Abstract
Two different condensin complexes make distinct contributions to metaphase chromosome architecture in vertebrate cells. We show here that the spatial and temporal distributions of condensins I and II are differentially regulated during the cell cycle in HeLa cells. Condensin II is predominantly nuclear during interphase and contributes to early stages of chromosome assembly in prophase. In contrast, condensin I is sequestered in the cytoplasm from interphase through prophase and gains access to chromosomes only after the nuclear envelope breaks down in prometaphase. The two complexes alternate along the axis of metaphase chromatids, but they are arranged into a unique geometry at the centromere/kinetochore region, with condensin II enriched near the inner kinetochore plate. This region-specific distribution of condensins I and II is severely disrupted upon depletion of Aurora B, although their association with the chromosome arm is not. Depletion of condensin subunits causes defects in kinetochore structure and function, leading to aberrant chromosome alignment and segregation. Our results suggest that the two condensin complexes act sequentially to initiate the assembly of mitotic chromosomes and that their specialized distribution at the centromere/kinetochore region may play a crucial role in placing sister kinetochores into the back-to-back orientation.
INTRODUCTION
The faithful segregation of duplicated genetic information into two daughter cells is central to cell proliferation. In eukaryotic cells, amorphous interphase chromatin is converted into individual chromosomes composed of a pair of cylindrical structures (sister chromatids) by metaphase. This process is followed by synchronous segregation of sister chromatids that initiates at the onset of anaphase. During the past decade, a variety of approaches have been combined to demonstrate that a large protein complex called condensin is one of the key regulators of chromosome behavior during mitosis (reviewed by Nasmyth, 2002; Swedlow and Hirano, 2003).
The condensin complex is composed of five subunits that are widely conserved among eukaryotic organisms from yeast to humans (Hirano et al., 1997; Sutani et al., 1999; Freeman et al., 2000; Schmiesing et al., 2000; Kimura et al., 2001). The two core subunits of condensin (CAP-E/SMC2 and CAP-C/SMC4) belong to a large family of chromosomal ATPases known as the structural maintenance of chromosomes (SMC) family. The SMC proteins participate in many aspects of higher order chromosome dynamics that include not only chromosome condensation but also sister chromatid cohesion and recombinational repair (reviewed by Hirano, 2002; Jessberger, 2002; Hagstrom and Meyer, 2003). The remaining subunits (CAP-D2, -G, and -H) are not related with SMCs, and share structural motifs with some components involved in cohesion (Neuwald and Hirano, 2000; Schleiffer et al., 2003). The holo-complex of condensin introduces positive superhelical tension into DNA in an ATP hydrolysis-dependent manner in vitro (Kimura and Hirano, 1997; Bazett-Jones et al., 2002), and this activity is believed to be important for initiating the assembly of mitotic chromosomes in the cell.
Functional assays using Xenopus laevis egg extracts demonstrated that the condensin complex is required for both chromosome assembly in metaphase (Hirano and Mitchison, 1994; Hirano et al., 1997) and segregation in anaphase (Wignall et al., 2003). Concurrently, genetic studies provided strong lines of evidence that condensin function is essential for proper segregation in a number of model organisms, including Schizossaccharomyces pombe (Saka et al., 1994; Sutani et al., 1999), Saccharomyces cerevisiae (Strunnikov et al., 1995; Bhalla et al., 2002; Lavoie et al., 2002), Caenorhabditis elegans (Lieb et al., 1998; Hagstrom et al., 2002), Drosophila melanogaster (Bhat et al., 1996; Steffensen et al., 2001; Coelho et al., 2003; Somma et al., 2003), and chicken DT40 cells (Hudson et al., 2003). The severity of defects in chromosome architecture observed in these condensin-deficient cells is, however, somewhat variable among different organisms or different mutations. The most commonly observed phenotype is the defect in sister chromatid resolution in metaphase, which in turn causes the formation of chromosome bridges in anaphase. Abnormal kinetochore-microtubule attachment is also observed in some cases, but the exact reason for this defect remains unclear.
The recent discovery of a second class of condensin (condensin II) in vertebrate cells provides an additional layer of complexity to this problem (Ono et al., 2003). Condensin II possesses the same pair of SMC core subunits as the canonical condensin complex (now referred to as condensin I), but contains a different set of non-SMC subunits (CAP-D3, -G2, and -H2). The two complexes show distinct distributions along the longitudinal axis of mitotic chromosomes, and small-interfering RNA (siRNA)-mediated depletion of condensin I- or II-specific subunits produces a distinct, highly characteristic defect in metaphase chromosome architecture. It remains to be determined how similarly or how differently the two condensin complexes are regulated during the cell cycle, and how each of them contributes to chromosome alignment and segregation in mitosis.
In this study, we show that condensins I and II display different kinetics in chromosomal association during the cell cycle, in particular, outside the window from prometaphase to anaphase. Condensin II associates with chromosomes within the nucleus in prophase, whereas condensin I gains access to chromosomes only after the nuclear envelope breaks down in prometaphase. In metaphase chromosomes, the two complexes display a unique distribution at the centromere/kinetochore region, which is distinct from their distribution along the chromosome arm. Depletion of individual condensin subunits produces characteristic defects in chromosome alignment and segregation, which are closely associated with aberrant kinetochore structure and function. Our results suggest that the two condensin complexes cooperate to provide a structural basis for specifying the back-to-back orientation of sister kinetochores.
MATERIALS AND METHODS
Antibodies
Rabbit polyclonal antisera were raised against synthetic peptides corresponding to the N-terminal amino acid sequence of human CENP-A (RRRSRKPEAPRRRS) and human Aurora B (SRRVLPPSALQSVA). Other rabbit polyclonal antibodies used in this study were anti-hCAP-C, -E, -G, -D2, and -H (Kimura et al., 2001); anti-hCAP-D3, -G2, and -H2 (Ono et al., 2003); anti-phosphoH3 (Kimura and Hirano, 2000); and anti-human CENP-E (Yen et al., 1991). Mouse monoclonal anti-human cyclin A and human CREST serum were provided by J. Gannon/T. Hunt and Y. Muro (Muro et al., 1990), respectively. The following mouse monoclonal antibodies were purchased from commercial sources and used for immunolabeling: anti-human cyclin B1 (Sc-245; Santa Cruz Biotechnology, Santa Cruz, CA), anti-human lamin B (Calbiochem, San Diego, CA), anti-human aurora B (anti-hAIM1; BD Biosciences, San Jose, CA), and anti-α-tubulin (Sigma-Aldrich, St. Louis, MO). All secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA).
RNA Interference and Monastrol Treatment
The siRNA duplexes specific to hCAP-C, -E, -G, and -G2 were described previously (Ono et al., 2003). The sequences of the sense strand for other condensin subunits used in this study are as follows:
hCAP-D2 (RD2-1, 5′-UCAGUAUGUUGUGCAAGAGTT-3′;
RD2-2, 5′-GAAGAUACUCUGGAAUUCCTT-3′), hCAP-D3
(RD3-1, 5′-CUGGAUUUCACAGAGACUGTT-3′; RD3-2,
5′-GCAGAGAUCAUAGAGACUGTT-3′), hCAP-H (RH-1, 5′-
GACUUUCCUCAGAAUGACGTT-3′; RH-2, 5′-
CAUUACUCCACCUGUAUCATT-3′), and hCAP-H2 (RH2-1, 5′-
GGAUUUCAGGAUGAACACGTT-3′; and RH2-2, 5′-
GCUGCAGGACUUCCACCAGTT-3′).
The sense sequence of the targeted region in the human Aurora B is 5′-GAGCCUGUCACCCCAUCUGTT-3′. HeLa cells were grown on coverslips coated with poly-l-lysine in DMEM containing 10% fetal bovine serum, and transfected with 120 nM RNA duplexes with Oligofectamine (Invitrogen, Carlsbad, CA) at 0 and 24 h at ∼20% starting confluence (Elbashir et al., 2001). Control cells were transfected with a mixture containing no siRNA. The cells were processed for further analyses at 24 h after the second round of transfection. The two duplexes designed for each condensin subunit depleted the target subunit with similar efficiency as judged by immunoblotting and produced almost identical morphological phenotypes. When necessary, 100 μM monastrol (Calbiochem) was added to siRNA-treated or control cells at 24 h after the second round of transfection and incubated for another 10 h before processing for immunofluorescent analysis.
Immunofluorescence
For immunofluorescence analysis of condensins, two different fixation methods were used. In the first method (referred to as postextraction), the cells were fixed with 2% formaldehyde in phosphate-buffered saline (PBS) (pH 7.4) at room temperature for 15 min and then permeabilized in 0.5% Triton X-100 in PBS for 5 min (Figures 1A and 2). In the second one (referred to as preextraction), the cells were permeabilized in 0.1% Triton X-100 in XBE2 (10 mM HEPES, pH 7.7, 2 mM MgCl2, 100 mM KCl, and 5 mM EGTA) at room temperature for 2 min and then fixed with 2% formaldehyde in XBE2 for 15 min (Figure 1B). To label the mitotic spindle and kinetochores, the cells were fixed with 100% methanol at -20°C for 10 min (Figures 5, B and C, and 6, B and C). Alternatively, the cells were treated with 60 mM KCl at room temperature for 30 min, fixed with 2% formaldehyde for 15 min, permeabilized in 0.5% Triton X-100 in PBS for 5 min, and then postfixed with 100% methanol at -20°C for 10 min (Figure 6A). Metaphase chromosome spreads were prepared by cytospin as described previously (Ono et al., 2003) and fixed with the postextraction method (Figures 3 and 4). When anti-condensin II antibodies were used, the spreads were fixed with the preextraction method with 0.1% Triton X-100. The inner kinetochore signal with anti-hCAP-H2 was most clearly observed when the preextraction method was combined with deconvolution microscopy (Figure 3A). A similar distribution also was detected with anti-hCAP-G2 under the same condition. The coverslips were incubated with antibodies and counterstained with 4,6-diamidino-2-phenylindole (DAPI) as described previously (Ono et al., 2003). They were mounted on slides with Vectachield (Vector Laboratories, Burlingame, CA) and examined with an Axioskop microscope (Carl Zeiss, Jena, Germany) equipped with a cooled charge-coupled device camera. Grayscale images were pseudocolored and merged using Adobe Photoshop.
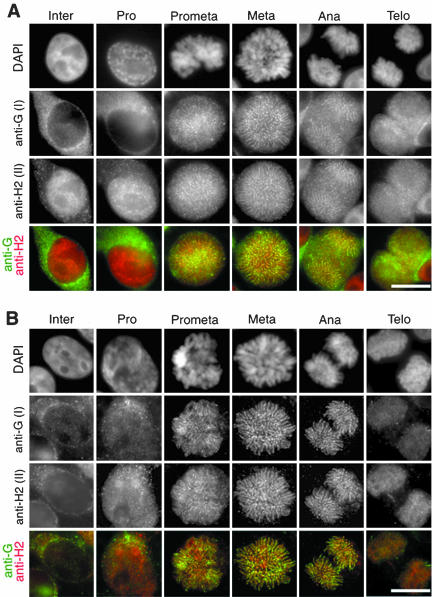
Figure 1.
Localization of condensins I and II during the cell cycle. (A) HeLa cells were fixed with the postextraction protocol (see MATERIALS AND METHODS) and labeled with biotinylated anti-hCAP-G/condensin I (anti-G) and anti-hCAP-H2/condensin II (anti-H2). Chromosomal DNA was stained with DAPI. Merged images of labeling with anti-hCAP-G (green) and anti-hCAP-H2 (red) are also shown. Bar, 10 μm. (B) HeLa cells were fixed with the preextraction protocol (see MATERIALS AND METHODS) and visualized as described in A. Bar, 10 μm.
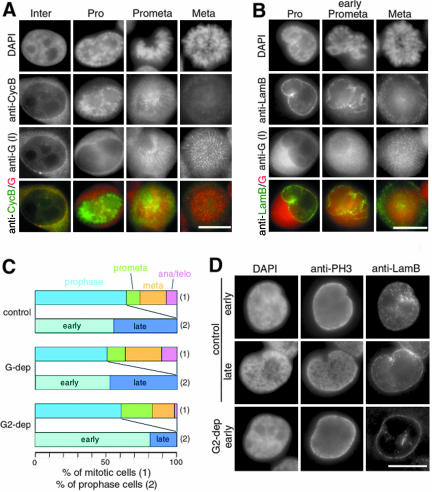
Figure 2.
Distinct distributions and functions of condensins I and II during early stages of mitosis. (A and B) HeLa cells were fixed with the postextraction protocol and labeled with anti-hCAP-G/condensin I (anti-G) and anti-cyclin B1 (A) or anti-lamin B (B). Chromosomal DNA was stained with DAPI. The bottom panels show merged images of labeling with anti-hCAP-G (red) and anti-cyclin B1 (green; A), or anti-lamin B (green; B). Bars, 10 μm. (C and D) HeLa cells were transfected with no siRNA (control) or siRNA specific to hCAP-G (G-dep) or hCAP-G2 (G2-dep) and were labeled with anti-phospho-H3 (anti-PH3) and anti-lamin B (anti-LamB). Chromosomal DNA was stained with DAPI. The frequency of each mitotic stage is expressed as a percentage of the mitotic population in C (1), and the frequency of the early or late category is expressed as a percentage of the prophase population in C (2). Examples of prophase cells in the two categories are shown in D. Bar, 10 μm.
Figure 5.
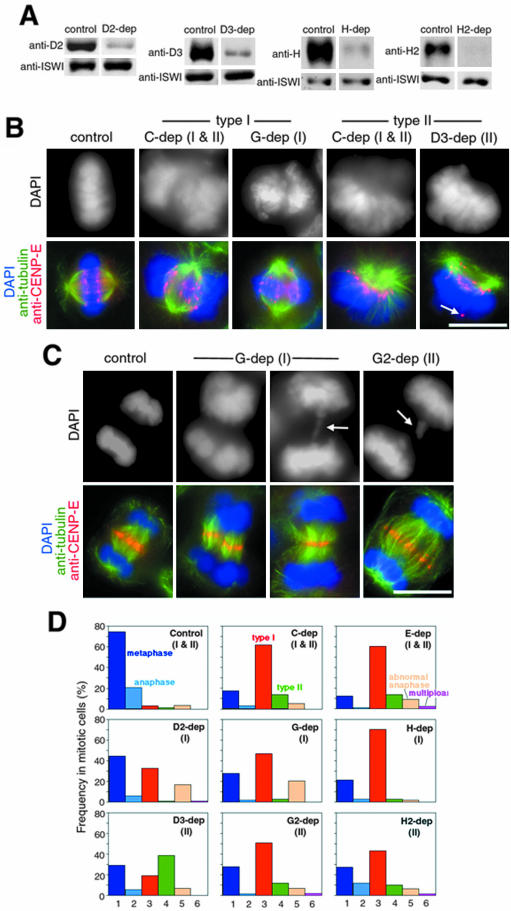
Alignment and segregation defects in condensin-depleted cells. (A) Whole cell lysates were prepared from HeLa cells treated with no siRNA (control) or siRNAs specific to hCAP-D2, -D3, -H, or -H2, and analyzed by immunoblotting with the indicated antibodies. ISWI was used as a loading control. The blots corresponding to the depletion of hCAP-E, -C, -G, and -G2 have been reported previously (Ono et al., 2003). (B) Representative examples of the segregation defects observed in the prometaphase- and metaphase-like populations. HeLa cells were transfected with no siRNA (control) or siRNAs specific to hCAP-C, -G, or -D3 (C-dep, G-dep, and D3-dep). After fixation, they were stained with DAPI (grayscale images, top; blue, bottom) and labeled with anti-α-tubulin (green, bottom) and anti-CENP-E (red, bottom). A delocalized CENP-E signal is indicated by arrow. Bar, 10 μm. (C) Representative examples of the segregation defects observed in anaphase-like populations. HeLa cells were transfected with no siRNA (control) or siRNAs to specific to hCAP-G (G-dep) or hCAP-G2 (G2-dep) and were subjected to immunofluorescence analysis as described above. Lagging chromosomes are indicated by arrows. Bar, 10 μm. (D) Quantification of the segregation defects. The frequency of each phenotype is expressed as a percentage of the mitotic population: normal metaphase (column 1); normal anaphase (2); the type I spindle defect (3); the type II spindle defect (4); abnormal anaphase, including chromatin bridges and lagging chromosomes (5); and multipolar spindles (6). More than 100 mitotic cells were analyzed in each depletion.
Figure 6.
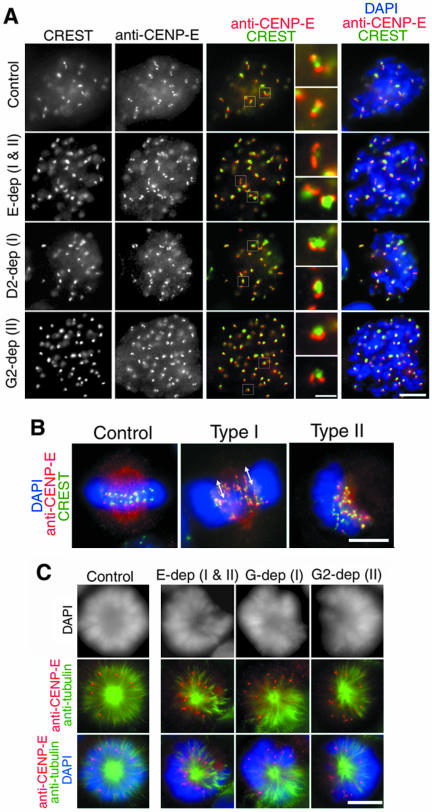
Kinetochore defects in condensin-depleted cells. (A) Metaphase cells from cultures transfected with no siRNA (control) or siRNAs specific to hCAP-E, -D2, or -G2 (E-dep, D2-dep, or G2-dep). After hypotonic treatment, the cells were fixed and stained with DAPI (blue, fifth column) and labeled with CREST serum (grayscale, first column; green, third to fifth column) and anti-hCENP-E (grayscale, second column; red, third to fifth column). Bar, 5 μm. The fourth column represents close-ups of the centromere/kinetochore region indicated by white boxes in the third column. Bar, 1 μm. (B) Representative examples of normal metaphase (control), the type I spindle defect (hCAP-E depletion) and the type II spindle defect (hCAP-D3 depletion). The cells were fixed without hypotonic treatment, labeled with anti-hCENP-E (red) and CREST serum (green) and stained with DAPI (blue). Arrows indicate stretched kinetochore signals. Bar, 5 μm. (C) HeLa cells transfected with no siRNA (control) or specific siRNAs (E-dep, G-dep and G2-dep) were treated with 100 μM monastrol for 10 h, labeled with anti-α-tubulin (green) and anti-CENP-E (red), and stained with DAPI (grayscale, top; blue, bottom), Bar, 5 μm.
Figure 3.
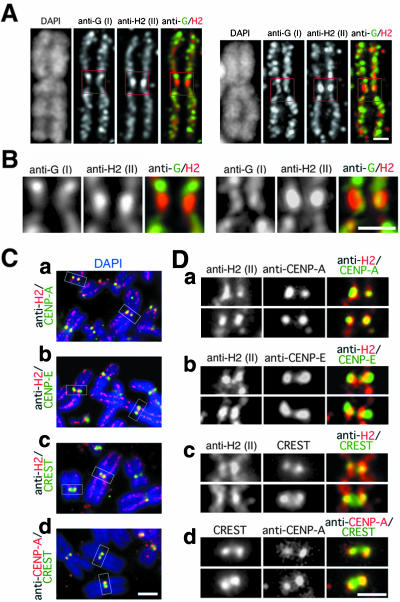
Condensins I and II display distinct localizations at the centromere/kinetochore region. (A) Metaphase chromosome spreads were prepared from HeLa cells and labeled with biotinylated anti-hCAPG/condensin I (anti-G) and anti-hCAP-H2/condensin II (anti-H2). Chromosomal DNA was stained with DAPI. Deconvoluted images of two representative chromosomes are shown. The right panels are merged images of labeling with anti-hCAP-G (green) and anti-hCAP-H2 (red). Bar, 1 μm. (B) Close-ups of the centromere/kinetochore regions indicated by red boxes in A. Bar, 1 μm. (C) Chromosome spreads were labeled with biotinylated anti-hCAP-H2 and anti-CENP-A (a), biotinylated anti-hCAP-H2 and anti-CENP-E (b), anti-hCAP-H2 and a CREST serum (c), or the CREST serum and anti-CENP-A (d). Merged images with chromosomal DNA stained with DAPI (blue) are shown. Bar, 2 μm. (D) Close-ups of the centromere/kinetochore regions indicated by white boxes in C. Bar, 1 μm.
Figure 4.
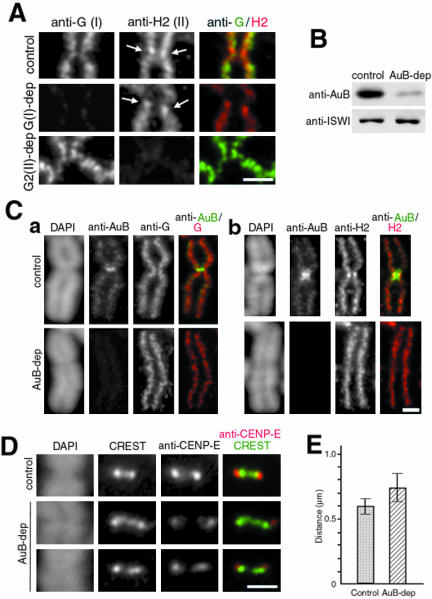
Specialized organization of condensins at the centromere/kinetochore region depends on Aurora B. (A) Metaphase chromosome spreads were prepared from HeLa cells transfected with no siRNA (control) or siRNA specific to hCAP-G/condensin I (G-dep) or hCAP-G2/condensin II (G2-dep) and were labeled with anti-hCAP-G (anti-G) and anti-hCAP-H2 (anti-H2). Arrows indicate the enrichment of the hCAP-H2 signal at the kinetochore region. Bar, 1 μm. (B) Whole cell lysates were prepared from HeLa cells treated with no siRNA (control) or Aurora B siRNA (AuB-dep) and analyzed by immunoblotting with the indicated antibodies. ISWI was used as a loading control. (C) Metaphase chromosome spreads were prepared from control and Aurora B-depleted cells and labeled with anti-Aurora B (anti-AuB) and anti-G (a) or anti-H2 (b). Chromosomal DNA was stained with DAPI. Merged images of labeling with anti-AuB and anti-G (a) or anti-H2 (b) are also shown. Bar, 1 μm. (D) Control or Aurora B-depleted chromosomes were stained with DAPI and labeled with CREST serum and anti-CENP-E. Bar, 1 μm. (E) Tip-to-tip distance of the CREST signals were measured in the control (0.59 ± 0.06 μm; n = 116) and Aurora B-depleted (0.73 ± 0.11 μm; n = 120) chromosomes.
Deconvolution Microscopy
To preserve chromosome structure, metaphase chromosome spreads were made by cytospin technique with a minor modification. In brief, mitotic cells collected by tapping culture dishes were treated with 75 mM KCl at 37°C for 30 min and then centrifuged onto coverslips at 1000 rpm for 2 min. After fixation with the preextraction method, the coverslips were simultaneously labeled with anti-hCAP-G and -H2. Chromosomal DNA was stained with DAPI. Three-dimensional image stacks of the chromosomes were acquired with a DeltaVision restoration microscope system (Applied Precision, Issaquah, WA) consisting of an Olympus IX70 inverted microscope equipped with a Plan Apo oil-immersion objective lens (60×, numerical aperture 1.40′ Olympus), and a Photometrics cooled charge-coupled device camera (Roper Scientific, Trenton, NJ). The image stacks of metaphase chromosomes with a Z-step size of 0.15 μm were subjected to constrained iterative (15 iterations) deconvolution (Chen et al., 1996) by using softWoRx software (Applied Precision).
Immunoblotting
Whole cell lysates were prepared from HeLa cells transfected with no siRNA or siRNA specific to each of condensin subunits or Aurora B at 24 h after the second round of transfection. Approximately 1 × 105 cells were suspended in 50 μl in 0.5× PBS and 1× SDS sample buffer, boiled for 5 min, and sonicated for 10 s. Aliquots (10-20 μl) were fractionated by SDS-PAGE and analyzed by immunoblotting by using antibodies against condensin subunits or Aurora B. ISWI, a subunit of chromatin remodeling complexes (MacCallum et al., 2002), was used as a loading control.
RESULTS
Condensins I and II Associate with and Dissociate from Chromosomes at Different Stages in Mitosis
To examine the kinetics of chromosomal association of condensins I and II during the cell cycle, HeLa cells were stained simultaneously with antibodies against hCAP-G (a condensin I-specific subunit) and hCAP-H2 (a condensin II-specific subunit). Here, we compared two different fixation protocols (see MATERIALS AND METHODS). In the first protocol (referred to as postextraction), the cells were first fixed with formaldehyde and then permeabilized with a nonionic detergent before being incubated with the antibodies. Under this condition, the majority of the signal with anti-hCAP-G was found in the cytoplasm in interphase and prophase (Figure 1A, Inter and Pro, anti-G). In striking contrast, the signal with anti-hCAP-H2 was primarily detected within the nucleus during these stages (Figure 1A, Inter and Pro, anti-H2). The signals obtained with anti-hCAP-G and anti-hCAP-H2 started to colocalize on chromosomes in prometaphase (Figure 1A, Prometa) and became concentrated on an axial structure within each chromatid during metaphase and anaphase (Figure 1A, Meta and Ana). The two signals observed along the chromatid axis did not completely overlap, consistent with the alternate distribution of condensin I and condensin II that we previously observed in metaphase chromosome spreads (Ono et al., 2003). In telophase, the axial localization of hCAP-G and -H2 became less clear as chromosomes decondensed (Figure 1A, Telo).
To visualize the population of condensins that is tightly bound to chromatin or chromosomes, we next used the second protocol (referred to as preextraction) in which the cells were first permeabilized with a nonionic detergent and then fixed with formaldehyde. As expected, the cytoplasmic signals observed with anti-hCAP-G in interphase and prophase cells were reduced under this condition (Figure 1B, Inter and Pro). The nuclear signal observed with anti-hCAP-H2 in interphase cells was also drastically reduced, but the signal in prophase nuclei was less affected. From prometaphase through anaphase, the axial distribution of hCAP-G and hCAP-H2 on chromosomes was visualized very clearly, due to removal of the cytoplasmic pool of condensins by preextraction (Figure 1B, Prometa, Meta, and Ana). In telophase, hCAP-H2 was more stably retained on chromosomes compared with hCAP-G (Figure 1B, Telo). The subcellular distribution of another condensin II-specific subunit, hCAP-G2, was indistinguishable from that of hCAP-H2 under both fixation conditions. Furthermore, the distribution of the SMC core subunits corresponded to the sum of the distributions of hCAP-G and hCAP-H2 as expected (our unpublished data). These results suggest that condensins I and II display distinct localizations during the cell cycle, in particular, outside the window from prometaphase to anaphase.
Distinct Distributions and Functions of Condensins I and II during Early Stages of Mitosis
To determine the localization of condensins I and II during early mitosis more precisely, we performed double labeling experiments using antibodies specific to cyclin B1, lamin B, and cyclin A. Consistent with a previous report (Pines and Hunter, 1991), cyclin B1 was cytoplasmic during interphase, was translocated to the nucleus in prophase, and was degraded after metaphase (Figure 2A, anti-CycB). We found that hCAP-G (condensin I) remained in the cytoplasm when cyclin B1 first entered the nucleus (Figure 2A, anti-G). The condensin I subunit gained access to chromosomes only after the nuclear envelope started to be broken down as judged by anti-lamin B labeling (Figure 2B). Unlike cyclin B1, however, cyclin A was nuclear in interphase and most of it disappeared at the time when condensin I bound to chromosomes by prometaphase (Supplemental Figure S1A). On the other hand, hCAP-H2 (condensin II) coexisted with cyclin A in the nucleus from interphase through prophase, and with cyclin B on chromosomes in prophase (Supplemental Figures S1B and S1C). These results suggest that cyclin A-cdk and cyclin B1-cdk1 may phosphorylate the two condensin complexes at different stages of the cell cycle and coordinate sequential structural changes of chromosomes from prophase to metaphase (see DISCUSSION).
To test the possibility that initial stages of chromosome assembly in prophase are supported by condensin II, HeLa cells were transfected with siRNAs specific to either hCAP-G or hCAP-G2, and labeled with antibodies against a phosphorylated form of histone H3 (phospho-H3) and lamin B. Although the control and transfected cells had a similar mitotic index as we described previously (Ono et al., 2003), we found that there were some variations in the frequency of cells at different stages of mitosis (Figure 2C [1]). We then focused on the prophase population that displays whole nuclear phospho-H3 labeling and has an intact nuclear envelope and classified it further into two categories on the basis of their nuclear morphology. In the “early” category, both DAPI and phospho-H3 signals were uniformly distributed throughout the nucleus with some signs of local heterogeneity (Figure 2D, control, early). In the “late” category, thread-like structures were clearly visualized by both DAPI staining and phospho-H3 labeling (Figure 2D, control, late). We noticed that, from the cultures transfected with no siRNA and with hCAP-G siRNA, 55% (n = 206) and 48% (n = 231) of prophase cells, respectively, were classified into the early category. In contrast, 81% (n = 194) of prophase cells belonged to the early category from the culture transfected with hCAP-G2 siRNA (Figure 2C [2]; Figure 2D, G2-dep). These results suggest that the initial stages of chromosome assembly within the prophase nucleus are compromised when the functional level of condensin II is reduced.
Condensins I and II Display a Unique Geometry at the Centromere/Kinetochore Region
We previously showed that condensins I and II distribute along the longitudinal axis of metaphase chromatids in a distinctive manner (Ono et al., 2003). To visualize the chromosomal localization of the two complexes in more detail, we labeled metaphase chromosome spreads with anti-hCAP-G and -H2 and analyzed them by deconvolution microscopy. Two representative examples of the labeling are shown in Figure 3A. The hCAP-G signal within each chromatid resembled a spiral (Figure 3A, anti-G). The axis labeled with anti-hCAP-H2 also displayed a spiral-like, yet somewhat irregular, appearance (Figure 3A, anti-H2). Merged images revealed an alternate distribution of the two signals, but it was not completely even along the chromatid axis (Figure 3A, anti-G/H2). The most prominent example of such local heterogeneity was observed at the primary constriction of each chromosome. In this region, the signal with anti-hCAP-G was thinner, less coiled, and more internal than the signal with anti-hCAP-H2 (Figure 3B, anti-G). Anti-hCAP-H2 intensely labeled paired dot-like structures in this region, which were likely to be located near the kinetochores (Figure 3B, anti-H2).
We then determined the localization of the hCAP-H2-enriched structure relative to known centromere/kinetochore markers (Figure 3, C and D). In terms of the lateral position, the pair of the hCAP-H2 signals largely overlapped with CENP-A, a component of the inner kinetochore plate (Palmer et al., 1991), whereas they were located internally with respect to CENP-E, a marker for the outer kinetochore plate (Yen et al., 1991). The two spots labeled with a CREST serum colocalized with hCAP-H2 at the kinetochore region (Figures 3Cc and 3Dc) as well as with CENP-A (Figures 3Cd and 3Dd). In many cases, however, the signals obtained with anti-hCAP-H2 were more extended in the axial directions compared with those of the other kinetochore markers.
Specialized Organization of Condensins I and II at the Centromere/Kinetochore Region Depends on Aurora B
Our previous results showed that targeting of condensins I and II to the chromosome arm is independent of each other both in HeLa cells and in Xenopus egg extracts (Ono et al., 2003).
We wished to test whether it was also the case at the centromere/kinetochore region. To this end, metaphase chromosome spreads were prepared from HeLa cells depleted of hCAP-G (condensin I) or hCAP-G2 (condensin II), and labeled with either anti-hCAP-G or anti-hCAP-H2 (Figure 4A). Whereas sister chromatids were swollen and centromeric cohesion was somewhat loosened in the chromosomes depleted of hCAP-G, the enrichment of hCAP-H2 at the kinetochore region was still detectable (Figure 4A, G-dep, anti-H2, arrows). Likewise, the hCAP-G signal in the primary constriction was not severely affected after depletion of hCAP-G2 (Figure 4A, G2-dep, anti-G).
We next tested whether Aurora B, a protein kinase that becomes enriched at the pericentromeric region in early mitosis (reviewed by Andrews et al., 2003; Carmena and Earnshaw, 2003), may contribute to specifying the unique geometry of the two condensin complexes at the centromere/kinetochore region. In metaphase chromosome spreads prepared from cells depleted of Aurora B (Figure 4B), localization of both hCAP-G and hCAP-H2 to the chromosomal arm was largely normal (Figure 4C). We noticed, however, that the primary constriction was severely disorganized or almost lost in these chromosomes. Unlike control chromosomes in which the two sister axes (as visualized with condensin antibodies) formed a typical X shape, the axes looked like parallel lines along the entire length of the chromosomes in the absence of Aurora B. Furthermore, the enrichment of hCAP-H2 at the kinetochore region was greatly diminished or no longer observed in these chromosomes (Figure 4Cb, AuB-dep, anti-H2). The signals labeled with CREST serum were stretched laterally (Figure 4D), providing additional evidence that the primary constriction was severely compromised in the Aurora B-depleted chromosomes: the tip-to-tip distance of the CREST signals in these chromosomes (0.73 ± 0.11 μm; n = 120) was significantly longer than that in the control chromosomes (0.59 ± 0.06 μm; n = 116) (Figure 4E). Finally, we found that the level of CENP-E located at the tips of the CREST signals was often reduced and somewhat variable from chromosomes from chromosomes after depletion of Aurora B (Figure 4D, red). These results suggest that Aurora B influences the specialized localization of condensins I and II at the centromere/kinetochore region, but not their bulk distribution along the chromosome arm.
Depletion of Condensin Subunits Leads to Characteristic Defects in Chromosome Alignment and Segregation
To evaluate the role of condensins in mitotic chromosome segregation, we depleted each of the eight subunits individually by treating HeLa cells with specific siRNAs. The depletion level of the target subunit in each case was at least 80-90% as judged by immunoblotting (Figure 5A; Ono et al., 2003). Although none of the depletions caused a noticeable accumulation of mitotic cells, immunofluorescent labeling with anti-tubulin and anti-CENP-E revealed highly characteristic segregation defects in many of the mitotic cells (Figure 5, B-D).
The defective phenotypes detected in prometa- and metaphase populations could be classified into two groups, which we referred to as type I and type II spindle defects. The type I defect represented the primary phenotype that was broadly observed after depletion of any one of the condensin subunits (Figure 5D). In these cells, the formation of a bipolar spindle was apparently normal, but the mass of chromosomes was largely displaced from the spindle axis and failed to align on the metaphase plate (Figure 5B, type I). The CENP-E signals were not aligned properly and were often found on the periphery of the spindle with a stretched appearance. The type II spindle defect was a less prominent phenotype found in cells depleted of the SMC core or condensin II-specific subunits (Figure 5D). This phenotype was barely detectable in condensin I-depleted cells. In this class of cells, the two poles were not fully separated from each other, and the mass of chromosomes was sprayed away from one side of the spindle (Figure 5B, type II). Most CENP-E signals were located on the convex side of the spindle, but some of them were often found in abnormal positions far away from the poles (Figure 5B, type II, bottom, arrow). Although this chromosome-spindle arrangement was reminiscent of that observed during normal prometaphase, such a population was extremely minor in control cells (Figure 5D, control). We also detected aberrant figures in the anaphase population of cells after depletion of condensin subunits. In some cases, the two masses of daughter chromosomes had asymmetrical shapes and were unequally distributed to the two poles (Figure 5C, second column from left). Lagging chromosomes or chromosome bridges were also frequently observed (Figure 5C, third and fourth columns from left, arrows). Transfer of CENP-E from the kinetochores to the midzone was largely normal in these cells.
Defective Kinetochore Structure and Function in Condensin-depleted Cells
To get more insight into the segregation defects described above, we next tested whether depletion of condensin subunits might affect kinetochore structure and function. To this end, control or siRNA-transfected cells were hypotonically treated, fixed, and immunolabeled with a CREST serum and anti-CENP-E. Representative examples of the labeling of metaphase cells are shown in Figure 6A. In the control cells, the signals of the two markers displayed a well-organized geometry, in which a pair of discrete CENP-E dots localized to the tips of a dumbbell-shaped CREST signal (Figure 6A, control). In the condensin-depleted cells, the targeting of the two markers to the centromere/kinetochore region was largely normal. However, the geometry of the two signals was often disrupted, producing a variety of disorganized structures. The most severe defects were observed in the cells depleted of hCAP-E, an SMC core subunit (Figure 6A, E-dep). The CREST signal was no longer dumbbell-shaped, displaying a somewhat expanded or roundish appearance. The pair of the CENP-E signals was often stretched or fused with each other and located on the same side of a CREST-positive structure. Less severe yet similar defects were observed in the cells depleted of condensin I- or condensin II-specific subunits (Figure 6A, D2-dep or G2-dep). These results suggest that depletion of condensin subunits destabilizes the structural organization of the centromere/kinetochore regions, resulting in alteration of the back-to-back orientation of sister kinetochores.
When the control cells were fixed and labeled under the standard condition in which spindle microtubules are preserved, CREST signals flanked by pairs of CENP-E signals were aligned on the metaphase plate (Figure 6B, control). In contrast, CREST signals were often stretched, together with CENP-E signals, along the spindle axis in the cells displaying the type I spindle defect (Figure 6B, type I). Such stretched signals were barely detectable in the cells displaying the type II defect (Figure 6B, type II).
To further understand the abnormal kinetochore-microtubule interactions observed in the condensin-depleted cells, we induced the formation of monopolar spindles by using monastrol, a chemical inhibitor of the kinesin motor Eg5 (Mayer et al., 1999). When mock-depleted cells were treated with monastrol, chromosomes and their kinetochores (as judged by labeling with anti-CENP-E) uniformly radiated from a single pole (Figure 6C, control). In contrast, when the same drug was applied to condensin-depleted cells, the pole-to-kinetochore distance became extremely variable and chromosomes were irregularly placed around the pole (Figure 6C, E-dep, G-dep, and G2-dep). These results show that kinetochore-microtubule interactions are compromised in condensin-depleted chromosomes even under a condition where bipolar spindle assembly is suppressed.
DISCUSSION
Spatial and Temporal Distribution of Condensins I and II during the Cell Cycle
Specific localization of condensin subunits to chromosomes during mitosis has been well established in many different organisms (reviewed by Swedlow and Hirano, 2003). Conflicting data, however, have been reported regarding the location of condensin subunits during interphase. For example, early studies showed that the SMC2/CAP-E subunit is primarily localized within the nucleus (Hirano and Mitchison, 1994; Saitoh et al., 1994), whereas other studies reported that SMC4 or non-SMC subunits (of condensin I) are largely cytoplasmic (Schmiesing et al., 2000; Steffensen et al., 2001; Ball et al., 2002). The recent discovery of condensin II (Ono et al., 2003) has prompted us to readdress and clarify this problem. In this study, we have used antibodies specific to condensins I and II and found that the distributions of the two condensin complexes are distinct outside the window from prometaphase to anaphase. The most drastic difference was observed during interphase when condensin I is largely cytoplasmic, whereas condensin II is located within the nucleus. Several lines of recent evidence from yeasts show that condensin subunits play important roles in checkpoint control and gene repression during interphase (Aono et al., 2002; Bhalla et al., 2002; Machin et al., 2004). Although such nonmitotic functions for condensins remain to be explored in vertebrate cells, our current results suggest that condensin II, rather than condensin I, may be the major form that contributes to the functional organization of the genome within the interphase nucleus.
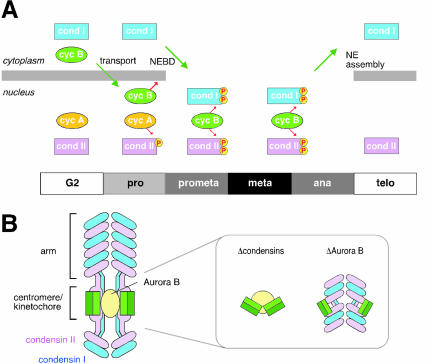
Our current data also provide new insights into the spatial and temporal regulation of mitotic chromosome assembly and disassembly. Mitosis-specific phosphorylation of condensin subunits has been suspected to regulate chromosomal targeting of the complex (Hirano et al., 1997). Furthermore, we showed that cyclin B1-cdk1 phosphorylates CAP-D2 and stimulates the supercoiling activity of condensin I in vitro (Kimura et al., 1998). Like CAP-D2, the CAP-D3 subunit of condensin II has a cluster of cdk-consensus sites in its C-terminal domain (Ono et al., 2003) and is phosphorylated in a mitosis-specific manner (Yeong et al., 2003). On the basis of the results from the immunolabeling and siRNA-mediated depletion experiments, one possibility is that a cyclin A-cdk complex phosphorylates condensin II in early prophase and triggers the initial structural changes of chromosomes within the nucleus (Figure 7A). Condensin I is sequestered in the cytoplasm until the end of prophase and gains access to chromosomes only after the nuclear envelope breaks down in prometaphase. At this stage, active cyclin B1-cdk1 is most likely to phosphorylate condensin I and to further modify condensin II, thereby regulating their coordinated actions at the late stages of chromosome assembly. Intriguingly, it has been reported that chromosome condensation in early prophase, but not that in late prophase, can be reversed by inducing a DNA damage (Rieder and Cole, 1998) and that cyclin A-cdk may be the target of this “prophase checkpoint” (Furuno et al., 1999). The sequential phosphorylation/targeting model for the action of condensins described above nicely fits the previous proposals and further predicts that targeting of condensin I to chromosomes may represent one of the key events that makes the final commitment to mitosis (Pines and Rieder, 2001). Clearly, future work is required to test and refine this model.
Figure 7.
Models. (A) Sequential phosphorylation/targeting model for the action of condensins. In G2 phase, condensin II localizes to the nucleus, whereas condensin I is sequestered in the cytoplasm. In prophase, phosphorylation of condensin II by cyclin A-cdk (cycA) initiates structural changes of chromosomes within the nucleus. Cyclin B (cycB) is transported into the nucleus and forms an active cyclin B-cdk1 complex to trigger nuclear envelope breakdown (NEBD). This allows condensin I to access the chromosomes in prometaphase, and the two condensin complexes, phosphorylated by cyclin B-cdk1, cooperate to support later stages of chromosome assembly from metaphase through anaphase. In telophase, condensin I is exported out of the reassembling nucleus whereas condensin II remains in the nucleus. (B) A model for the distinct distributions of condensin I (blue) and condensin II (magenta) along the chromosome arm and at the centromere/kinetochore region. The inner and outer kinetochore plates are shown by light and dark green, respectively. The back-to-back orientation of the kinetochores is compromised in condensin-depleted chromosomes (inset, left). Depletion of Aurora B causes disruption of the specialized geometry of condensins I and II at the centromere/kinetochore region (inset, right).
Global and Region-specific Localization of Condensins I and II in Metaphase Chromosomes
Deconvolution analysis of metaphase chromosomes labeled with antibodies against condensins I and II confirms their distinct distribution along the chromatid axis that we described previously (Ono et al., 2003). More importantly, it unveils a unique organization of the two condensin complexes at the centromere/kinetochore region (Figure 7B). The axial distribution of condensin I at this region is less coiled than that at the arm region, often displaying a pair of loosely juxtaposed lines. A subpopulation of condensin II is concentrated on paired spots that lie close to the inner kinetochore plate and are located on the outer sides of the two condensin I-labeled lines. Such enrichment of condensin II at the kinetochore region is not unique to HeLa cells but is also found in X. laevis, both in somatic chromosomes (Ono, unpublished data) and embryonic chromosomes assembled in the cell-free extracts (Losada, unpublished data). We propose that this specific arrangement of condensins I and II provides a structural basis for building centromeric chromatin and plays an important role in establishing and/or stabilizing the back-to-back orientation of the sister kinetochores (see below).
It has been reported that Aurora B is required for chromosomal association of some of the condensin subunits in Drosophila (Giet and Glover, 2001) and C. elegans (Hagstrom et al., 2002; Kaitna et al., 2002). In contrast, other studies have shown that depletion or inhibition of Aurora B has little, if any, impact on bulk association of the SMC subunits with chromosomes in yeast and vertebrate cells (Losada et al., 2002; MacCallum et al., 2002; Hauf et al., 2003; Lavoie et al., 2004). Our current work extends this conclusion and demonstrates that neither condensin I nor condensin II requires Aurora B for localization to the chromosome arm. We find, however, that depletion of Aurora B disrupts the unique geometry of condensins I and II at the centromere/kinetochore region and causes loss of the primary constriction in metaphase chromosomes. Such a drastic effect on chromosome structure was surprising although it had been shown that dominant negative inhibition or depletion of aurora B delocalizes some proteins from the centromere/kinetochore region (Murata-Hori and Wang, 2002; Andrews et al., 2004). Conversely, depletion of condensin subunits barely affected the enrichment of Aurora B at the pericentromeric region (Supplemental Figure S2). It is possible that Aurora B directly phosphorylates condensin subunits in a region-specific manner and helps establish the structural integrity of the centromere/kinetochore region. In fact, one of the condensin subunits (Ycg1/CAP-G) is phosphorylated in an Ipl1/aurora-dependent manner in S. cerevisiae, although the functional significance of this phosphorylation remains unknown (Lavoie et al., 2004). Alternatively, the specialized distribution of condensins may be regulated indirectly by other substrates of Aurora B functioning at this region, such as topoisomerase II (Morrison et al., 2002) and CENP-A (Zeitlin et al., 2001; Kunitoku et al., 2003).
Defects in Kinetochore Structure and Function in Condensin-depleted Cells
One prominent phenotype observed in condensin-deficient cells in many different organisms is the massive amount of chromosome bridges in anaphase (Saka et al., 1994; Bhat et al., 1996; Steffensen et al., 2001; Coelho et al., 2003; Hudson et al., 2003). Kinetochores seem to be functional in these cells, attempting to pull chromatin to the opposite poles without success. It is reasonable to assume that the primary source of this phenotype is poor resolution and/or abnormal compaction of sister chromatids in the preceding metaphase. In striking contrast, the most frequently detected phenotype in the current study was the defect in metaphase chromosome alignment (the type I spindle defect), which cannot be easily explained by the model described above. Several lines of evidence support the idea that the primary source of this alignment defect is structural and functional distortions of the centromere/kinetochore region. First, condensins I and II are arranged into a very specific and unique geometry in this region. Notably, condensin II seems to underlie the inner kinetochore plate or be a part of it. Second, depletion of condensin subunits induces structural alterations in the centromere/kinetochore region and causes aberrant interactions with the mitotic spindle. The stretched kinetochores observed in the type I spindle defect are likely to be caused by their merotelic attachment to microtubules. This mode of attachment would be another source of lagging chromosomes in anaphase as has been demonstrated previously (Cimini et al., 2001, 2002). On the other hand, the type II spindle defects may be produced by syntelic or monotelic attachments accompanied by a partial delay at a prometaphase-like stage. It remains to be determined why this phenotype is more frequently found in condensin II-depleted cells than in condensin I-depleted cells. Third and finally, chromosomes depleted of condensins fail to interact properly with monopolar spindles produced in the presence of monastrol. The defective phenotype reported here is distinct from that observed when the chromokinesin Kid is inhibited under a similar condition (Levesque and Compton, 2001), suggesting that polar ejection forces operate, at least partially, on the chromosome arm depleted of condensin subunits. Consistently, an apparently normal level of Kid was detectable in these chromosomes as judged by immunofluorescence (Ono, unpublished data).
To our knowledge, this is the first study to demonstrate that the two condensin complexes in vertebrate cells display a specialized geometry at the centromere/kinetochore region and play a crucial role in placing sister kinetochores into the back-to-back orientation. It should be mentioned, however, that some indications of kinetochore defects have previously been reported in other organisms such as S. cerevisiae (Lavoie et al., 2000; Ouspenski et al., 2000), C. elegans (Hagstrom et al., 2002; Stear and Roth, 2002), and X. laevis (Wignall et al., 2003). Among them, Stear and Roth (2002) described the most compelling example of merotelic attachment of C. elegans chromosomes deficient in Hcp6, one of the non-SMC subunits of condensin II. Because the chromosomes in this organism seem to lack condensin I (Ono et al., 2003) and have a very unique holocentric structure, it is not straightforward to compare this work with our current study in HeLa cells. It is nevertheless intriguing to note that the condensin II subunits in C. elegans become concentrated at the centromere/kinetochore region by metaphase in an Aurora B-dependent manner (Hagstrom et al., 2002; Kaitna et al., 2002; Stear and Roth, 2002). Thus, the C. elegans chromosomes have many molecular properties in common with the mammalian centromeres. Structural and functional comparison of monocentric and holocentric chromosomes in the future would undoubtedly help enhance our understanding of chromosome biology.
Supplementary Material
Acknowledgments
We are grateful to J. Gannon/T. Hunt (Cancer Research UK, London, England), T.J. Yen (Fox Chase Cancer Center, Philadelphia, PA), D. Compton (Dartmouth Medical School, Hanover, NH), and Y. Muro (Nagoya University, Nagoya, Japan) for antibodies. We thank A. Losada (Cold Spring Harbor Laboratory) for preparing antibodies against human CENP-A and Aurora B, J. Swedlow (University of Dundee, Dundee, United Kingdom) for the sequence information of Aurora B siRNA, and members of the Hirano laboratory for critically reading the manuscript. T.O. is on leave from Aichi Human Service Center, Japan, and is supported in part by the Mochida Memorial Foundation for Medical and Pharmaceutical Research and the Robertson Research Fund. This work was supported by grants from the National Institutes of Health to D.L.S. and T.H.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-03-0242. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-03-0242.
Abbreviations used: CAP, chromosome-associated polypeptide; siRNA, small interfering RNA; SMC, structural maintenance of chromosomes.
Online version of this article contains supporting material. Online version is available at www.molbiolcell.org.
References
- Andrews, P.D., Knatko, E., Moore, W.J., and Swedlow, J.R. (2003). Mitotic mechanics: the auroras come into view. Curr. Opin. Cell Biol. 15, 672-683. [DOI] [PubMed] [Google Scholar]
- Andrews, P.D., Ovechkina, Y., Morrice, N., Wagenbach, M., Duncan, K., Wordeman, L., and Swedlow, J.R. (2004). Aurora B regulates MCAK at the mitotic centromere. Dev. Cell 6, 253-268. [DOI] [PubMed] [Google Scholar]
- Aono, N., Sutani, T., Tomonaga, T., Mochida, S., and Yanagida, M. (2002). Cnd2 has dual roles in mitotic condensation and interphase. Nature 417, 197-202. [DOI] [PubMed] [Google Scholar]
- Ball, Jr., A.R., Schmiesing, J.A., Zhou, C., Gregson, H.C., Okada, Y., Doi, T., and Yokomori, K. (2002). Identification of a chromosome-targeting domain in the human condensin subunit CNAP1/hCAP-D2/Eg7. Mol. Cell. Biol. 22, 5769-5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazett-Jones, D.P., Kimura, K., and Hirano, T. (2002). Efficient supercoiling of DNA by a single condensin complex as revealed by electron spectroscopic imaging. Mol. Cell 9, 1183-1190. [DOI] [PubMed] [Google Scholar]
- Bhalla, N., Biggins, S., and Murray, A.W. (2002). Mutation of YCS4, a budding yeast condensin subunit, affects mitotic and nonmitotic chromosome behavior. Mol. Biol. Cell 13, 632-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat, M.A., Philp, A.V., Glover, D.M., and Bellen, H.J. (1996). Chromatid segregation at anaphase requires the barren product, a novel chromosome associated protein that interacts with topoisomerase II. Cell 87, 1103-1114. [DOI] [PubMed] [Google Scholar]
- Carmena, M., and Earnshaw, W.C. (2003). The cellular geography of aurora kinases. Nat. Rev. Mol. Cell. Biol. 4, 842-854. [DOI] [PubMed] [Google Scholar]
- Chen, H., Hughs, D.D., Chan, T.A., Sedat, J.W., and Agard, D.A. (1996). IVE (Image Visualization Environment): a software platform for all three-dimensional microscopy applications. J. Struct. Biol. 116, 56-60. [DOI] [PubMed] [Google Scholar]
- Cimini, D., Fioravanti, D., Salmon, E.D., and Degrassi, F. (2002). Merotelic kinetochore orientation versus chromosome mono-orientation in the origin of lagging chromosomes in human primary cells. J. Cell Sci. 115, 507-515. [DOI] [PubMed] [Google Scholar]
- Cimini, D., Howell, B., Maddox, P., Khodjakov, A., Degrassi, F., and Salmon, E.D. (2001). Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells. J. Cell Biol. 153, 517-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho, P., Queiroz-Mechado, J., and Sunkel, C.E. (2003). Condensin-dependent localisation of topoisomerase II to an axial chromosomal structure is required for sister chromatid resolution during mitosis. J. Cell Sci. 116, 4763-4776. [DOI] [PubMed] [Google Scholar]
- Elbashir, S.M., Harborth, J., Lendeckel, W., Yalcin, A., Weber, K., and Tuschl, T. (2001). Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411, 494-498. [DOI] [PubMed] [Google Scholar]
- Freeman, L., Aragon-Alcaide, L., and Strunnikov, A.V. (2000). The condensin complex governs chromosome condensation and mitotic transmission of rDNA. J. Cell Biol. 149, 811-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuno, N., den Elzen, N., and Pines, J. (1999). Human cyclin A is required for mitosis until mid prophase. J. Cell Biol. 147, 295-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giet, R., and Glover, D.M. (2001). Drosophila Aurora B kinase is required for histone H3 phosphorylation and condensin recruitment during chromosome condensation and to organize the central spindle during cytokinesis. J. Cell Biol. 152, 669-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagstrom, K.A., Holmes, V.F., Cozzarelli, N.R., and Meyer, B.J. (2002). C. elegans condensin promotes mitotic chromosome architecture, centromere organization, and sister chromatid segregation during mitosis and meiosis. Genes Dev. 16, 729-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagstrom, K.A., and Meyer, B.J. (2003). Condensin and cohesin: more than chromosome compactor and glue. Nat. Rev. Genet. 4, 520-534. [DOI] [PubMed] [Google Scholar]
- Hauf, S., Cole, R.W., LaTerra, S., Zimmer, C., Schnapp, G., Walter, R., Heckel, A., van Meel, J., Rieder, C.L., and Peters, J.-M. (2003). The small molecule Hesperadin reveals a role for aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J. Cell Biol. 161, 281-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano, T. (2002). The ABCs of SMC proteins: two-armed ATPases for chromosome condensation, cohesion and repair. Genes Dev. 16, 399-414. [DOI] [PubMed] [Google Scholar]
- Hirano, T., Kobayashi, R., and Hirano, M. (1997). Condensins, chromosome condensation protein complexes containing XCAP-C, XCAP-E and a Xenopus homolog of the Drosophila Barren protein. Cell 89, 511-521. [DOI] [PubMed] [Google Scholar]
- Hirano, T., and Mitchison, T.J. (1994). A heterodimeric coiled-coil protein required for mitotic chromosome condensation in vitro. Cell 79, 449-458. [DOI] [PubMed] [Google Scholar]
- Hudson, D.F., Vagnarelli, P., Gassmann, R., and Earnshaw, W.C. (2003). Condensin is required for nonhistone protein assembly and structural integrity of vertebrate chromosomes. Dev. Cell 5, 323-336. [DOI] [PubMed] [Google Scholar]
- Jessberger, R. (2002). The many functions of SMC proteins in chromosome dynamics. Nat. Rev. Mol. Cell. Biol. 3, 767-778. [DOI] [PubMed] [Google Scholar]
- Kaitna, S., Pasierbek, P., Jantsch, M., Loidl, J., and Glotzer, M. (2002). The aurora B kinase, AIR-2, regulates kinetochores during mitosis and is required for separation of homologous chromosomes during meiosis. Curr. Biol. 12, 798-812. [DOI] [PubMed] [Google Scholar]
- Kimura, K., Cuvier, O., and Hirano, T. (2001). Chromosome condensation by a human condensin complex in Xenopus egg extracts. J. Biol. Chem. 276, 5417-5420. [DOI] [PubMed] [Google Scholar]
- Kimura, K., and Hirano, T. (1997). ATP-dependent positive supercoiling of DNA by 13S condensin: a biochemical implication for chromosome condensation. Cell 90, 625-634. [DOI] [PubMed] [Google Scholar]
- Kimura, K., and Hirano, T. (2000). Dual roles of the 11S regulatory subcomplex in condensin functions. Proc. Natl. Acad. Sci. USA 97, 11972-11977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, K., Hirano, M., Kobayashi, R., and Hirano, T. (1998). Phosphorylation and activation of 13S condensin by Cdc2 in vitro. Science 282, 487-490. [DOI] [PubMed] [Google Scholar]
- Kunitoku, N., Sasayama, T., Marumoto, T., Zhang, D., Honda, S., Kobayashi, O., Hatakeyama, K., Ushio, Y., Saya, H., and Hirota, T. (2003). CENP-A phosphorylation by Aurora A in prophase is required for enrichment of Aurora B at inner centromeres and for kinetochore function. Dev. Cell 5, 853-864. [DOI] [PubMed] [Google Scholar]
- Lavoie, B.D., Hogan, E., and Koshland, D. (2002). In vivo dissection of the chromosome condensation machinery: reversibility of condensation distinguishes contributions of condensin and cohesin. J. Cell Biol. 156, 805-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie, B.D., Hogan, E., and Koshland, D. (2004). In vivo requirements for rDNA chromosome condensation reveal two cell-cycle-regulated pathways for mitotic chromosome folding. Genes Dev. 18, 76-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie, B.D., Tuffo, K.M., Oh, S., Koshland, D., and Holm, C. (2000). Mitotic chromosome condensation requires Brn1p, the yeast homolog of Barren. Mol. Biol. Cell 11, 1293-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque, A.A., and Compton, D.A. (2001). The chromokinesin kid is necessary for chromosome arm orientation and oscillation, but not congression, on mitotic spindles. J. Cell Biol. 154, 1135-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb, J.D., Albrecht, M.R., Chuang, P.-T., and Meyer, B.J. (1998). MIX-1, an essential component of the C. elegans mitotic machinery executes X-chromosome dosage compensation. Cell 92, 265-277. [DOI] [PubMed] [Google Scholar]
- Losada, A., Hirano, M., and Hirano, T. (2002). Cohesin release is required for sister chromatid resolution, but not for condensin-mediated compaction, at the onset of mitosis. Genes Dev. 16, 3004-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCallum, D.E., Losada, A., Kobayashi, R., and Hirano, T. (2002). ISWI remodeling complexes in Xenopus egg extracts: identification as major chromosomal components that are regulated by INCENP-aurora B. Mol. Biol. Cell 13, 25-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machin, F., Paschos, K., Jarmuz, A., Torres-Rosell, J., Pade, C., and Aragon, L. (2004). Condensin regulates rDNA silencing by modulating nucleolar Sir2p. Curr. Biol. 14, 125-130. [PubMed] [Google Scholar]
- Mayer, T.U., Kapoor, T.M., Haggarty, S.J., King, R.W., Shchreiber, S.L., and Mitchison, T.J. (1999). Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science 286, 971-974. [DOI] [PubMed] [Google Scholar]
- Morrison, C., Henzing, A.J., Jensen, O.N., Osheroff, N., Dodson, H., Kandels-Lewis, S.E., Adams, R.R., and Earnshaw, W.C. (2002). Proteomitc analysis of human metaphase chromosomes reveals topoisomerase II α as an Aurora B substrate. Nucleic Acids Res. 30, 5318-5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata-Hori, M., and Wang, Y.L. (2002). The kinase activity of Aurora B is required for kinetochore-microtubule interactions during mitosis. Curr. Biol. 12, 894-899. [DOI] [PubMed] [Google Scholar]
- Muro, Y., Sugimoto, K., Okazaki, T., and Ohashi, M. (1990). The heterogeneity of anti-centromere antibodies in immunoblotting analysis. J. Rheumatol. 17, 1042-1047. [PubMed] [Google Scholar]
- Nasmyth, K. (2002). Segregating sister genomes: the molecular biology of chromosome separation. Science 297, 559-565. [DOI] [PubMed] [Google Scholar]
- Neuwald, A.F., and Hirano, T. (2000). HEAT repeats associated with condensins, cohesins and other chromosome-related complexes. Genome Res. 10, 1445-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono, T., Losada, A., Hirano, M., Myers, M.P., Neuwald, A.F., and Hirano, T. (2003). Differential contributions of condensin I and condensin II to mitotic chromosome architecture in vertebrate cells. Cell 115, 109-121. [DOI] [PubMed] [Google Scholar]
- Ouspenski, I.I., Cabello, O.A., and Brinkley, B.R. (2000). Chromosome condensation factor Brn1p is required for chromatid separation in mitosis. Mol. Biol. Cell 11, 1305-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer, D.K., O'Day, K., Trong, H.L., Charbonneau, H., and Margolis, R.L. (1991). Purification of the centromere-specific protein CENP-A and demonstration that it is a distinctive histone. Proc. Natl. Acad. Sci. USA 88, 3734-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines, J., and Hunter, T. (1991). Human cyclins A and B1 are differentially located in the cell and undergo cell cycle-dependent nuclear transport. J. Cell Biol. 115, 1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines, J., and Rieder, C.L. (2001). Re-staging mitosis: a contemporary view of mitotic progression. Nat. Cell Biol. 3, E3-E6. [DOI] [PubMed] [Google Scholar]
- Rieder, C.L., and Cole, R.W. (1998). Entry into mitosis in vertebrate somatic cells is guarded by a chromosome damage checkpoint that reverses the cell cycle when triggered during early but not late prophase. J. Cell Biol. 142, 1013-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh, N., Goldberg, I.G., Wood, E.R., and Earnshaw, W.C. (1994). ScII: an abundant chromosome scaffold protein is a member of a family of putative ATPases with an unusual predicted tertiary structure. J. Cell Biol. 127, 303-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saka, Y., Sutani, T., Yamashita, Y., Saitoh, S., Takeuchi, M., Nakaseko, Y., and Yanagida, M. (1994). Fission yeast cut3 and cut14, members of a ubiquitous protein family, are required for chromosome condensation and segregation in mitosis. EMBO J. 13, 4938-4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleiffer, A., Kaitna, S., Maurer-Stroh, S., Glotzer, M., Nasmyth, K., and Eisenhaber, F. (2003). Kleisins: a superfamily of bacterial and eukaryotic SMC protein partners. Mol. Cell 11, 571-575. [DOI] [PubMed] [Google Scholar]
- Schmiesing, J.A., Gregson, H.C., Zhou, S., and Yokomori, K. (2000). A human condensin complex containing hCAP-C-hCAP-E and CNAP1, a homolog of Xenopus XCAP-D2, colocalizes with phosphorylated histone H3 during the early stage of mitotic chromosome condensation. Mol. Cell. Biol. 20, 6996-7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somma, M.P., Fasulo, B., Siriaco, G., and Centi, G. (2003). Chromosome condensation defects in barren RNA-interfered Drosophila cells. Genetics 165, 1607-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stear, J.H., and Roth, M.B. (2002). Characterization of HCP-6, a C. elegans protein required to prevent chromosome twisting and merotelic attachment. Genes Dev. 16, 1498-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen, S., Coelho, P.A., Cobbe, N., Vass, S., Costa, M., Hassan, B., Prokopenko, S.N., Bellen, H., Heck, M.M.S., and Sunkel, C.E. (2001). A role for Drosophila SMC4 in the resolution of sister chromatids in mitosis. Curr. Biol. 11, 295-307. [DOI] [PubMed] [Google Scholar]
- Strunnikov, A.V., Hogan, E., and Koshland, D. (1995). SMC2, a Saccharomyces cerevisiae gene essential for chromosome segregation and condensation, defines a subgroup within the SMC family. Genes Dev. 9, 587-599. [DOI] [PubMed] [Google Scholar]
- Sutani, T., Yuasa, T., Tomonaga, T., Dohmae, N., Takio, K., and Yanagida, M. (1999). Fission yeast condensin complex: essential roles of non-SMC subunits for condensation and cdc2 phosphorylation of Cut3/SMC4. Genes Dev. 13, 2271-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedlow, J.R., and Hirano, T. (2003). The making of the mitotic chromosome: modern insights into classical questions. Mol. Cell 11, 557-569. [DOI] [PubMed] [Google Scholar]
- Wignall, S.M., Deehan, R., Maresca, T.J., and Heald, R. (2003). The condensin complex is required for proper spindle assembly and chromosome segregation in Xenopus egg extracts. J. Cell Biol. 161, 1041-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen, T.J., Compton, D.A., Wise, D., Zinkowski, R.P., Brinkley, B.R., Earnshaw, W.C., and Cleveland, D.W. (1991). CENP-E, a novel human centromere-associated protein required for progression from metaphase to anaphase. EMBO J. 10, 1245-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeong, F.M., et al. (2003). Identification of a subunit of a novel kleisin-beta/SMC complex as a potential substrate of protein phosphatase 2A. Curr. Biol. 13, 2058-2064. [DOI] [PubMed] [Google Scholar]
- Zeitlin, S.G., Shelby, R.D., and Sullivan, K.F. (2001). CENP-A is phosphorylated by Aurora B kinase and plays an unexpected role in completion of cytokinesis. J. Cell Biol. 155, 1147-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.