Abstract
Excessive extracellular matrix degradation caused by the hyperfunction of matrix metalloproteinases (MMPs) has been implicated in the failure of pressure ulcers healing. EMMPRIN, as a widely expressed protein, has emerged as an important regulator of MMP activity. We hypothesize that EMMPRIN affects the process of pressure ulcer healing by modulating MMP activity. In the rat pressure ulcer model, the expression of EMMPRIN in ulcers detected by Western blot was elevated compared with that observed in normal tissue. To investigate the role of EMMPRIN in regulating ulcer healing, specific antibodies against EMMPRIN were used via direct administration on the pressure ulcer. Local blockage of EMMPRIN resulted in a poor ulcer healing process compared with control ulcers, which was the opposite of our expectation. Furthermore, inhibiting EMMPRIN minimally impacted MMP activity. However, the collagen content in the pressure ulcer was reduced in the EMMPRIN treated group. Angiogenesis and the expression of angiogenic factors in pressure ulcers were also reduced by EMMPRIN local blockage. The results in the present study indicate a novel effect of EMMPRIN in the regulation of pressure ulcer healing by controlling the collagen contents and angiogenesis rather than MMPs activity.
Keywords: Pressure ulcer, EMMPRIN, CD147, matrix metalloproteinases, collagen, angiogenesis
Introduction
Pressure ulcers are common in the aging and paralyzed populations. Conventional treatments of pressure ulcers focus on prevention, and the external interventions focus on the wound-healing process [1]. However, as chronic wounds, pressure ulcers show a wide complexity in the healing process. A successful wound healing process involves inflammation, cell proliferation, extracellular matrix (ECM) deposition and remodeling [2]. The degradation of the extracellular matrix (ECM) relay affects the matrix metalloproteinases (MMPs), which are secreted by various cells types as proenzymes and are activated by membrane type MMPs or by serine proteases. However, excessive extracellular matrix degradation caused by the hyperfunction of MMPs has been implicated in the failure of pressure ulcer healing [3]. Therefore, alleviating the detrimental effects of MMPs may promote the healing process of pressure ulcers.
Extracellular matrix metalloproteinase inducer (EMMPRIN), also referred to CD147, is a widely expressed membrane protein [4]. It was initially found on the surface of tumor cells and is expressed on numerous cell types and serves as a cell surface receptor for multiple ligands, such as cyclophilin A, monocarboxylate transporter, integrins, and EMMPRIN itself. Binding to these various partners enables EMMPRIN to induce MMPs and mediate various functions [5]. Given the role of EMMPRIN in regulating MMP expression, we hypothesize that EMMPRIN could affect the process of pressure ulcer healing by modulating MMP activity. In the present study, we addressed whether EMMPRIN is expressed in pressure ulcers and whether anti-EMMPRIN intervention promotes wound healing in a rat model of chronic skin pressure ulcers.
Materials and methods
Animal model establishment
All animal experiments conformed to the Guide for the Care and Use of Laboratory Animals, published by the U.S. National Institute of Health (NIH Publication No. 85-23, revised 1996). A total of 48 male Sprague-Dawley rats (10-11 weeks old; 150-200 g; purchased from Chongqing, China) were used. Animals were randomized to an EMMPRIN antibody-treated group and a physiological saline-treated (control) group. The magnet compression model that was previously reported was used [6]. Briefly, rats were anesthetized by an intraperitoneal administration of 50 mg/kg pentobarbital. The dorsal hair was shaved, and the area was cleansed with 70% alcohol. A 3-cm full-thickness skin incision was made on each rat’s dorsal region, and an autoclaved magnet disk was placed under the skin caudal to the incision, where it was held in place by the surrounding fascia. The incision was closed with size 4/0 polysorb sutures. The rats were left to emerge from anesthesia and were individually housed in non-magnetic cages. After a constant 2 h of clamping with another magnet disk on the skin, the outer magnet was removed for 0.5 h, and the clamping/remove cycle was repeated 5 times daily for 5 consecutive days. The compressed area was left uncovered.
EMMPRIN antibody treatment
Anti-rat EMMPRIN antibody (Santa, Cruz, USA) was used for the antibody treatment regimens (diluted with autoclaved saline to 10 μg/ml). After 5 days of compressing, diluted antibody solution (0.1 ml) was injected intradermally around the wounds, and the wound surface were treated with topical spray (0.1 ml/cm2, twice per week, 3 weeks). The control group was given isopyknic saline. After 15 minutes of air-drying to allow the adsorption of the antibody, the wounds were dressed with a non-adherent dressing next to the wound surface, and dry gauze was used to fill the ulcer crater. At the end of treatment, animals were anesthetized, and the skin of the ulcer was harvested.
Evaluation of ulcer stage and ulcer closure percentage
Ulcer stage was evaluated according to the grading system of the National Pressure Ulcer Advisory Panel (NPUAP) [7]. In this system, pressure ulcers are classified as superficial pressure ulcers with intact skin (Grade 1); partial-thickness skin loss involving the epidermis, dermis, or both (Grade 2); full-thickness skin loss involving damage to subcutaneous tissue (Grade 3); or full-thickness skin loss with extensive destruction of the underlying tissue (Grade 4). The closed ulcers percentage at day 21 was counted in both the EMMPRIN antibody-treated and control groups. Closed ulcer percentage = (initial ulcer area-ulcer area on day 21)/initial ulcer area × 100%.
Evaluation of angiogenesis
The ulcer site on the skin was fixed and embedded in paraffin. Cross sections were stained with hematoxylin and eosin (HE). To evaluate angiogenesis, CD34 (a vascular endothelial cell marker, Santa Cruz) was used to detect ulcer tissues biopsied on day 7, 14 and 21 after the removal of compression. The microvessel density was evaluated by direct counting of the positive signal in every high power field (× 400) on 6 fields per slide.
Collection of wound fluids and biopsies
On day 14 after removing the compression, wound fluid exudates were collected from the dressing covering the ulcer and were frozen immediately at -80°C until being analyzed. A 4 mm punch biopsy of tissues was collected adjacent to the edge of the pressure ulcer and was stored at -80°C.
Estimation of the hydroxyproline content
Hydroxyproline content was analyzed on day 14 after removing the compression, as described earlier [8]. Briefly, homogenates of wounded tissue were hydrolyzed in 6 M HCl (50 mg tissue/1 ml 6 M HCl) at 100°C for 24 hours. The remaining HCl was neutralized with 2.5 M NaOH and was diluted 10-fold with deionized H2O. Chloramine T (0.05 M Sigma-Aldrich) solution was added to the neutralized/diluted solution isometric (100 ml), and the mixture was incubated for 20 minutes at room temperature. Then, 100 ml of perchloric acid solution (3.15 M) was added and incubated for 5 minutes at room temperature. A volume of 100 mL of 20% P-dimethylaminobenzaldehyde (Sigma-Aldrich) was added, and the final mixture was incubated for 20 min at 60°C. Absorbance was measured at 560 nm using a microplate reader. The hydroxyproline content was determined according to a standard curve and was normalized to a milligram of wounded tissue homogenate for data analysis.
Gelatin zymogram analysis
The activity of MMP-2 and MMP-9 in the fluid exudates and homogenized biopsies from pressure ulcers on day 14 after removing the compression were assessed using a gelatin zymogram, as described previously [9]. The protein concentration was determined using a BCA assay, and 50 µg of protein was loaded onto precast gelatin zymogram gel (7.5% Tris-glycine gels with 1 g/L gelatin). Following electrophoresis, the gels were washed with renaturing buffer (2.5% Triton X-100, w/v) for 30 minutes at 370°C, and the gels were then incubated with developing buffer (50 mM Tris-HCl, 200 mM NaCl, 5 mM CaCl2, 0.2% Brij 35, w/v) on a rotary shaker at 370°C for 24 hours to allow the MMPs to digest. The gels were stained with 0.1% Coomassie blue R-250 and then destained with 12.5% trichloroacetic acid. Then, the gel was visualized under a chemiluminescence imaging system (Bio-Rad, USA), and images were captured.
Western blot analysis
The expression of EMMPEIN, VEGF, and PDGF-A in pressure ulcers was assessed by western blot. The protein concentration was determined using a BCA assay, and 50 µg samples were separated on 10% and 15% Tris-glycine gels (Beyotime, China) and were transferred to PVDF membranes (Millipore). Membranes were probed with antibodies against EMMPRIN (1:200), VEGF (1:500), PDGF-A (1:200) and β-actin (1:1000) overnight at 4°C, followed by 1 h incubation with HRP-conjugated secondary antibody, and were visualized with enhanced chemiluminescence following the manufacturer’s instructions. Densitometric signals were quantified by Quantity One software.
Statistical analysis
The results are expressed as the mean ± SE. SPSS16.0 software was used for statistical analysis. The independent samples t test was used for quantitative continuous data, and the chi-square test was used for nominal data arising from pressure ulcer grade. Values of P < 0.05 were considered statistically significant.
Results
Expression of EMMPRIN was elevated in pressure ulcers
In preparation for our studies investigating pressure ulcers, rats underwent magnet compression on the skin. After the model was established, we assessed EMMPRIN expression in the pressure ulcer lesions. EMMPRIN was expressed in normal rat skin as detected by immunoblotting, and the EMMPRIN levels were increased substantially in the pressure ulcers of rat skin on day 7 after compression removal (Figure 1).
Figure 1.
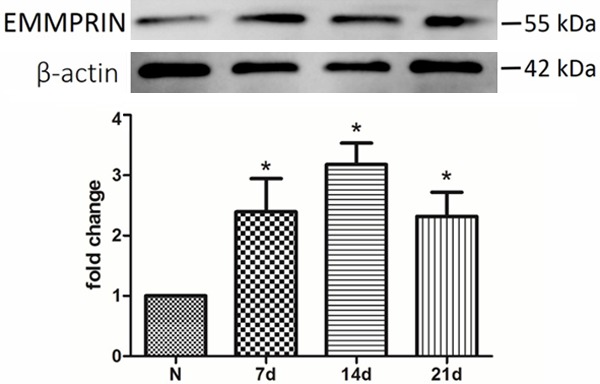
EMMPRIN expression was increased in pressure ulcers. Western blot analysis showed that the expression of EMMPRIN was detectable both in normal and pressure ulcer tissue and was significantly higher in pressure ulcers. N = 3, *P < 0.05 compared with normal tissue.
EMMPRIN antibody treatment prolonged ulcer healing
The grades of ulcers were determined according to the NPUAP grading system. As shown in Table 1, the ulcer grades reached the maximum at day 14 in both groups. Of the 48 ulcers, there were only 8 (16.7%) superficial ulcers (less than grade 2); however, only 3 (62.5%) of these were EMMPRIN antibody-treatment ulcers. In addition, the grades of ulcers in the EMMPRIN antibody-treated group were significantly higher than in the control group at days 14 and 21 (P < 0.05). On day 21, 31.2% (15) of ulcers were completely closed, 26.7% (4) of which were EMMPRIN antibody treated.
Table 1.
Grading of pressure ulcers according to the NPUAP pressure ulcer grading system
| Days after the removal of compression | Ulcer grade | |
|---|---|---|
|
| ||
| Control | EMMPRIN antibody treated | |
| 1 | 1.38 ± 0.49 (n = 24) | 1.42 ± 0.50 (n = 24) |
| 3 | 1.58 ± 0.58 (n = 24) | 1.54 ± 0.72 (n = 24) |
| 7 | 1.70 ± 0.69 (n = 24) | 1.58 ± 0.72 (n = 24) |
| 14 | 3.08 ± 1.06 (n = 24) | 3.84 ± 1.01 (n = 24)* |
| 21 | 0.92 ± 0.50 (n = 24) | 2.62 ± 0.58 (n = 24)* |
Data were presented as mean ± SE.
P < 0.05 compared with control.
EMMPRIN antibody treatment reduced angiogenesis in the ulcers
In view of angiogenesis playing an important role in the wound healing process, we evaluated the microvessel density in ulcer tissues by immunohistochemistry using an antibody targeted to vascular endothelial cells marker CD 34 (Figure 2). EMMPRIN antibody-treated ulcers showed lower angiogenesis than that of the controls on days 7, 14 and 21 (Table 2).
Figure 2.
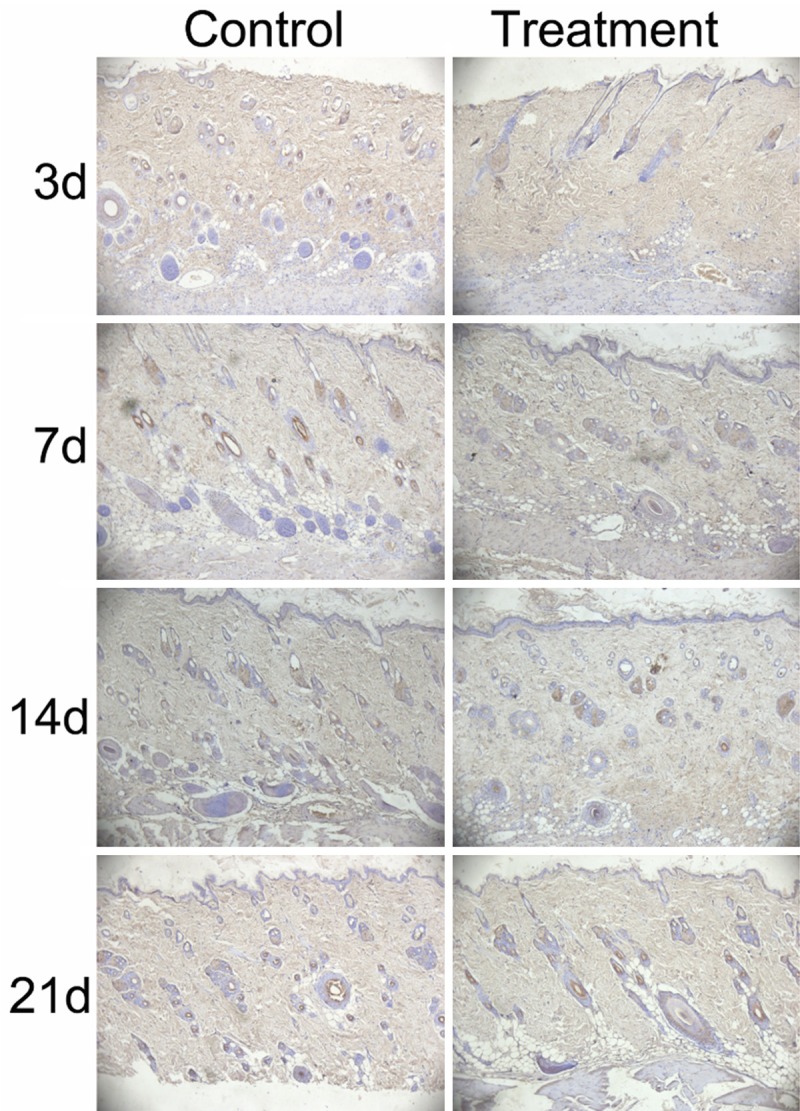
EMMPRIN antibody treatment reduced angiogenesis in the ulcers (Left): Representative angiogenesis of control ulcers by endothelial cell marker (CD34) immunohistochemistry; (Right): Representative angiogenesis of EMMPRIN antibody treatment ulcers. Magnification = × 100.
Table 2.
Microvessel density of pressure ulcers after the removal of compression
| Group | 3 d | 7 d | 14 d | 21 d |
|---|---|---|---|---|
| Control | 21.36 ± 5.32 | 47.38 ± 6.12 | 62.48 ± 7.25 | 49.46 ± 6.39 |
| EMMPRIN antibody treatment | 19.24 ± 4.63 | 24.68 ± 5.38* | 32.38 ± 6.84* | 28.46 ± 8.37* |
Data were presented as mean ± SE.
P < 0.05 compared with control.
EMMPRIN antibody treatment did not affect the content of collagen in the pressure ulcers
Collagen biosynthesis in the pressure ulcers was assessed by detecting the hydroxyproline content. The results showed that there was no significant difference of hydroxyproline content in the EMMPRIN antibody treated group and the control group (9.26 ± 2.14 vs. 8.74 ± 1.68 μg/mg wounded tissue homogenate).
EMMPRIN antibody treatment did not affect the activity of gelatinase in pressure ulcers
The results of the gelatinases spectrum analysis showed that the activity of MMP-2 and MMP-9 in the homogenates of wounds biopsied from pressure ulcers were increased compared to the homogenates from normal tissue, and the activity reached the maximum on day 14 (Figure 3A). However, on day 14 after removal of compression, the activity of MMP-2 and MMP-9 of wounds biopsied from pressure ulcers in the EMMPRIN antibody treated group was slightly lower than that of the control group, but the difference was not statistically significant (Figure 3B). Accordingly, the activity of MMP-9 in wound fluid exudates of the EMMPRIN antibody treated group was slightly higher than that in the control group, but the difference was not statistically significant. MMP-2 was not detected in the wound fluid exudates (Figure 3C).
Figure 3.
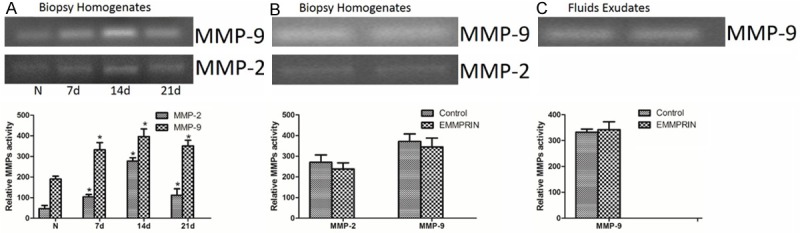
EMMPRIN antibody did not affect the activity of gelatinase in pressure ulcers. The gelatinases spectrum analysis was used to determine the activity of MMP-9 and MMP-2. The intensity of the band was quantified. A: The activity of MMP-2 and MMP-9 was gradually increased in pressure ulcers compared to normal tissue. B: The activity of gelatinase in biopsy homogenates had no significant difference between EMMPRIN antibody treatment and the control group. C: The activity of MMP-9 in fluid exudates had no significant difference between EMMPRIN antibody treatment and the control group; the activity of MMP-2 in fluid exudates was not detectable. N = 3,*P < 0.05 compared with normal tissue.
EMMPRIN antibody treatment inhibited the expression of angiogenic factors
As mentioned above, EMMPEIN antibody treated ulcers showed lower angiogenesis, so we observed the level of angiogenic factors (VEGF and PDGF-A) in the pressure ulcers. The western blot analysis showed that the expression of VEGF and PDGF-A gradually increased over time in the homogenates of wounds biopsied from pressure ulcers compared to the homogenates from normal tissue (Figure 4). However, on day 14 after removal of compression, the expression of VEGF and PDGF-A in EMMPRIN antibody ulcers was significantly lower compared to the control group.
Figure 4.
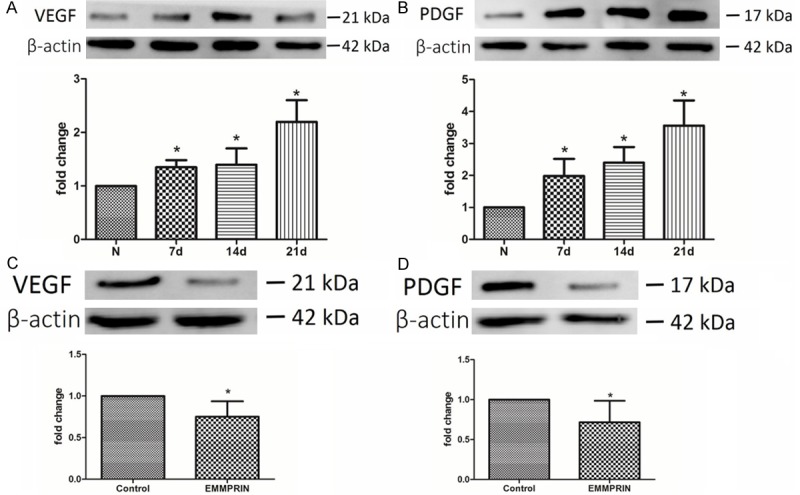
EMMPRIN antibody inhibited the expression of VEGF and PDGF-A. A, B: Western blot analysis showed that the expression of VEGF and PDGD-A in pressure was increased along with the pressure ulcer healing compare to normal tissue. N = 3, *P < 0.05 compared with normal tissue. C, D: On day 14 after the removal of compression, the expression levels of VEGF and PDGD-A were lower in the EMMPRIN treated group. N = 3, *P < 0.05 compared with control.
Discussion
EMMPRIN has emerged as an important regulator for MMPs activity in cancer progression. Considering the correlation of MMPS with wound healing, we hypothesize that EMMPRIN is involved in pressure ulcer healing. The present data showed the first evidence that 1) EMMPRIN expression was up-regulated in pressure ulcers; 2) inhibiting EMMPRIN activity by specific antibody prolongs the pressure ulcer healing; 3) inhibiting EMMPRIN activity reduces the collagen content in pressure ulcers; and 4) inhibiting EMMPRIN activity also reduces the angiogenesis and the level of angiogenic factors. Together, our data implicate an EMMPRIN correlative with pressure ulcer healing.
Despite research and hypotheses [10] indicating that EMMPRIN may be involved in wound healing, evidence from in vivo studies is still lacking. In this study, we found that in a rat pressure ulcer model, the expression of EMMPRIN was up-regulated along with the process of ulcer healing. The results are consistent with previous investigative studies in chronic ulcerated corneas [10].
Because there is no EMMPRIN null animal model, specific antibodies against EMMPRIN are widely used to block EMMPRIN function in many pathogeneses, such as cancer [11], asthma [12], and multiple sclerosis [13]. Thus, to investigate the role of EMMPRIN in regulating pressure ulcer healing, we used EMMPRIN antibody to inhibit the activity of this protein. We hypothesized that inhibiting EMMPRIN would promote the pressure ulcer healing by reducing the MMP activity. However, pressure ulcers receiving EMMPRIN antibody treatment exhibited poor healing quality. Furthermore, EMMPRIN antibody treatment did not affect the activity of MMP-2 and MMP-9. MMP expression can be induced in several cell types (keratinocytes, fibroblasts, endothelial cells and in inflammatory cells, such as monocytes, lymphocytes and macrophages) in response to cytokines, hormones, oncogenes and cell contact with ECM or other cell types [14]. In the process of pressure ulcer healing, most of the cell types mentioned above, such as fibroblasts, monocytes, lymphocytes and macrophages, were activated by soluble cytokines/growth factors; thus, the main regulating mechanism of MMPs in pressure ulcers may rely on the soluble factors rather than EMMPRIN, which is why EMMPRIN antibody treatment only minimally impacted MMPs activity.
Collagen content is a wisely used indicator for wound healing quality; it is primarily determined by the balance of synthesis and degradation. In this present study, we found that EMMPRIN antibody treatment reduced the content of collagen in pressure ulcers, independent of MMP activity, which was implicated in collagen degradation. Thus, EMMPRIN may play a role in regulating the collagen content by controlling the collagen synthesis process. However, more research is required to determine the exact relationship between EMMPRIN and collagen metabolism.
Angiogenesis is another indicator of wound healing. In the present study, blocking EMMPRIN significantly reduced the microvessel density in pressure ulcers; accordingly, expression of angiogenic growth factors (VEGF and PDGF-A) also decreased. Thus, the possible mechanism by which EMMPRIN antibody treatment reduces angiogenesis is (at least partly) attributed to the impacted expression of these growth factors observed in the present study. Fibroblasts are the key original source of growth factors and collagen; we presume that EMMPRIN may participate in regulating fibroblast function. This finding agrees with the data of Huet et al. [10] that EMMPRIN promoted fibroblast differentiation, but more research is required to illuminate the relationship between EMMPRIN and these growth factors.
In conclusion, in the process of pressure healing, the expression of EMMPRIN was up regulated. Inhibiting EMMPRIN by a specific antibody reduced the collagen contents and angiogenesis in pressure ulcers but only minimally impacted MMP activity, which led to the poor healing quality. More study is required to illuminate the intrinsic mechanism of EMMPRIN in regulating collagen metabolism and angiogenesis, which has important implications for the control of pressure ulcer healing.
Acknowledgements
This study was supported in part by Fuling Science and Technology Commission project (FLKJ, 2012ABB1103).
Disclosure of conflict of interest
None.
References
- 1.Dini V, Bertone M, Romanelli M. Prevention and management of pressure ulcers. Dermatol Ther. 2006;19:356–364. doi: 10.1111/j.1529-8019.2006.00094.x. [DOI] [PubMed] [Google Scholar]
- 2.Hackam DJ, Ford HR. Cellular, biochemical, and clinical aspects of wound healing. Surg Infect (Larchmt) 2002;3(Suppl 1):S23–35. doi: 10.1089/sur.2002.3.s1-23. [DOI] [PubMed] [Google Scholar]
- 3.Norgauer J, Hildenbrand T, Idzko M, Panther E, Bandemir E, Hartmann M, Vanscheidt W, Herouy Y. Elevated expression of extracellular matrix metalloproteinase inducer (CD147) and membrane-type matrix metalloproteinases in venous leg ulcers. Br J Dermatol. 2002;147:1180–1186. doi: 10.1046/j.1365-2133.2002.05025.x. [DOI] [PubMed] [Google Scholar]
- 4.Biswas C, Zhang Y, DeCastro R, Guo H, Nakamura T, Kataoka H, Nabeshima K. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995;55:434–439. [PubMed] [Google Scholar]
- 5.Iacono KT, Brown AL, Greene MI, Saouaf SJ. CD147 immunoglobulin superfamily receptor function and role in pathology. Exp Mol Pathol. 2007;83:283–295. doi: 10.1016/j.yexmp.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stadler I, Zhang RY, Oskoui P, Whittaker MS, Lanzafame RJ. Development of a simple, noninvasive, clinically relevant model of pressure ulcers in the mouse. J Invest Surg. 2004;17:221–227. doi: 10.1080/08941930490472046. [DOI] [PubMed] [Google Scholar]
- 7.Lardenoye JW, Thiefaine JA, Breslau PJ. Assessment of incidence, cause, and consequences of pressure ulcers to evaluate quality of provided care. Dermatol Surg. 2009;35:1797–1803. doi: 10.1111/j.1524-4725.2009.01293.x. [DOI] [PubMed] [Google Scholar]
- 8.Woessner JF Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 9.Fini ME, Girard MT. The pattern of metalloproteinase expression by corneal fibroblasts is altered by passage in cell culture. J Cell Sci. 1990;97:373–383. doi: 10.1242/jcs.97.2.373. [DOI] [PubMed] [Google Scholar]
- 10.Huet E, Vallee B, Szul D, Verrecchia F, Mourah S, Jester JV, Hoang-Xuan T, Menashi S, Gabison EE. Extracellular matrix metalloproteinase inducer/CD147 promotes myofibroblast differentiation by inducing alpha-smooth muscle actin expression and collagen gel contraction: implications in tissue remodeling. FASEB J. 2008;22:1144–1154. doi: 10.1096/fj.07-8748com. [DOI] [PubMed] [Google Scholar]
- 11.Dean NR, Newman JR, Helman EE, Zhang W, Safavy S, Weeks DM, Cunningham M, Snyder LA, Tang Y, Yan L, McNally LR, Buchsbaum DJ, Rosenthal EL. Anti-EMMPRIN monoclonal antibody as a novel agent for therapy of head and neck cancer. Clin Cancer Res. 2009;15:4058–4065. doi: 10.1158/1078-0432.CCR-09-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gwinn WM, Damsker JM, Falahati R, Okwumabua I, Kelly-Welch A, Keegan AD, Vanpouille C, Lee JJ, Dent LA, Leitenberg D, Bukrinsky MI, Constant SL. Novel approach to inhibit asthma-mediated lung inflammation using anti-CD147 intervention. J Immunol. 2006;177:4870–4879. doi: 10.4049/jimmunol.177.7.4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agrawal SM, Silva C, Tourtellotte WW, Yong VW. EMMPRIN: a novel regulator of leukocyte transmigration into the CNS in multiple sclerosis and experimental autoimmune encephalomyelitis. J Neurosci. 2011;31:669–677. doi: 10.1523/JNEUROSCI.3659-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan C, Boyd DD. Regulation of matrix metalloproteinase gene expression. J Cell Physiol. 2007;211:19–26. doi: 10.1002/jcp.20948. [DOI] [PubMed] [Google Scholar]


