Abstract
Procarboxypeptidase B is converted to enzymatically active carboxypeptidase B by limited proteolysis catalysed by trypsin, removing the long N-terminal activation segment of 95 amino acids. The three-dimensional crystal structure of procarboxypeptidase B from porcine pancreas has been determined at 2.3 A resolution and refined to a crystallographic R-factor of 0.169. The functional determinants of its enzymatic inactivity and of its activation by limited proteolysis have thus been unveiled. The activation segment folds in a globular region with an open sandwich antiparallel-alpha antiparallel-beta topology and in a C terminal alpha-helix which connects it to the enzyme moiety. The globular region (A7-A82) shields the preformed active site, and establishes specific interactions with residues important for substrate recognition. AspA41 forms a salt bridge with Arg145, which in active carboxypeptidase binds the C-terminal carboxyl group of substrate molecules. The connecting region occupies the putative extended substrate binding site. The scissile peptide bond cleaved by trypsin during activation is very exposed. Its cleavage leads to the release of the activation segment and to exposure of the substrate binding site. An open-sandwich folding has been observed in a number of other proteins and protein domains. One of them is the C-terminal fragment of L7/L12, a ribosomal protein from Escherichia coli that displays a topology similar to the activation domain of procarboxypeptidase.
Full text
PDF



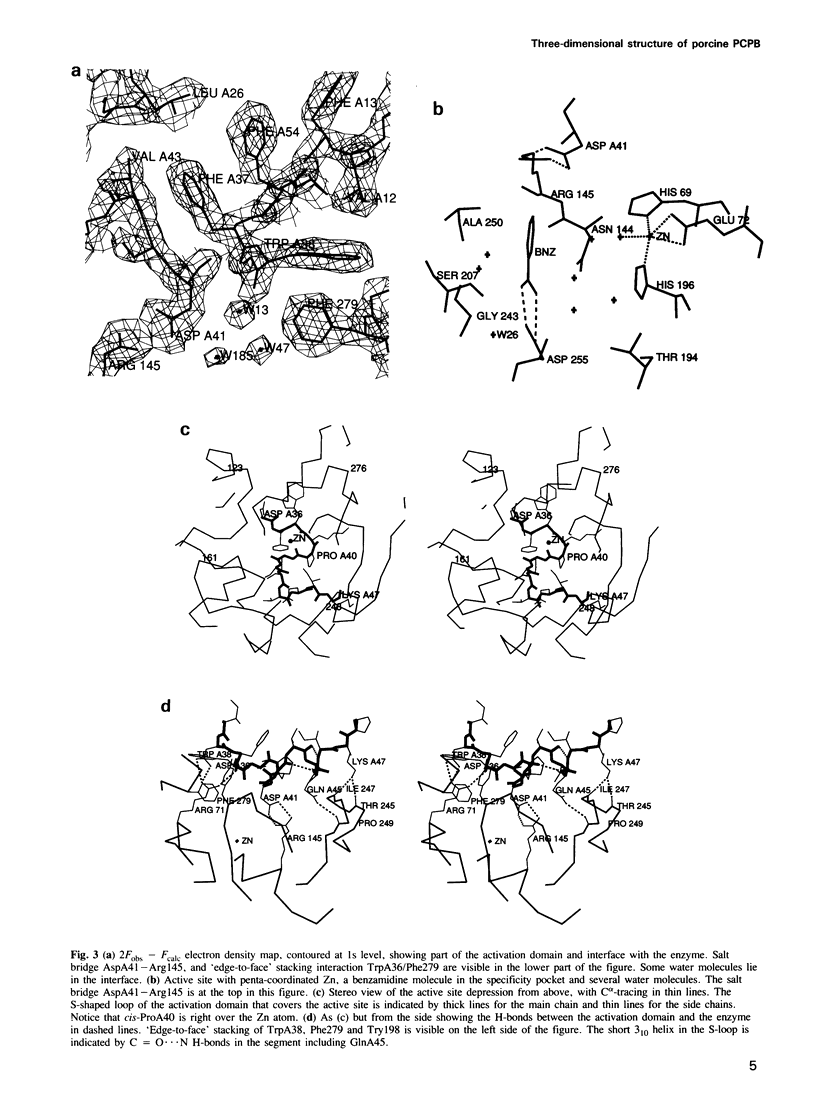




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramowitz N., Schechter I., Berger A. On the size of the active site in proteases. II. Carboxypeptidase-A. Biochem Biophys Res Commun. 1967 Dec 29;29(6):862–867. doi: 10.1016/0006-291x(67)90299-9. [DOI] [PubMed] [Google Scholar]
- Clauser E., Gardell S. J., Craik C. S., MacDonald R. J., Rutter W. J. Structural characterization of the rat carboxypeptidase A1 and B genes. Comparative analysis of the rat carboxypeptidase gene family. J Biol Chem. 1988 Nov 25;263(33):17837–17845. [PubMed] [Google Scholar]
- Freer S. T., Kraut J., Robertus J. D., Wright H. T., Xuong N. H. Chymotrypsinogen: 2.5-angstrom crystal structure, comparison with alpha-chymotrypsin, and implications for zymogen activation. Biochemistry. 1970 Apr 28;9(9):1997–2009. doi: 10.1021/bi00811a022. [DOI] [PubMed] [Google Scholar]
- Fricker L. D. Carboxypeptidase E. Annu Rev Physiol. 1988;50:309–321. doi: 10.1146/annurev.ph.50.030188.001521. [DOI] [PubMed] [Google Scholar]
- Gardell S. J., Craik C. S., Hilvert D., Urdea M. S., Rutter W. J. Site-directed mutagenesis shows that tyrosine 248 of carboxypeptidase A does not play a crucial role in catalysis. Nature. 1985 Oct 10;317(6037):551–555. doi: 10.1038/317551a0. [DOI] [PubMed] [Google Scholar]
- Gebhard W., Schube M., Eulitz M. cDNA cloning and complete primary structure of the small, active subunit of human carboxypeptidase N (kininase 1). Eur J Biochem. 1989 Jan 2;178(3):603–607. doi: 10.1111/j.1432-1033.1989.tb14488.x. [DOI] [PubMed] [Google Scholar]
- Hol W. G., Halie L. M., Sander C. Dipoles of the alpha-helix and beta-sheet: their role in protein folding. Nature. 1981 Dec 10;294(5841):532–536. doi: 10.1038/294532a0. [DOI] [PubMed] [Google Scholar]
- Kim H., Lipscomb W. N. Crystal structure of the complex of carboxypeptidase A with a strongly bound phosphonate in a new crystalline form: comparison with structures of other complexes. Biochemistry. 1990 Jun 12;29(23):5546–5555. doi: 10.1021/bi00475a019. [DOI] [PubMed] [Google Scholar]
- Lacko A. G., Neurath H. Studies on procarboxypeptidase A and carboxypeptidase A of the spiny pacific dogfish (Squalus acanthias). Biochemistry. 1970 Nov 24;9(24):4680–4690. doi: 10.1021/bi00826a010. [DOI] [PubMed] [Google Scholar]
- Lattman E. Diffraction methods for biological macromolecules. Use of the rotation and translation functions. Methods Enzymol. 1985;115:55–77. doi: 10.1016/0076-6879(85)15007-x. [DOI] [PubMed] [Google Scholar]
- Leijonmarck M., Liljas A. Structure of the C-terminal domain of the ribosomal protein L7/L12 from Escherichia coli at 1.7 A. J Mol Biol. 1987 Jun 5;195(3):555–579. doi: 10.1016/0022-2836(87)90183-5. [DOI] [PubMed] [Google Scholar]
- Levin Y., Skidgel R. A., Erdös E. G. Isolation and characterization of the subunits of human plasma carboxypeptidase N (kininase i). Proc Natl Acad Sci U S A. 1982 Aug;79(15):4618–4622. doi: 10.1073/pnas.79.15.4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath H. Evolution of proteolytic enzymes. Science. 1984 Apr 27;224(4647):350–357. doi: 10.1126/science.6369538. [DOI] [PubMed] [Google Scholar]
- Neurath H. Proteolytic processing and physiological regulation. Trends Biochem Sci. 1989 Jul;14(7):268–271. doi: 10.1016/0968-0004(89)90061-3. [DOI] [PubMed] [Google Scholar]
- Quiocho F. A., Lipscomb W. N. Carboxypeptidase A: a protein and an enzyme. Adv Protein Chem. 1971;25:1–78. doi: 10.1016/s0065-3233(08)60278-8. [DOI] [PubMed] [Google Scholar]
- Reeck G. R., Neurath H. Isolation and characterization of pancreatic procarboxypeptidase B and carboxypeptidase B of the African lungfish. Biochemistry. 1972 Oct 10;11(21):3947–3955. doi: 10.1021/bi00771a018. [DOI] [PubMed] [Google Scholar]
- Rees D. C., Lewis M., Lipscomb W. N. Refined crystal structure of carboxypeptidase A at 1.54 A resolution. J Mol Biol. 1983 Aug 5;168(2):367–387. doi: 10.1016/s0022-2836(83)80024-2. [DOI] [PubMed] [Google Scholar]
- Rees D. C., Lipscomb W. N. Binding of ligands to the active site of carboxypeptidase A. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5455–5459. doi: 10.1073/pnas.78.9.5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. C., Lipscomb W. N. Refined crystal structure of the potato inhibitor complex of carboxypeptidase A at 2.5 A resolution. J Mol Biol. 1982 Sep 25;160(3):475–498. doi: 10.1016/0022-2836(82)90309-6. [DOI] [PubMed] [Google Scholar]
- Remington S., Wiegand G., Huber R. Crystallographic refinement and atomic models of two different forms of citrate synthase at 2.7 and 1.7 A resolution. J Mol Biol. 1982 Jun 15;158(1):111–152. doi: 10.1016/0022-2836(82)90452-1. [DOI] [PubMed] [Google Scholar]
- Reynolds D. S., Gurley D. S., Stevens R. L., Sugarbaker D. J., Austen K. F., Serafin W. E. Cloning of cDNAs that encode human mast cell carboxypeptidase A, and comparison of the protein with mouse mast cell carboxypeptidase A and rat pancreatic carboxypeptidases. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9480–9484. doi: 10.1073/pnas.86.23.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez C., Brayton K. A., Brownstein M., Dixon J. E. Rat preprocarboxypeptidase H. Cloning, characterization, and sequence of the cDNA and regulation of the mRNA by corticotropin-releasing factor. J Biol Chem. 1989 Apr 5;264(10):5988–5995. [PubMed] [Google Scholar]
- Scheele G. A. Two-dimensional gel analysis of soluble proteins. Charaterization of guinea pig exocrine pancreatic proteins. J Biol Chem. 1975 Jul 25;250(14):5375–5385. [PubMed] [Google Scholar]
- Schmid M. F., Herriott J. R. Structure of carboxypeptidase B at 2-8 A resolution. J Mol Biol. 1976 May 5;103(1):175–190. doi: 10.1016/0022-2836(76)90058-9. [DOI] [PubMed] [Google Scholar]
- Segundo B. S., Martínez M. C., Vilanova M., Cuchillo C. M., Avilés F. X. The severed activation segment of porcine pancreatic procarboxypeptidase A is a powerful inhibitor of the active enzyme. Isolation and characterisation of the activation peptide. Biochim Biophys Acta. 1982 Sep 22;707(1):74–80. doi: 10.1016/0167-4838(82)90398-3. [DOI] [PubMed] [Google Scholar]
- Skidgel R. A. Basic carboxypeptidases: regulators of peptide hormone activity. Trends Pharmacol Sci. 1988 Aug;9(8):299–304. doi: 10.1016/0165-6147(88)90015-6. [DOI] [PubMed] [Google Scholar]
- Skidgel R. A., Davis R. M., Tan F. Human carboxypeptidase M. Purification and characterization of a membrane-bound carboxypeptidase that cleaves peptide hormones. J Biol Chem. 1989 Feb 5;264(4):2236–2241. [PubMed] [Google Scholar]
- Tan F., Chan S. J., Steiner D. F., Schilling J. W., Skidgel R. A. Molecular cloning and sequencing of the cDNA for human membrane-bound carboxypeptidase M. Comparison with carboxypeptidases A, B, H, and N. J Biol Chem. 1989 Aug 5;264(22):13165–13170. [PubMed] [Google Scholar]
- Titani K., Ericsson L. H., Walsh K. A., Neurath H. Amino-acid sequence of bovine carboxypeptidase B. Proc Natl Acad Sci U S A. 1975 May;72(5):1666–1670. doi: 10.1073/pnas.72.5.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendrell J., Avilés F. X., Genescà E., San Segundo B., Soriano F., Méndez E. Primary structure of the activation segment of procarboxypeptidase A from porcine pancreas. Biochem Biophys Res Commun. 1986 Dec 15;141(2):517–523. doi: 10.1016/s0006-291x(86)80203-0. [DOI] [PubMed] [Google Scholar]
- Vendrell J., Cuchillo C. M., Avilés F. X. The tryptic activation pathway of monomeric procarboxypeptidase A. J Biol Chem. 1990 Apr 25;265(12):6949–6953. [PubMed] [Google Scholar]
- Vendrell J., Wider G., Avilés F. X., Wüthrich K. Sequence-specific 1H NMR assignments and determination of the secondary structure for the activation domain isolated from pancreatic procarboxypeptidase B. Biochemistry. 1990 Aug 14;29(32):7515–7522. doi: 10.1021/bi00484a600. [DOI] [PubMed] [Google Scholar]
- Vilanova M., Burgos F. J., Cuchillo C. M., Avilés F. X. Urea-gradient gel electrophoresis studies on the association of procarboxypeptidases A and B, proproteinase E, and their tryptic activation products. FEBS Lett. 1985 Oct 28;191(2):273–277. doi: 10.1016/0014-5793(85)80023-5. [DOI] [PubMed] [Google Scholar]
- Zisapel N., Sokolovsky M. On the interaction of esters and peptides with carboxypeptidase B. Eur J Biochem. 1975 Jun;54(2):541–547. doi: 10.1111/j.1432-1033.1975.tb04167.x. [DOI] [PubMed] [Google Scholar]



