Abstract
Galectin-1 (GAL-1) belongs to the family of β-galactoside-binding lectins. It regulates cell-cell and cell-matrix interactions, the immune response, apoptosis, cell cycle, RNA splicing and neoplastic transformation. We investigate the effect of heart manipulation secondary to cardiac surgery on the level of GAL-1 in murine heart and plasma. Male C57B6/J mice were used for adopted model of cardiac surgery. Heart samples were processed for immunohistochemical and immunofluorescent labeling, Enzyme linked immunosorbent assay and quantitative RT-PCR to identify GAL-1 levels in the heart and plasma during the first 24 hours following cardiac surgery. There is significant increase of GAL-1 in the LV at 30 minutes (P<0.000), 60 minutes (P<0.001), 4 hour (P<0.003), and 24 hour (P<0.003) time points of surgically operated groups compared to non-operated control group, while GAL-1 mRNA levels in any of the surgically operated groups are not significantly different from the non-operated group suggesting extracardiac origin of this raise of GAL-1. There is significant increase of GAL-1 in the plasma at 30 minutes (P<0.000), 60 minutes (P<0.009), 4 hour (P<0.043), and 24 hour (P<0.000) time points of surgically operated groups compared to non-operated control group. In conclusion, GAL-1 is valuable biomarker of surgical stress.
Keywords: Heart, surgical stress, galectin-1
Introduction
Galectin-1 (GAL-1) belongs to the family of β-galactoside-binding lectins [1]. GAL-1 is a 14.5-kDa protein which forms lattice-like complexes with receptors that participate in recognition of cell-matrix [2-5]. GAL-1 regulates cell-cell and cell-matrix interactions, the immune response, apoptosis, cell cycle, RNA splicing and neoplastic transformation [5-11]. Intracellular GAL-1 has been shown to be present mainly in cells cytosol and less likely in nuclei depending upon various types and conditions of the cells [12]. GAL-1 is endogenous to the heart but its upregulation in the myocardium has been reported in literature following myocardial infarction [13,14]. Surgical stress is the body reaction to surgical manipulation and characterized by activation of the sympathetic nervous system, endocrine responses as well as immunological and haematological changes leading to disturbances of metabolic and physiological processes that induce changes in the acute phase, inflammatory, hormonal, and genomic responses of the body [15]. Open-heart surgical procedures are associated with an inflammatory response that is largely the result of surgical trauma [16,17]. In this study we investigate the effect of heart manipulation secondary to cardiac surgery on the level of GAL-1 in murine heart and plasma.
Material and methods
Animal groups
Male C57B6/J mice were used for the surgical procedure for the following time points: 30 minutes, 60 minutes, 4- hour and 24- hour post operation. Samples from non-operated animals (Naïve) were also studied. The Animal Research Ethics Committee of the College of Medicine and Health Sciences, UAE University have approved all experimental procedures; Protocol No.A12/10.
Murine surgical procedure
C57B6/J mice (male, age: 12-16 weeks; wt: 20-25 g) were anesthetized by an intraperitoneal injection of a combination of Ketamine (100 mg/kg) and Xylazine (10 mg/kg). The mice were then intubated and ventilated. We adopt a mouse model similar to open-heart surgery to fulfill the requirements for inclusion criteria of this project. The inclusion criteria includes opening of the chest wall, removal of pericardium, and one passing a stitch needle in the left ventricle (LV) and taking it out in intubated ventilated anaesthetized mice to be followed by closure of chest and skin and scarification at 30 minutes, 60 minutes, 4- hour and 24- hour postoperatively. The adopted surgical procedure is similar to the sham myocardial infarction surgery done on the mice as described elsewhere [18-20].
Briefly, the chest was opened with a lateral incision at the 4th intercostal space on the left side of the sternum and the chest wall was retracted. The pericardial sac was removed and an 8-0 silk suture was passed in the myocardium at the anterior surface of the heart for one second and then removed. The chest wall then was closed by approximating the third and fourth ribs with one or two interrupted sutures, the muscles returned back to their original position and the skin closed with 4-0 prolene suture. The animal was gently disconnected from the ventilator and spontaneous breathing was seen immediately. Postoperative analgesic (Butorphanol 2 mg/kg, s/c, 6 hourly) was given at the end of the procedure. According to the experimental protocol mice were sacrificed at desired time points. Blood was collected in EDTA vacutainers and the heart was resected, washed in ice cold phosphate buffered saline (PBS), the right ventricle and both atria were dissected away and LV was immediately frozen in liquid nitrogen and later stored in -80°C freezer. Collected blood was centrifuged at 3000 RPM for 15 minutes. The plasma was collected, alliquoted and stored at -80°C until further analysis. Heart samples from the same time points were also fixed in 10% buffered formal-saline for 24 hours. Heart samples from the same time points were also fixed in 10% buffered formal-saline for 24 hours and in RNA stabilization solution to be processed later.
RNA isolation and quantitative reverse transcriptase PCR
The mouse LV heart samples were homogenized in TRI Reagent (Ambion), and RNA was isolated by phenol-chloroform method [21]. RNA pellet was resuspended in nuclease-free water and stored at -80°C. RNA was quantified using a NanoDrop spectrophotometer and reverse transcribed using High-Capacity cDNA Reverse Transcription kit (Part #4368814, PN 4374967; Applied Biosystems Inc, Foster City, CA, USA) according to the recommended protocol. cDNA was diluted 1:10 and amplified by real-time PCR using target-specific TaqMan gene expression assays using the 7500 Real- Time PCR system (Applied Biosystems). Each well was run in duplicate for gene of interest and reference primer 18S rRNA (Applied Biosystems). The primers and probes for GAL-1 (Mm00839408_g1) and 18S rRNA (Mm03928990_g1) were purchased from Applied Biosystems. Data acquisition was done by using 7500 software v2.0.6 (Applied Biosystems). Analysis was carried out using the comparative Ct method. The level of GAL-1 expression was normalized to 18S rRNA, and fold changes were calculated relative to expression in sham-operated LV heart tissue using the formula 2-ΔΔCt. Many aspects of Minimum Information for Publication of Quantitative Real-Time PCR Experiments guidelines were taken into consideration for methods and analysis [22].
Sample processing for protein extraction
Total protein was extracted from heart samples by homogenizing on ice and then centrifugation at 14000 RPM for 15 minutes at 4°C. Supernatant was collected, alliquoted and stored at -80°C until further analysis. Total protein concentration was determined by BCA protein assay method (Thermo Scientific Pierce BCA Protein Assay Kit).
Sample processing for histology
Each heart was sectioned, casseted and fixed directly in 10% buffered formalin. Sections were dehydrated in increasing concentrations of ethanol, cleared with xylene and embedded with paraffin. Three-μm sections were prepared from paraffin blocks and stained with haematoxylin and eosin. The stained sections were evaluated by the histopathologist that participated in this project.
Immunohistochemistry
Five- μm sections were cut, dewaxed and rehydrated with graded alcohol. Pre-treatment procedure to unmask the antigens was performed in a microwave oven for 10 minutes. Sections were incubated overnight with anti- GAL-1 (rabbit antimouse polyclonal antibody 1:2500, Davids Biotechnologie GmbH, Germany) antibodies at 4°C. After conjugation with primary antibodies, sections were incubated with biotin-labeled secondary antibody (Thermo Scientific, USA) for 20 minutes at room temperature. Finally, sections were incubated with streptavidin-peroxidase complex for 20 minutes at room temperature (Thermo Scientific, USA), DAB chromogen (Thermo Scientific, USA) added and counter staining done with haematoxylin. Appropriate positive and negative controls were used.
Immunofluorescent labeling
5 µm sections were deparaffinized and rehydrated with graded alcohol. Sections were placed in EnVision™ FLEX Target Retrieval Solution with a high PH (PH 9) (DAKO Cytomation, Denmark) in a water bath at 80°C for one hour. Later they were incubated with anti-GAL-1 (rabbit anti-mouse polyclonal antibody 1:50, Davids Biotechnologie GmbH, Germany) overnight at room temperature. Sections were subsequently incubated with Donkey anti-rabbit Alexa Fluor 488, (Invitrogen, USA, 1: 50). Finally, sections were mounted in water-soluble mounting media and viewed with Olympus Fluorescent microscope.
Enzyme linked immunosorbent assay
Left ventricular myocardial and plasma concentration of GAL-1 was determined using DuoSet enzyme linked immunosorbent assay (ELISA) Development kit (R&D Systems, Minneapolis, MN, USA) for sandwich ELISA, using standard procedure according to the manufacturer’s instructions.
Statistical analysis
Statistical analysis is done using IBM SPSS Statistics version 20. Data are presented in mean ± S.E. Statistically significant differences (P<0.05) were calculated between each surgically operated group and the non- operated naïve group by students t test.
Results
Galectin-1 in response to surgical stress in the heart
GAL-1 concentration in LV tissue of non-operated control animal group was measured to be 66.32±4.69 ng/mg. GAL-1 concentration in LV tissue of surgically operated mice at different time points of surgical stress are presented in Table 1. There is significant increase of GAL-1 in LV at all tested time points compared to non-operated control group (Figure 1). In normal heart, GAL-1 is mainly seen to be expressed by cardiomyocytes and endothelial cells (Figure 2A-C). The expression is more pronounced in the right ventricle as compared to the left ventricle (Figure 2A). In the LV, GAL-1 is mainly expressed by endothelial cells and few cardiomyocytes (Figure 2C). A very characteristic staining pattern is seen in immunofluorescent labeling of GAL-1, which gives a mesh-like staining pattern of myocardium. While in the surgical operated groups at 30 minute, 60 minute, 4 hour and 24 hour time points, we have increase in the expression of GAL-1 by cardiomyocytes and endothelial cells in the LV (Figure 2F-O) as compared to the non-operated group (Figure 2A-C)
Table 1.
Galectin-1 levels in ng/mg of total protein at different time points after surgical operation
| Groups | N | Mean (ng/mg) | Std Dev | Std Error | P value |
|---|---|---|---|---|---|
| Non-operated control | 6 | 68.21 | 12.42 | 5.07 | |
| 30 min post surgery | 6 | 116.75 | 18.76 | 7.66 | 0.000* |
| 60 min post surgery | 6 | 93.35 | 6.42 | 2.62 | 0.001* |
| 4 hours post surgery | 6 | 92.28 | 8.78 | 3.58 | 0.003* |
| 24 hours post surgery | 6 | 88.28 | 3.03 | 1.24 | 0.003* |
Shows P<0.05 in surgically operated vs non-operated control groups.
Figure 1.
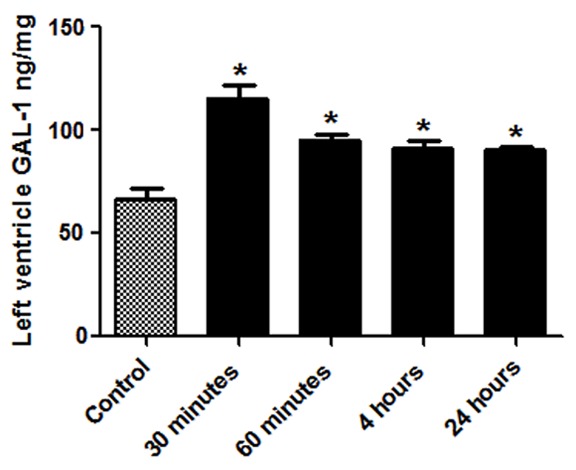
Graphical representation of the Galectin-1 levels in the LV at different post operated time points. *Shows P<0.05 in non-operated control vs surgically operated groups.
Figure 2.
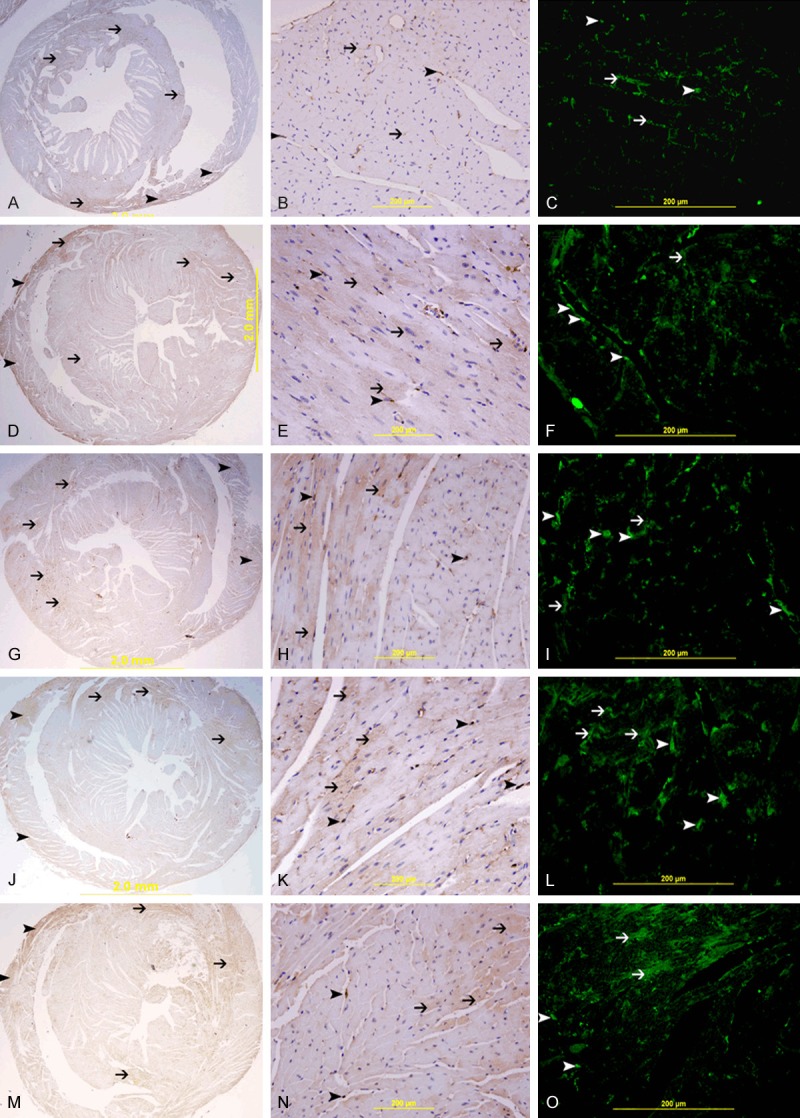
Galectin-1 in the heart. A. Low power view of heart from non-operated control group showing low and patchy expression of GAL-1, mainly in the right ventricle (arrow head) and interventricular septum (thin arrow), Immunoperoxidase streptavidin-biotin method. B. High power view of left ventricle tissue from non-operated control group showing spotty low expression of GAL-1 mainly in endothelial cells (arrow head) and cardiomyocytes sarcolemmal membrane (thin arrow), Immunoperoxidase streptavidin-biotin method. C. High power view of left ventricle tissue from non-operated control group showing spotty low expression of GAL-1 mainly in endothelial cells (arrow head) and cardiomyocytes sarcolemmal membrane (thin arrow) giving a mesh-like pattern, Alexa Fluor 488, immunofluorescent technique. D, G, J, M. Low power views of heart from surgically operated groups at 30 min, 60 min, 4 hour, 24 hour post-operatively time points showing higher patchy expression of GAL-1in the left ventricle and interventricular septum (thin arrow), the right ventricle is also showing expression of GAL-1 (arrow head), Immunoperoxidase streptavidin-biotin method. E, H, K, N. High power views of left ventricle from surgically operated groups at 30 min, 60 min, 4 hour, 24 hour post-operatively time points showing higher patchy expression of GAL-1 in cardiomyocytes (thin arrow) and endothelial cells (arrow head), Immunoperoxidase streptavidin-biotin method. F, I, L, O. High power views of left ventricle from surgically operated groups at 30 min, 60 min, 4 hour, 24 hour post-operatively time points showing higher patchy expression of GAL-1 in cardiomyocytes (thin arrow) and endothelial cells (arrow head) of left ventricle, Alexa Fluor 488, immunofluorescent technique.
GAL-1 mRNA expression in post surgery groups
GAL-1 mRNA levels in all surgically operated groups are not significantly different from the non-operated group (Table 2).
Table 2.
GAL-1 mRNA fold expression in the left ventricle following surgery
| Groups | N | Mean mRNA Fold Expression | Std Dev | Std Error | P value |
|---|---|---|---|---|---|
| Non-operated control | 6 | 1.041 | 0.31 | 0.12 | |
| 30 min post surgery | 6 | 0.78 | 0.27 | 0.11 | 0.15 |
| 60 min post surgery | 6 | 1.23 | 0.25 | 0.10 | 0.26 |
| 4 hours post surgery | 6 | 1.32 | 0.22 | 0.09 | 0.11 |
| 24 hours post surgery | 6 | 0.95 | 0.13 | 0.05 | 0.50 |
Plasma Galectin-1 in response to surgical stress
The concentration of GAL-1 in plasma of non-operated control animals was measured to be 8.71 ng/ml. The level of GAL-1 in surgically operated groups is shown in Table 3. There are significant higher levels of GAL-1 in the surgically operated groups compared to the non-operated controls (Figure 3).
Table 3.
Plasma Galectin-1 levels in ng/ml at different time points after sham operation
| Groups | N | Mean (ng/ml) | Std Dev | Std Error | P value |
|---|---|---|---|---|---|
| Non-operated control | 6 | 8.71 | 2.04 | 0.83 | |
| 30 min post surgery | 8 | 14.52 | 2.21 | 0.74 | 0.000* |
| 60 min post surgery | 8 | 13.83 | 3.92 | 1.38 | 0.009* |
| 4 hours post surgery | 6 | 10.64 | 2.51 | 1.02 | 0.043* |
| 24 hours post surgery | 6 | 17.86 | 2.63 | 0.93 | 0.000* |
Shows P<0.05 in surgically operated vs non-operated control groups.
Figure 3.
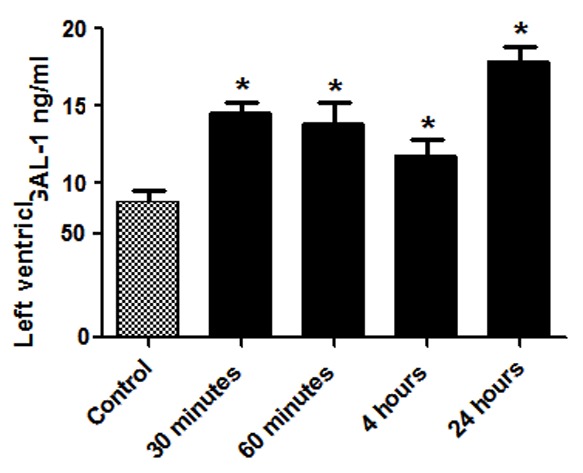
Graphical representation of the Galectin-1 levels in the plasma at different post operated time points. *Shows P<0.05 in non-operated control vs surgically operated groups.
Discussion
The major observation in this study is that GAL-1 levels in the heart and plasma are significantly raised in response to cardiac surgeries in mice. Our adopted murine model of cardiac surgery includes thoracotomy, pericardiectomy and a minor surgical injury to the LV tissue induced by passing the stitch needle once in the LV. The observed increase in GAL-1 levels in the plasma and heart tissue suggests that GAL-1 may be part of the surgical stress response proteins.
It is well known that surgical trauma results in derangements of metabolic and physiological processes that can disrupt the inflammatory, acute phase, hormonal, and genomic responses [15]. Surgical stress response is triggered by the nervous system that sends impulses from the injured site to the hypothalamus. The hypothalamus either stimulates or inhibits the release of pituitary hormones. Pituitary hormones induce the release of the stress hormone, cortisol [23]. Cortisol, glucagon, catecholamines, and some inflammatory cytokines are the major players in the stress response to surgery. They are responsible for tachycardia, nervousness and hypermetabolic state, which itself lead to increased stress on the heart. Open heart surgery is associated with acute perioperative changes in plasma levels of cortisol [24].
GAL-1 has been linked to stress in a research study by Iwamoto et al. [25]. In this study GAL-1 levels in the serum were found to be significantly higher in animals subjected to stress by restraining the animals. The pattern of change was found to be similar to that of corticosterone. A very interesting observation made in the same study was that the increase of GAL-1 was prevented by a neurotoxin, 6-hydroxydopamine (6-OHDA), which destroys the noradrenergic nerve terminals, suggesting that the stress-induced increase of GAL-1 is regulated by the sympathetic nervous system.
The heart is densely innervated by sympathetic nerve fibers [26]. The increase in GAL-1 levels in the LV tissue of the operated group may be due to the general effect of sympathetic stimulation of the nerve terminal of the heart in response to surgery. This idea is further supported by the fact that we observed no changes in LV GAL-1 mRNA levels between non-operated control group and all the postoperative groups. This suggests that the initial increase in GAL-1 in heart tissue and subsequently the plasma may not be due to the GAL-1 transcribed in the heart tissue but due to GAL-1 production by the sympathetic nerve endings in the heart.
In surgically operated groups, we observed that GAL-1 immunoreactivity was focally increased in the cytoplasm of cardiomyocytes in the LV. In this case the increase in GAL-1 can be due to the effect of injury to the myocardium sustained during the operation. Also the effect of pericardiectomy and thoracotomy on the heart and plasma should be taken into account. The idea of increase GAL-1 at the site of injury is not new. GAL-1 was found to be increased at the site of skeletal muscle injury [27]. GAL-1 can promote both myoblast fusion and axonal growth following muscle injury and play a role in regenerating skeletal muscles. Immunostaining analysis of GAL-1 in injured spinal cords also showed GAL-1 -expressing cells assembled around the lesion site [28]. We propose therefore, that the increase in GAL-1 levels in the heart may partly be due to the focal injury to the cardiomyocytes and partly due to the sympathetic stimulation of the heart as part of the surgical stress response.
The pattern of GAL-1 levels at different time points following cardiac surgery is also very important. We have seen a sharp increase in GAL-1 levels after 30 minutes post operatively and later remains elevated, but towards the 24 hour time point it starts going down. This pattern also points towards GAL-1 as an acute stress player which increases in concentration locally immediately following injury but as the body’s homeostatic mechanism takes effect its level starts to come down. However, we do not see the same pattern in the plasma where we have observed higher levels at 24 hours post operation. A possible explanation for this phenomenon may be related to the fact that GAL-1 is expressed in activated T-Cells [29] and major surgery is one factor that enhances the early phase of T lymphocyte activation [30] which may have led to this increase in plasma GAL-1 at 24 hours postoperatively.
We believe that there should be a purpose for this rise in GAL-1 at very early time postoperatively. GAL-1 is cardio protective as it plays a critical role in controlling acute inflammation [31], apoptosis [32,33] and neovascularization [34]. Its upregulation possibly can be a part of survival mechanism to protect the heart after surgical insult. GAL-1 is also part of the hypoxia-regulated transcriptome [14,35], and a component of the contractile apparatus in cardiomyocytes [36] suggesting a potential role in modulating cardiac function. GAL-1 up regulation in the heart after surgical manipulation can have important implications in the postoperative recovery phase [37,38]. The therapeutic administration or modulation of its expression in cardiac tissue can present as a potential novel strategy to prevent postoperative complications.
In conclusion, GAL-1 is valuable biomarker of surgical stress. More work is needed to clarify further the role of GAL-1 as a stress marker for surgical trauma particularly related to open heart procedures. This work has opened a small window for looking at GAL-1 from a new perspective.
Acknowledgements
The authors would like to thank The National Research Foundation and the United Arab Emirates University for their support of this project (Grant 31MO76).
Disclosure of conflict of interest
None.
References
- 1.Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994;269:20807–10. [PubMed] [Google Scholar]
- 2.Poirier F. Roles of galectins in vivo. Biochem Soc Symp. 2002:95–103. doi: 10.1042/bss0690095. [DOI] [PubMed] [Google Scholar]
- 3.Poirier F, Timmons PM, Chan CT, Guénet JL, Rigby PW. Expression of the L14 lectin during mouse embryogenesis suggests multiple roles during pre- and post-implantation development. Development. 1992;115:143–55. doi: 10.1242/dev.115.1.143. [DOI] [PubMed] [Google Scholar]
- 4.Poirier F, Robertson EJ. Normal development of mice carrying a null mutation in the gene encoding the L14 S-type lectin. Development. 1993;119:1229–36. doi: 10.1242/dev.119.4.1229. [DOI] [PubMed] [Google Scholar]
- 5.Stillman BN, Mischel PS, Baum LG. New roles for galectins in brain tumors--from prognostic markers to therapeutic targets. Brain Pathol. 2005;15:124–32. doi: 10.1111/j.1750-3639.2005.tb00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auvynet C, Moreno S, Melchy E, Coronado-Martínez I, Montiel JL, Aguilar-Delfin I, Rosenstein Y. Galectin-1 promotes human neutrophil migration. Glycobiology. 2013;23:32–42. doi: 10.1093/glycob/cws128. [DOI] [PubMed] [Google Scholar]
- 7.Colnot C, Ripoche MA, Scaerou F, Foulis D, Poirier F. Galectins in mouse embryogenesis. Biochem Soc Trans. 1996;24:141–6. doi: 10.1042/bst0240141. [DOI] [PubMed] [Google Scholar]
- 8.Liu FT, Patterson RJ, Wang JL. Intracellular functions of galectins. Biochim Biophys Acta. 2002;1572:263–73. doi: 10.1016/s0304-4165(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 9.Park JW, Voss PG, Grabski S, Wang JL, Patterson RJ. Association of galectin-1 and galectin-3 with Gemin4 in complexes containing the SMN protein. Nucleic Acids Res. 2001;29:3595–602. doi: 10.1093/nar/29.17.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perillo NL, Pace KE, Seilhamer JJ, Baum LG. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378:736–9. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- 11.Scott K, Weinberg C. Galectin-1: a bifunctional regulator of cellular proliferation. Glycoconj J. 2004;19:467–77. doi: 10.1023/B:GLYC.0000014076.43288.89. [DOI] [PubMed] [Google Scholar]
- 12.Camby I, Le Mercier M, Lefranc F, Kiss R. Galectin-1: a small protein with major functions. Glycobiology. 2006;16:137R–57R. doi: 10.1093/glycob/cwl025. [DOI] [PubMed] [Google Scholar]
- 13.Seropian IM, Cerliani JP, Toldo S, Van Tassell BW, Ilarregui JM, González GE, Matoso M, Salloum FN, Melchior R, Gelpi RJ, Stupirski JC, Benatar A, Gómez KA, Morales C, Abbate A, Rabinovich GA. Galectin-1 controls cardiac inflammation and ventricular remodeling during acute myocardial infarction. Am J Pathol. 2013;182:29–40. doi: 10.1016/j.ajpath.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Salam S, Hashmi S. Galectin-1 in early acute myocardial infarction. PLoS One. 2014;9:e86994. doi: 10.1371/journal.pone.0086994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finnerty CC, Mabvuure NT, Ali A, Kozar RA, Herndon DN. The surgically induced stress response. JPEN J Parenter Enteral Nutr. 2013;37(Suppl 5):21S–9S. doi: 10.1177/0148607113496117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall RI, Smith MS, Rocker G. The systemic inflammatory response to cardiopulmonary bypass: pathophysiological, therapeutic, and pharmacological considerations. Anesth Analg. 1997;85:766–82. doi: 10.1097/00000539-199710000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Prondzinsky R, Knüpfer A, Loppnow H, Redling F, Lehmann DW, Stabenow I, Witthaut R, Unverzagt S, Radke J, Zerkowski HR, Werdan K. Surgical trauma affects the proinflammatory status after cardiac surgery to a higher degree than cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2005;129:760–6. doi: 10.1016/j.jtcvs.2004.07.052. [DOI] [PubMed] [Google Scholar]
- 18.Michael LH, Entman ML, Hartley CJ, Youker KA, Zhu J, Hall SR, Hawkins HK, Berens K, Ballantyne CM. Myocardial ischemia and reperfusion: a murine model. Am J Physiol. 1995;269:H2147–54. doi: 10.1152/ajpheart.1995.269.6.H2147. [DOI] [PubMed] [Google Scholar]
- 19.Hashmi S, Al-Salam S. Loss of dystrophin staining in cardiomyocytes: a novel method for detection early myocardial infarction. Int J Clin Exp Pathol. 2013;6:249–57. [PMC free article] [PubMed] [Google Scholar]
- 20.Hashmi S, Al-Salam S. Galectin-3 is expressed in the myocardium very early post-myocardial infarction. Cardiovasc Pathol. 2015;24:213–23. doi: 10.1016/j.carpath.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Chomczynski P, Mackey K. Short technical reports. Modification of the TRI reagent procedure for isolation of RNA from polysaccharide- and proteoglycan-rich sources. Biotechniques. 1995;19:942–5. [PubMed] [Google Scholar]
- 22.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–22. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 23.Gore DC, Jahoor F, Wolfe RR, Herndon DN. Acute response of human muscle protein to catabolic hormones. Ann Surg. 1993;218:679–84. doi: 10.1097/00000658-199321850-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoda MR, El-Achkar H, Schmitz E, Scheffold T, Vetter HO, De Simone R. Systemic stress hormone response in patients undergoing open heart surgery with or without cardiopulmonary bypass. Ann Thorac Surg. 2006;82:2179–86. doi: 10.1016/j.athoracsur.2006.06.087. [DOI] [PubMed] [Google Scholar]
- 25.Iwamoto M, Taguchi C, Sasaguri K, Kubo KY, Horie H, Yamamoto T, Onozuka M, Sato S, Kadoya T. The Galectin-1 level in serum as a novel marker for stress. Glycoconj J. 2010;27:419–25. doi: 10.1007/s10719-010-9288-z. [DOI] [PubMed] [Google Scholar]
- 26.Kimura K, Ieda M, Fukuda K. Development, maturation, and transdifferentiation of cardiac sympathetic nerves. Circ Res. 2012;110:325–36. doi: 10.1161/CIRCRESAHA.111.257253. [DOI] [PubMed] [Google Scholar]
- 27.Kami K, Senba E. Galectin-1 is a novel factor that regulates myotube growth in regenerating skeletal muscles. Curr Drug Targets. 2005;6:395–405. doi: 10.2174/1389450054021918. [DOI] [PubMed] [Google Scholar]
- 28.Kurihara D, Ueno M, Tanaka T, Yamashita T. Expression of galectin-1 in immune cells and glial cells after spinal cord injury. Neurosci Res. 2010;66:265–70. doi: 10.1016/j.neures.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Fuertes MB, Molinero LL, Toscano MA, Ilarregui JM, Rubinstein N, Fainboim L, Zwirner NW, Rabinovich GA. Regulated expression of galectin-1 during T-cell activation involves Lck and Fyn kinases and signaling through MEK1/ERK, p38 MAP kinase and p70S6 kinase. Mol Cell Biochem. 2004;267:177–85. doi: 10.1023/b:mcbi.0000049376.50242.7f. [DOI] [PubMed] [Google Scholar]
- 30.Shimaoka M, Hosotsubo K, Sugimoto M, Sakaue G, Taenaka N, Yoshiya I, Kiyono H. The influence of surgical stress on T cells: enhancement of early phase lymphocyte activation. Anesth Analg. 1998;87:1431–5. doi: 10.1097/00000539-199812000-00043. [DOI] [PubMed] [Google Scholar]
- 31.Rabinovich GA, Sotomayor CE, Riera CM, Bianco I, Correa SG. Evidence of a role for galectin-1 in acute inflammation. Eur J Immunol. 2000;30:1331–9. doi: 10.1002/(SICI)1521-4141(200005)30:5<1331::AID-IMMU1331>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 32.Rabinovich GA, Alonso CR, Sotomayor CE, Durand S, Bocco JL, Riera CM. Molecular mechanisms implicated in galectin-1-induced apoptosis: activation of the AP-1 transcription factor and downregulation of Bcl-2. Cell Death Differ. 2000;7:747–53. doi: 10.1038/sj.cdd.4400708. [DOI] [PubMed] [Google Scholar]
- 33.Rabinovich GA, Daly G, Dreja H, Tailor H, Riera CM, Hirabayashi J, Chernajovsky Y. Recombinant galectin-1 and its genetic delivery suppress collagen-induced arthritis via T cell apoptosis. J Exp Med. 1999;190:385–98. doi: 10.1084/jem.190.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thijssen VL, Barkan B, Shoji H, Aries IM, Mathieu V, Deltour L, Hackeng TM, Kiss R, Kloog Y, Poirier F, Griffioen AW. Tumor cells secrete galectin-1 to enhance endothelial cell activity. Cancer Res. 2010;70:6216–24. doi: 10.1158/0008-5472.CAN-09-4150. [DOI] [PubMed] [Google Scholar]
- 35.Hashmi S, Al-Salam S. Hypoxia-inducible factor-1 alpha in the heart: a double agent? Cardiol Rev. 2012;20:268–73. doi: 10.1097/CRD.0b013e31826287f6. [DOI] [PubMed] [Google Scholar]
- 36.Levi G, Tarrab-Hazdai R, Teichberg VI. Prevention and therapy with electrolectin of experimental autoimmune myasthenia gravis in rabbits. Eur J Immunol. 1983;13:500–7. doi: 10.1002/eji.1830130613. [DOI] [PubMed] [Google Scholar]
- 37.Offner H, Celnik B, Bringman TS, Casentini-Borocz D, Nedwin GE, Vandenbark AA. Recombinant human beta-galactoside binding lectin suppresses clinical and histological signs of experimental autoimmune encephalomyelitis. J Neuroimmunol. 1990;28:177–84. doi: 10.1016/0165-5728(90)90032-i. [DOI] [PubMed] [Google Scholar]
- 38.Cunha-Neto E, Coelho V, Guilherme L, Fiorelli A, Stolf N, Kalil J. Autoimmunity in Chagas’ disease. Identification of cardiac myosin-B13 Trypanosoma cruzi protein crossreactive T cell clones in heart lesions of a chronic Chagas’ cardiomyopathy patient. J Clin Invest. 1996;98:1709–12. doi: 10.1172/JCI118969. [DOI] [PMC free article] [PubMed] [Google Scholar]


