Abstract
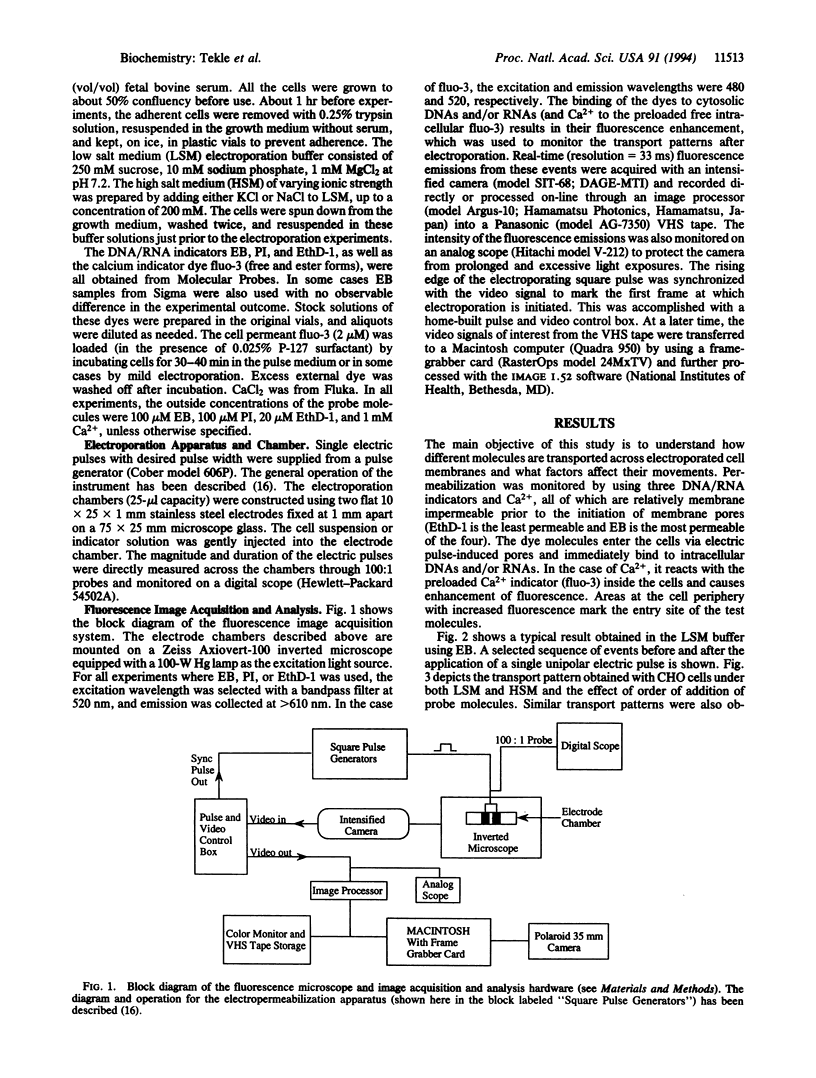
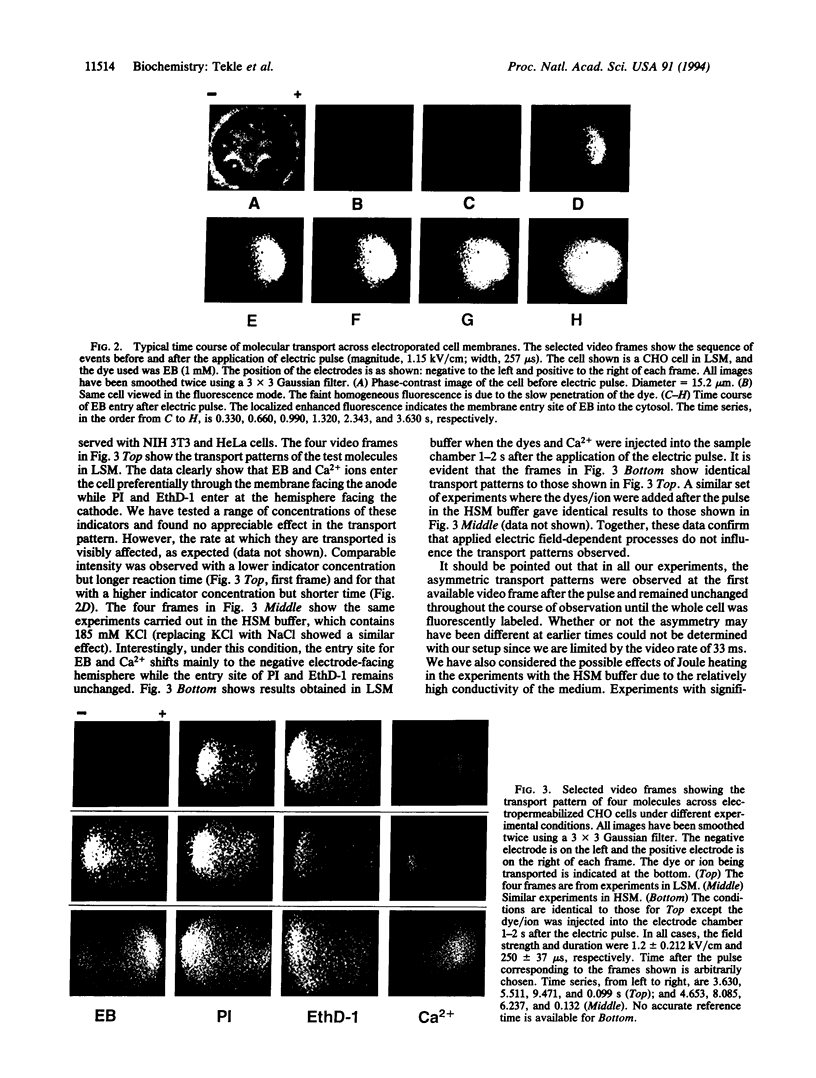
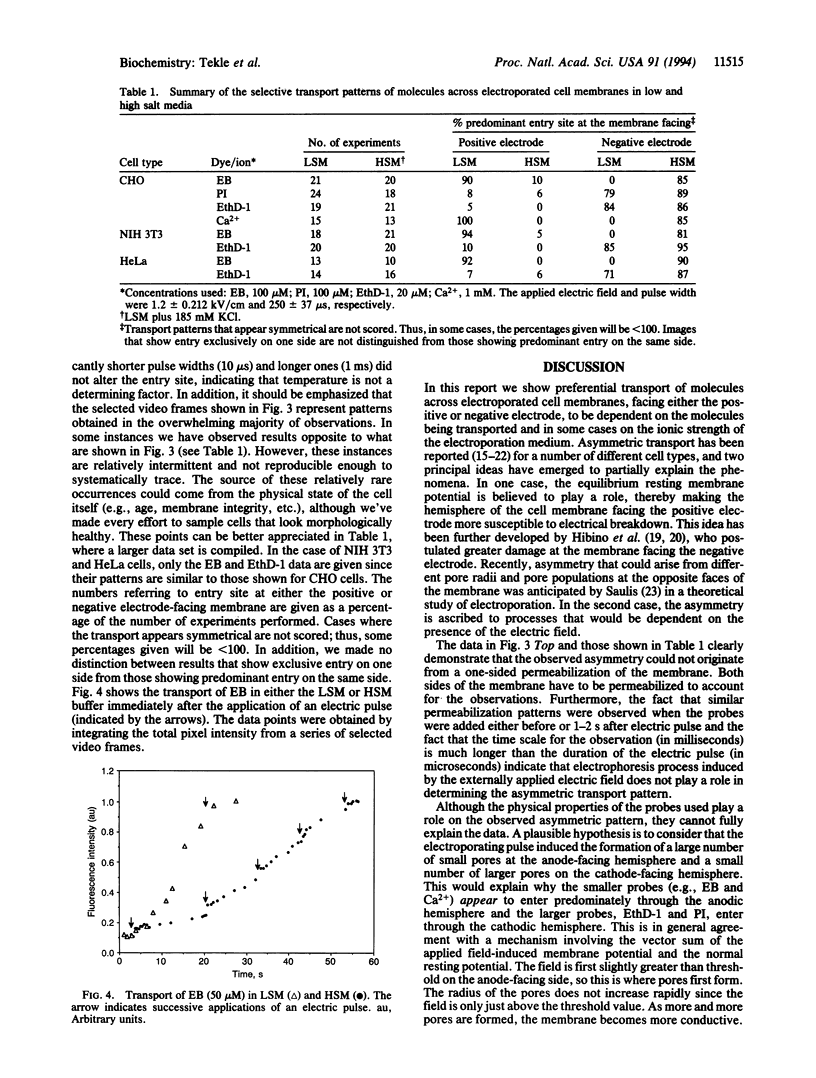
Transport of a divalent cation (Ca2+) and three DNA indicators [ethidium bromide (EB), propidium iodide (PI), and ethidium homodimer (EthD-1)] across electroporated membranes of several mammalian cell lines was found to be selective and asymmetrical. In low salt medium, Ca2+ and EB were preferentially transported across the anodefacing cell membrane while PI and EthD-1 predominately entered at the site facing the cathode. In high salt medium, the entry site for Ca2+ and EB was reversed to the cathode-facing hemisphere while it remained unchanged for PI and EthD-1. In all these experiments, the observed transport patterns remained unaffected whether the dyes (or ion) were present during or added after the electroporating pulse. The data suggest that asymmetric pores are created on both sides of the membrane facing the electrodes, with smaller pore size (but greater in number) on the anode side and larger pores (with a lower population) on the cathode side. Furthermore, the rate of resealing of the membrane pores is significantly enhanced in high ionic strength medium, thus affecting the entry site. The asymmetric transport pattern is neither caused by electrophoresis induced by the externally applied electric field nor due to one-sided membrane breakdown as previously believed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dimitrov D. S., Sowers A. E. Membrane electroporation--fast molecular exchange by electroosmosis. Biochim Biophys Acta. 1990 Mar;1022(3):381–392. doi: 10.1016/0005-2736(90)90289-z. [DOI] [PubMed] [Google Scholar]
- Ehrenberg B., Farkas D. L., Fluhler E. N., Lojewska Z., Loew L. M. Membrane potential induced by external electric field pulses can be followed with a potentiometric dye. Biophys J. 1987 May;51(5):833–837. doi: 10.1016/S0006-3495(87)83410-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross D., Loew L. M., Webb W. W. Optical imaging of cell membrane potential changes induced by applied electric fields. Biophys J. 1986 Aug;50(2):339–348. doi: 10.1016/S0006-3495(86)83467-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino M., Itoh H., Kinosita K., Jr Time courses of cell electroporation as revealed by submicrosecond imaging of transmembrane potential. Biophys J. 1993 Jun;64(6):1789–1800. doi: 10.1016/S0006-3495(93)81550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino M., Shigemori M., Itoh H., Nagayama K., Kinosita K., Jr Membrane conductance of an electroporated cell analyzed by submicrosecond imaging of transmembrane potential. Biophys J. 1991 Jan;59(1):209–220. doi: 10.1016/S0006-3495(91)82212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinosita K., Jr, Ashikawa I., Saita N., Yoshimura H., Itoh H., Nagayama K., Ikegami A. Electroporation of cell membrane visualized under a pulsed-laser fluorescence microscope. Biophys J. 1988 Jun;53(6):1015–1019. doi: 10.1016/S0006-3495(88)83181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight D. E., Scrutton M. C. Gaining access to the cytosol: the technique and some applications of electropermeabilization. Biochem J. 1986 Mar 15;234(3):497–506. doi: 10.1042/bj2340497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrle W., Hampp R., Zimmermann U. Electric pulse induced membrane permeabilization. Spatial orientation and kinetics of solute efflux in freely suspended and dielectrophoretically aligned plant mesophyll protoplasts. Biochim Biophys Acta. 1989 Jan 30;978(2):267–275. doi: 10.1016/0005-2736(89)90124-7. [DOI] [PubMed] [Google Scholar]
- Potter H. Electroporation in biology: methods, applications, and instrumentation. Anal Biochem. 1988 Nov 1;174(2):361–373. doi: 10.1016/0003-2697(88)90035-8. [DOI] [PubMed] [Google Scholar]
- Rossignol D. P., Decker G. L., Lennarz W. J., Tsong T. Y., Teissie J. Induction of calcium-dependent, localized cortical granule breakdown in sea-urchin eggs by voltage pulsation. Biochim Biophys Acta. 1983 Dec 19;763(4):346–355. doi: 10.1016/0167-4889(83)90096-4. [DOI] [PubMed] [Google Scholar]
- Sowers A. E. Fusion events and nonfusion contents mixing events induced in erythrocyte ghosts by an electric pulse. Biophys J. 1988 Oct;54(4):619–626. doi: 10.1016/S0006-3495(88)82997-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teissié J., Rols M. P. An experimental evaluation of the critical potential difference inducing cell membrane electropermeabilization. Biophys J. 1993 Jul;65(1):409–413. doi: 10.1016/S0006-3495(93)81052-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekle E., Astumian R. D., Chock P. B. Electro-permeabilization of cell membranes: effect of the resting membrane potential. Biochem Biophys Res Commun. 1990 Oct 15;172(1):282–287. doi: 10.1016/s0006-291x(05)80206-2. [DOI] [PubMed] [Google Scholar]
- Tekle E., Astumian R. D., Chock P. B. Electroporation by using bipolar oscillating electric field: an improved method for DNA transfection of NIH 3T3 cells. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4230–4234. doi: 10.1073/pnas.88.10.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsong T. Y. Electroporation of cell membranes. Biophys J. 1991 Aug;60(2):297–306. doi: 10.1016/S0006-3495(91)82054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann U. Electrical breakdown, electropermeabilization and electrofusion. Rev Physiol Biochem Pharmacol. 1986;105:176–256. [PubMed] [Google Scholar]