Abstract
The Raf protein kinases are major effectors of Ras GTPases and key components of the transcriptional response to serum factors, acting at least in part through the extracellular signal-regulated kinase/mitogen-activated protein kinase pathway. It has recently been suggested that Raf also may trigger other as yet uncharacterized signaling pathways. Here, we have used cDNA microarrays to dissect changes in gene expression induced by activation of inducible c-Raf-1 constructs in human mammary epithelial and ovarian epithelial cells. The majority of Raf-induced transcriptional responses are shown to be blocked by pharmacological inhibition of the Raf substrate mitogen-activated protein kinase kinase, indicating that potential mitogen-activated protein kinase kinase-independent Raf signaling pathways have no significant influence on gene expression. In addition, we used epidermal growth factor receptor inhibitory drugs to address the contribution of autocrine signaling by Raf-induced EGF family proteins to the Raf transcriptional response. At least one-half of the transcription induced by Raf activation requires epidermal growth factor (EGF) receptor function The EGF receptor-independent component of the Raf transcriptional response is entirely up-regulation of gene expression, whereas the EGF receptor-dependent component is an equal mixture of up- and down-regulation. The use of transcriptional profiling in this way allows detailed analysis of the architecture of signaling pathways to be undertaken.
INTRODUCTION
The dominantly acting oncogene that is implicated most frequently in human cancer is RAS, which is activated by mutation in ∼25% of malignancies (Bos, 1989). Ras proteins exert their transforming influence through a number of effector enzymes, including the serine/threonine kinase Raf, the lipid kinase phosphoinositide 3-kinase (PI 3-kinase) and the guanine nucleotide exchange factor Ral-GDS (Marshall, 1996; Downward, 1998). As a result of point mutation or growth factor treatment of cells, Ras enters the active GTP-bound state, which interacts with Raf proteins. Formation of this complex leads, together with other stimulatory signals, to increase in the kinase activity of Raf (Marais and Marshall, 1996). Once activated, Raf phosphorylates and activates its major substrates, the dual specificity kinases mitogen-activated protein kinase kinase (MEK)1 and MEK2, which go on to phosphorylate and activate the mitogen-activated protein (MAP) kinases ERK1 and ERK2 (Kolch, 2000). Raf acting through MEK and ERK has been found to play a critical role in the control of cell cycle progression (Marshall, 1999), can induce protection against various apoptotic stimuli (Bonni et al., 1999; Erhardt et al., 1999; Kazama and Yonehara, 2000; Le Gall et al., 2000), and has numerous other effects on cell behavior.
The activation of the ERK/MAP kinase pathway by Raf induces phosphorylation and stimulation of several transcription factors, including Elk1, SRF, ATF2, and Jun (Treisman, 1996). Raf activation thus leads to profound changes in cellular gene expression (Schulze et al., 2001), which can account for many of its biological effects. However, ERKs also target proteins other than transcription factors, for example, phospholipase A2 (Sa et al., 1995) and the kinase p90Rsk2, which can phosphorylate the proapoptotic protein BAD (Bonni et al., 1999), so some of the effects of ERK/MAP kinase do not require transcriptional changes. To date, what has been less clear is whether Raf has effects on cell behavior, either transcriptional or not, that are independent of its best-characterized substrates, MEK1 and MEK2. Raf might be able to phosphorylate proteins other than MEKs, but although several other direct in vitro substrates of Raf have been reported in the past (Li and Sedivy, 1993; Wang et al., 1996a), none of these have yet been convincingly demonstrated to be physiologically significant. In addition, Raf could have effects that do not depend on its kinase activity but are mediated by other possible functions, such as the ability to act as an adaptor or scaffold protein to bring other proteins into proximity with each other. Recent data from mice in which the c-Raf-1 gene has been deleted have led to speculation that Raf-1 might have an important antiapoptotic function that is independent of its kinase activity (Hüser et al., 2001; Mikula et al., 2001). The possibility that Raf might signal through mechanisms that are independent of MEKs is of considerable practical importance because of the advanced development of a pharmacological inhibitor of MEK, CI-1040, for use in cancer therapy.
The ability of Raf activation to strongly induce autocrine expression of the epidermal growth factor (EGF)-like growth factors such as heparin-binding-EGF, transforming growth factor-α, and amphiregulin (de Larco and Todaro, 1978; McCarthy et al., 1995; Schulze et al., 2001) raises the possibility that a component of the biological effects of Raf activation are mediated by an autocrine loop through the EGF receptor. EGF itself activates the Raf/MAP kinase pathway but also leads to activation of PI 3-kinase, signal transducer and activator of transcriptions, and phospholipase Cγ. Setting up an EGF autocrine loop downstream of the MAP kinase pathway might therefore considerably extend the complexity of the transcriptional response to Raf activation, especially in cells that are relatively sensitive to the effects of EGF. Autocrine loops of this kind have been implicated in transformation by Ras and Raf (de Larco and Todaro, 1978; Sibilia et al., 2000; Schulze et al., 2001).
To address the importance of MEK and EGF receptor activities in what is probably the most significant aspect of the Raf response, the induction of gene expression, we have used cDNA microarrays to analyze the transcriptional changes induced upon activation of inducible Raf-1 constructs in human epithelial cells and how this is affected by selective pharmacological inhibitors. Our results show that almost the entire Raf-induced transcriptional response is inhibited by pharmacological inhibitors of MEK, suggesting that if Raf does control MEK-independent signaling pathways, they do not impact on gene expression. In addition, a significant portion of Raf-induced transcription requires EGF receptor function, emphasizing the importance of autocrine signaling.
MATERIALS AND METHODS
Cell Culture, Retroviral Infection, and Transfection
MCF-10A cells were grown in Ham's nutrient mixture F-12/DMEM (1:1) containing 5% horse serum and 10 μg/ml insulin, 20 ng/ml EGF, 5 μg/ml hydrocortisone, and 100 ng/ml cholera toxin (full medium). Minimal medium consisted of Ham's nutrient mixture F-12/DMEM (1:1) containing 5% horse serum. 4-Hydroxytamoxifen (4-OHT) was purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in ethanol to obtain a 0.5 mM stock solution. PD98059 and PD168393 were purchased from Calbiochem (San Diego, CA).
Human ovarian surface epithelial cells containing the HPV-E6/E7 open reading frames (HOSE642-1cells) were kindly provided by Samuel Mok (Harvard Medical School, Boston, MA). HOSE cells were maintained in mixture of MCDB105 and M199 (1:1) supplemented with 15% fetal bovine serum, 1× penicillin/streptomycin, and 4 mM glutamine.
Retroviral vectors pBabe-puro-EGFP-ΔRaf:hbER (Bosch et al., 1997) or corresponding empty vectors were packaged in GP+E cells and used to infect MCF-10A cells expressing the ecotropic retrovirus receptor. Cells were selected with 2.5 μg/ml puromycin or 0.5 mg/ml G418 for 2 wk and sorted twice for enhanced green fluorescent protein expression by fluorescence-activated cell sorting.
MCF-10A V12Ras-expressing cells have been described previously (Schulze et al., 2001).
HOSE642-1 cells were infected with the retroviral vector pBabe-hygro-hTERT (8). After selection with 30 μg/ml hygromycin, pools of hTERT-positive HOSE cells were cotransfected with pcDNA6/TR and pcDNA4/TO (Invitrogen, Carlsbad, CA), with pcDNA4/TO containing Raf1-CAAX or as an empty vector. HOSE-TREX Raf1-CAAX cell clones were selected using 5 μg/ml blasticidin and 250 μg/ml zeocin.
For transient transfections, MCF-10A ΔRaf-ER cells were seeded in six-well plates, grown for 24 h, and transfected using Effectene (QIAGEN, Valencia, CA). Twenty-four hours posttransfection, cells were washed and medium was replaced with minimal medium with or without addition of 4-OHT. Cells were lysed after a further 24 h and CAT activity was determined using the chloramphenicol acetyltransferase enzyme-linked immunosorbent assay (Roche Diagnostics, Indianapolis, IN).
Antibodies and Immunoblotting
Anti-phospho-p42/p44 Erk1/2 (T202, Y204) and anti-phospho-EGFR (Y1173) were purchased from Cell Signaling Technology (Beverly, MA). Cells were lysed in buffer containing 1% Triton X-100 or in SDS sample buffer. Proteins were separated by SDS-PAGE and blotted onto polyvinylidene difluoride membrane, incubated with antibody solutions, and detected using enhanced chemiluminescence.
RNA Preparation and Array Hybridization
Total RNA was prepared using TRIzol reagent (Invitrogen) following the manufacturer's protocol. Total RNA from control and experimental sample was labeled by oligo-dT17-primed first strand cDNA synthesis in the presence of Cy3-dUTP or Cy5-dUTP (Amersham Biosciences, Piscataway, NJ), respectively. Labeled cDNA was purified using AutoSeq G-50 columns and hybridized for 16h to human cDNA microarrays (Sanger human 10K version 1.2.1, having 10,000 probes representing 6000 different genes). All hybridizations were performed at least in duplicates with opposite Cy3/Cy5 labeling. For detailed description of the cDNA clones, the preparation of the microarrays and the protocols used in sample preparation, array hybridization, washing, and handling, see http://www.sanger.ac.uk/Projects/Microarrays/.
Data Analysis
Images were quantified using the histogram method of GSI-Lumonics QuantArray software by defining the 60th to 95th percentile of pixels as signal and the 5th to 30th percentile as background. Channels were normalized by global normalization of total signal intensity. Normalized intensity values were imported into Silicon Genetics Genespring software for subsequent analysis. Intensity values were then normalized to the control hybridization, which represented a self-to-self comparison of the control sample.
Genes significantly regulated in response to Raf-ER activation, Raf1-CAAX induction, or EGF treatment were identified by applying a restriction on fold change of 1.5- to 2-fold followed by statistical group comparison of the normalized intensity ratios of the different time points to the control sample (p < = 0.05; Welch analysis of variance [ANOVA]). The Benjamini and Hochberg false discovery rate was applied as multiple testing correction. To rule out effects of tamoxifen or doxycycline, RNA from vector transfected MCF10-A or HOSE cells treated with 100 nM 4-OHT or 1 nM doxycycline, respectively, was compared in a control experiment (our unpublished data).
Intensity ratios of the selected genes were used to perform a cluster analysis using a Pearson correlation.
Probes were annotated according to the Hver1.2.1mfg30 annotation provided by the Sanger Microarray facility (for more information, see http://www.sanger.ac.uk/Projects/Microarrays/informatics/annotation.shtml).
RESULTS
Changes in Gene Expression Induced by ΔRaf-ER Activation in Human Breast Epithelial Cells Are Dependent on MEK Activity
To investigate Raf-induced transcriptional regulation, we used MCF-10A cells, a normal spontaneously immortalized human lumenal mammary epithelial cell line (Soule et al., 1990). These cells were infected with retroviruses encoding a Raf/estrogen receptor/green fluorescent protein fusion (ΔRaf-ER) whose kinase activity is stimulatable by 4-OHT but not natural estrogens (Bosch et al., 1997). Addition of 4-OHT to MCF-10A ΔRaf-ER cells induced rapid and sustained stimulation of ERK/MAP kinase phosphorylation, indicating activation of the fusion protein (Schulze et al., 2001). The strength of ERK/MAPK phosphorylation after 24 h of 4-OHT treatment in MCF-10A ΔRaf-ER cells, although somewhat weaker, is comparable with that in MCF-10A cells expressing an activated mutant of the H-Ras protein (MCF-10A V12Ras; Supplementary Figure 1).
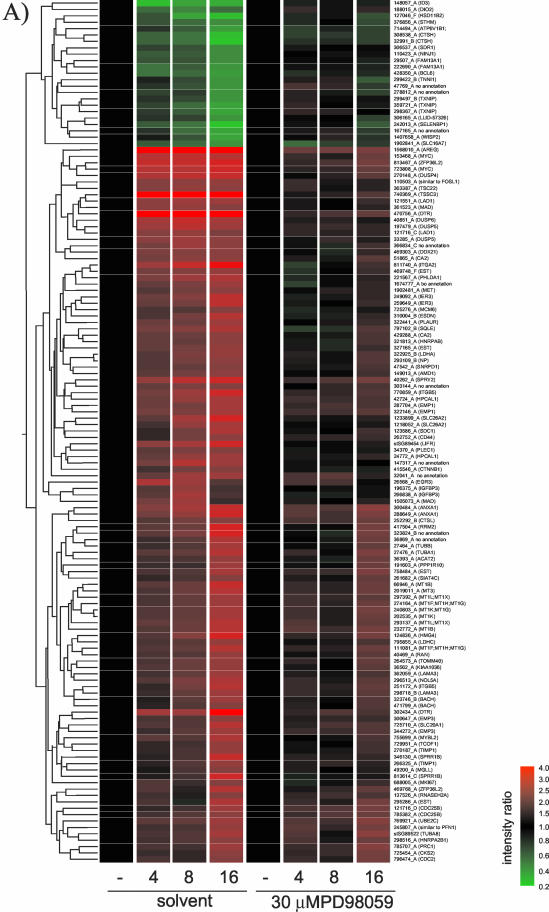
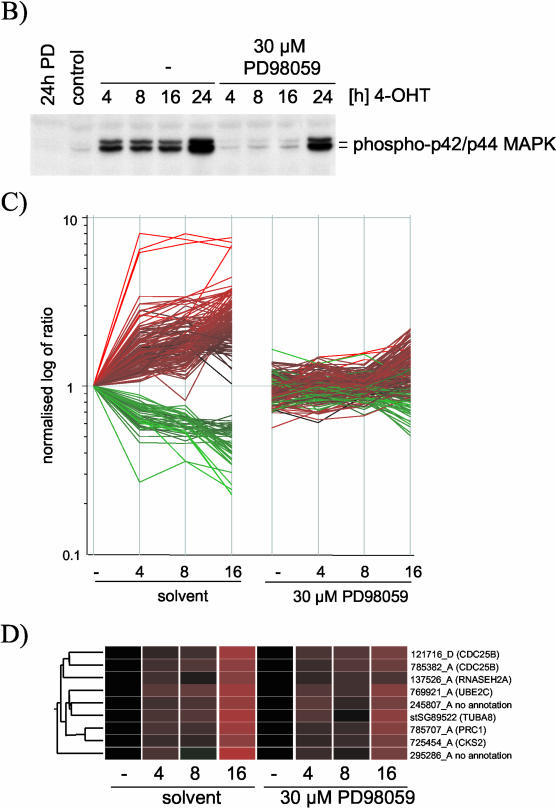
MCF-10A ΔRaf-ER cells were incubated in low serum (“minimal”) medium for 24 h, and Raf activity was induced with 100 nM 4-OHT for the last 16, 8, 4, or 0 h before lysis. In parallel, cells also were treated with the selective MEK inhibitor PD98059 at 30 μM. After lysis, analysis of gene expression profiles was performed using human cDNA microarrays representing some 6000 genes. Figure 1A shows mean intensity ratios of biological duplicates with inverted Cy3/Cy5 labeling hybridization replicates relative to the untreated control for 135 probes that were significantly altered by Raf induction. Genes were sorted by hierarchical clustering into sets with similar expression profiles. For the majority of these genes, treatment with PD98059 strongly inhibited the induction of expression seen after Raf activation. For a number of genes, it was observed that although Raf-induced expression was strongly suppressed by PD98059 at the 4- and 8-h time points, by 16 h some Raf-induced changes in gene expression were becoming apparent. This suggested that the effectiveness of PD98059 as a MEK inhibitor might be declining by this time, presumably due to metabolism or hydrolysis of the drug. Alternatively, the declining effectiveness of PD98059 could be due to the fact that ΔRaf-ER protein levels increase with time of induction with 4-OHT due to a protein stabilization effect (Lehmann et al., 2000). To investigate this possibility, the activation states of ERK1 and ERK2, the known substrates for MEK, were checked using a phosphospecific antibody. As shown in Figure 1B, a low level of ERK phosphorylation was visible above the control levels in PD98059-treated cells by 8, and more clearly 16, h of induction. At 24 h, strong ERK phosphorylation was seen, indicating that there is a limited time span to the inhibition of MEK by PD98059. It should be noted that the level of ERK phosphorylation in the cells treated with 4-OHT plus PD98059 never falls below the basal level found in serum-starved cells, making it unlikely that PD98059 might be affecting the output from parallel pathways due to elimination of basal ERK signaling.
Figure 1.
Changes in gene expression induced by ΔRaf-ER activation in MCF-10A cells are dependent on MEK activity. (A) MCF-10A ΔRaf-ER cells were treated with 100 nM 4-OHT or solvent (ethanol) for 4, 8, or 16 h in minimal medium in the presence or absence of 30 μM PD98059. Relative mRNA abundance was measured by comparative hybridization of experimental and control samples to human cDNA microarrays. Hybridizations were performed in quadruplicates with inverted Cy3/Cy5 labeling. Mean intensity ratios relative to the solvent treated control (-) for 135 probes that detected significant changes in gene expression upon Raf-ER activation were used to generate hierarchical clusters of genes with similar expression profiles. Probes are identified by their unique Sanger IDs as well as HUGO names of the corresponding gene (mapped to ENSEMBL). For a detailed description of the clones including GenBank and Unigene accession numbers, see Table 1 (supplementary data). EST, sequence maps to an ENSEMBL EST gene; no annotation, sequence does not map to any expressed sequence. (B) Phosphorylation of p42ERK2/p44ERK1 detected with a phospho-specific antibody by using cytoplasmic lysates from cells prepared in parallel to the experiment shown in A. To demonstrate decreased activity of PD98059 over time, a 24-h time point was included in this experiment. (C) Graphical representation of the data shown in Figure 1. The vertical axis shows the log of normalized intensity ratios for all Raf-responsive probes. The horizontal axis shows the time of treatment with 4-OHT in the presence or absence of PD98059. (D) Nine of the 135 Raf-regulated probes represented in 1A that do not show a significant difference in their expression in the presence or absence of PD98059. For a detailed description of the clones, see Table 1 (supplementary data).
Figure 1C shows a graphical representation of the normalized intensity ratios of the 135 probes shown in Figure 1A. It is evident that the majority of the Raf-induced probes are clearly MEK dependent. To identify any genes that may show regulation by Raf in the presence of PD98059, we performed a statistical group comparison between the samples from untreated cells and from PD98059-treated cells. Only nine probes (representing 6 genes) were considered to show no significant differences in their expression pattern between the two sample sets (Figure 1D). However, almost all of these cases represent genes that are induced at the later time points, and they show a somewhat attenuated induction in the presence of PD98059 at 16 h, compatible with the notion that these genes were induced by low levels of MEK activity that escaped inhibition. It therefore seems that the transcriptional response to Raf activation in MCF-10A cells is likely to be entirely sensitive to PD98059.
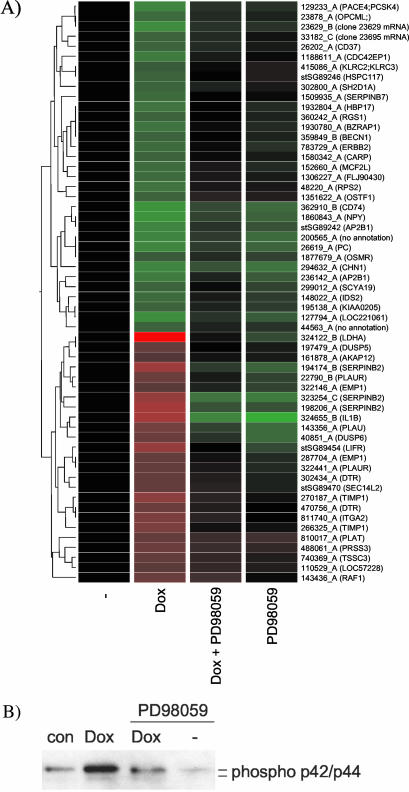
To ensure that the apparent MEK dependence of Raf-induced gene transcription was not an artifact specific to the ΔRaf-ER construct, we analyzed the function of a different inducible Raf construct. Raf-CAAX is full-length c-Raf-1 that is activated as a consequence of fusion to an H-Ras isoprenylation sequence resulting in constitutive localization to the plasma membrane (Leevers et al., 1994). We expressed this in immortalized HOSE cells (Tsao et al., 1995) under the control of a tetracycline-inducible promoter. HOSE-TREx Raf-CAAX cells were serum starved for 8 h before induction of Raf-CAAX expression for a further 16 h with doxycycline in the presence or absence of 50 μM PD98059. The expression of 51 Raf-responsive genes is shown in Figure 2A. The suppression of ERK phosphorylation by the inhibitors is shown in Figure 2B. All of the Raf-induced gene expression changes show a more than 1.5-fold inhibition by PD98059 treatment (Figure 2A).
Figure 2.
Changes in gene expression induced by induction of Raf1-CAAX expression in HOSE cells are dependent on MEK activity. (A) HOSE-TREX Raf1-CAAX cells were starved in medium containing 0.5% horse serum for 8 h and subsequently treated with 1 nM doxycycline (dox) for 16 h in the presence or absence of 50 μM PD98059. Relative mRNA abundance was measured by comparative hybridization of experimental and control samples from two independent experiments to human cDNA microarrays. Mean Intensity ratios relative to the solvent treated control (-) for 58 probes that show significant changes in gene expression upon Raf activation were used to generate hierarchical clusters of genes with similar expression profiles. For a detailed description of the genes, see Table 2 (supplementary data). (B) Phosphorylation of p42ERK2/p44ERK1 detected with a phospho-specific antibody by using cytoplasmic lysates from cells prepared in parallel to the experiment shown in A.
It therefore seems that all the changes in gene expression induced by Raf-1, whether in the form ΔRaf-ER or Raf-CAAX and whether in human breast or ovarian epithelial cells, are mediated by pathways requiring the activity of enzymes inhibited by PD98059. It is, however, at least a theoretical possibility that genes that are not represented among the 6000 on these microarrays may behave differently.
It has been reported that Raf-induced activation of nuclear factor-κB (NF-κB) is independent of MEK function (Baumann et al., 2000). To investigate whether parts of the transcriptional response to Raf activation in MCF-10A cells could be attributed to NF-κB, we analyzed the behavior of known NF-κB target genes. We found 61 genes that have been reported to be regulated by NF-κB in the literature (Pahl, 1999) to be present on the DNA microarray used here. Of these, only seven are also regulated in response to Raf activation in MCF-10A cells (our unpublished data). In all cases induction of expression by Raf activation is completely blocked in the presence of PD98059, indicating that activation of these genes is dependent on MEK. In addition, ΔRaf-ER failed to activate a NF-κB reporter construct in these cells (Supplementary Figure 2). Thus, it is unlikely that activation of NF-κB plays a major role in the transcriptional response to Raf activation in MCF-10A cells under the conditions used here. It has been reported before that Raf-dependent transformation of NIH3T3 cells requires NF-κB, and activation of NF-κB by a Raf-1 construct, which is activated by deletion of the N-terminal regulatory domain (Raf-BXB), has been shown to induce NF-κB through an autocrine loop involving interleukin-1 (Vale et al., 2001). It is important to note that although capable of transforming NIH 3T3 cells (Bosch et al., 1997), ΔRaf-ER failed to induce growth in soft agar in MCF-10A cells (Schulze et al., 2001). This most likely reflects differences between human and murine cell lines but also indicates that activation of NF-κB by Raf may be cell type dependent.
Involvement of EGF Receptor Autocrine Signaling in Transcriptional Responses to Raf
Activation of Raf is known to induce MEK-mediated expression and secretion of growth factors of the EGF family (McCarthy et al., 1995). These have been shown to be responsible for some of the biological consequences of Raf activation, such as protection of MCF-10A cells from detachment-induced apoptosis (Schulze et al., 2001). However, the importance of autocrine signaling in the overall transcriptional response to Raf activation remains unclear. We therefore used MCF-10A ΔRaf-ER cells to determine what effect inhibition of autocrine signaling through the EGF receptor had on the Raf transcriptional response.
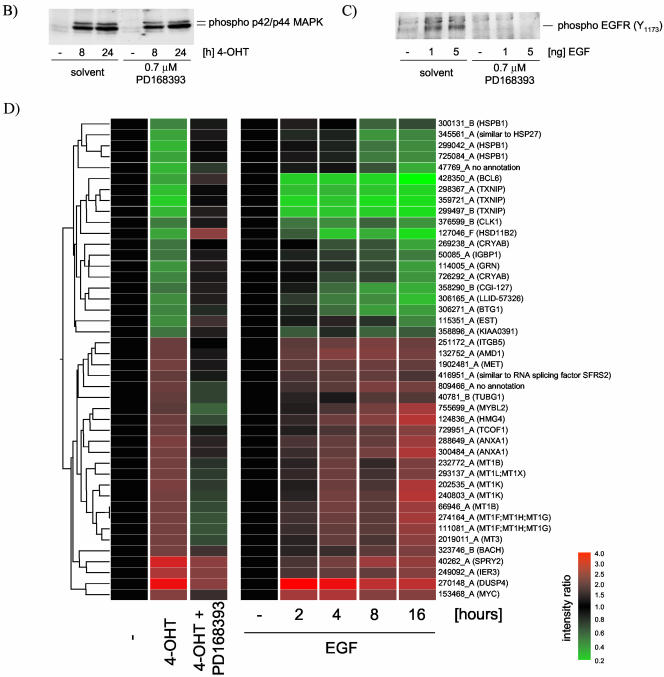
The PD168393 compound has been shown to selectively inhibit EGFR and ErbB2 tyrosine kinase activity by irreversibly binding to the kinase domain (Fry et al., 1998). Although it has been used previously at a concentration of 2 μM, we chose the lower concentration of 0.7 μM as being sufficient to inhibit EGFR phosphorylation in response to stimulation with soluble EGF without affecting activation of MAPK in response to ΔRaf-ER activation in MCF-10A cells (Figure 3, B and C, respectively).
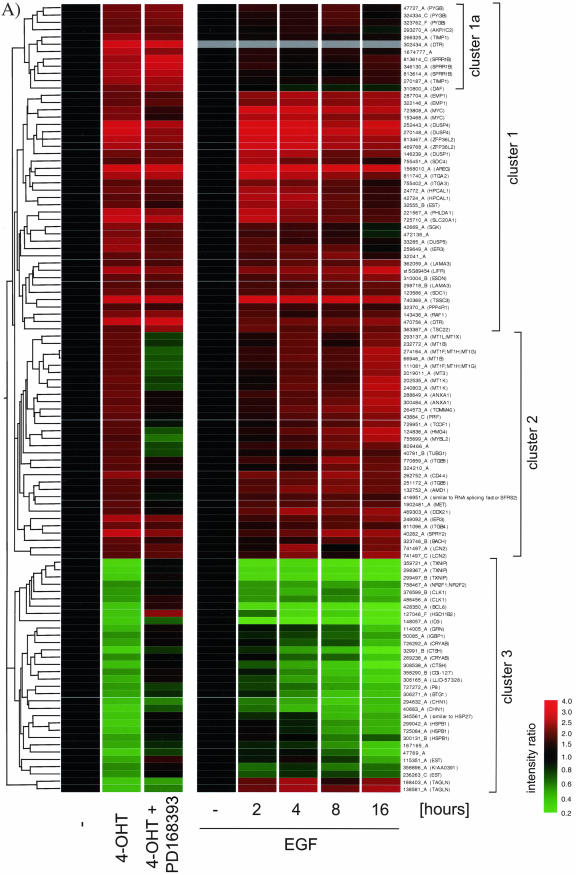
Figure 3.
A large proportion of the transcriptional response to Raf activation is dependent on autocrine activation of the EGF receptor. (A) MCF-10A ΔRaf-ER cells were treated with 100 nM 4-OHT or solvent (control and 0 h) for 24 h in minimal medium in the presence (4-OHT + PD168393) or absence (4-OHT) of 0.7 μM PD168393 in minimal medium. In a parallel experiment, parental MCF-10A cells were grown in minimal medium for 8 h and subsequently treated with 20 ng/ml EGF for 2, 4, 8, or 16 h. Relative mRNA abundance was measured by comparative hybridization of experimental and control samples from two independent experiments to human cDNA microarrays. Mean Intensity ratios relative to the solvent treated control (-) for 108 probes that show significant changes in gene expression upon Raf-ER activation were used to generate hierarchical clusters of genes with similar expression profiles. Note that the results for probe 302434_A (DTR) are absent from the EGF induction data (gray bars). For a detailed description of the clones see Table 3 (supplementary data). (B) Time course of 4-OHT treatment in MCF-10A ΔRaf-ER cells in the presence or absence of PD16839. Phosphorylation of p42ERK2/p44ERK1 was detected using a phospho-specific antibody. (C) MCF-10A cells were treated with 0.7 μu PD 168393 or solvent for 24 h in minimal medium and subsequently stimulated with EGF. Phosphorylation of EGFR was detected using a phospho-specific antibody. (D) EGFR dependent Raf-responsive genes: 44 of the 108 Raf-regulated probes shown in A that show a significant difference in their response to Raf activation in the presence or absence of PD168393 (p < 0.05; Welch ANOVA). (E) EGFR independent Raf-responsive genes: 24 of the 108 Raf-responsive genes shown in A that show no significant difference in their response to Raf activation in the presence or absence of PD16839. Probes that show some difference (p < 0.5; Welch ANOVA) were selected and subtracted from all Raf-responsive probes. The remaining 24 probes were considered to show very similar expression levels between the two samples. Cluster 1 indicates a group of genes that shows up-regulation in response to Raf activation but no change in response to EGF treatment.
Figure 3A shows the effect on gene expression in response to activation of ΔRaf-ER by treating cells with 4-OHT for 24 h in the presence or absence of the selective EGF receptor tyrosine kinase inhibitor PD168393. One hundred eight probes showed significant changes in expression in response to Raf activation in this experiment. In a parallel experiment, cells were treated with soluble EGF for 2, 4, 8, and 16 h, and the expression profile of the 108 Raf-responsive probes is also shown in Figure 3A. Cluster analysis reveals that these probes can be divided into three major groups. Cluster 1 contains genes that are up-regulated in response to Raf activation but are mostly not affected by EGFR inhibition. Clusters 2 and 3 contain genes that are regulated by Raf and are sensitive to EGFR inhibition. The majority of Raf-responsive genes are also regulated by treatment with soluble EGF. Only 13 probes are induced by ΔRaf-ER activation but not by EGF treatment. The seven distinct genes represented by these probes are likely to require the particularly sustained or strong activation of Raf that this construct can provide, rather than the more transient activation of Raf caused by EGF treatment.
To more specifically identify the set of Raf-responsive genes with epidermal growth factor receptor (EGFR)-dependent regulation, we performed a statistical group comparison between the samples in which Raf was activated in the presence or absence of PD168393. Forty-four of these (41% of the total) show a significant difference in expression in cells treated with the EGF receptor inhibitor compared with Raf activation alone (Welch/ANOVA p < 0.05). These are re-clustered and plotted separately in Figure 3D. All of these genes are also induced in cells treated with EGF alone, in the majority of cases within 4 h. It is therefore likely that these changes in gene expression are induced as a result of Raf induction of the activity of the EGF receptor and are not direct targets of the Raf/MAP kinase pathway. It is conceivable, however, that activation of these genes is a direct result of MAP kinase activation but also requires basal activation of EGFR signaling. Although we cannot formally rule out this possibility, it should be noted that basal level of ERK phosphorylation is not affected by inhibition of EGFR (Figure 3B).
Twenty-four of the 108 Raf-induced probes (22% of the total) show no change in expression in cells treated with the EGF receptor tyrosine kinase inhibitor PD168393 (Figure 3E). When these probes were reclustered, it was apparent that about two-thirds (16 of 24) are also induced by EGF treatment: these are likely to be more direct targets of the Raf/MAP kinase pathway for which EGF receptor function is sufficient (as when cells are treated with EGF) but not necessary (if Raf is activated directly as with ΔRaf-ER). Only eight of the EGFR-independent Raf-responsive probes are not induced by EGF treatment alone (cluster1 marked in Figure 3E).
The remaining 40 probes could not be assigned to either of the two groups with significant statistical confidence.
Comparing Figure 3D and 3E, it becomes obvious that the EGFR-independent target genes are all up-regulated after Raf activation. By contrast, the EGFR-dependent Raf target genes are divided between 24 probes that are up-regulated and 20 that are down-regulated. We conclude that it is likely that all Raf-induced down-regulation of gene expression in this system is dependent on the EGF receptor autocrine loop, with EGF receptor-independent Raf-induced gene expression being exclusively up-regulation.
Timing of Raf-induced Gene Expression of EGFR-dependent and -independent Genes
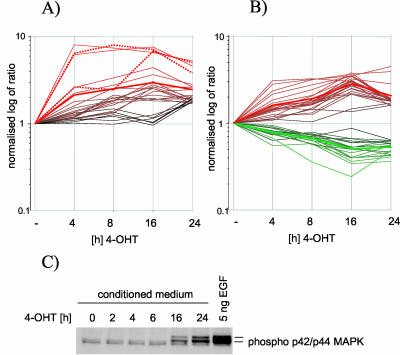
To investigate whether the timing of response to Raf activation differs between EGFR-dependent and -independent genes, we compared the expression profiles of the two groups of genes identified by the experiment shown in Figure 3 in a time course of 4-OHT treatment of MCF-10A ΔRaf-ER cells. Figure 4A shows a graphical representation of the normalized intensity ratios of 44 EGFR-dependent and 24 EGFR-independent probes over time. It seems that the EGFR-independent genes are induced somewhat more rapidly than the EGFR-dependent probes. However, there is no major delay in the induction of the EGFR-dependent genes detectable in the time course performed here, which starts at 4 h. We then investigated at what time after Raf activation soluble EGF-like growth factors can be detected. Conditioned medium from MCF-10A ΔRaf-ER cells treated with 4-OHT for 2, 4, 6, 16, and 24 h was used to stimulate parental cells that had been starved in minimal medium for 24 h. Figure 4C shows that after 16 h, levels of secreted factors are sufficient to activate EGFR signaling, as shown by phosphorylation of ERK/MAP kinase. However, induction of HB-EGF/DTR gene expression is already almost maximal after 4 h (dashed line in Figure 4A), making it likely that the protein is expressed at this time as well. The failure to activate EGFR by conditioned medium is most likely due to low levels of secreted proteins. It has been shown before that the autocrine response can be rapidly induced (McCarthy et al., 1995).
Figure 4.
Differences in timing of the induction of EGFR dependent and independent genes by Raf. EGFR-dependent and -independent genes do not show a significantly different timing in their response to Raf activation. Graphical representation of the expression profiles of EGFR independent (A) and EGFR dependent genes (B) after 4, 8, 16, and 24 h of ΔRaf-ER activation. The vertical axis represents the log of normalized intensity ratios, whereas the horizontal axis shows time of treatment with 4-OHT. Bold red and green lines represent the average intensity ratio of all up- or down-regulated probes at the different time points. Expression profiles of two probes representing HB-EGF (DTR) are shown as dotted lines in A. (C) To detect the onset of secretion of soluble EGF-like growth factors into the medium, MCF-10A ΔRaf-ER cells were treated with 4-OHT in minimal medium for 2, 4, 26, or 24 h. The medium conditioned by these cells was used to induce EGFR activation in parental MCF-10A cells. Parental cells were lysed after 10 min of treatment with conditioned medium and analyzed for p42ERK2/p44ERK1 phosphorylation by using phospho-specific antibodies. MAP kinase phosphorylation in parental MCF-10A cells after stimulation with 5 ng/ml soluble EGF is shown as control.
Similarity between the Transcriptional Response to ΔRaf-ER Activation and Treatment with Soluble EGF
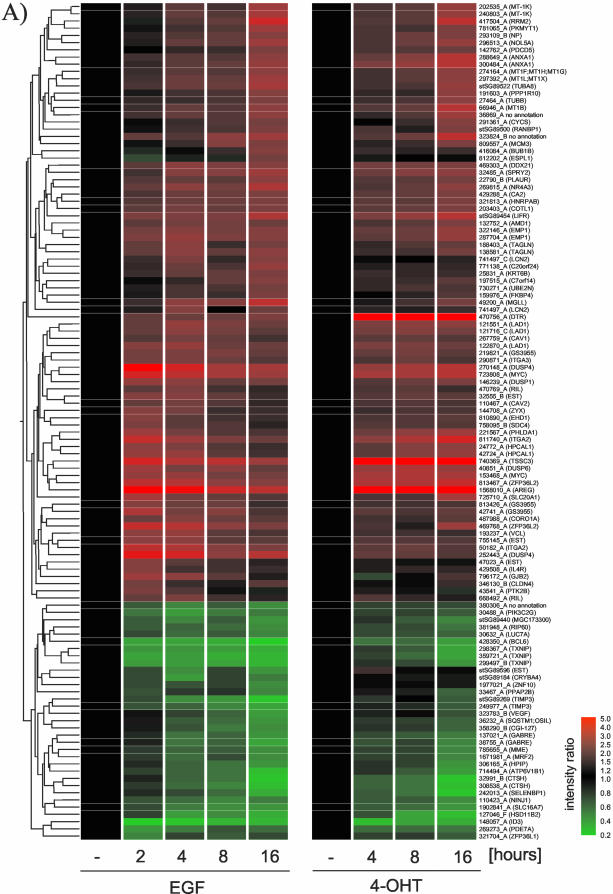
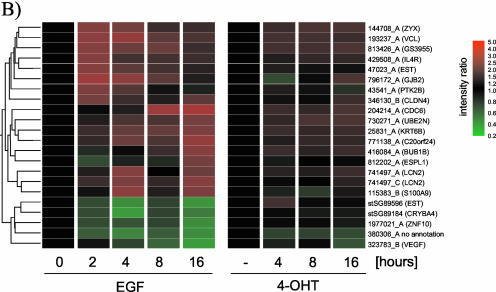
The data presented in Figure 3 indicate that a large proportion of Raf-responsive genes are also regulated by EGF treatment. To identify whether EGF induces genes that are not induced by Raf, we compared expression profiles of 116 probes that show significant regulation in response to EGF treatment with a time course of ΔRaf-ER activation. Figure 5A shows that the majority of EGF regulated genes show a very similar expression pattern in response to Raf activation. However, statistical group comparison revealed 22 probes that are regulated in an EGF-specific manner.
Figure 5.
The transcriptional response to Raf activation and EGF treatment are very similar. (A) Expression data from MCF-10A cells treated with soluble EGF (experiment described in Figure 3) and MCF-10A ΔRaf-ER cells treated with 4-OHT for the indicated times (experiment described in Figure 1) were compared. Mean intensity ratios of 116 probes that show significant changes in gene expression in response to EGF treatment (p > 0.05, Welch ANOVA) were used to create hierarchical clusters of genes with similar expression profiles. For a detailed description of the clones, see Table 4 (supplementary data). (B) Twenty-two of the 116 EGF-responsive probes do not show a response to Raf activation.
The high similarity between the two responses is clearly expected because activation of the Raf/MAP kinase pathway is a major pathway downstream of EGFR. In addition, Raf-independent EGFR effector pathways are activated through the autocrine loop that is induced by ΔRaf-ER activation. These data provide further evidence for the importance of autocrine signaling in the cellular response to activation of the Ras/Raf pathway.
DISCUSSION
The signal transduction pathways controlled by c-Raf-1 and its close relatives A-Raf and B-Raf have been intensively studied, with a large body of evidence accumulating to support the central importance of Raf activation of the mitogen-activated protein kinases ERK1 and ERK2 through its ability to phosphorylate and activate the dual specificity kinases MEK1 and MEK2. Many, although not all, of the targets of ERK are transcription factors or regulators of transcription factors. Activation of Raf has been shown to have profound and rapid effects on gene expression. Although it has been assumed by many that all Raf-1 signaling is mediated through MEK and ERK, a certain amount of evidence to the contrary has arisen recently. A major goal of this report was to use global analysis of gene expression by cDNA microarrays to investigate whether there were signs of MEK-independent signaling in the transcriptional profile induced by activated Raf-1.
A number of reports have claimed that Raf-1 is able to signal independently of MEK. One pathway downstream of Raf-1 that has been discussed as being MEK independent is the activation of NF-κB. Several reports have suggested that activation of NF-κB is essential for cell transformation mediated by activated Ras or Raf-1 (Finco et al., 1997; Baumann et al., 2000a; Vale et al., 2001). One of these reports shows evidence that, in transient transfection assays, NF-κB and IkappaB-kinase 2 activation by Raf-BXB (lacking the amino terminal negative regulatory domain) is not affected by dominant negative mutants of MEK. Instead, they found that Raf-mediated activation of NF-κB was blocked by a dominant negative form of the membrane shuttle kinase MEKK1 (Baumann et al., 2000). Another study showed that a mutation in the Raf-1 protein (T481A) that disrupts its ability to bind to MEK1 is still able to activate NF-κB reporters (Pearson et al., 2000). In addition, two reports indicate that Raf-1-dependent activation of NF-κB involves autocrine signaling either through interleukin-1 (Vale et al., 2001) or EGF receptor signaling (Troppmair et al., 1998).
It is very likely that activation of NF-κB may involve different mechanisms in different cell types. However, it is also striking that all studies mentioned above used transient transfection of activated Raf-1. In the MCF-10A cells used here that stably express ΔRaf-ER, we did not observe any activation of a NF-κB reporter construct in response to Raf activation. In addition, activation of ΔRaf-ER was not sufficient to induce growth in soft agar. This does not seem to be restricted to the ΔRaf-ER construct because expression of an oncogenic mutant of B-Raf (B-RafV599E) also did not induce soft agar growth in these cells (A.Schulze, unpublished observation). This is consistent with the finding that activation of the Raf/MEK/MAP kinase pathway by oncogenic Ras is necessary but not sufficient for transformation of human epithelial cell lines (Oldham et al., 1996; Gire et al., 1999; Pruitt et al., 2002).
A second MEK-independent target for Raf-1 that has been proposed is ASK1 (Chen et al., 2001). This is a MAP kinase kinase kinase capable of activating c-Jun NH2-terminal kinase and p38 (Wang et al., 1996b; Ichijo et al., 1997), stress-activated protein kinases that are implicated in induction of apoptosis under certain circumstances. Inhibition of ASK1 induced apoptosis by coexpression of wild-type Raf-1 in a transient transfection assay in HeLa cells was insensitive to the MEK inhibitor PD98059. This action of Raf-1 was found to be independent of the kinase activity of Raf-1 but mediated by direct interaction with the amino-terminal region of ASK1. Although the binding site on Raf-1 has not been mapped, it is possible that it is not present in the ΔRaf-ER construct used here. Because inactivation of ASK1 was independent of Raf-1 kinase activity, it is also possible that the effect would not be obvious in the experimental situation used here where only the kinase activity of Raf is induced port-translationally but the protein is constitutively expressed. It seems also likely that inactivation of ASK1 by Raf-1 may be more apparent in Fas induced apoptosis or in situations in which cells are exposed to environmental stress such as reactive oxygen species or heat shock.
Two studies of mice in which the c-raf-1 gene has been knocked out have also provided evidence for MEK-independent signaling downstream of Raf-1 (Hüser et al., 2001; Mikula et al., 2001). These mice were growth retarded and die in midgestation. They showed elevated levels of apoptosis in many embryonic tissues. Hematopoietic and fibroblastic cells from these mice showed increased sensitivity to a number of apoptotic stimuli. However, regulation of MEK and ERK in these cells seemed to be normal, leading the authors to conclude that B-Raf was able to control MEK and ERK adequately in the Raf-1 knockout cells and that the effect of Raf-1 on apoptosis did not require MEK and ERK signaling. Hüser et al. (2001) also made mice in which a mutant form of Raf-1, Y340F/Y341F (“FF”) had been knocked in. This mutant Raf-1 allowed normal development of the mice and reversed the apoptotic phenotype in fibroblasts, but it was not able to activate MEK in an in vitro assay.
In both studies, the conclusion that Raf-1 acts independent of MEK is somewhat circumstantial. It cannot be excluded that there are subtle differences in the activation of MEK and ERK in Raf-1 -/- mouse embryo fibroblasts that are not detected in the assays performed: for example, only high doses of growth factors are applied and only for relatively short time courses. Under other conditions, possibly more relevant to the in vivo situation, B-Raf may not be able to fully compensate for Raf-1 in the activation of MEK. For the control of apoptosis, it may be that basal levels of ERK activity are critical and these are less easily measured. The data with the Raf-1 FF mutant (Hüser et al., 2001) need to be interpreted with caution because this mutant has been reported to be able to phosphorylate and activate MEK in a number of assays (Fabian et al., 1993; Bosch et al., 1997; Woods et al., 1997). These issues could be resolved by the generation of c-Raf-1 kinase dead mutant knockin mice.
One issue that needs to be emphasized is that whereas our data show no sign of MEK-independent transcriptional regulation downstream of Raf-1, they do not rule out direct effects that do not impact on transcription. It is possible that Raf-1 targets an antiapoptotic mechanism that does not regulate transcription, at least not under the conditions used here. Slight changes in gene expression that are effective only over time could have a major impact in vivo but are below detection limit in vitro. It also has to be stated that any potential MEK-independent Raf-1 targets were not among in the 6000 or so human genes represented on the microarray used in our study.
Another potential limitation of this study is that the data presented here on the role of MEK in Raf-1 signaling rely entirely on the use of the MEK inhibitory drug PD98059. These compounds have been reported to be highly specific for MEK1 and MEK2 (Alessi et al., 1995; Dudley et al., 1995; Favata et al., 1998), differing from most kinase inhibitors in not targeting the conserved ATP-binding site. However, more recently evidence has been reported that these drugs are able to inhibit the related kinase MEK5 (Kamakura et al., 1999), which is an activator of ERK5. It cannot therefore be ruled out that some of the PD98059-sensitive effects of Raf-1 activation on transcription seen here are due to MEK5 and ERK5 signaling and not just MEK1/2 and ERK1/2 signaling. On the other hand, we were unable to see any effects of Raf-1 activation on ERK5 phosphorylation in the ΔRaf-ER MCF-10A system (P.Warne and J.Downward, unpublished observations). However, the use of a related MEK-inhibitor (CI-1040) in clinical trials for cancer therapy (Herrera and Sebolt-Leopold, 2002) makes their mechanism of action of interest per se.
Our data also demonstrate that a significant proportion of the Raf-1-induced transcriptional changes are dependent on the function of the EGF receptor. Changes in gene expression of ∼40% of Raf responsive genes can be blocked by the EGF receptor inhibitor PD168393. This is most likely to reflect the effect of a Raf-induced EGF autocrine loop. EGF-like growth factors, namely, HB-EGF, amphiregulin, and transforming growth factor-α have been shown to be transcriptional targets of the Raf/MAP kinase pathway (McCarthy et al., 1995; Schulze et al., 2001). From the experiments shown in this study, we cannot formally exclude that some of the EGF receptor-dependent Raf-1-responsive genes are controlled by parallel pathways that require basal EGF receptor kinase activity in addition to activation of the Raf/MAP kinase pathway. However, PD168393 had little effect on transcription in the absence of Raf-1 or EGF stimulation (A.Schulze and J.Downward, unpublished observations), suggesting that such effects may be marginal.
The fact that Raf-1 stimulation can induce an EGF autocrine loop might lead one to expect that the effects of Raf-1 activation and EGF stimulation should be identical. Although the overall effect of EGF stimulation is clearly similar to Raf-1 activation, there are some differences, with EGF inducing a number of genes that are not significantly affected by Raf-1 induction (as shown in Figure 5B). Presumably this reflects the fact that induction of expression of autocrine EGF family members such as HB-EGF is likely to have a very different effect on cells from acute stimulation with saturating levels of soluble EGF. In the latter case, EGF receptors are maximally stimulated initially then rapidly down-regulate. In the former situation, levels of EGF ligand build up slowly over prolonged periods. Furthermore, these ligands are initially presented as membrane bound molecules at the cell surface, which are subsequently proteolytically processed to soluble forms (Goishi et al., 1995). Membrane-bound ligand has different signaling properties compared with the soluble form and may also be down-regulated with different kinetics. As a consequence, the amplitude and time course of autocrine EGF family signaling is likely to be profoundly different from that of added soluble EGF signaling, resulting in both qualitative and quantitative differences in transcriptional activity. In addition some of the EGF family members produced show different specificity for members of the EGF receptor family from EGF itself; for example, HB-EGF is a better activator of erbB4 than is EGF (Jones et al., 1999).
An interesting feature of the EGF receptor-dependent component of the Raf-1-induced transcriptional response is that it seems to include all of the Raf-1-induced down-regulation of gene expression. The EGF receptor-independent Raf-1 transcriptional response is all up-regulation, whereas the EGF receptor-dependent Raf-1 transcriptional response is about one-half up-regulation and half down-regulation. It is possible that some of these genes are targets of transcription factors such as the FoxO family of Forkhead factors that are known to be negatively regulated by PI 3-kinase, which is activated downstream of the EGF receptor. Indeed, two of the down-regulated EGF receptor-dependent genes, bcl-6 and IGFBP1, are known targets of FoxO transcription factors (Tang et al., 1999, 2002). In addition, three of the remaining genes contain FoxO binding sites in their promoter regions as determined by sequence analysis (A.Schulze and J.Downward, unpublished data). It will be interesting to investigate whether more of these genes are indeed regulated by a PI3-kinase/Akt/Forkhead pathway. Other pathways that regulate transcription downstream of the EGF receptor involve the signal transducer and activator of transcription family of transcription factors. Using sequence analysis tools, we are currently investigating whether we can identify differences in the presence of binding sites for transcription factors that are overrepresented in the different groups of coregulated genes.
The use of transcriptional profiling in the way described here allows a detailed analysis of the architecture of signaling pathways to be undertaken, allowing the study of bifurcation points and autocrine signaling, and potentially also feedback loops and cross-talk between pathways. In our study, although restricted to about one-fifth of the total number of human genes, we did not find any evidence for a major MEK-independent Raf effector pathway that impacts on transcription. Furthermore, the strong similarity between the response to Raf-1 activation and EGF treatment and the high fraction of EGF receptor-dependent responses underlines the importance of autocrine signaling in Ras/Raf/MAP kinase signaling.
Supplementary Material
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-11-0807. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-11-0807.
Online version of this article contains supporting material. Online version is available at www.molbiolcell.org.
References
- Alessi, D.R., Cuenda, A., Cohen, P., Dudley, D.T., and Saltiel, A.R. (1995). PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 270, 27489-27494. [DOI] [PubMed] [Google Scholar]
- Baumann, B., Weber, C.K., Troppmair, J., Whiteside, S., Israel, A., Rapp, U.R., and Wirth, T. (2000). Raf induces NF-kappaB by membrane shuttle kinase MEKK1, a signaling pathway critical for transformation. Proc. Natl. Acad. Sci. USA 97, 4615-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonni, A., Brunet, A., West, A.E., Datta, S.R., Takasu, M.A., and Greenberg, M.E. (1999). Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 286, 1358-1362. [DOI] [PubMed] [Google Scholar]
- Bos, J.L. (1989). ras oncogenes in human cancer: a review. Cancer Res. 49, 4682-4689. [PubMed] [Google Scholar]
- Bosch, E., Cherwinski, H., Peterson, D., and McMahon, M. (1997). Mutations of critical amino acids affect the biological and biochemical properties of oncogenic A-Raf and Raf-1. Oncogene 15, 1021-1033. [DOI] [PubMed] [Google Scholar]
- Chen, J., Fujii, K., Zhang, L., Roberts, T., and Fu, H. (2001). Raf-1 promotes cell survival by antagonizing apoptosis signal-regulating kinase 1 through a MEK-ERK independent mechanism. Proc. Natl. Acad. Sci. USA 98, 7783-7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Larco, J.E., and Todaro, G.J. (1978). Growth factors from murine sarcoma virus-transformed cells. Proc. Natl. Acad. Sci. USA 75, 4001-4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward, J. (1998). Ras signalling and apoptosis. Curr. Opin. Genet. Dev. 8, 49-54. [DOI] [PubMed] [Google Scholar]
- Dudley, D.T., Pang, L., Decker, S.J., Bridges, A.J., and Saltiel, A.R. (1995). A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc. Natl. Acad. Sci. USA 92, 7686-7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt, P., Schremser, E.J., and Cooper, G.M. (1999). B-Raf inhibits programmed cell death downstream of cytochrome c release from mitochondria by activating the MEK/Erk pathway. Mol. Cell. Biol. 19, 5308-5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian, J.R., Daar, I.O., and Morrison, D.K. (1993). Critical tyrosine residues regulate the enzymatic and biological activity of Raf-1 kinase. Mol. Cell. Biol. 13, 7170-7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favata, M.F., et al. (1998). Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 273, 18623-18632. [DOI] [PubMed] [Google Scholar]
- Finco, T.S., Westwick, J.K., Norris, J.L., Beg, A.A., Der, C.J., and Baldwin, A.S., Jr. (1997). Oncogenic Ha-Ras-induced signaling activates NF-kappaB transcriptional activity, which is required for cellular transformation. J. Biol. Chem. 272, 24113-24116. [DOI] [PubMed] [Google Scholar]
- Fry, D.W., Bridges, A.J., Denny, W.A., Doherty, A., Greis, K.D., Hicks, J.L., Hook, K.E., Keller, P.R., Leopold, W.R., Loo, J.A., et al. (1998). Specific, irreversible inactivation of the epidermal growth factor receptor and erbB2, by a new class of tyrosine kinase inhibitor. Proc. Natl. Acad. Sci. USA 95, 12022-12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gire, V., Marshall, C.J., and Wynford-Thomas, D. (1999). Activation of mitogen-activated protein kinase is necessary but not sufficient for proliferation of human thyroid epithelial cells induced by mutant Ras. Oncogene 18, 4819-4832. [DOI] [PubMed] [Google Scholar]
- Goishi, K., Higashiyama, S., Klagsbrun, M., Nakano, N., Umata, T., Ishikawa, M., Mekada, E., and Taniguchi, N. (1995). Phorbol ester induces the rapid processing of cell surface heparin-binding EGF-like growth factor: conversion from juxtacrine to paracrine growth factor activity. Mol. Biol. Cell 6, 967-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera, R., and Sebolt-Leopold, J.S. (2002). Unraveling the complexities of the Raf/MAP kinase pathway for pharmacological intervention. Trends Mol. Med. 8, S27-S31. [DOI] [PubMed] [Google Scholar]
- Hüser, M., Luckett, J., Chiloeches, A., Mercer, K., Iwobi, M., Giblett, S., Sun, X.M., Brown, J., Marais, R., and Pritchard, C. (2001). MEK kinase activity is not necessary for Raf-1 function. EMBO J. 20, 1940-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichijo, H., Nishida, E., Irie, K., ten Dijke, P., Saitoh, M., Moriguchi, T., Takagi, M., Matsumoto, K., Miyazono, K., and Gotoh, Y. (1997). Induction of apoptosis by A.S.K1, a mammalian M.A.P.K.K.K. that activates S.A.P.K/J.N.K. and p38 signaling pathways. Science 275, 90-94. [DOI] [PubMed] [Google Scholar]
- Jones, J.T., Akita, R.W., and Sliwkowski, M.X. (1999). Binding specificities and affinities of EGF domains for ErbB receptors. FEBS Lett. 447, 227-231. [DOI] [PubMed] [Google Scholar]
- Kamakura, S., Moriguchi, T., and Nishida, E. (1999). Activation of the protein kinase ERK5/BMK1 by receptor tyrosine kinases. Identification and characterization of a signaling pathway to the nucleus. J. Biol. Chem. 274, 26563-26571. [DOI] [PubMed] [Google Scholar]
- Kazama, H., and Yonehara, S. (2000). Oncogenic K-Ras and basic fibroblast growth factor prevent Fas-mediated apoptosis in fibroblasts through activation of mitogen-activated protein kinase. J. Cell Biol. 148, 557-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolch, W. (2000). Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem. J. 351, 289-305. [PMC free article] [PubMed] [Google Scholar]
- Le Gall, M., Chambard, J.C., Breittmayer, J.P., Grall, D., Pouyssegur, J., and Van Obberghen-Schilling, E. (2000). The p42/p44 MAP kinase pathway prevents apoptosis induced by anchorage and serum removal. Mol. Biol. Cell 11, 1103-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leevers, S.J., Paterson, H.F., and Marshall, C.J. (1994). Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature 369, 411-414. [DOI] [PubMed] [Google Scholar]
- Lehmann, K., Janda, E., Pierreux, C.E., Rytomaa, M., Schulze, A., McMahon, M., Hill, C.S., Beug, H., and Downward, J. (2000). Raf induces TGFbeta production while blocking its apoptotic but not invasive responses: a mechanism leading to increased malignancy in epithelial cells. Genes Dev. 14, 2610-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S., and Sedivy, J.M. (1993). Raf-1 protein kinase activates the NF-kappa B transcription factor by dissociating the cytoplasmic NF-kappa B-I kappa B complex. Proc. Natl. Acad. Sci. USA 90, 9247-9251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais, R., and Marshall, C.J. (1996). Control of the ERK MAP kinase cascade by Ras and Raf. Cancer Surv. 27, 101-125. [PubMed] [Google Scholar]
- Marshall, C. (1999). How do small GTPase signal transduction pathways regulate cell cycle entry? Curr. Opin. Cell Biol. 11, 732-736. [DOI] [PubMed] [Google Scholar]
- Marshall, C.J. (1996). Ras effectors. Curr. Opin. Cell Biol. 8, 197-204. [DOI] [PubMed] [Google Scholar]
- McCarthy, S.A., Samuels, M.L., Pritchard, C.A., Abraham, J.A., and McMahon, M. (1995). Rapid induction of heparin-binding epidermal growth factor/diphtheria toxin receptor expression by Raf and Ras oncogenes. Genes Dev. 9, 1953-1964. [DOI] [PubMed] [Google Scholar]
- Mikula, M., Schreiber, M., Husak, Z., Kucerova, L., Ruth, J., Wieser, R., Zatloukal, K., Beug, H., Wagner, E.F., and Baccarini, M. (2001). Embryonic lethality and fetal liver apoptosis in mice lacking the c-raf-1 gene. EMBO J. 20, 1952-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham, S.M., Clark, G.J., Gangarosa, L.M., Coffey, R.J., Jr., and Der, C.J. (1996). Activation of the Raf-1/MAP kinase cascade is not sufficient for Ras transformation of RIE-1 epithelial cells. Proc. Natl. Acad. Sci. USA 93, 6924-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahl, H.L. (1999). Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 18, 6853-6866. [DOI] [PubMed] [Google Scholar]
- Pearson, G., Bumeister, R., Henry, D.O., Cobb, M.H., and White, M.A. (2000). Uncoupling Raf1 from MEK1/2 impairs only a subset of cellular responses to Raf activation. J. Biol. Chem. 275, 37303-37306. [DOI] [PubMed] [Google Scholar]
- Pruitt, K., Pruitt, W.M., Bilter, G.K., Westwick, J.K., and Der, C.J. (2002). Raf-independent deregulation of p38 and JNK mitogen-activated protein kinases are critical for Ras transformation. J. Biol. Chem. 277, 31808-31817. [DOI] [PubMed] [Google Scholar]
- Sa, G., Murugesan, G., Jaye, M., Ivashchenko, Y., and Fox, P.L. (1995). Activation of cytosolic phospholipase A2 by basic fibroblast growth factor via a p42 mitogen-activated protein kinase-dependent phosphorylation pathway in endothelial cells. J. Biol. Chem. 270, 2360-2366. [DOI] [PubMed] [Google Scholar]
- Schulze, A., Lehmann, K., Jefferies, H.B., McMahon, M., and Downward, J. (2001). Analysis of the transcriptional program induced by Raf in epithelial cells. Genes Dev. 15, 981-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibilia, M., Fleischmann, A., Behrens, A., Stingl, L., Carroll, J., Watt, F.M., Schlessinger, J., and Wagner, E.F. (2000). The EGF receptor provides an essential survival signal for SOS-dependent skin tumor development. Cell 102, 211-220. [DOI] [PubMed] [Google Scholar]
- Soule, H.D., Maloney, T.M., Wolman, S.R., Peterson, W.D., Jr., Brenz, R., McGrath, C.M., Russo, J., Pauley, R.J., Jones, R.F., and Brooks, S.C. (1990). Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 50, 6075-6086. [PubMed] [Google Scholar]
- Tang, E.D., Nunez, G., Barr, F.G., and Guan, K.L. (1999). Negative regulation of the forkhead transcription factor FKHR by Akt. J. Biol. Chem. 274, 16741-16746. [DOI] [PubMed] [Google Scholar]
- Tang, T.T., Dowbenko, D., Jackson, A., Toney, L., Lewin, D.A., Dent, A.L., and Lasky, L.A. (2002). The forkhead transcription factor AFX activates apoptosis by induction of the BCL-6 transcriptional repressor. J. Biol. Chem. 277, 14255-14265. [DOI] [PubMed] [Google Scholar]
- Treisman, R. (1996). Regulation of transcription by MAP kinase cascades. Curr. Opin. Cell Biol. 8, 205-215. [DOI] [PubMed] [Google Scholar]
- Troppmair, J., Hartkamp, J., and Rapp, U.R. (1998). Activation of NF-kappa B by oncogenic Raf in HEK 293 cells occurs through autocrine recruitment of the stress kinase cascade. Oncogene 17, 685-690. [DOI] [PubMed] [Google Scholar]
- Tsao, S.W., Mok, S.C., Fey, E.G., Fletcher, J.A., Want, T.S., Chew, E.C., Muto, M.G., Knapp, R.C., and Berkowitz, R.S. (1995). Characterization of human ovarian surface epithelial cells immortalized by human papilloma viral oncogenes (HPV-E6E7 ORFs). Exp. Cell Res. 218, 499-507. [DOI] [PubMed] [Google Scholar]
- Vale, T., Ngo, T.T., White, M.A., and Lipsky, P.E. (2001). Raf-induced transformation requires an interleukin 1 autocrine loop. Cancer Res 61, 602-607. [PubMed] [Google Scholar]
- Wang, H.G., Rapp, U.R., and Reed, J.C. (1996a). Bcl-2 targets the protein kinase Raf-1 to mitochondria. Cell 87, 629-638. [DOI] [PubMed] [Google Scholar]
- Wang, X.S., Diener, K., Jannuzzi, D., Trollinger, D., Tan, T.H., Lichenstein, H., Zukowski, M., and Yao, Z. (1996b). Molecular cloning and characterization of a novel protein kinase with a catalytic domain homologous to mitogen-activated protein kinase kinase kinase. J. Biol. Chem. 271, 31607-31611. [DOI] [PubMed] [Google Scholar]
- Woods, D., Parry, D., Cherwinski, H., Bosch, E., Lees, E., and McMahon, M. (1997). Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21Cip1. Mol. Cell. Biol. 17, 5598-5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.