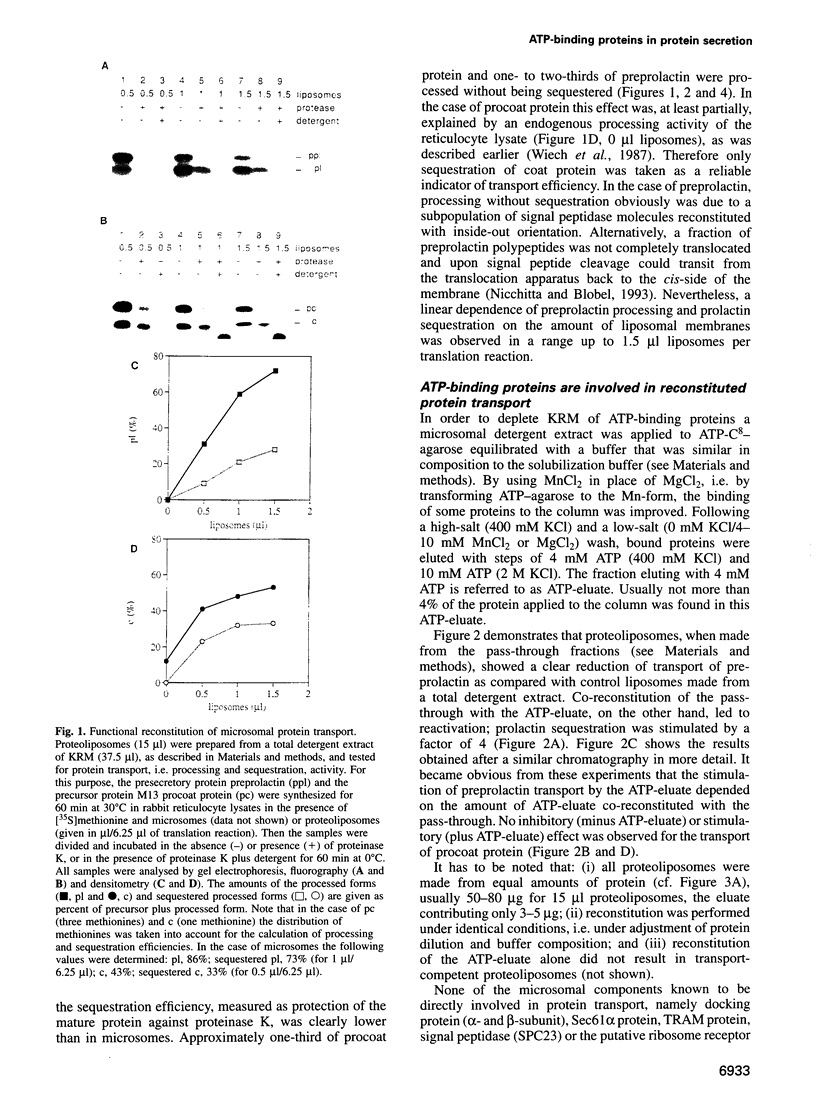
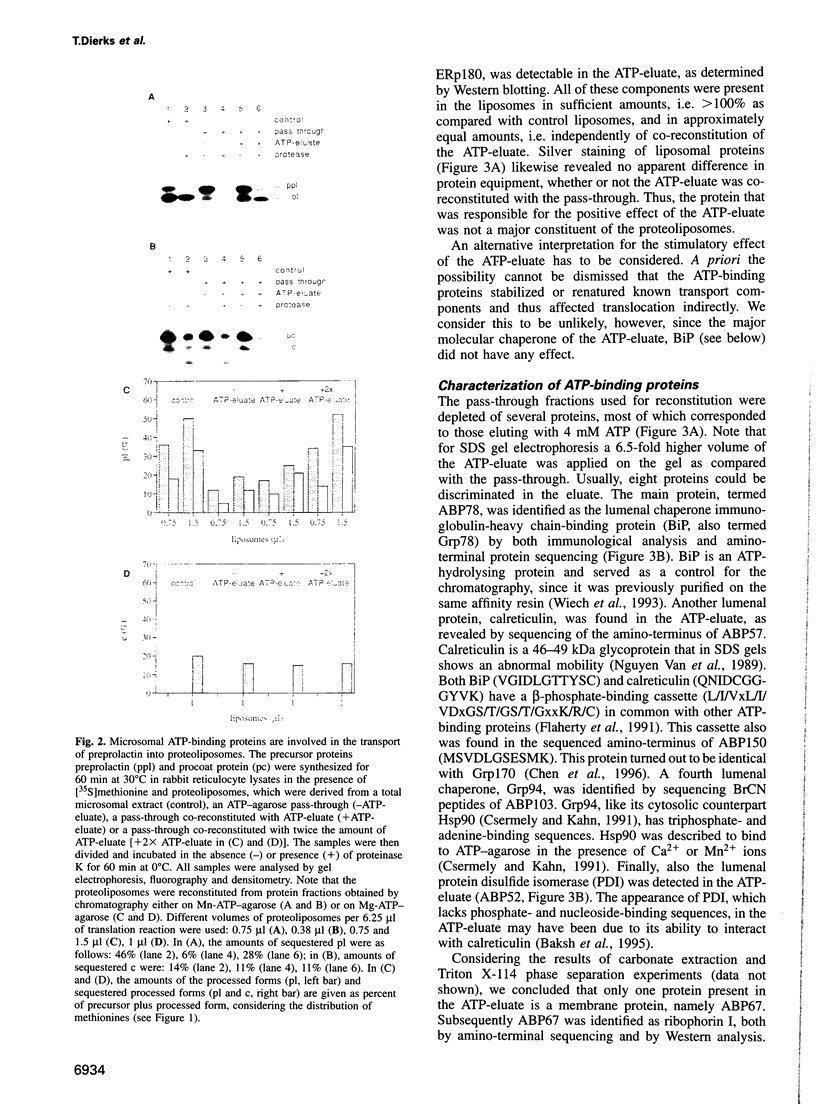
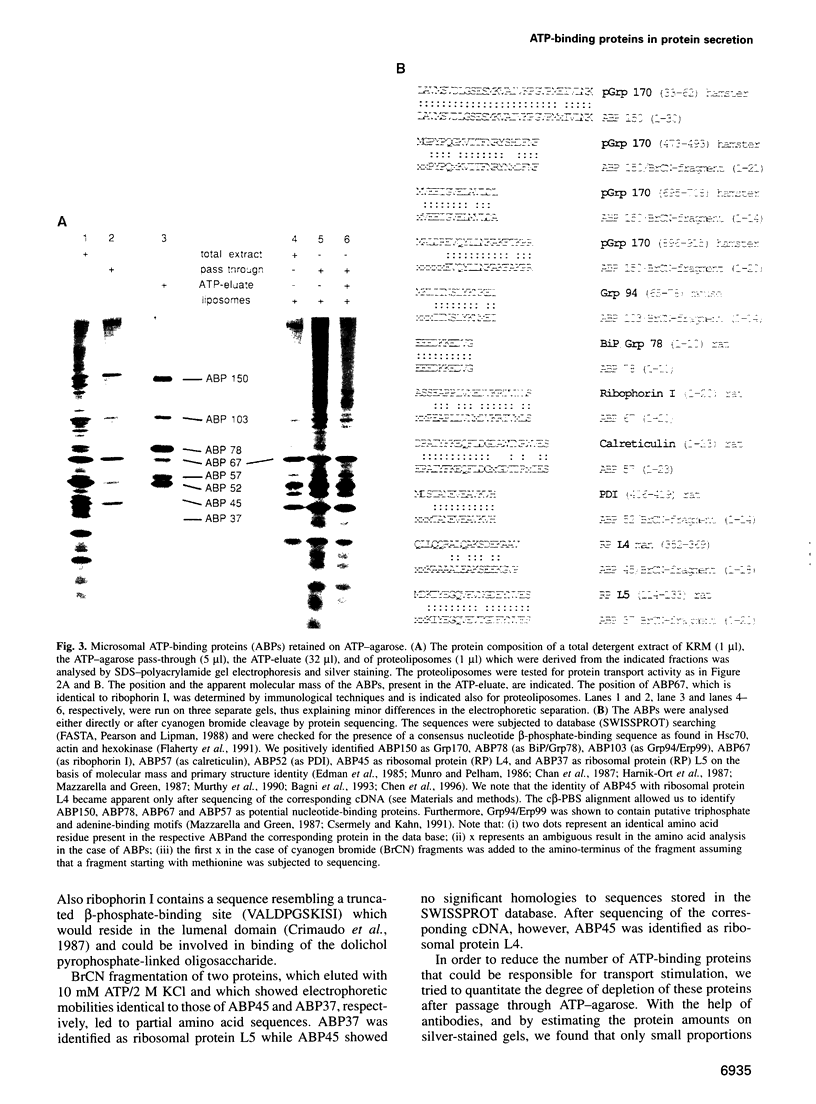
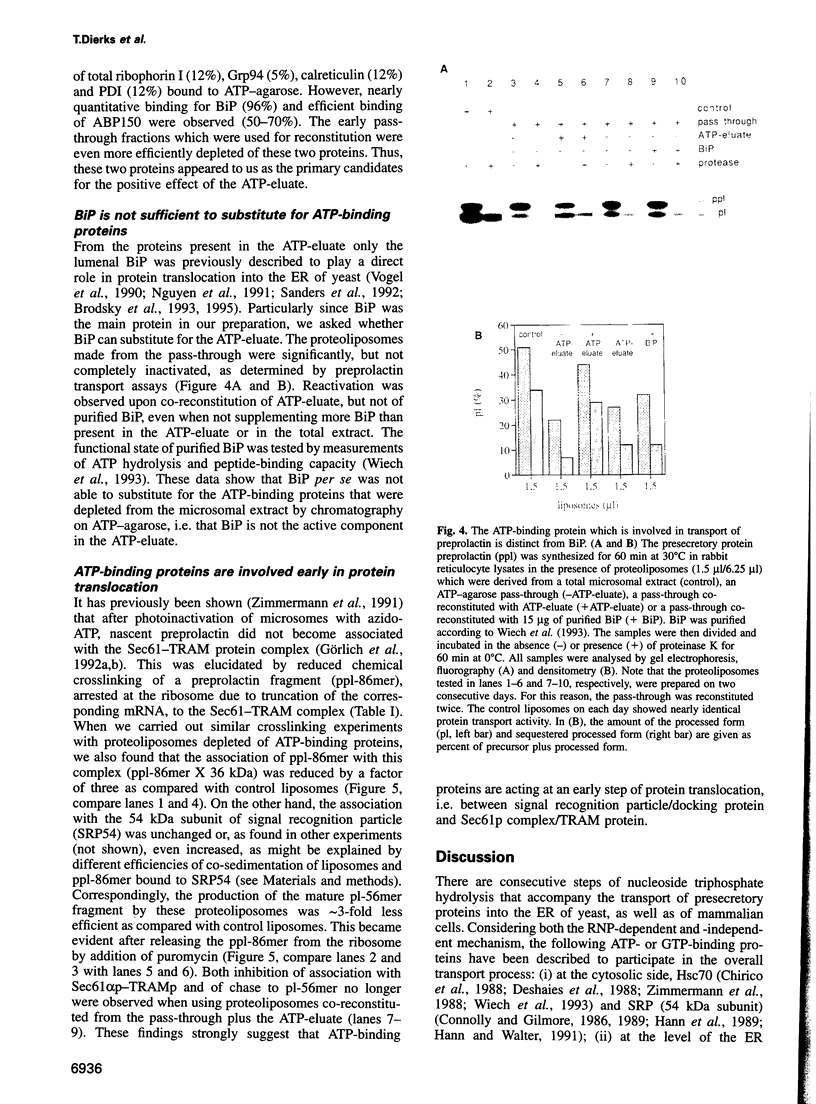
Abstract
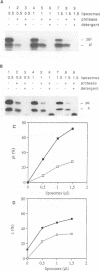
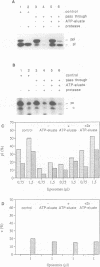
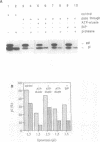
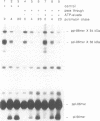
Protein transport into the mammalian endoplasmic reticulum depends on nucleoside triphosphates. Photoaffinity labelling of microsomes with azido-ATP prevents protein transport at the level of association of precursor proteins with the components of the transport machinery, Sec61alpha and TRAM proteins. The same phenotype of inactivation was observed after depleting a microsomal detergent extract of ATP-binding proteins by passage through ATP-agarose and subsequent reconstitution of the pass-through into proteoliposomes. Transport was restored by co-reconstitution of the ATP eluate. This eluate showed eight distinct bands in SDS gels. We identified five lumenal proteins (Grp170, Grp94, BiP/Grp78, calreticulin and protein disulfide isomerase), one membrane protein (ribophorin I) and two ribosomal proteins (L4 and L5). In addition to BiP (Grp78), Grp170 was most efficiently retained on ATP-agarose. Purified BiP did not stimulate transport activity. Sequence analysis revealed a striking similarity of Grp170 and the yeast microsomal protein Lhs1p which was recently shown to be involved in protein transport into yeast microsomes. We suggest that Grp170 mediates efficient insertion of polypeptides into the microsomal membrane at the expense of nucleoside triphosphates.
Full text
PDF

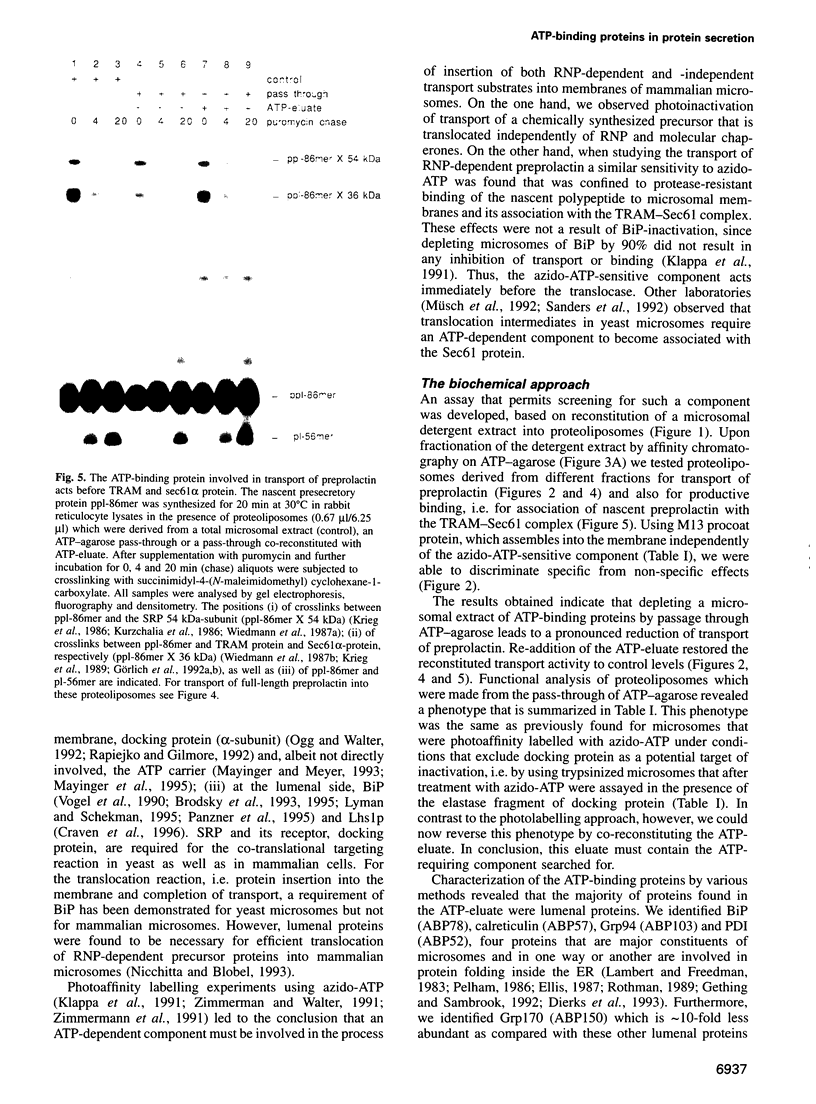





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacher G., Lütcke H., Jungnickel B., Rapoport T. A., Dobberstein B. Regulation by the ribosome of the GTPase of the signal-recognition particle during protein targeting. Nature. 1996 May 16;381(6579):248–251. doi: 10.1038/381248a0. [DOI] [PubMed] [Google Scholar]
- Bagni C., Mariottini P., Annesi F., Amaldi F. Human ribosomal protein L4: cloning and sequencing of the cDNA and primary structure of the protein. Biochim Biophys Acta. 1993 Dec 14;1216(3):475–478. doi: 10.1016/0167-4781(93)90017-8. [DOI] [PubMed] [Google Scholar]
- Baksh S., Burns K., Andrin C., Michalak M. Interaction of calreticulin with protein disulfide isomerase. J Biol Chem. 1995 Dec 29;270(52):31338–31344. doi: 10.1074/jbc.270.52.31338. [DOI] [PubMed] [Google Scholar]
- Bernstein H. D., Poritz M. A., Strub K., Hoben P. J., Brenner S., Walter P. Model for signal sequence recognition from amino-acid sequence of 54K subunit of signal recognition particle. Nature. 1989 Aug 10;340(6233):482–486. doi: 10.1038/340482a0. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brightman S. E., Blatch G. L., Zetter B. R. Isolation of a mouse cDNA encoding MTJ1, a new murine member of the DnaJ family of proteins. Gene. 1995 Feb 14;153(2):249–254. doi: 10.1016/0378-1119(94)00741-a. [DOI] [PubMed] [Google Scholar]
- Brodsky J. L., Goeckeler J., Schekman R. BiP and Sec63p are required for both co- and posttranslational protein translocation into the yeast endoplasmic reticulum. Proc Natl Acad Sci U S A. 1995 Oct 10;92(21):9643–9646. doi: 10.1073/pnas.92.21.9643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky J. L., Hamamoto S., Feldheim D., Schekman R. Reconstitution of protein translocation from solubilized yeast membranes reveals topologically distinct roles for BiP and cytosolic Hsc70. J Cell Biol. 1993 Jan;120(1):95–102. doi: 10.1083/jcb.120.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulleid N. J., Freedman R. B. Defective co-translational formation of disulphide bonds in protein disulphide-isomerase-deficient microsomes. Nature. 1988 Oct 13;335(6191):649–651. doi: 10.1038/335649a0. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chan Y. L., Lin A., McNally J., Wool I. G. The primary structure of rat ribosomal protein L5. A comparison of the sequence of amino acids in the proteins that interact with 5 S rRNA. J Biol Chem. 1987 Sep 15;262(26):12879–12886. [PubMed] [Google Scholar]
- Chen X., Easton D., Oh H. J., Lee-Yoon D. S., Liu X., Subjeck J. The 170 kDa glucose regulated stress protein is a large HSP70-, HSP110-like protein of the endoplasmic reticulum. FEBS Lett. 1996 Feb 12;380(1-2):68–72. doi: 10.1016/0014-5793(96)00011-7. [DOI] [PubMed] [Google Scholar]
- Chirico W. J., Waters M. G., Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988 Apr 28;332(6167):805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Connolly T., Gilmore R. Formation of a functional ribosome-membrane junction during translocation requires the participation of a GTP-binding protein. J Cell Biol. 1986 Dec;103(6 Pt 1):2253–2261. doi: 10.1083/jcb.103.6.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly T., Gilmore R. The signal recognition particle receptor mediates the GTP-dependent displacement of SRP from the signal sequence of the nascent polypeptide. Cell. 1989 May 19;57(4):599–610. doi: 10.1016/0092-8674(89)90129-3. [DOI] [PubMed] [Google Scholar]
- Connolly T., Rapiejko P. J., Gilmore R. Requirement of GTP hydrolysis for dissociation of the signal recognition particle from its receptor. Science. 1991 May 24;252(5009):1171–1173. doi: 10.1126/science.252.5009.1171. [DOI] [PubMed] [Google Scholar]
- Craven R. A., Egerton M., Stirling C. J. A novel Hsp70 of the yeast ER lumen is required for the efficient translocation of a number of protein precursors. EMBO J. 1996 Jun 3;15(11):2640–2650. [PMC free article] [PubMed] [Google Scholar]
- Crimaudo C., Hortsch M., Gausepohl H., Meyer D. I. Human ribophorins I and II: the primary structure and membrane topology of two highly conserved rough endoplasmic reticulum-specific glycoproteins. EMBO J. 1987 Jan;6(1):75–82. doi: 10.1002/j.1460-2075.1987.tb04721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csermely P., Kahn C. R. The 90-kDa heat shock protein (hsp-90) possesses an ATP binding site and autophosphorylating activity. J Biol Chem. 1991 Mar 15;266(8):4943–4950. [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Dierks T., Klappa P., Wiech H., Zimmermann R. The role of molecular chaperones in protein transport into the endoplasmic reticulum. Philos Trans R Soc Lond B Biol Sci. 1993 Mar 29;339(1289):335–341. doi: 10.1098/rstb.1993.0032. [DOI] [PubMed] [Google Scholar]
- Edman J. C., Ellis L., Blacher R. W., Roth R. A., Rutter W. J. Sequence of protein disulphide isomerase and implications of its relationship to thioredoxin. Nature. 1985 Sep 19;317(6034):267–270. doi: 10.1038/317267a0. [DOI] [PubMed] [Google Scholar]
- Ellis J. Proteins as molecular chaperones. 1987 Jul 30-Aug 5Nature. 328(6129):378–379. doi: 10.1038/328378a0. [DOI] [PubMed] [Google Scholar]
- Feldheim D., Rothblatt J., Schekman R. Topology and functional domains of Sec63p, an endoplasmic reticulum membrane protein required for secretory protein translocation. Mol Cell Biol. 1992 Jul;12(7):3288–3296. doi: 10.1128/mcb.12.7.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty K. M., McKay D. B., Kabsch W., Holmes K. C. Similarity of the three-dimensional structures of actin and the ATPase fragment of a 70-kDa heat shock cognate protein. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):5041–5045. doi: 10.1073/pnas.88.11.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Gilmore R., Blobel G., Walter P. Protein translocation across the endoplasmic reticulum. I. Detection in the microsomal membrane of a receptor for the signal recognition particle. J Cell Biol. 1982 Nov;95(2 Pt 1):463–469. doi: 10.1083/jcb.95.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore R., Walter P., Blobel G. Protein translocation across the endoplasmic reticulum. II. Isolation and characterization of the signal recognition particle receptor. J Cell Biol. 1982 Nov;95(2 Pt 1):470–477. doi: 10.1083/jcb.95.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlich D., Hartmann E., Prehn S., Rapoport T. A. A protein of the endoplasmic reticulum involved early in polypeptide translocation. Nature. 1992 May 7;357(6373):47–52. doi: 10.1038/357047a0. [DOI] [PubMed] [Google Scholar]
- Görlich D., Prehn S., Hartmann E., Kalies K. U., Rapoport T. A. A mammalian homolog of SEC61p and SECYp is associated with ribosomes and nascent polypeptides during translocation. Cell. 1992 Oct 30;71(3):489–503. doi: 10.1016/0092-8674(92)90517-g. [DOI] [PubMed] [Google Scholar]
- Görlich D., Rapoport T. A. Protein translocation into proteoliposomes reconstituted from purified components of the endoplasmic reticulum membrane. Cell. 1993 Nov 19;75(4):615–630. doi: 10.1016/0092-8674(93)90483-7. [DOI] [PubMed] [Google Scholar]
- Hann B. C., Poritz M. A., Walter P. Saccharomyces cerevisiae and Schizosaccharomyces pombe contain a homologue to the 54-kD subunit of the signal recognition particle that in S. cerevisiae is essential for growth. J Cell Biol. 1989 Dec;109(6 Pt 2):3223–3230. doi: 10.1083/jcb.109.6.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann B. C., Walter P. The signal recognition particle in S. cerevisiae. Cell. 1991 Oct 4;67(1):131–144. doi: 10.1016/0092-8674(91)90577-l. [DOI] [PubMed] [Google Scholar]
- Harnik-Ort V., Prakash K., Marcantonio E., Colman D. R., Rosenfeld M. G., Adesnik M., Sabatini D. D., Kreibich G. Isolation and characterization of cDNA clones for rat ribophorin I: complete coding sequence and in vitro synthesis and insertion of the encoded product into endoplasmic reticulum membranes. J Cell Biol. 1987 Apr;104(4):855–863. doi: 10.1083/jcb.104.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann E., Sommer T., Prehn S., Görlich D., Jentsch S., Rapoport T. A. Evolutionary conservation of components of the protein translocation complex. Nature. 1994 Feb 17;367(6464):654–657. doi: 10.1038/367654a0. [DOI] [PubMed] [Google Scholar]
- Hegde R. S., Lingappa V. R. Sequence-specific alteration of the ribosome-membrane junction exposes nascent secretory proteins to the cytosol. Cell. 1996 Apr 19;85(2):217–228. doi: 10.1016/s0092-8674(00)81098-3. [DOI] [PubMed] [Google Scholar]
- High S., Andersen S. S., Görlich D., Hartmann E., Prehn S., Rapoport T. A., Dobberstein B. Sec61p is adjacent to nascent type I and type II signal-anchor proteins during their membrane insertion. J Cell Biol. 1993 May;121(4):743–750. doi: 10.1083/jcb.121.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High S., Görlich D., Wiedmann M., Rapoport T. A., Dobberstein B. The identification of proteins in the proximity of signal-anchor sequences during their targeting to and insertion into the membrane of the ER. J Cell Biol. 1991 Apr;113(1):35–44. doi: 10.1083/jcb.113.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnen W., Ward L. D., Reid G. E., Moritz R. L., Simpson R. J. Internal amino acid sequencing of proteins by in situ cyanogen bromide cleavage in polyacrylamide gels. Biochem Biophys Res Commun. 1990 Jan 15;166(1):139–145. doi: 10.1016/0006-291x(90)91922-f. [DOI] [PubMed] [Google Scholar]
- Jungnickel B., Rapoport T. A. A posttargeting signal sequence recognition event in the endoplasmic reticulum membrane. Cell. 1995 Jul 28;82(2):261–270. doi: 10.1016/0092-8674(95)90313-5. [DOI] [PubMed] [Google Scholar]
- Kellaris K. V., Bowen S., Gilmore R. ER translocation intermediates are adjacent to a nonglycosylated 34-kD integral membrane protein. J Cell Biol. 1991 Jul;114(1):21–33. doi: 10.1083/jcb.114.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher D. J., Kreibich G., Gilmore R. Oligosaccharyltransferase activity is associated with a protein complex composed of ribophorins I and II and a 48 kd protein. Cell. 1992 Apr 3;69(1):55–65. doi: 10.1016/0092-8674(92)90118-v. [DOI] [PubMed] [Google Scholar]
- Klappa P., Mayinger P., Pipkorn R., Zimmermann M., Zimmermann R. A microsomal protein is involved in ATP-dependent transport of presecretory proteins into mammalian microsomes. EMBO J. 1991 Oct;10(10):2795–2803. doi: 10.1002/j.1460-2075.1991.tb07828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klappa P., Zimmermann M., Dierks T., Zimmermann R. Components and mechanisms involved in transport of proteins into the endoplasmic reticulum. Subcell Biochem. 1993;21:17–40. doi: 10.1007/978-1-4615-2912-5_2. [DOI] [PubMed] [Google Scholar]
- Klappa P., Zimmermann M., Zimmermann R. The membrane proteins TRAMp and sec61 alpha p may be involved in post-translational transport of presecretory proteins into mammalian microsomes. FEBS Lett. 1994 Mar 21;341(2-3):281–287. doi: 10.1016/0014-5793(94)80473-7. [DOI] [PubMed] [Google Scholar]
- Krieg U. C., Johnson A. E., Walter P. Protein translocation across the endoplasmic reticulum membrane: identification by photocross-linking of a 39-kD integral membrane glycoprotein as part of a putative translocation tunnel. J Cell Biol. 1989 Nov;109(5):2033–2043. doi: 10.1083/jcb.109.5.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg U. C., Walter P., Johnson A. E. Photocrosslinking of the signal sequence of nascent preprolactin to the 54-kilodalton polypeptide of the signal recognition particle. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8604–8608. doi: 10.1073/pnas.83.22.8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzchalia T. V., Wiedmann M., Girshovich A. S., Bochkareva E. S., Bielka H., Rapoport T. A. The signal sequence of nascent preprolactin interacts with the 54K polypeptide of the signal recognition particle. Nature. 1986 Apr 17;320(6063):634–636. doi: 10.1038/320634a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambert N., Freedman R. B. Structural properties of homogeneous protein disulphide-isomerase from bovine liver purified by a rapid high-yielding procedure. Biochem J. 1983 Jul 1;213(1):225–234. doi: 10.1042/bj2130225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffer L., Garcia P. D., Harkins R. N., Coussens L., Ullrich A., Walter P. Topology of signal recognition particle receptor in endoplasmic reticulum membrane. 1985 Nov 28-Dec 4Nature. 318(6044):334–338. doi: 10.1038/318334a0. [DOI] [PubMed] [Google Scholar]
- Lin H. Y., Masso-Welch P., Di Y. P., Cai J. W., Shen J. W., Subjeck J. R. The 170-kDa glucose-regulated stress protein is an endoplasmic reticulum protein that binds immunoglobulin. Mol Biol Cell. 1993 Nov;4(11):1109–1119. doi: 10.1091/mbc.4.11.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman S. K., Schekman R. Interaction between BiP and Sec63p is required for the completion of protein translocation into the ER of Saccharomyces cerevisiae. J Cell Biol. 1995 Dec;131(5):1163–1171. doi: 10.1083/jcb.131.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayinger P., Bankaitis V. A., Meyer D. I. Sac1p mediates the adenosine triphosphate transport into yeast endoplasmic reticulum that is required for protein translocation. J Cell Biol. 1995 Dec;131(6 Pt 1):1377–1386. doi: 10.1083/jcb.131.6.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayinger P., Meyer D. I. An ATP transporter is required for protein translocation into the yeast endoplasmic reticulum. EMBO J. 1993 Feb;12(2):659–666. doi: 10.1002/j.1460-2075.1993.tb05699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzarella R. A., Green M. ERp99, an abundant, conserved glycoprotein of the endoplasmic reticulum, is homologous to the 90-kDa heat shock protein (hsp90) and the 94-kDa glucose regulated protein (GRP94). J Biol Chem. 1987 Jun 25;262(18):8875–8883. [PubMed] [Google Scholar]
- Meyer D. I., Dobberstein B. A membrane component essential for vectorial translocation of nascent proteins across the endoplasmic reticulum: requirements for its extraction and reassociation with the membrane. J Cell Biol. 1980 Nov;87(2 Pt 1):498–502. doi: 10.1083/jcb.87.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D. I., Dobberstein B. Identification and characterization of a membrane component essential for the translocation of nascent proteins across the membrane of the endoplasmic reticulum. J Cell Biol. 1980 Nov;87(2 Pt 1):503–508. doi: 10.1083/jcb.87.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D. I., Krause E., Dobberstein B. Secretory protein translocation across membranes-the role of the "docking protein'. Nature. 1982 Jun 24;297(5868):647–650. doi: 10.1038/297647a0. [DOI] [PubMed] [Google Scholar]
- Meyer D. I. Protein translocation into the endoplasmic reticulum: a light at the end of the tunnel. Trends Cell Biol. 1991 Dec;1(6):154–159. doi: 10.1016/0962-8924(91)90016-3. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986 Jul 18;46(2):291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- Murthy K. K., Banville D., Srikant C. B., Carrier F., Holmes C., Bell A., Patel Y. C. Structural homology between the rat calreticulin gene product and the Onchocerca volvulus antigen Ral-1. Nucleic Acids Res. 1990 Aug 25;18(16):4933–4933. doi: 10.1093/nar/18.16.4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müsch A., Wiedmann M., Rapoport T. A. Yeast Sec proteins interact with polypeptides traversing the endoplasmic reticulum membrane. Cell. 1992 Apr 17;69(2):343–352. doi: 10.1016/0092-8674(92)90414-8. [DOI] [PubMed] [Google Scholar]
- Nguyen T. H., Law D. T., Williams D. B. Binding protein BiP is required for translocation of secretory proteins into the endoplasmic reticulum in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1565–1569. doi: 10.1073/pnas.88.4.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicchitta C. V., Blobel G. Assembly of translocation-competent proteoliposomes from detergent-solubilized rough microsomes. Cell. 1990 Jan 26;60(2):259–269. doi: 10.1016/0092-8674(90)90741-v. [DOI] [PubMed] [Google Scholar]
- Nicchitta C. V., Blobel G. Lumenal proteins of the mammalian endoplasmic reticulum are required to complete protein translocation. Cell. 1993 Jun 4;73(5):989–998. doi: 10.1016/0092-8674(93)90276-v. [DOI] [PubMed] [Google Scholar]
- Ogg S. C., Poritz M. A., Walter P. Signal recognition particle receptor is important for cell growth and protein secretion in Saccharomyces cerevisiae. Mol Biol Cell. 1992 Aug;3(8):895–911. doi: 10.1091/mbc.3.8.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzner S., Dreier L., Hartmann E., Kostka S., Rapoport T. A. Posttranslational protein transport in yeast reconstituted with a purified complex of Sec proteins and Kar2p. Cell. 1995 May 19;81(4):561–570. doi: 10.1016/0092-8674(95)90077-2. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986 Sep 26;46(7):959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- Perara E., Rothman R. E., Lingappa V. R. Uncoupling translocation from translation: implications for transport of proteins across membranes. Science. 1986 Apr 18;232(4748):348–352. doi: 10.1126/science.3961485. [DOI] [PubMed] [Google Scholar]
- Rapiejko P. J., Gilmore R. Protein translocation across the ER requires a functional GTP binding site in the alpha subunit of the signal recognition particle receptor. J Cell Biol. 1992 May;117(3):493–503. doi: 10.1083/jcb.117.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport T. A. Transport of proteins across the endoplasmic reticulum membrane. Science. 1992 Nov 6;258(5084):931–936. doi: 10.1126/science.1332192. [DOI] [PubMed] [Google Scholar]
- Rothblatt J. A., Deshaies R. J., Sanders S. L., Daum G., Schekman R. Multiple genes are required for proper insertion of secretory proteins into the endoplasmic reticulum in yeast. J Cell Biol. 1989 Dec;109(6 Pt 1):2641–2652. doi: 10.1083/jcb.109.6.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E. Polypeptide chain binding proteins: catalysts of protein folding and related processes in cells. Cell. 1989 Nov 17;59(4):591–601. doi: 10.1016/0092-8674(89)90005-6. [DOI] [PubMed] [Google Scholar]
- Römisch K., Webb J., Herz J., Prehn S., Frank R., Vingron M., Dobberstein B. Homology of 54K protein of signal-recognition particle, docking protein and two E. coli proteins with putative GTP-binding domains. Nature. 1989 Aug 10;340(6233):478–482. doi: 10.1038/340478a0. [DOI] [PubMed] [Google Scholar]
- Sadler I., Chiang A., Kurihara T., Rothblatt J., Way J., Silver P. A yeast gene important for protein assembly into the endoplasmic reticulum and the nucleus has homology to DnaJ, an Escherichia coli heat shock protein. J Cell Biol. 1989 Dec;109(6 Pt 1):2665–2675. doi: 10.1083/jcb.109.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders S. L., Whitfield K. M., Vogel J. P., Rose M. D., Schekman R. W. Sec61p and BiP directly facilitate polypeptide translocation into the ER. Cell. 1992 Apr 17;69(2):353–365. doi: 10.1016/0092-8674(92)90415-9. [DOI] [PubMed] [Google Scholar]
- Savitz A. J., Meyer D. I. 180-kD ribosome receptor is essential for both ribosome binding and protein translocation. J Cell Biol. 1993 Feb;120(4):853–863. doi: 10.1083/jcb.120.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz A. J., Meyer D. I. Identification of a ribosome receptor in the rough endoplasmic reticulum. Nature. 1990 Aug 9;346(6284):540–544. doi: 10.1038/346540a0. [DOI] [PubMed] [Google Scholar]
- Schlenstedt G., Gudmundsson G. H., Boman H. G., Zimmermann R. A large presecretory protein translocates both cotranslationally, using signal recognition particle and ribosome, and post-translationally, without these ribonucleoparticles, when synthesized in the presence of mammalian microsomes. J Biol Chem. 1990 Aug 15;265(23):13960–13968. [PubMed] [Google Scholar]
- Tajima S., Lauffer L., Rath V. L., Walter P. The signal recognition particle receptor is a complex that contains two distinct polypeptide chains. J Cell Biol. 1986 Oct;103(4):1167–1178. doi: 10.1083/jcb.103.4.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrift R. N., Andrews D. W., Walter P., Johnson A. E. A nascent membrane protein is located adjacent to ER membrane proteins throughout its integration and translation. J Cell Biol. 1991 Mar;112(5):809–821. doi: 10.1083/jcb.112.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiruppathi C., Alpers D. H., Seetharam B. Phase separation of rat intestinal brush border membrane proteins using Triton X-114. Anal Biochem. 1986 Mar;153(2):330–335. doi: 10.1016/0003-2697(86)90100-4. [DOI] [PubMed] [Google Scholar]
- Toyn J., Hibbs A. R., Sanz P., Crowe J., Meyer D. I. In vivo and in vitro analysis of ptl1, a yeast ts mutant with a membrane-associated defect in protein translocation. EMBO J. 1988 Dec 20;7(13):4347–4353. doi: 10.1002/j.1460-2075.1988.tb03333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van P. N., Peter F., Söling H. D. Four intracisternal calcium-binding glycoproteins from rat liver microsomes with high affinity for calcium. No indication for calsequestrin-like proteins in inositol 1,4,5-trisphosphate-sensitive calcium sequestering rat liver vesicles. J Biol Chem. 1989 Oct 15;264(29):17494–17501. [PubMed] [Google Scholar]
- Vogel J. P., Misra L. M., Rose M. D. Loss of BiP/GRP78 function blocks translocation of secretory proteins in yeast. J Cell Biol. 1990 Jun;110(6):1885–1895. doi: 10.1083/jcb.110.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Preparation of microsomal membranes for cotranslational protein translocation. Methods Enzymol. 1983;96:84–93. doi: 10.1016/s0076-6879(83)96010-x. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Purification of a membrane-associated protein complex required for protein translocation across the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7112–7116. doi: 10.1073/pnas.77.12.7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J Cell Biol. 1981 Nov;91(2 Pt 1):557–561. doi: 10.1083/jcb.91.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum. II. Signal recognition protein (SRP) mediates the selective binding to microsomal membranes of in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):551–556. doi: 10.1083/jcb.91.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Ibrahimi I., Blobel G. Translocation of proteins across the endoplasmic reticulum. I. Signal recognition protein (SRP) binds to in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):545–550. doi: 10.1083/jcb.91.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanker E. E., Sun Y., Savitz A. J., Meyer D. I. Functional characterization of the 180-kD ribosome receptor in vivo. J Cell Biol. 1995 Jul;130(1):29–39. doi: 10.1083/jcb.130.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts C., Wickner W., Zimmermann R. M13 procoat and a pre-immunoglobulin share processing specificity but use different membrane receptor mechanisms. Proc Natl Acad Sci U S A. 1983 May;80(10):2809–2813. doi: 10.1073/pnas.80.10.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel D., Flügge U. I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984 Apr;138(1):141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- Wiech H., Buchner J., Zimmermann M., Zimmermann R., Jakob U. Hsc70, immunoglobulin heavy chain binding protein, and Hsp90 differ in their ability to stimulate transport of precursor proteins into mammalian microsomes. J Biol Chem. 1993 Apr 5;268(10):7414–7421. [PubMed] [Google Scholar]
- Wiech H., Klappa P., Zimmerman R. Protein export in prokaryotes and eukaryotes. Theme with variations. FEBS Lett. 1991 Jul 22;285(2):182–188. doi: 10.1016/0014-5793(91)80800-i. [DOI] [PubMed] [Google Scholar]
- Wiech H., Sagstetter M., Müller G., Zimmermann R. The ATP requiring step in assembly of M13 procoat protein into microsomes is related to preservation of transport competence of the precursor protein. EMBO J. 1987 Apr;6(4):1011–1016. doi: 10.1002/j.1460-2075.1987.tb04853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedmann M., Kurzchalia T. V., Bielka H., Rapoport T. A. Direct probing of the interaction between the signal sequence of nascent preprolactin and the signal recognition particle by specific cross-linking. J Cell Biol. 1987 Feb;104(2):201–208. doi: 10.1083/jcb.104.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedmann M., Kurzchalia T. V., Hartmann E., Rapoport T. A. A signal sequence receptor in the endoplasmic reticulum membrane. 1987 Aug 27-Sep 2Nature. 328(6133):830–833. doi: 10.1038/328830a0. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Zimmerman D. L., Walter P. An ATP-binding membrane protein is required for protein translocation across the endoplasmic reticulum membrane. Cell Regul. 1991 Oct;2(10):851–859. doi: 10.1091/mbc.2.10.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman D. L., Walter P. Reconstitution of protein translocation activity from partially solubilized microsomal vesicles. J Biol Chem. 1990 Mar 5;265(7):4048–4053. [PubMed] [Google Scholar]
- Zimmermann R., Sagstetter M., Lewis M. J., Pelham H. R. Seventy-kilodalton heat shock proteins and an additional component from reticulocyte lysate stimulate import of M13 procoat protein into microsomes. EMBO J. 1988 Sep;7(9):2875–2880. doi: 10.1002/j.1460-2075.1988.tb03144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R., Zimmermann M., Mayinger P., Klappa P. Photoaffinity labeling of dog pancreas microsomes with 8-azido-ATP inhibits association of nascent preprolactin with the signal sequence receptor complex. FEBS Lett. 1991 Jul 29;286(1-2):95–99. doi: 10.1016/0014-5793(91)80949-4. [DOI] [PubMed] [Google Scholar]