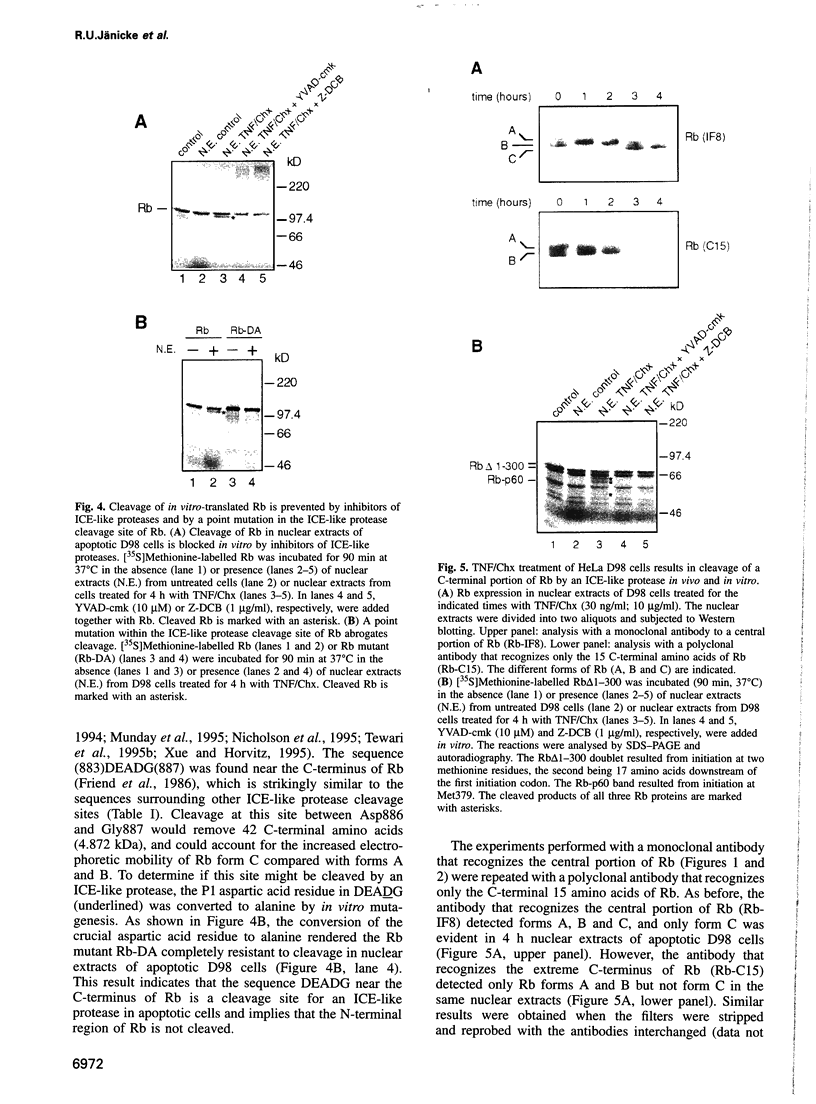
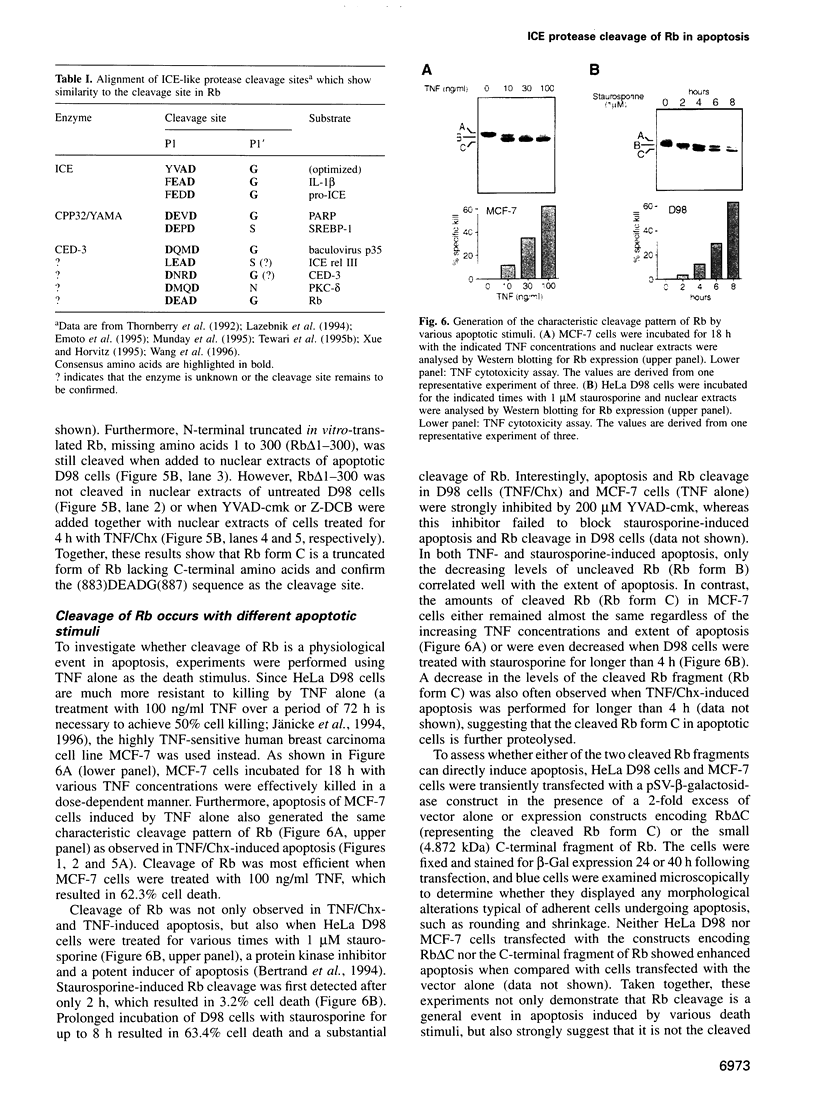
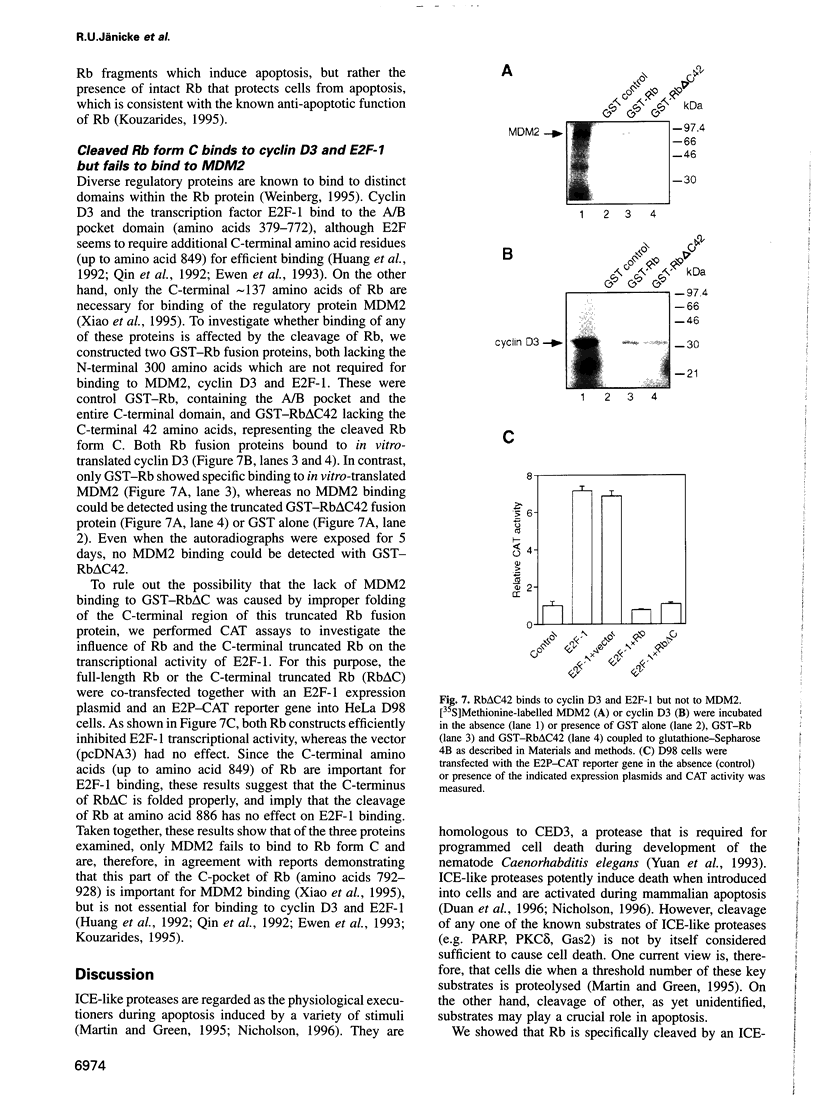
Abstract
Interleukin 1beta-converting enzyme-like (ICE-like) proteases are important mediators of apoptosis in diverse cell types and organisms. However, the role of these proteases in apoptosis cannot be satisfactorily explained on the basis of the physiological functions of their known substrates. Here we show that the C-terminal 42 amino acid peptide of the retinoblastoma (Rb) protein, an important cell cycle regulator with a known anti-apoptotic function, is specifically cleaved off by an ICE-like protease in tumour necrosis factor (TNF)- and staurosporine-induced apoptosis. Cleavage of Rb induced by TNF was blocked in vivo and in vitro by two specific inhibitors of ICE-like proteases, and in vitro by a point mutation (Asp886 to Ala) within the ICE-like protease cleavage site of Rb, (883)DEAD(886). An antibody raised against the C-terminal 15 amino acid peptide of Rb recognized the full-length but not the cleaved form of Rb. The extent of Rb cleavage correlated directly with TNF-induced apoptosis in all tumour cell lines examined. Cleaved Rb bound cyclin D3 and inhibited the transcriptional activity of E2F-1, but failed to bind to the regulatory protein MDM2, which has been implicated in apoptosis. As Rb suppresses cell death and its C-terminus has important regulatory functions, our results suggest that Rb cleavage is an important event in apoptosis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almasan A., Yin Y., Kelly R. E., Lee E. Y., Bradley A., Li W., Bertino J. R., Wahl G. M. Deficiency of retinoblastoma protein leads to inappropriate S-phase entry, activation of E2F-responsive genes, and apoptosis. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5436–5440. doi: 10.1073/pnas.92.12.5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An B., Dou Q. P. Cleavage of retinoblastoma protein during apoptosis: an interleukin 1 beta-converting enzyme-like protease as candidate. Cancer Res. 1996 Feb 1;56(3):438–442. [PubMed] [Google Scholar]
- Bertrand R., Solary E., O'Connor P., Kohn K. W., Pommier Y. Induction of a common pathway of apoptosis by staurosporine. Exp Cell Res. 1994 Apr;211(2):314–321. doi: 10.1006/excr.1994.1093. [DOI] [PubMed] [Google Scholar]
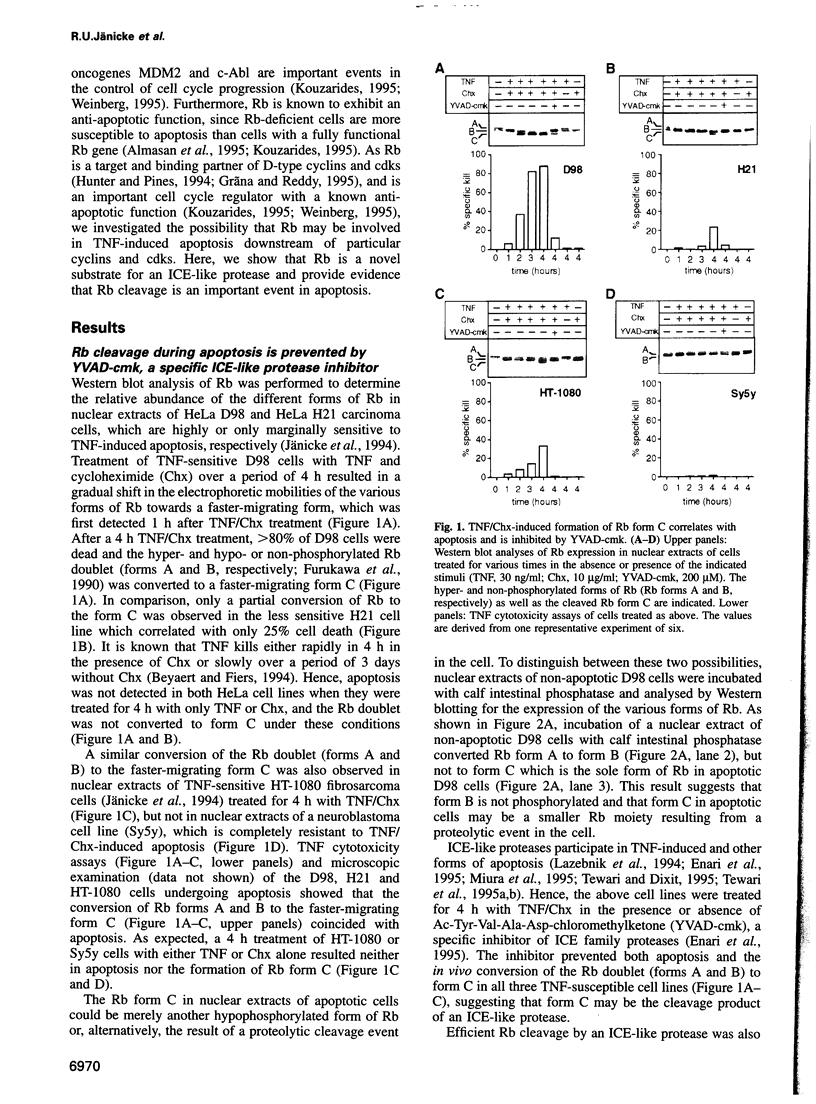
- Beyaert R., Fiers W. Molecular mechanisms of tumor necrosis factor-induced cytotoxicity. What we do understand and what we do not. FEBS Lett. 1994 Feb 28;340(1-2):9–16. doi: 10.1016/0014-5793(94)80163-0. [DOI] [PubMed] [Google Scholar]
- Brancolini C., Benedetti M., Schneider C. Microfilament reorganization during apoptosis: the role of Gas2, a possible substrate for ICE-like proteases. EMBO J. 1995 Nov 1;14(21):5179–5190. doi: 10.1002/j.1460-2075.1995.tb00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casciola-Rosen L. A., Anhalt G. J., Rosen A. DNA-dependent protein kinase is one of a subset of autoantigens specifically cleaved early during apoptosis. J Exp Med. 1995 Dec 1;182(6):1625–1634. doi: 10.1084/jem.182.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Wu X., Lin J., Levine A. J. mdm-2 inhibits the G1 arrest and apoptosis functions of the p53 tumor suppressor protein. Mol Cell Biol. 1996 May;16(5):2445–2452. doi: 10.1128/mcb.16.5.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnaiyan A. M., Orth K., O'Rourke K., Duan H., Poirier G. G., Dixit V. M. Molecular ordering of the cell death pathway. Bcl-2 and Bcl-xL function upstream of the CED-3-like apoptotic proteases. J Biol Chem. 1996 Mar 1;271(9):4573–4576. doi: 10.1074/jbc.271.9.4573. [DOI] [PubMed] [Google Scholar]
- Clarke A. R., Maandag E. R., van Roon M., van der Lugt N. M., van der Valk M., Hooper M. L., Berns A., te Riele H. Requirement for a functional Rb-1 gene in murine development. Nature. 1992 Sep 24;359(6393):328–330. doi: 10.1038/359328a0. [DOI] [PubMed] [Google Scholar]
- Duan H., Chinnaiyan A. M., Hudson P. L., Wing J. P., He W. W., Dixit V. M. ICE-LAP3, a novel mammalian homologue of the Caenorhabditis elegans cell death protein Ced-3 is activated during Fas- and tumor necrosis factor-induced apoptosis. J Biol Chem. 1996 Jan 19;271(3):1621–1625. doi: 10.1074/jbc.271.3.1621. [DOI] [PubMed] [Google Scholar]
- Emoto Y., Manome Y., Meinhardt G., Kisaki H., Kharbanda S., Robertson M., Ghayur T., Wong W. W., Kamen R., Weichselbaum R. Proteolytic activation of protein kinase C delta by an ICE-like protease in apoptotic cells. EMBO J. 1995 Dec 15;14(24):6148–6156. doi: 10.1002/j.1460-2075.1995.tb00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enari M., Hug H., Nagata S. Involvement of an ICE-like protease in Fas-mediated apoptosis. Nature. 1995 May 4;375(6526):78–81. doi: 10.1038/375078a0. [DOI] [PubMed] [Google Scholar]
- Ewen M. E., Sluss H. K., Sherr C. J., Matsushime H., Kato J., Livingston D. M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993 May 7;73(3):487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- Fotedar R., Flatt J., Gupta S., Margolis R. L., Fitzgerald P., Messier H., Fotedar A. Activation-induced T-cell death is cell cycle dependent and regulated by cyclin B. Mol Cell Biol. 1995 Feb;15(2):932–942. doi: 10.1128/mcb.15.2.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
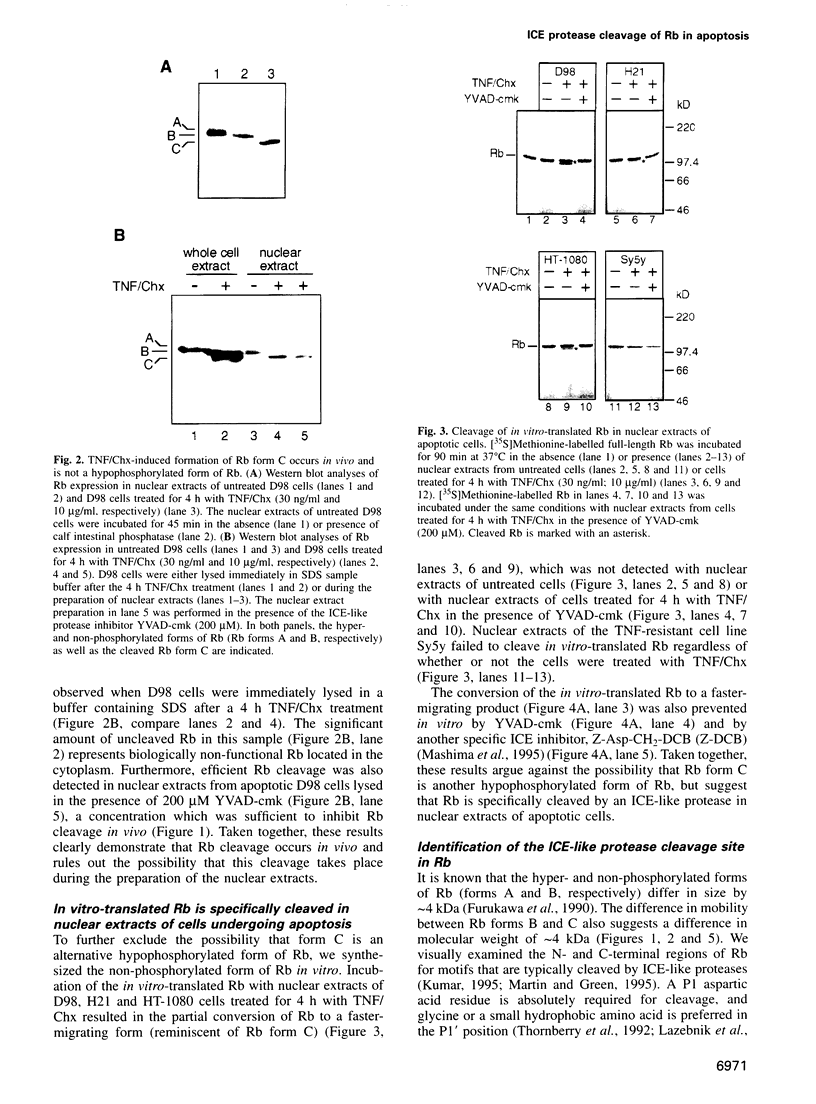
- Furukawa Y., DeCaprio J. A., Freedman A., Kanakura Y., Nakamura M., Ernst T. J., Livingston D. M., Griffin J. D. Expression and state of phosphorylation of the retinoblastoma susceptibility gene product in cycling and noncycling human hematopoietic cells. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2770–2774. doi: 10.1073/pnas.87.7.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graña X., Reddy E. P. Cell cycle control in mammalian cells: role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs). Oncogene. 1995 Jul 20;11(2):211–219. [PubMed] [Google Scholar]
- Hansen M. F., Morgan R., Sandberg A. A., Cavenee W. K. Structural alterations at the putative retinoblastoma locus in some human leukemias and preleukemia. Cancer Genet Cytogenet. 1990 Oct 1;49(1):15–23. doi: 10.1016/0165-4608(90)90159-8. [DOI] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Hoang A. T., Cohen K. J., Barrett J. F., Bergstrom D. A., Dang C. V. Participation of cyclin A in Myc-induced apoptosis. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):6875–6879. doi: 10.1073/pnas.91.15.6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Shin E., Sheppard K. A., Chokroverty L., Shan B., Qian Y. W., Lee E. Y., Yee A. S. The retinoblastoma protein region required for interaction with the E2F transcription factor includes the T/E1A binding and carboxy-terminal sequences. DNA Cell Biol. 1992 Sep;11(7):539–548. doi: 10.1089/dna.1992.11.539. [DOI] [PubMed] [Google Scholar]
- Hunter T., Pines J. Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell. 1994 Nov 18;79(4):573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- Jacks T., Fazeli A., Schmitt E. M., Bronson R. T., Goodell M. A., Weinberg R. A. Effects of an Rb mutation in the mouse. Nature. 1992 Sep 24;359(6393):295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- Jarvis W. D., Kolesnick R. N., Fornari F. A., Traylor R. S., Gewirtz D. A., Grant S. Induction of apoptotic DNA damage and cell death by activation of the sphingomyelin pathway. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):73–77. doi: 10.1073/pnas.91.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänicke R. U., Lee F. H., Porter A. G. Nuclear c-Myc plays an important role in the cytotoxicity of tumor necrosis factor alpha in tumor cells. Mol Cell Biol. 1994 Sep;14(9):5661–5670. doi: 10.1128/mcb.14.9.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänicke R. U., Lin X. Y., Lee F. H., Porter A. G. Cyclin D3 sensitizes tumor cells to tumor necrosis factor-induced, c-Myc-dependent apoptosis. Mol Cell Biol. 1996 Oct;16(10):5245–5253. doi: 10.1128/mcb.16.10.5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S., Barnett G. H., Hara H., Morimura T., Takeuchi J. MDM2 protein confers the resistance of a human glioblastoma cell line to cisplatin-induced apoptosis. Oncogene. 1995 May 18;10(10):2001–2006. [PubMed] [Google Scholar]
- Kouzarides T. Functions of pRb and p53: what's the connection? Trends Cell Biol. 1995 Dec;5(12):448–450. doi: 10.1016/s0962-8924(00)89109-6. [DOI] [PubMed] [Google Scholar]
- Kranenburg O., van der Eb A. J., Zantema A. Cyclin D1 is an essential mediator of apoptotic neuronal cell death. EMBO J. 1996 Jan 2;15(1):46–54. [PMC free article] [PubMed] [Google Scholar]
- Kumar S. ICE-like proteases in apoptosis. Trends Biochem Sci. 1995 May;20(5):198–202. doi: 10.1016/s0968-0004(00)89007-6. [DOI] [PubMed] [Google Scholar]
- Lazebnik Y. A., Kaufmann S. H., Desnoyers S., Poirier G. G., Earnshaw W. C. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 1994 Sep 22;371(6495):346–347. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- Lee E. Y., Chang C. Y., Hu N., Wang Y. C., Lai C. C., Herrup K., Lee W. H., Bradley A. Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature. 1992 Sep 24;359(6393):288–294. doi: 10.1038/359288a0. [DOI] [PubMed] [Google Scholar]
- Li P., Allen H., Banerjee S., Franklin S., Herzog L., Johnston C., McDowell J., Paskind M., Rodman L., Salfeld J. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995 Feb 10;80(3):401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Satoh M. S., Poirier G. G., Klungland A. Post-translational modification of poly(ADP-ribose) polymerase induced by DNA strand breaks. Trends Biochem Sci. 1995 Oct;20(10):405–411. doi: 10.1016/s0968-0004(00)89089-1. [DOI] [PubMed] [Google Scholar]
- Martin S. J., Green D. R. Protease activation during apoptosis: death by a thousand cuts? Cell. 1995 Aug 11;82(3):349–352. doi: 10.1016/0092-8674(95)90422-0. [DOI] [PubMed] [Google Scholar]
- Mashima T., Naito M., Kataoka S., Kawai H., Tsuruo T. Aspartate-based inhibitor of interleukin-1 beta-converting enzyme prevents antitumor agent-induced apoptosis in human myeloid leukemia U937 cells. Biochem Biophys Res Commun. 1995 Apr 26;209(3):907–915. doi: 10.1006/bbrc.1995.1584. [DOI] [PubMed] [Google Scholar]
- Miura M., Friedlander R. M., Yuan J. Tumor necrosis factor-induced apoptosis is mediated by a CrmA-sensitive cell death pathway. Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8318–8322. doi: 10.1073/pnas.92.18.8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T., Krajewski S., Krajewska M., Wang H. G., Lin H. K., Liebermann D. A., Hoffman B., Reed J. C. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994 Jun;9(6):1799–1805. [PubMed] [Google Scholar]
- Munday N. A., Vaillancourt J. P., Ali A., Casano F. J., Miller D. K., Molineaux S. M., Yamin T. T., Yu V. L., Nicholson D. W. Molecular cloning and pro-apoptotic activity of ICErelII and ICErelIII, members of the ICE/CED-3 family of cysteine proteases. J Biol Chem. 1995 Jun 30;270(26):15870–15876. doi: 10.1074/jbc.270.26.15870. [DOI] [PubMed] [Google Scholar]
- Nicholson D. W. ICE/CED3-like proteases as therapeutic targets for the control of inappropriate apoptosis. Nat Biotechnol. 1996 Mar;14(3):297–301. doi: 10.1038/nbt0396-297. [DOI] [PubMed] [Google Scholar]
- Qin X. Q., Chittenden T., Livingston D. M., Kaelin W. G., Jr Identification of a growth suppression domain within the retinoblastoma gene product. Genes Dev. 1992 Jun;6(6):953–964. doi: 10.1101/gad.6.6.953. [DOI] [PubMed] [Google Scholar]
- Shi L., Nishioka W. K., Th'ng J., Bradbury E. M., Litchfield D. W., Greenberg A. H. Premature p34cdc2 activation required for apoptosis. Science. 1994 Feb 25;263(5150):1143–1145. doi: 10.1126/science.8108732. [DOI] [PubMed] [Google Scholar]
- Shimizu T., O'Connor P. M., Kohn K. W., Pommier Y. Unscheduled activation of cyclin B1/Cdc2 kinase in human promyelocytic leukemia cell line HL60 cells undergoing apoptosis induced by DNA damage. Cancer Res. 1995 Jan 15;55(2):228–231. [PubMed] [Google Scholar]
- Singh P., Coe J., Hong W. A role for retinoblastoma protein in potentiating transcriptional activation by the glucocorticoid receptor. Nature. 1995 Apr 6;374(6522):562–565. doi: 10.1038/374562a0. [DOI] [PubMed] [Google Scholar]
- Tewari M., Beidler D. R., Dixit V. M. CrmA-inhibitable cleavage of the 70-kDa protein component of the U1 small nuclear ribonucleoprotein during Fas- and tumor necrosis factor-induced apoptosis. J Biol Chem. 1995 Aug 11;270(32):18738–18741. doi: 10.1074/jbc.270.32.18738. [DOI] [PubMed] [Google Scholar]
- Tewari M., Dixit V. M. Fas- and tumor necrosis factor-induced apoptosis is inhibited by the poxvirus crmA gene product. J Biol Chem. 1995 Feb 17;270(7):3255–3260. doi: 10.1074/jbc.270.7.3255. [DOI] [PubMed] [Google Scholar]
- Tewari M., Quan L. T., O'Rourke K., Desnoyers S., Zeng Z., Beidler D. R., Poirier G. G., Salvesen G. S., Dixit V. M. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995 Jun 2;81(5):801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- Thornberry N. A., Bull H. G., Calaycay J. R., Chapman K. T., Howard A. D., Kostura M. J., Miller D. K., Molineaux S. M., Weidner J. R., Aunins J. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992 Apr 30;356(6372):768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- Wang J. Y., Knudsen E. S., Welch P. J. The retinoblastoma tumor suppressor protein. Adv Cancer Res. 1994;64:25–85. doi: 10.1016/s0065-230x(08)60834-9. [DOI] [PubMed] [Google Scholar]
- Wang X., Zelenski N. G., Yang J., Sakai J., Brown M. S., Goldstein J. L. Cleavage of sterol regulatory element binding proteins (SREBPs) by CPP32 during apoptosis. EMBO J. 1996 Mar 1;15(5):1012–1020. [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Q., Auer B., Stingl L., Berghammer H., Haidacher D., Schweiger M., Wagner E. F. Mice lacking ADPRT and poly(ADP-ribosyl)ation develop normally but are susceptible to skin disease. Genes Dev. 1995 Mar 1;9(5):509–520. doi: 10.1101/gad.9.5.509. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. The retinoblastoma protein and cell cycle control. Cell. 1995 May 5;81(3):323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- Welch P. J., Wang J. Y. A C-terminal protein-binding domain in the retinoblastoma protein regulates nuclear c-Abl tyrosine kinase in the cell cycle. Cell. 1993 Nov 19;75(4):779–790. doi: 10.1016/0092-8674(93)90497-e. [DOI] [PubMed] [Google Scholar]
- Welch P. J., Wang J. Y. Disruption of retinoblastoma protein function by coexpression of its C pocket fragment. Genes Dev. 1995 Jan 1;9(1):31–46. doi: 10.1101/gad.9.1.31. [DOI] [PubMed] [Google Scholar]
- Xiao Z. X., Chen J., Levine A. J., Modjtahedi N., Xing J., Sellers W. R., Livingston D. M. Interaction between the retinoblastoma protein and the oncoprotein MDM2. Nature. 1995 Jun 22;375(6533):694–698. doi: 10.1038/375694a0. [DOI] [PubMed] [Google Scholar]
- Xue D., Horvitz H. R. Inhibition of the Caenorhabditis elegans cell-death protease CED-3 by a CED-3 cleavage site in baculovirus p35 protein. Nature. 1995 Sep 21;377(6546):248–251. doi: 10.1038/377248a0. [DOI] [PubMed] [Google Scholar]
- Yuan J., Shaham S., Ledoux S., Ellis H. M., Horvitz H. R. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell. 1993 Nov 19;75(4):641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- Zheng X. M., Wang Y., Pallen C. J. Cell transformation and activation of pp60c-src by overexpression of a protein tyrosine phosphatase. Nature. 1992 Sep 24;359(6393):336–339. doi: 10.1038/359336a0. [DOI] [PubMed] [Google Scholar]