Abstract
Epidemiological studies suggested that obesity increases the risk of colorectal cancer (CRC). The genetic connection between CRC and obesity is multifactorial and inconclusive. In this study, we hypothesize that the study of shared comorbid diseases between CRC and obesity can offer unique insights into common genetic basis of these two diseases. We constructed a comorbidity network based on mining health data for millions of patients. We developed a novel approach and extracted the diseases that play critical roles in connecting obesity and CRC in the comorbidity network. Our approach was able to prioritize metabolic syndrome and diabetes, which are known to be associated with obesity and CRC through insulin resistance pathways. Interestingly, we found that osteoporosis was highly associated with the connection between obesity and CRC. Through gene expression meta-analysis, we identified novel genes shared among CRC, obesity and osteoporosis. Literature evidences support that these genes may contribute in explaining the genetic overlaps between obesity and CRC.
Keywords: comorbidity network, colorectal cancer, obesity, osteoporosis, association rule mining, gene expression
I. INTRODUCTION
Comorbidity studies often detect unexpected disease links [1] and offer novel insights into the genetic mechanisms of diseases [2, 3]. A number of epidemiological studies suggest that obesity increases the risk of colorectal cancer (CRC) [4–6]. Based on these evidences of co-occurrence, many genetic factors have been proposed to explain the role of obesity in the development of CRC. For example, both animal and human studies have demonstrated that the increased release of insulin and reduced insulin signaling play roles in obesity and colorectal carcinogenesis [7–9]. Experiments also show that obesity leads to altered level of adipocytokines, such as Adiponectin [10–12] and leptin [13, 14], which may either prevent or foster carcinogenesis.
The mechanism for the association between obesity and CRC is multifactorial and inconclusive [6, 15, 16]. Shared comorbidities between obesity and CRC can provide unique insights into the common genetic basis for the two diseases. For example, type 2 diabetes is highly correlated with obesity and was identified as a risk factor for CRC [17]. A few studies then discovered that genetic factors of insulin resistance, which occur in type 2 diabetes, contribute in explaining the role of obesity in CRC [18]. However, both obesity and CRC are heterogeneous conditions. Over 40% of the obese population is not characterized by the presence of insulin resistance [19]. We hypothesize that systems approaches to studying the diseases that are phenotypically-significant to both CRC and obesity may offer new insights into the common molecular mechanisms between the two interconnected diseases.
Systematic comorbidity studies have been conducted previously, but mostly focused on pairwise comorbidities and their genetic overlaps. Rhetsky et al. developed a statistical model to estimate the co-occurrence relationship for each pair of 160 diseases [20], and demonstrated that comorbidities are genetically linked. Park et al. [21] and Hidalgo et al. [22] detected the comorbidities pairs from the Medicare claims (which only contain senior patients ages 65 or older) with statistical measures. Roque et al. mined pairwise disease correlations using similar measures from medical records of a psychiatric hospital [23]. Recently, we extracted comorbidity patterns from a publically accessible database, which contains disease records for millions of patients at all ages, using an association rule mining approach [24, 25].
In this study, we constructed a disease comorbidity network based on our previous work. We developed a novel approach to detect diseases that have strong connections with both obesity and CRC in the comorbidity network. Specifically, we extracted the local network consisting of all the paths between obesity and CRC, and prioritized the nodes (diseases) that play critical roles in maintaining the connection between the two diseases (Fig. 1). Substantial literature evidences can support that the top ranked diseases have associations with both obesity and CRC. We investigated the gene expression profiles of a prioritized comorbid disease to facilitate detecting novel genetic basis underlying the link between obesity and CRC. Our approach is generalizable to study the genetic basis for other disease associations.
Fig. 1.
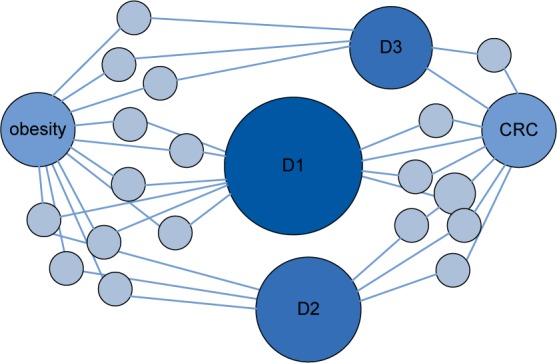
Approach to detect the diseases that have strong connections with both obesity and CRC in the comorbidity network. Nodes D1, D2 and D3 were prioritized because they play important roles in maintaining the network structure and the connection
II. MATERIALS AND METHODS
Fig. 2 shows the three steps of our approach. We first mined disease comorbidity relationships from large amounts of patient records and constructed a disease comorbidity network. We then extracted the local comorbidity cluster for obesity and CRC and prioritize the candidate comorbidity that plays a critical role in connecting the two diseases. Finally we conducted gene expression meta-analysis to identify common genes shared by obesity, CRC and the prioritized comorbidity.
Fig. 2.
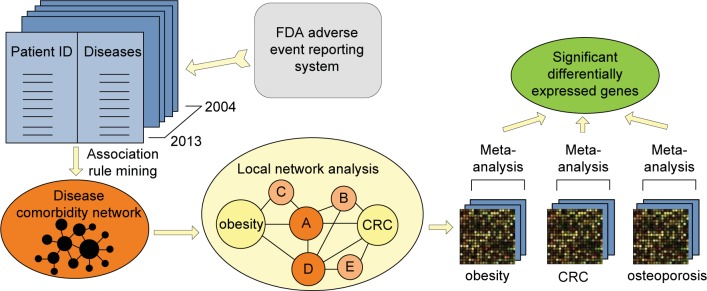
Our approach contains three steps: (1) We constructed a comorbidity network based on data mining; (2) we extracted the local network that contains paths from obesity to CRC, and analyzed the local network to pin point the strong comorbidity for both obesity and CRC; (3) we conducted gene expression meta-analysis to identify common genes shared among obesity, CRC and the comorbidity.
A. Construct Disease Comorbidity Network
We mined disease comorbidity relationships from the FDA adverse event reporting system. The database contains records (2004–2013) of 3,354,043 patients (male and female at all age levels) and 10,112 disorders. Our previous studies [24, 25] have demonstrated that this database is useful in mining comorbidity patterns among diverse patient populations.
We applied the association rule mining approach to detect disease comorbidity relationships from the patient-disease pairs. Association rule mining can flexibly detect strong co-occurrence relationships among sets of diseases, and alleviates the intrinsic bias of traditional comorbidity measures (such as relative risk and φ-correlation) towards rare diseases [24, 25].
We constructed an undirected and unweighted comorbidity network based on the result of association rule mining, which is a list of patterns between two sets of diseases, represented in the form x→y. We collected all diseases in the set x and y in each pattern, assuming they have comorbidity relationships with each other, and established an edge between each pair of diseases in xUy to construct the comorbidity network [24].
B. Prioritize the Diseases That Have Strong Associations with Both Obesity and CRC
We extracted the local network consisting of the paths from obesity to CRC in the disease comorbidity network. The local network thus includes the nodes that may represent different aspects of the relationship between obesity and CRC. We implemented breath first search to enumerate the paths, and limited the paths within four steps.
Then we ranked the nodes in the local network, except obesity and CRC, based on how important they are in maintaining the local network structure and the connection between obesity and CRC. We used the degree and betweenness centrality to characterize the importance of each node in the flowing of the network. The degree of a node becomes higher if more paths between obesity and CRC pass through this node. The betweeness evaluates the number of times that the node acts as the bridge along the shortest paths. Removing the nodes with highest degree or betweenness can easily break down the connection between obesity and CRC. We investigated the top ranked diseases based on both ranking methods, and used the unexpected ones to guide the detection of genetic associations between obesity and CRC.
C. Identify Gene Overlaps Through Gene Expression Meta-analysis
We chose a top ranked disease on the path between obesity and CRC, and then conducted gene expression meta-analysis for the prioritized disease, obesity and CRC, respectively, to detect new genetic explanations for the relationship between obesity and CRC. Gene expression normalized data (SOFT files) were downloaded from NCBI GEO omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) using the R package GEOquery [28]. Then, we performed microarray meta-analyses for each disease independently using the R package MetaDE [29]. MetaDE implements meta-analysis methods for differential expression analysis, and we used the Fisher’s method. Significant differentially expressed genes (DEGs) were selected as those displaying a FDR corrected p-value <0.05. Last, we extracted the common significant genes for the three diseases.
III. RESULTS
A. Local Disease Comorbidity Network Models the Connection Between Obesity and CRC
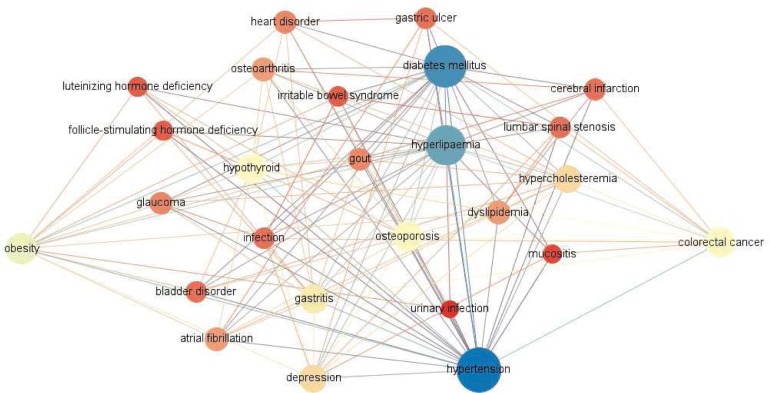
We extracted 7006 comorbidity association rules with the confidence larger than 50% from the patient records across ten years. The comorbidity network based on these rules contains 771 nodes and 15,667 edges. Fig. 3 shows the local network consisting of all the 119 paths (no longer than four steps) from obesity to CRC. A total of 24 nodes in the local network are the candidate diseases, which have associations with both obesity and CRC, and may indicate different aspects of the relationship between the two diseases.
Fig. 3.
The local network that contains all paths from obesity to colorectal cancer in the comorbidity network.
B. Osteoporosis Shows High Comorbidity Associations with Both CRC and Obesity
Table 1 shows the top five nodes sorted by degree and betweenness in the local network. In either way of ranking, hypertension, diabetes and hyperlipaemia were in top three and closely related with both obesity and CRC. Substantial literature evidences support that the metabolic syndrome components, hypertension and hyperlipaemia, as well as diabetes have association with obesity and CRC through insulin resistance in substantial literature [6–9, 18]. These three disorders also independently increase the risk of CRC and colorectal adenoma [6, 17, 18]. The top ranked comorbidities demonstrated the validity of our network analysis approach.
TABLE I.
TOP FIVE DISEASE NODES IN THE LOCAL NETWORK THAT CONTAINS ALL PATHS FROM OBESITY TO COLORECTAL CANCER. THE DISEASES WERE RANKED BY DEGREE AND BETWEENNESS, RESPECTIVELY.
| Rank | Ranked by degree | Ranked by betweenness | ||
|---|---|---|---|---|
| Nodes | Degree | Nodes | Betweenness | |
| 1 | Hypertension | 26 | Hypertension | 60.2 |
| 2 | Diabetes mellitus | 24 | Diabetes mellitus | 55.9 |
| 3 | Hyperlipaemia | 22 | Hyperlipaemia | 35.2 |
| 4 | Osteoporosis | 14 | Osteoporosis | 12.3 |
| 5 | Hypothyroid | 14 | Hypothyroid | 9.5 |
Significantly, osteoporosis was ranked highly by both centrality ranking methods. Epidemiological studies suggested an inverse association between bone mineral density and CRC [30], colon cancer among postmenopausal women [31], and colorectal adenoma [32]. On the other hand, patients of obesity and osteoporosis may share common genetic and environmental factors [33]. Different from previous studies, our result shows that osteoporosis is crucial for the association between CRC and obesity. Fig. 4 shows the paths of obesity-osteoporosis-CRC. We further investigate the gene expression profiles of osteoporosis patients to gain novel insight of the genetic basis for the link between obesity and CRC.
Fig. 4.
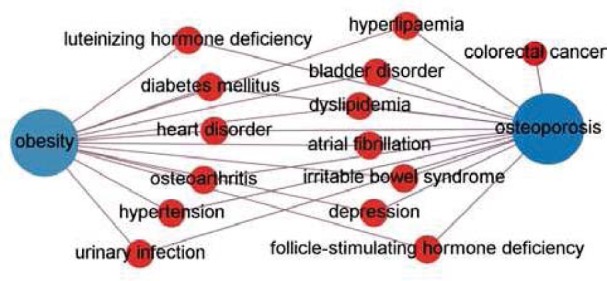
The paths from obesity to colorectal cancer that pass through osteoporosis.
C. Innovative Genes Shared Among Osteoporosis, Obesity and CRC Were Detected Using Gene Expression Meta-analysis
We downloaded five microarray series (GSE4017, GSE9348, GSE4183, GSE8671, GSE20916) for CRC, three (GSE48964, GSE29718, GSE55205) for obesity and three (GSE7429, GSE2208, GSE7158) for osteoporosis. Through meta-analysis, we obtained 9058 significant differentially expressed genes for CRC, 275 for obesity and 91 for osteoporosis. CRC and obesity shared a total of 192 genes. Among them, we found genes on insulin signaling pathways, such as PDK1, PRKAG2 and PDE3B, and adipocytokines, such as IL6 and IL8.
The three diseases osteoporosis, obesity and CRC shared six genes. Table II lists the genes and literature evidences, which support their relationships with each of the three diseases. Among them, FOS, JUN, and FOSB are oncogenes. FOS and JUN are known on the insulin signaling pathway. FOSB is on the AP1 pathway, which is associated with the proliferation of colon cancer cells [55]. Several studies suggested that overexpression of FOSB increases the responding of high fat reward while decreases energy expenditure and promotes adiposity [40, 56].
TABLE II.
COMMON GENES SHARED BY OBESITY, CRC AND OSTEOPOROSIS, AND PLAUSIBLE EVIDENCE SUPPORTING THEIR RELATIONSHIPS WITH THE THREE DISEASES.
| GENES | OBESITY | CRC | OSTEOPOROSIS |
|---|---|---|---|
| PPP1R15A* | In the bone morphogenetic protein (BMP) signaling pathway, which regulates appetite [34] | Mutations in the BMP pathway are related with colorectal carcinogenesis [35] | In the bone morphogenetic protein signaling pathway, which are associated with bone-related diseases, such as osteoporosis [36] |
| FOS | diet-induced obesity is accompanied by alteration of FOS expression [37] | Proto-oncogene, in the KEGG pathway of colorectal cancer [38] | Mice lacking c-fos develop severe osteopetrosis [39] |
| FOSB | positive association between maternal obesity [40] | Oncogene, regulators of cell proliferation, has a debatable impact on CRC patient survival [41] | Overexpression of FosB increases bone formation [42] |
| HADHA* | Associated with multiple fatty acid metabolism pathways [43] | Unknown. Associated with breast cancer [44] | Unknown. |
| JUN | The c-Jun NH2-terminal Kinase Promotes Insulin Resistance [45] | Proto-oncogene, in the KEGG pathway of colorectal cancer [38] | Associated with osteogenesis [46, 47] |
| NRIP1* | Down-regulated in obese subjects, may suggest a compensatory mechanism to favor energy expenditure and reduce fat accumulation in obesity states [48] | Unknown. Involved in regulation of E2F1, an oncogene [49] | Modulates transcriptional activity of the estrogen receptor. Interact with ESR1 and ESR2 in osteoporosis [50] |
novel genes not involving insulin resistance pathways
Interestingly, we found several genes not involving insulin signaling. Gene PPP1R15A is in the bone morphogenetic protein signaling (BMP) pathway and its superfamily, the TGF beta signaling pathway. The mutation of BMP pathway has been found in patients with juvenile polyposis, which is rare syndrome with an increased risk for developing CRC [51, 52]. Mutations in TGF beta signaling also have been found susceptibility to CRC through genome-wide association studies [53]. A recent mouse experiment also showed that the BMP pathway regulates brown adipogenesis, energy expenditure and appetite, thus is highly associated with diet-induced obesity [54]. These evidences support our result. Further investigation is required to confirm and elucidate the role of the BMP pathway in the connection between obesity and CRC.
Gene NRIP1 regulates the estrogen receptor. Its interaction with sex hormone receptors plays a role in both obesity [48] and osteoporosis [50]. Its relationship with CRC is unclear yet, but studies suggested that estrogen may have protective effect on CRC [57]. Gene HADHA is on multiple pathways of fatty acid metabolism. But its role in CRC and osteoporosis is unknown yet.
To identify the common genes among obesity, CRC and osteoporosis, we currently analyzed the gene expression data, which can be noisy. While we found literature evidences to support the detected genes and their relationships with both obesity and CRC, these candidate genes need further investigations, for example, through mouse model experiments.
IV. DISCUSSIONS AND CONCLUSIONS
The genetic connection between CRC and obesity is multifactorial and inconclusive. In this study, we developed a comorbidity network analysis approach, which suggested that osteoporosis is important for the connection between obesity and CRC. We identified common genes among obesity, CRC and osteoporosis, and found these genes are associated with the regulation of sex hormone receptors and growth factors inducing bone formation. These genes are candidates in explaining the genetic overlaps between obesity and CRC.
Our comorbidity network may be not inclusive and biased toward the diseases whose drugs have high toxicity. The FDA adverse event reporting system collects data from medical product manufacturers, health professionals, and the public. The diseases without drug treatments are not included in the data, and the disease comorbidity relationships were often under-estimated in practice based on these data. In this study, we developed a network analysis approach to compensate the bias of the comorbidity data. In the future, including more complete patient disease data may facilitate the detection of new interesting comorbidities other than osteoporosis for obesity and CRC.
In addition, we currently detect comorbidities based on disease co-occurrence. The co-occurrence patterns may indicate the increase of the risk between two diseases in a mutual way. Incorporating more comprehensive patient-level data, such as time series data, may help refine the disease relationships and control confounding factors.
Acknowledgments
Our research was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under award number DP2HD084068, the training grant in computational genomic epidemiology of cancer (CoGEC) (R25 CA094186-06)
Contributor Information
Li Li, Departments of Family Medicine and Community Health, Epidemiology and Biostatistics, Case Western Reserve University, Cleveland, OH, USA, li.li@uhhospitals.org.
Rong Xu, Division of Medical Informatics, Case Western Reserve University Cleveland, Ohio, USA, rxx@case.edu.
REFERENCES
- [1].Oti M, Huynen MA, Brunner HG. Phenome connections. Trends Genet. 24(3):103–106. doi: 10.1016/j.tig.2007.12.005. [DOI] [PubMed] [Google Scholar]
- [2].Blair DR, Lyttle CS, Mortensen JM, et al. A nondegenerate code of deleterious variants in mendelian Loci contributes to complex disease risk. Cell. 2013;155(1):70–80. doi: 10.1016/j.cell.2013.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Avery CL, He Q, North KE, et al. A phenomics-based strategy identifies loci on APOC1, BRAP, and PLCG1 associated with metabolic syndrome phenotype domains. PLoS genetics. 2011;7(10):e1002322. doi: 10.1371/journal.pgen.1002322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. New England Journal of Medicine. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- [5].Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut. 2013;62(6):933–947. doi: 10.1136/gutjnl-2013-304701. [DOI] [PubMed] [Google Scholar]
- [6].Khaodhiar L, McCowen KC, Blackburn GL. Obesity and its comorbid conditions. Clinical cornerstone. 1999;2(3):17–31. doi: 10.1016/s1098-3597(99)90002-9. [DOI] [PubMed] [Google Scholar]
- [7].Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nature Reviews Cancer. 2008;8(12):915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- [8].LeRoith D, Roberts CT., Jr The insulin-like growth factor system and cancer. Cancer letters. 2003;195(2):127–137. doi: 10.1016/s0304-3835(03)00159-9. [DOI] [PubMed] [Google Scholar]
- [9].Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. The Lancet. 2004;363(9418):1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- [10].Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: a review of current evidence. Endocrine reviews. 2012;33(4):547–594. doi: 10.1210/er.2011-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].An W, Bai Y, Deng SX, Gao J, Ben QW, Cai QC, Li ZS. Adiponectin levels in patients with colorectal cancer and adenoma: a meta-analysis. European Journal of Cancer Prevention. 2012;21(2):126–133. doi: 10.1097/CEJ.0b013e32834c9b55. [DOI] [PubMed] [Google Scholar]
- [12].Wei EK, Giovannucci E, Fuchs CS, Willett WC, Mantzoros CS. Low plasma adiponectin levels and risk of colorectal cancer in men: a prospective study. Journal of the National Cancer Institute. 2005;97(22):1688–1694. doi: 10.1093/jnci/dji376. [DOI] [PubMed] [Google Scholar]
- [13].Pärstattin RP, Söderberg S, Biessy C, Ardnor B, Kaaks GHR, Olsson T. Plasma leptin and colorectal cancer risk: a prospective study in Northern Sweden. Oncology reports. 2003;10:2015–2021. [PubMed] [Google Scholar]
- [14].Tamakoshi K, Toyoshima H, Wakai K, Kojima M, Suzuki K, Watanabe Y, Tamakoshi A. Leptin is associated with an increased female colorectal cancer risk: a nested case-control study in Japan. Oncology. 2005;68(4–6):454–461. doi: 10.1159/000086988. [DOI] [PubMed] [Google Scholar]
- [15].Zhu Y, Michelle Luo T, Jobin C, Young HA. Gut microbiota and probiotics in colon tumorigenesis. Cancer letters. 2011;309(2):119–127. doi: 10.1016/j.canlet.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Danese E, Montagnana M, Minicozzi AM, Bonafini S, Ruzzenente O, Gelati M, Guidi GC. The role of resistin in colorectal cancer. Clinica Chimica Acta. 2012;413(7):760–764. doi: 10.1016/j.cca.2012.01.019. [DOI] [PubMed] [Google Scholar]
- [17].Berster JM, Göke B. Type 2 diabetes mellitus as risk factor for colorectal cancer. Archives of physiology and biochemistry. 2008;114(1):84–98. doi: 10.1080/13813450802008455. [DOI] [PubMed] [Google Scholar]
- [18].Komninou D, Ayonote A, Richie JP, Rigas B. Insulin resistance and its contribution to colon carcinogenesis. Experimental Biology and Medicine. 2003;228(4):396–405. doi: 10.1177/153537020322800410. [DOI] [PubMed] [Google Scholar]
- [19].Karelis AD. Metabolically healthy but obese individuals. Lancet. 2008;372(9646):1281–1283. doi: 10.1016/S0140-6736(08)61531-7. [DOI] [PubMed] [Google Scholar]
- [20].Rzhetsky A, Wajngurt D, Park N, Zheng T. Probing genetic overlap among complex human phenotypes. Proc Natl Acad Sci USA. 2007;104:11694–9. doi: 10.1073/pnas.0704820104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Park J, Lee DS, Christakis NA, Barabasi AL. The impact of cellular networks on disease comorbidity. Mol Syst Biol. 2009;5:262. doi: 10.1038/msb.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hidalgo CA, Blumm N, Barabási AL, Christakis NA. A dynamic network approach for the study of human phenotypes. PLoS Comput Biol. 2009;5:e1000353. doi: 10.1371/journal.pcbi.1000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Roque FS, Jensen PB, Schmock H, et al. Using electronic patient records to discover disease correlations and stratify patient cohorts. PLoS Comput Biol. 2011;7(8):e1002141. doi: 10.1371/journal.pcbi.1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chen Y, Xu R. Bioinformatics Research and Applications. Springer International Publishing; 2014. Network Analysis of Human Disease Comorbidity Patterns Based on Large-Scale Data Mining; pp. 243–254. [Google Scholar]
- [25].Chen Y, Xu R. Mining cancer-specific disease comorbidities from a large observational database, Cancer Informatics (in press) 2014 doi: 10.4137/CIN.S13893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Capobianco E, Lio P. Comorbidity: a multidimensional approach. Trends in molecular medicine. 2013;19(9):515–521. doi: 10.1016/j.molmed.2013.07.004. [DOI] [PubMed] [Google Scholar]
- [27].Cramer AO, Waldorp LJ, van der Maas HL, Borsboom D. Comorbidity: a network perspective. Behavioral and Brain Sciences. 2010;33(2–3):137–150. doi: 10.1017/S0140525X09991567. [DOI] [PubMed] [Google Scholar]
- [28].Davis S, Meltzer P. GEOquery: a bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics. 2007;14:1846–1847. doi: 10.1093/bioinformatics/btm254. [DOI] [PubMed] [Google Scholar]
- [29].Wang X, Kang DD, Shen K, Song C, Lu S, Chang LC, Tseng GC. An R package suite for microarray meta-analysis in quality control, differentially expressed gene analysis and pathway enrichment detection. Bioinformatics. 2012;28(19):2534–2536. doi: 10.1093/bioinformatics/bts485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nelson RL, Turyk M, Kim J, Persky V. Bone mineral density and the subsequent risk of cancer in the NHANES I follow-up cohort. BMC Cancer. 2002;2:22–31. doi: 10.1186/1471-2407-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ganry O, Lapotre-Ledoux B, Fardellone P, Dubreuil A. Bone mass density, subsequent risk of colon cancer and survival in postmenopausal women. Eur J Epidemiol. 2008;23:467–73. doi: 10.1007/s10654-008-9256-0. [DOI] [PubMed] [Google Scholar]
- [32].Nock NL, Patrick-Melin A, Cook M, Thompson C, Kirwan JP, Li L. Higher bone mineral density is associated with a decreased risk of colorectal adenomas. International Journal of Cancer. 2011;129(4):956–964. doi: 10.1002/ijc.25722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhao LJ, Liu YJ, Liu PY, Hamilton J, Recker RR, Deng HW. Relationship of obesity with osteoporosis. The Journal of Clinical Endocrinology & Metabolism. 2007;92(5):1640–1646. doi: 10.1210/jc.2006-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Townsend KL, Suzuki R, Huang TL, Jing E, Schulz TJ, Lee K, Tseng YH. Bone morphogenetic protein 7 (BMP7) reverses obesity and regulates appetite through a central mTOR pathway. The FASEB Journal. 2012;26(5):2187–2196. doi: 10.1096/fj.11-199067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hardwick JC, Kodach LL, Offerhaus GJ, Van den Brink GR. Bone morphogenetic protein signalling in colorectal cancer. Nature Reviews Cancer. 2008;8(10):806–812. doi: 10.1038/nrc2467. [DOI] [PubMed] [Google Scholar]
- [36].Chen G, Deng C, Li YP. TGF-β and BMP signaling in osteoblast differentiation and bone formation. International journal of biological sciences. 2012;8(2):272. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Parker JA, McCullough KA, Field BCT, Minnion JS, Martin NM, Ghatei MA, Bloom SR. Glucagon and GLP-1 inhibit food intake and increase c-fos expression in similar appetite regulating centres in the brainstem and amygdala. International Journal of Obesity. 2013;37(10):1391–1398. doi: 10.1038/ijo.2012.227. [DOI] [PubMed] [Google Scholar]
- [38].Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic acids research. 2000;28(1):27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Okada S, Wang ZQ, Grigoriadis AE, Wagner EF, von Rüden T. Mice lacking c-fos have normal hematopoietic stem cells but exhibit altered B-cell differentiation due to an impaired bone marrow environment. Molecular and cellular biology. 1994;14(1):382–390. doi: 10.1128/mcb.14.1.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Thakali KM, Saben J, Faske JB, Lindsey F, Gomez-Acevedo H, Lowery CL, Jr, Shankar K. Maternal Pre-Gravid Obesity Changes Gene Expression Profiles Towards Greater Inflammation and Reduced Insulin Sensitivity in Umbilical Cord. Pediatric research. 2014 doi: 10.1038/pr.2014.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Pfannschmidt J, Bade S, Hoheisel J, Muley T, Dienemann H, Herpel E. Identification of immunohistochemical prognostic markers for survival after resection of pulmonary metastases from colorectal carcinoma. The Thoracic and cardiovascular surgeon. 2009;57(7):403–408. doi: 10.1055/s-0029-1185820. [DOI] [PubMed] [Google Scholar]
- [42].Sabatakos G, Sims NA, Chen J, Aoki K, Kelz MB, Amling M, Baron R. Overexpression of ΔFosB transcription factor (s) increases bone formation and inhibits adipogenesis. Nature medicine. 2000;6(9):985–990. doi: 10.1038/79683. [DOI] [PubMed] [Google Scholar]
- [43].Liberzon A, Subramanian A, Pinchback R, Thorvaldsdóttir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27(12):1739–1740. doi: 10.1093/bioinformatics/btr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mamtani M, Kulkarni H. Association of HADHA expression with the risk of breast cancer: targeted subset analysis and meta-analysis of microarray data. BMC research notes. 2012;5(1):25. doi: 10.1186/1756-0500-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH2-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser307. Journal of Biological Chemistry. 2000;275(12):9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- [46].Lewinson D, Rachmiel A, Rihani-Bisharat S, Kraiem Z, Schenzer P, Korem S, Rabinovich Y. Stimulation of Fos-and Jun-related genes during distraction osteogenesis. Journal of Histochemistry & Cytochemistry. 2003;51(9):1161–1168. doi: 10.1177/002215540305100906. [DOI] [PubMed] [Google Scholar]
- [47].Krzeszinski JY, Wei W, Huynh H, Jin Z, Wang X, Chang TC, Wan Y. miR-34a blocks osteoporosis and bone metastasis by inhibiting osteoclastogenesis and Tgif2. Nature. 2014;512(7515):431–435. doi: 10.1038/nature13375. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [48].Catalán V, Gómez-Ambrosi J, Lizanzu A, Rodríguez A, Silva C, Rotellar F, Frühbeck G. RIP140 Gene and protein expression levels are downregulated in visceral adipose tissue in human morbid obesity. Obesity surgery. 2009;19(6):771–776. doi: 10.1007/s11695-009-9834-6. [DOI] [PubMed] [Google Scholar]
- [49].Docquier A, Harmand PO, Fritsch S, Chanrion M, Darbon JM, Cavaillès V. The transcriptional coregulator RIP140 represses E2F1 activity and discriminates breast cancer subtypes. Clinical Cancer Research. 2010;16(11):2959–2970. doi: 10.1158/1078-0432.CCR-09-3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Morón FJ, Mendoza N, Vázquez F, Molero E, Quereda F, Salinas A, Ruiz A. Multilocus analysis of estrogen-related genes in Spanish postmenopausal women suggests an interactive role of< i> ESR1,< i> ESR2 and< i> NRIP1 genes in the pathogenesis of osteoporosis. Bone. 2006;39(1):213–221. doi: 10.1016/j.bone.2005.12.079. [DOI] [PubMed] [Google Scholar]
- [51].Howe JR, Bair JL, Sayed MG, Anderson ME, Mitros FA, Petersen GM, Vogelstein B. Germline mutations of the gene encoding bone morphogenetic protein receptor 1A in juvenile polyposis. Nature genetics. 2001;28(2):184–187. doi: 10.1038/88919. [DOI] [PubMed] [Google Scholar]
- [52].Brosens LA, van Hattem A, Hylind LM, Iacobuzio-Donahue C, Romans KE, Axilbund J, Giardiello FM. Risk of colorectal cancer in juvenile polyposis. Gut. 2007;56(7):965–967. doi: 10.1136/gut.2006.116913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Bellam N, Pasche B. Cancer Genetics. Springer US: 2010. TGF-β signaling alterations and colon cancer; pp. 85–103. [DOI] [PubMed] [Google Scholar]
- [54].Townsend KL, Suzuki R, Huang TL, Jing E, Schulz TJ, Lee K, Tseng YH. Bone morphogenetic protein 7 (BMP7) reverses obesity and regulates appetite through a central mTOR pathway. The FASEB Journal. 2012;26(5):2187–2196. doi: 10.1096/fj.11-199067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ashida R, Tominaga K, Sasaki E, Watanabe T, Fujiwara Y, Oshitani N, Arakawa T. AP-1 and colorectal cancer. Inflammopharmacology. 2005;13(1–3):113–125. doi: 10.1163/156856005774423935. [DOI] [PubMed] [Google Scholar]
- [56].Vialou V, Cui H, Perello M, Mahgoub M, Yu HG, Rush AJ, Lutter M. A role for ΔFosB in calorie restriction-induced metabolic changes. Biological psychiatry. 2011;70(2):204–207. doi: 10.1016/j.biopsych.2010.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Barzi A, Lenz AM, Labonte MJ, Lenz HJ. Molecular pathways: estrogen pathway in colorectal cancer. Clinical Cancer Research. 2013;19(21):5842–5848. doi: 10.1158/1078-0432.CCR-13-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]