Abstract
The small nuclear GTP binding protein Ran is required for transport of nuclear proteins through the nuclear pore complex (NPC). Although it is known that GTP hydrolysis by Ran is essential for this reaction, it has been unclear whether additional energy-consuming steps are also required. To uncouple the energy requirements for Ran from other nucleoside triphosphatases, we constructed a mutant derivative of Ran that has an altered nucleotide specificity from GTP to xanthosine 5' triphosphate. Using this Ran mutant, we demonstrate that nucleotide hydrolysis by Ran is sufficient to promote efficient nuclear protein import in vitro. Under these conditions, protein import could no longer be inhibited with non-hydrolysable nucleotide analogues, indicating that no Ran-independent energy-requiring steps are essential for the protein translocation reaction through the NPC. We further provide evidence that nuclear protein import requires Ran in the GDP form in the cytoplasm. This suggests that a coordinated exchange reaction from Ran-GDP to Ran-GTP at the pore is necessary for translocation into the nucleus.
Full text
PDF

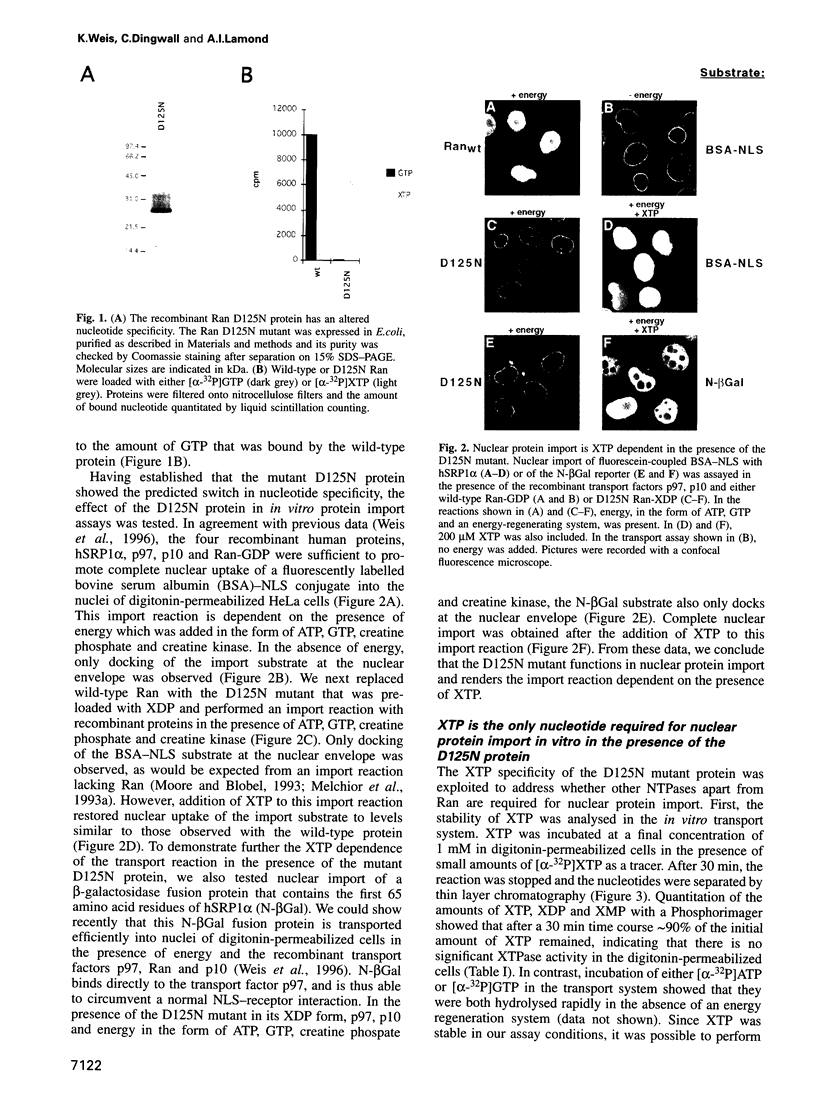




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam S. A., Gerace L. Cytosolic proteins that specifically bind nuclear location signals are receptors for nuclear import. Cell. 1991 Sep 6;66(5):837–847. doi: 10.1016/0092-8674(91)90431-w. [DOI] [PubMed] [Google Scholar]
- Adam S. A., Marr R. S., Gerace L. Nuclear protein import in permeabilized mammalian cells requires soluble cytoplasmic factors. J Cell Biol. 1990 Sep;111(3):807–816. doi: 10.1083/jcb.111.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff F. R., Klebe C., Kretschmer J., Wittinghofer A., Ponstingl H. RanGAP1 induces GTPase activity of nuclear Ras-related Ran. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2587–2591. doi: 10.1073/pnas.91.7.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff F. R., Krebber H., Kempf T., Hermes I., Ponstingl H. Human RanGTPase-activating protein RanGAP1 is a homologue of yeast Rna1p involved in mRNA processing and transport. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1749–1753. doi: 10.1073/pnas.92.5.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff F. R., Krebber H., Smirnova E., Dong W., Ponstingl H. Co-activation of RanGTPase and inhibition of GTP dissociation by Ran-GTP binding protein RanBP1. EMBO J. 1995 Feb 15;14(4):705–715. doi: 10.1002/j.1460-2075.1995.tb07049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff F. R., Ponstingl H. Catalysis of guanine nucleotide exchange on Ran by the mitotic regulator RCC1. Nature. 1991 Nov 7;354(6348):80–82. doi: 10.1038/354080a0. [DOI] [PubMed] [Google Scholar]
- Bischoff F. R., Ponstingl H. Mitotic regulator protein RCC1 is complexed with a nuclear ras-related polypeptide. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10830–10834. doi: 10.1073/pnas.88.23.10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi N. C., Adam E. J., Adam S. A. Sequence and characterization of cytoplasmic nuclear protein import factor p97. J Cell Biol. 1995 Jul;130(2):265–274. doi: 10.1083/jcb.130.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett A. H., Koepp D. M., Schlenstedt G., Lee M. S., Hopper A. K., Silver P. A. Rna1p, a Ran/TC4 GTPase activating protein, is required for nuclear import. J Cell Biol. 1995 Sep;130(5):1017–1026. doi: 10.1083/jcb.130.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes P., Ye Z. S., Baltimore D. RAG-1 interacts with the repeated amino acid motif of the human homologue of the yeast protein SRP1. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7633–7637. doi: 10.1073/pnas.91.16.7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuomo C. A., Kirch S. A., Gyuris J., Brent R., Oettinger M. A. Rch1, a protein that specifically interacts with the RAG-1 recombination-activating protein. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6156–6160. doi: 10.1073/pnas.91.13.6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasso M. RCC1 in the cell cycle: the regulator of chromosome condensation takes on new roles. Trends Biochem Sci. 1993 Mar;18(3):96–101. doi: 10.1016/0968-0004(93)90161-f. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Kandels-Lewis S., Séraphin B. A family of Ran binding proteins that includes nucleoporins. Proc Natl Acad Sci U S A. 1995 Aug 1;92(16):7525–7529. doi: 10.1073/pnas.92.16.7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Nuclear targeting sequences--a consensus? Trends Biochem Sci. 1991 Dec;16(12):478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- Enenkel C., Blobel G., Rexach M. Identification of a yeast karyopherin heterodimer that targets import substrate to mammalian nuclear pore complexes. J Biol Chem. 1995 Jul 14;270(28):16499–16502. doi: 10.1074/jbc.270.28.16499. [DOI] [PubMed] [Google Scholar]
- Floer M., Blobel G. The nuclear transport factor karyopherin beta binds stoichiometrically to Ran-GTP and inhibits the Ran GTPase activating protein. J Biol Chem. 1996 Mar 8;271(10):5313–5316. doi: 10.1074/jbc.271.10.5313. [DOI] [PubMed] [Google Scholar]
- Forbes D. J. Structure and function of the nuclear pore complex. Annu Rev Cell Biol. 1992;8:495–527. doi: 10.1146/annurev.cb.08.110192.002431. [DOI] [PubMed] [Google Scholar]
- Goldberg M. W., Allen T. D. Structural and functional organization of the nuclear envelope. Curr Opin Cell Biol. 1995 Jun;7(3):301–309. doi: 10.1016/0955-0674(95)80083-2. [DOI] [PubMed] [Google Scholar]
- Görlich D., Henklein P., Laskey R. A., Hartmann E. A 41 amino acid motif in importin-alpha confers binding to importin-beta and hence transit into the nucleus. EMBO J. 1996 Apr 15;15(8):1810–1817. [PMC free article] [PubMed] [Google Scholar]
- Görlich D., Kostka S., Kraft R., Dingwall C., Laskey R. A., Hartmann E., Prehn S. Two different subunits of importin cooperate to recognize nuclear localization signals and bind them to the nuclear envelope. Curr Biol. 1995 Apr 1;5(4):383–392. doi: 10.1016/s0960-9822(95)00079-0. [DOI] [PubMed] [Google Scholar]
- Görlich D., Mattaj I. W. Nucleocytoplasmic transport. Science. 1996 Mar 15;271(5255):1513–1518. doi: 10.1126/science.271.5255.1513. [DOI] [PubMed] [Google Scholar]
- Görlich D., Prehn S., Laskey R. A., Hartmann E. Isolation of a protein that is essential for the first step of nuclear protein import. Cell. 1994 Dec 2;79(5):767–778. doi: 10.1016/0092-8674(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Görlich D., Vogel F., Mills A. D., Hartmann E., Laskey R. A. Distinct functions for the two importin subunits in nuclear protein import. Nature. 1995 Sep 21;377(6546):246–248. doi: 10.1038/377246a0. [DOI] [PubMed] [Google Scholar]
- Hattori M., Tsukamoto N., Nur-e-Kamal M. S., Rubinfeld B., Iwai K., Kubota H., Maruta H., Minato N. Molecular cloning of a novel mitogen-inducible nuclear protein with a Ran GTPase-activating domain that affects cell cycle progression. Mol Cell Biol. 1995 Jan;15(1):552–560. doi: 10.1128/mcb.15.1.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper A. K., Traglia H. M., Dunst R. W. The yeast RNA1 gene product necessary for RNA processing is located in the cytosol and apparently excluded from the nucleus. J Cell Biol. 1990 Aug;111(2):309–321. doi: 10.1083/jcb.111.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang Y. W., Miller D. L. A mutation that alters the nucleotide specificity of elongation factor Tu, a GTP regulatory protein. J Biol Chem. 1987 Sep 25;262(27):13081–13085. [PubMed] [Google Scholar]
- Imamoto N., Shimamoto T., Kose S., Takao T., Tachibana T., Matsubae M., Sekimoto T., Shimonishi Y., Yoneda Y. The nuclear pore-targeting complex binds to nuclear pores after association with a karyophile. FEBS Lett. 1995 Jul 24;368(3):415–419. doi: 10.1016/0014-5793(95)00699-a. [DOI] [PubMed] [Google Scholar]
- Imamoto N., Shimamoto T., Takao T., Tachibana T., Kose S., Matsubae M., Sekimoto T., Shimonishi Y., Yoneda Y. In vivo evidence for involvement of a 58 kDa component of nuclear pore-targeting complex in nuclear protein import. EMBO J. 1995 Aug 1;14(15):3617–3626. doi: 10.1002/j.1460-2075.1995.tb00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovine M. K., Watkins J. L., Wente S. R. The GLFG repetitive region of the nucleoporin Nup116p interacts with Kap95p, an essential yeast nuclear import factor. J Cell Biol. 1995 Dec;131(6 Pt 2):1699–1713. doi: 10.1083/jcb.131.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaurralde E., Mattaj I. W. RNA export. Cell. 1995 Apr 21;81(2):153–159. doi: 10.1016/0092-8674(95)90323-2. [DOI] [PubMed] [Google Scholar]
- Kang C., Sun N., Honzatko R. B., Fromm H. J. Replacement of Asp333 with Asn by site-directed mutagenesis changes the substrate specificity of Escherichia coli adenylosuccinate synthetase from guanosine 5'-triphosphate to xanthosine 5'-triphosphate. J Biol Chem. 1994 Sep 30;269(39):24046–24049. [PubMed] [Google Scholar]
- Klebe C., Bischoff F. R., Ponstingl H., Wittinghofer A. Interaction of the nuclear GTP-binding protein Ran with its regulatory proteins RCC1 and RanGAP1. Biochemistry. 1995 Jan 17;34(2):639–647. doi: 10.1021/bi00002a031. [DOI] [PubMed] [Google Scholar]
- Klebe C., Nishimoto T., Wittinghofer F. Functional expression in Escherichia coli of the mitotic regulator proteins p24ran and p45rcc1 and fluorescence measurements of their interaction. Biochemistry. 1993 Nov 9;32(44):11923–11928. doi: 10.1021/bi00095a023. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küssel P., Frasch M. Pendulin, a Drosophila protein with cell cycle-dependent nuclear localization, is required for normal cell proliferation. J Cell Biol. 1995 Jun;129(6):1491–1507. doi: 10.1083/jcb.129.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounsbury K. M., Richards S. A., Perlungher R. R., Macara I. G. Ran binding domains promote the interaction of Ran with p97/beta-karyopherin, linking the docking and translocation steps of nuclear import. J Biol Chem. 1996 Feb 2;271(5):2357–2360. doi: 10.1074/jbc.271.5.2357. [DOI] [PubMed] [Google Scholar]
- Melchior F., Gerace L. Mechanisms of nuclear protein import. Curr Opin Cell Biol. 1995 Jun;7(3):310–318. doi: 10.1016/0955-0674(95)80084-0. [DOI] [PubMed] [Google Scholar]
- Melchior F., Guan T., Yokoyama N., Nishimoto T., Gerace L. GTP hydrolysis by Ran occurs at the nuclear pore complex in an early step of protein import. J Cell Biol. 1995 Nov;131(3):571–581. doi: 10.1083/jcb.131.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior F., Paschal B., Evans J., Gerace L. Inhibition of nuclear protein import by nonhydrolyzable analogues of GTP and identification of the small GTPase Ran/TC4 as an essential transport factor. J Cell Biol. 1993 Dec;123(6 Pt 2):1649–1659. doi: 10.1083/jcb.123.6.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior F., Weber K., Gerke V. A functional homologue of the RNA1 gene product in Schizosaccharomyces pombe: purification, biochemical characterization, and identification of a leucine-rich repeat motif. Mol Biol Cell. 1993 Jun;4(6):569–581. doi: 10.1091/mbc.4.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. S., Blobel G. Purification of a Ran-interacting protein that is required for protein import into the nucleus. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10212–10216. doi: 10.1073/pnas.91.21.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. S., Blobel G. The GTP-binding protein Ran/TC4 is required for protein import into the nucleus. Nature. 1993 Oct 14;365(6447):661–663. doi: 10.1038/365661a0. [DOI] [PubMed] [Google Scholar]
- Moore M. S., Blobel G. The two steps of nuclear import, targeting to the nuclear envelope and translocation through the nuclear pore, require different cytosolic factors. Cell. 1992 Jun 12;69(6):939–950. doi: 10.1016/0092-8674(92)90613-h. [DOI] [PubMed] [Google Scholar]
- Moroianu J., Blobel G., Radu A. Previously identified protein of uncertain function is karyopherin alpha and together with karyopherin beta docks import substrate at nuclear pore complexes. Proc Natl Acad Sci U S A. 1995 Mar 14;92(6):2008–2011. doi: 10.1073/pnas.92.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroianu J., Hijikata M., Blobel G., Radu A. Mammalian karyopherin alpha 1 beta and alpha 2 beta heterodimers: alpha 1 or alpha 2 subunit binds nuclear localization signal and beta subunit interacts with peptide repeat-containing nucleoporins. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6532–6536. doi: 10.1073/pnas.92.14.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehrbass U., Blobel G. Role of the nuclear transport factor p10 in nuclear import. Science. 1996 Apr 5;272(5258):120–122. doi: 10.1126/science.272.5258.120. [DOI] [PubMed] [Google Scholar]
- Newmeyer D. D., Forbes D. J. Nuclear import can be separated into distinct steps in vitro: nuclear pore binding and translocation. Cell. 1988 Mar 11;52(5):641–653. doi: 10.1016/0092-8674(88)90402-3. [DOI] [PubMed] [Google Scholar]
- O'Neill R. E., Palese P. NPI-1, the human homolog of SRP-1, interacts with influenza virus nucleoprotein. Virology. 1995 Jan 10;206(1):116–125. doi: 10.1016/s0042-6822(95)80026-3. [DOI] [PubMed] [Google Scholar]
- Palacios I., Weis K., Klebe C., Mattaj I. W., Dingwall C. RAN/TC4 mutants identify a common requirement for snRNP and protein import into the nucleus. J Cell Biol. 1996 May;133(3):485–494. doi: 10.1083/jcb.133.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschal B. M., Gerace L. Identification of NTF2, a cytosolic factor for nuclear import that interacts with nuclear pore complex protein p62. J Cell Biol. 1995 May;129(4):925–937. doi: 10.1083/jcb.129.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers M. A., Forbes D. J. Cytosolic factors in nuclear transport: what's importin? Cell. 1994 Dec 16;79(6):931–934. doi: 10.1016/0092-8674(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Powers T., Walter P. Reciprocal stimulation of GTP hydrolysis by two directly interacting GTPases. Science. 1995 Sep 8;269(5229):1422–1424. doi: 10.1126/science.7660124. [DOI] [PubMed] [Google Scholar]
- Radu A., Blobel G., Moore M. S. Identification of a protein complex that is required for nuclear protein import and mediates docking of import substrate to distinct nucleoporins. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1769–1773. doi: 10.1073/pnas.92.5.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexach M., Blobel G. Protein import into nuclei: association and dissociation reactions involving transport substrate, transport factors, and nucleoporins. Cell. 1995 Dec 1;83(5):683–692. doi: 10.1016/0092-8674(95)90181-7. [DOI] [PubMed] [Google Scholar]
- Richardson W. D., Mills A. D., Dilworth S. M., Laskey R. A., Dingwall C. Nuclear protein migration involves two steps: rapid binding at the nuclear envelope followed by slower translocation through nuclear pores. Cell. 1988 Mar 11;52(5):655–664. doi: 10.1016/0092-8674(88)90403-5. [DOI] [PubMed] [Google Scholar]
- Rush M. G., Drivas G., D'Eustachio P. The small nuclear GTPase Ran: how much does it run? Bioessays. 1996 Feb;18(2):103–112. doi: 10.1002/bies.950180206. [DOI] [PubMed] [Google Scholar]
- Saitoh H., Dasso M. The RCC1 protein interacts with Ran, RanBP1, hsc70, and a 340-kDa protein in Xenopus extracts. J Biol Chem. 1995 May 5;270(18):10658–10663. doi: 10.1074/jbc.270.18.10658. [DOI] [PubMed] [Google Scholar]
- Scheffzek K., Klebe C., Fritz-Wolf K., Kabsch W., Wittinghofer A. Crystal structure of the nuclear Ras-related protein Ran in its GDP-bound form. Nature. 1995 Mar 23;374(6520):378–381. doi: 10.1038/374378a0. [DOI] [PubMed] [Google Scholar]
- Schlenstedt G., Saavedra C., Loeb J. D., Cole C. N., Silver P. A. The GTP-bound form of the yeast Ran/TC4 homologue blocks nuclear protein import and appearance of poly(A)+ RNA in the cytoplasm. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):225–229. doi: 10.1073/pnas.92.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet D. J., Gerace L. A GTPase distinct from Ran is involved in nuclear protein import. J Cell Biol. 1996 Jun;133(5):971–983. doi: 10.1083/jcb.133.5.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet D. J., Gerace L. Taking from the cytoplasm and giving to the pore: soluble transport factors in nuclear protein import. Trends Cell Biol. 1995 Dec;5(12):444–447. doi: 10.1016/s0962-8924(00)89108-4. [DOI] [PubMed] [Google Scholar]
- Tachibana T., Imamoto N., Seino H., Nishimoto T., Yoneda Y. Loss of RCC1 leads to suppression of nuclear protein import in living cells. J Biol Chem. 1994 Oct 7;269(40):24542–24545. [PubMed] [Google Scholar]
- Török I., Strand D., Schmitt R., Tick G., Török T., Kiss I., Mechler B. M. The overgrown hematopoietic organs-31 tumor suppressor gene of Drosophila encodes an Importin-like protein accumulating in the nucleus at the onset of mitosis. J Cell Biol. 1995 Jun;129(6):1473–1489. doi: 10.1083/jcb.129.6.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijland A., Parmeggiani A. Toward a model for the interaction between elongation factor Tu and the ribosome. Science. 1993 Feb 26;259(5099):1311–1314. doi: 10.1126/science.8446899. [DOI] [PubMed] [Google Scholar]
- Weis K., Mattaj I. W., Lamond A. I. Identification of hSRP1 alpha as a functional receptor for nuclear localization sequences. Science. 1995 May 19;268(5213):1049–1053. doi: 10.1126/science.7754385. [DOI] [PubMed] [Google Scholar]
- Weis K., Ryder U., Lamond A. I. The conserved amino-terminal domain of hSRP1 alpha is essential for nuclear protein import. EMBO J. 1996 Apr 15;15(8):1818–1825. [PMC free article] [PubMed] [Google Scholar]
- Wittinghofer A., Pai E. F. The structure of Ras protein: a model for a universal molecular switch. Trends Biochem Sci. 1991 Oct;16(10):382–387. doi: 10.1016/0968-0004(91)90156-p. [DOI] [PubMed] [Google Scholar]
- Wu J., Matunis M. J., Kraemer D., Blobel G., Coutavas E. Nup358, a cytoplasmically exposed nucleoporin with peptide repeats, Ran-GTP binding sites, zinc fingers, a cyclophilin A homologous domain, and a leucine-rich region. J Biol Chem. 1995 Jun 9;270(23):14209–14213. doi: 10.1074/jbc.270.23.14209. [DOI] [PubMed] [Google Scholar]
- Yano R., Oakes M., Yamaghishi M., Dodd J. A., Nomura M. Cloning and characterization of SRP1, a suppressor of temperature-sensitive RNA polymerase I mutations, in Saccharomyces cerevisiae. Mol Cell Biol. 1992 Dec;12(12):5640–5651. doi: 10.1128/mcb.12.12.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama N., Hayashi N., Seki T., Panté N., Ohba T., Nishii K., Kuma K., Hayashida T., Miyata T., Aebi U. A giant nucleopore protein that binds Ran/TC4. Nature. 1995 Jul 13;376(6536):184–188. doi: 10.1038/376184a0. [DOI] [PubMed] [Google Scholar]