Abstract
Importance
Exposure to ambient particulate matter during pregnancy has been suggested as a risk factor for preterm birth. However results from limited epidemiologic studies have been inconclusive. Very few studies have been conducted in areas with high air pollution levels.
Objective
We investigated the hypothesis that high level exposure to particulate matter with aerodynamic diameter no larger than 10µm (PM10) during pregnancy increases the risk of preterm birth.
Methods
A birth cohort study was carried out between 2010–2012 in Lanzhou, China, including 8,969 singleton live births with available information on daily PM10 levels from four monitoring stations, individual exposures during pregnancy were calculated using inverse-distance weighting based on both home and work addresses. Unconditional logistic regression modeling was used to examine the associations between PM10 exposure and risk of preterm birth and its clinical subtypes.
Results
Increased risk of very preterm birth was associated with exposure to PM10 during the last two months of pregnancy (OR, 1.07; 95%CI, 1.02–1.13 per 10µg/m3 increase for last four weeks before delivery; 1.09; 1.02–1.15 for last six weeks before delivery; 1.10; 1.03–1.17 for last eight weeks before delivery). Compared to the U.S. National Ambient Air Quality Standard (150µg/m3), higher exposure level (≥150µg/m3) of PM10 during entire pregnancy was associated with an increased risk of preterm birth (1.48; 1.22–1.81) and the association was higher for medically indicated preterm birth (1.80, 1.24–2.62) during entire pregnancy and for very preterm during last 6 weeks before delivery (2.03, 1.11–3.72).
Conclusions and relevance
Our study supports the hypothesis that exposure to high levels of ambient PM10 increases the risk of preterm birth. Our study also suggests that the risk may vary by clinical subtypes of preterm birth and exposure time windows. Our findings are relevant for health policy makers from China and other regions with high levels of air pollution to facilitate the efforts of reducing air pollution level in order to protect public health.
Keywords: China, Epidemiology, PM10, Preterm birth, Birth cohort, Air pollution
1. Introduction
A recent systematic analysis of major global health risk factors has indicated that ambient particulate matter (PM) pollution is among the top public health risks and contributes annually to over 3.1 million premature deaths worldwide with 1.2 million occurring in China (Lim et al.,2012). The health burden from ambient PM has declined from 1990 to 2010 in many parts of the world, however, the health burden from ambient PM in China has increased (Greenberg et al.,2011). While the Global Burden of Disease Project estimated mortality by age and sex for regions of the world, it identified a literature gap in air pollution studies, for which most of the existing epidemiological studies investigated ambient PM and health outcomes were conducted in areas with relatively low concentrations of ambient PM. There is an urgent need to elucidate health consequences in regions with high ambient PM such as in China.
Preterm birth (PB) is a leading cause of neonatal morbidity and mortality (Mathews and MacDorman,2006). It has also been linked to adult chronic diseases including cardiovascular diseases, diabetes, and certain forms of cancers (Falah et al.,2013). A rising trend of PB has been observed worldwide during the past decade (WHO,2009). A solution to this growing problem is a priority for Millennium Development Goal 4 by the World Health Organization (WHO) (WHO,2012b), as PB continues to emerge as a major public health concern. Several risk factors have been suggested to be associated with PB risk, including younger or older maternal age, alcohol consumption, cigarette smoking, preeclampsia, diabetes, birth defects, infections during pregnancy, and problems with uterus or cervix, etc. (Goldenberg et al.,2008; Shiono PH,1995). However, these factors cannot explain all PBs.
While environmental chemical exposures have been suggested as potential risk factors for PB, such as phthalate diesters (Ferguson et al.,2014), organochlorine pesticides, (Kadhel et al.,2014; Longnecker et al.,2001), and polychlorinated biphenyls (PCBs) (Helmfrid et al.,2012; Taylor et al.,1984), recent evidence suggests that ambient air pollution may play a role in PB (Brauer et al.,2008; Dadvand et al.,2013; Darrow et al.,2009; Hannam et al.,2014; Hansen et al.,2006; Hyder et al.,2014; Jiang et al.,2007; Kim et al.,2007; Lee et al.,2013; Pereira et al.,2014; Ritz et al.,2000; Rojas-Rueda et al.,2013; Rudra et al.,2011; Sagiv et al.,2005; Schifano et al.,2013; Suh et al.,2009; van den Hooven et al.,2012; Wilhelm and Ritz,2005; Wilhelm et al.,2011; Yorifuji et al.,2011; Zhao et al.,2011). Further, air pollution has been associated with other adverse birth outcomes such as low birth weight, small for gestational age, and birth defects (Ha et al.,2014; Harris et al.,2014; Hwang et al.,2011; Vinikoor-Imler et al.,2014).
While a majority of the preterm birth studies suggested that maternal exposure to high levels of PM with aerodynamic diameter no larger than 2.5µm (PM2.5) or 10µm (PM10) during pregnancy was a risk factor for PB (Brauer et al.,2008; Dadvand et al.,2013; Hansen et al.,2006; Hyder et al.,2014; Jiang et al.,2007; Kim et al.,2007; Lee et al.,2013; Pereira et al.,2014; Ritz et al.,2000; Rojas-Rueda et al.,2013; Sagiv et al.,2005; Schifano et al.,2013; Suh et al.,2009; van den Hooven et al.,2012; Wilhelm and Ritz,2005; Wilhelm et al.,2011; Zhao et al.,2011), others found no association (Darrow et al.,2009; Hannam et al.,2014; Rudra et al.,2011). Most of these studies were conducted in Europe and United States where the air pollution levels are generally low. In addition, the majority of early studies were based on registry or administrative databases (Brauer et al.,2008; Darrow et al.,2009; Hannam et al.,2014; Hansen et al.,2006; Hyder et al.,2014; Jiang et al.,2007; Pereira et al.,2014; Ritz et al.,2000; Rojas-Rueda et al.,2013; Sagiv et al.,2005; Suh et al.,2009; Wilhelm and Ritz,2005; Wilhelm et al.,2011; Zhao et al.,2011). These databases, while useful, have limitations including lack of detailed information on confounding factors. Further, use of such databases introduces potential exposure misclassification as residential address at delivery is used to assign exposure, as data on work addresses and residential mobility during pregnancy are often unavailable. In light of the inconsistent results linking ambient PM to PB and very few studies conducted in high PM level areas, we conducted a birth cohort study in Lanzhou, China with detailed information on both home and work addresses as well as potential confounders to investigate the association between PM10 and the risk of PB.
2. Material and methods
2.1. Study population
The study population has been described previously (Qiu et al.,2014). In brief, pregnant women who came to the Gansu Provincial Maternity & Child Care Hospital (GPMCCH) in Lanzhou, China for delivery in 2010–2012, who were 18 years or older with a gestational age of ≥ 20 weeks and without mental illness were eligible. A total of 10,542 (73.4%) women participated in the study. All study procedures were approved by the Human Investigation Committees at the GPMCCH and Yale University. After obtaining written consent, an in-person interview was conducted at the hospital by trained study interviewers using a standardized and structured questionnaire to collect information on demographics, reproductive and medical history, lifestyle factors, occupation, and residential history. The majority of women (84%) were interviewed within one to three days after delivery, while others were interviewed within 2 days before delivery. Information on birth outcomes and maternal complications were abstracted from the medical records.
2.2. Exposure Assessment
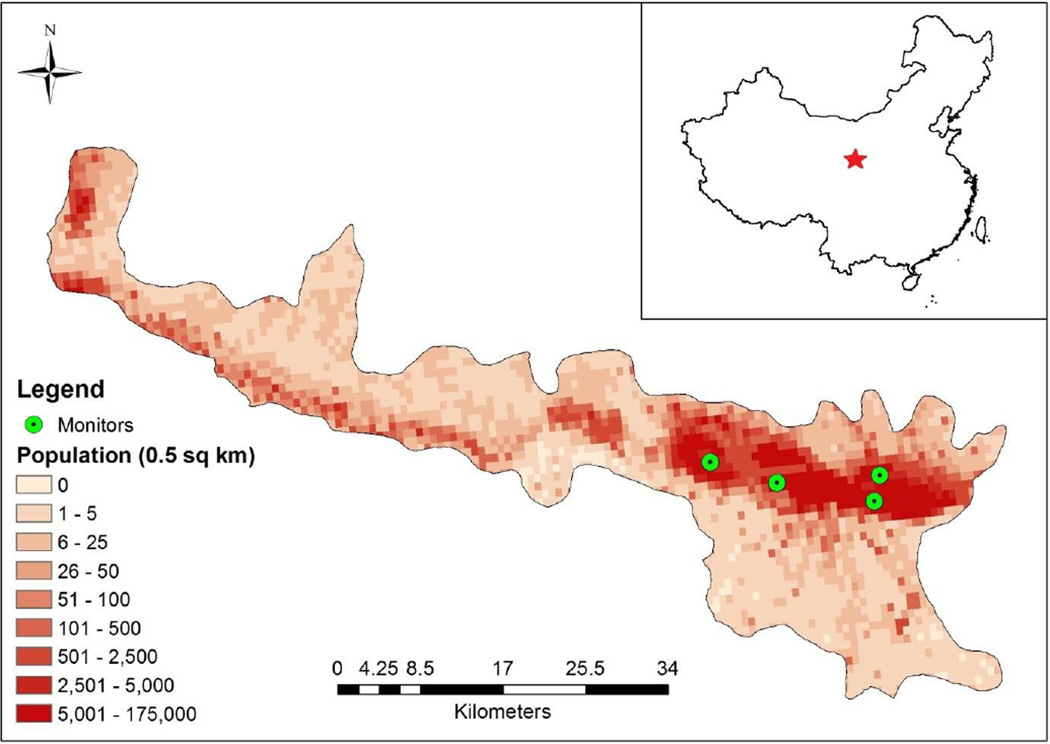
Data on ambient air pollutants were obtained from the Gansu Provincial Environmental Monitoring Central Station, which collects 24-hr average concentration for PM10, sulfur dioxide (SO2), and nitrogen dioxide (NO2) through an automated data reporting system from four monitoring stations in Lanzhou. The 24-hour average PM10 was measured for the period April 1, 2009 to December 31, 2012 for two stations, and January 1, 2011 to December 31, 2012 for the two additional stations. The monitors were located in the southern part of Lanzhou in the metropolitan area with high population density (Figure 1) (Y Zhang et al.,2014). Though the distance from the participant’s home and work addresses to the nearest monitors ranged from 0.1 to 88.5 km (mean: 5.0 km, median: 3.3 km), the majority (90%) of participants lived within 5.5 km from the nearest monitors. Values from these monitors were used to represent community-level exposure for Lanzhou, to investigate the association between outdoor air exposure and PB.
Figure 1.
Population of Lanzhou City center and placement of monitors
Each subject’s residences throughout pregnancy, as measured by move-in and move-out dates, and work addresses were collected. We used the earth online sharing website provided by Google (www.earthol.com) to obtain longitude and latitude coordinates for each subject's home and work addresses. Individuals who resided outside of Lanzhou during pregnancy were excluded from analysis (N=1,344) due to lack of information on air pollution. We calculated daily PM10 concentration at each subject’s home and work addresses using 1) the nearest monitor, 2) all four monitors with the inverse-distance weighting approach, and 3) the two monitors in operation the full study period (April 2009 to December 2010) and inverse distance weighting.
For each subject we calculated the overall exposure level during pregnancy by considering exposure time at home and work. Since the regular working hours are about 8 hours/day, we used a time-weighted approach to calculate daily PM10 on weekdays for each subject (i.e., two-thirds of exposure at home address and one-third exposure at work address). Weekend exposures were based on home address. Residential mobility was considered as time-weighted averaging to account for changes in residence during pregnancy. Finally, the daily exposures were averaged over seven exposure windows based on a priori decisions: entire pregnancy; last four, six, or eight weeks before delivery; and each trimester. Exposures for each subject to NO2 and SO2 were generated in the same manner as PM10. Exposures to PM10 was analyzed as a continues variable and as a binary variable meeting or exceeding the 24-h, health-based U.S. National Ambient Air Quality Standard (NAAQS) (150µg/m3, equivalent to the China NAAQS Grade II level) (EPA,2012).
2.3. Preterm Birth
PB (case) was defined as delivery prior to 37 completed weeks of gestation (Beck et al.,2010). Gestational age at delivery was calculated in completed weeks from the first day of the last menstrual period. According to gestational age, PB can be categorized into moderate preterm (32 to 36 completed weeks of gestation), very preterm (28 to 31 completed weeks), and extremely preterm (<28 completed weeks) (WHO,2012a). Very preterm and extremely preterm were combined as very PB to increase statistical power. Term birth (control) was defined as delivery at 37 or more completed weeks of gestation. PB was further classified as medically indicated (iatrogenic) PB and spontaneous PB with or without premature rupture of membrane (Goldenberg et al.,2008). Medically indicated preterm, which relates to maternal or fetal complications, includes placenta abruption, placenta previa, placental accreta, pregnancy hypertension and preeclampsia, intrauterine growth restriction, oligohydramnion, uterine rupture, and pre-gestational diabetes.
2.4. Statistical Analysis
After excluding multiple births (n=323) and still births (n=53), the final sample size was 8,969. Univariate-analysis (χ2 test) was conducted to examine the distributions of selected characteristics for cases and controls. Unconditional logistic regression model was used to calculate the odds ratios (OR) and 95% confidence intervals (CI) for association between PM10 exposure during pregnancy and risk of PB and its clinical subtypes, adjusting for maternal age (<25, 25–35, >35 years), education levels (<college, ≥college), family monthly income per capita (<3000, ≥3000RMB), active smoking (yes, no), and passive smoking (yes, no) during pregnancy, season of conception (fall/winter, summer/spring), parity (primiparous, multiparous), previous PB (yes, no), cooking fuel (gas/electricity, coal/biomass, others), and daily average temperature over pregnancy (continuous variable). For the final model selection, we set multivariate regression models adjusting each risk estimate for all putative risk factors included one at the time, and a forward step-wise single regression model including all putative risk factors. We examined the association by different exposure windows: entire pregnancy; last four, six, or eight weeks before delivery; and each trimester. Models with and without adjustment for SO2 and NO2 were fitted, and similar associations were observed, so the results from single-pollutant model were presented. All analyses were performed using SAS software, version 9.3 (SAS Institute, Inc., Cary, NC).
3. Results
Of 8,969 singleton live births, 677 (7.5%) were preterm and 8,292 were term births. Among PBs, moderate and very PBs were 571 (84.3%) and 106 (15.7%) respectively. Medically indicated PBs (n=185) accounted for 27.3% of PBs while spontaneous PBs (n=492) accounted for 72.7% of all cases. The concentrations of exposure to PM10 using different approaches (nearest monitors vs. inverse-distance weighting) were similar (Table 1).
Table 1.
Descriptive statistics of PM10 (µg/m3) at various pregnancy periods
| Pregnancy period | PM10a Mean(SD) |
PM10b Mean(SD) |
|---|---|---|
| Entire pregnancy | 142.1(17.6) | 140.5(21.7) |
| 1st trimester | 140.1(42.3) | 139.7(40.8) |
| 2nd trimester | 144.7(39.7) | 141.5(39.7) |
| 3rd trimester | 141.5(40.8) | 138.0(39.7) |
| Last 4 weeks before delivery | 139.5(51.7) | 137.8(53.9) |
| Last 6 weeks before delivery | 139.7(48.5) | 137.2(49.5) |
| Last 8 weeks before delivery | 140.1(46.0) | 137.1(45.8) |
Abbreviation: PM, particulate matter
Calculated by using inverse-distance weighting approach
Calculated by using the nearest monitor
Compared to women who delivered term babies, women who delivered preterm babies were more likely to be older, have lower education and less income, and be unemployed during pregnancy (Table 2). The percentages of women who had preeclampsia, who were multiparous, and who were overweight were higher among the PB group than term birth group. Women who had PB were more likely to use biomass or coal as cooking fuels, have previous pregnancies with PB, and have cesarean delivery. No significant differences in season of conception, and smoking were observed between the preterm and term groups. Distributions of the selected characteristics between the cases and controls in the current study population were similar to the distributions in the overall population (Qiu et al.,2014).
Table 2.
Distributions of selected characteristics between cases and control
| Characteristics | Cases (n=677) | Controls (n=8292) | P value |
|---|---|---|---|
| N (%) | N (%) | ||
| Maternal age (years) | |||
| <30 | 394 (58.2) | 5300 (63.9) | .003 |
| ≥30 | 283 (41.8) | 2992 (36.1) | |
| Highest education level | |||
| < College | 356 (52.6) | 2987 (36.0) | <.001 |
| ≥College | 321 (47.4) | 5305 (64.0) | |
| Family monthly income (RMB per capita) | |||
| <3,000 | 456 (67.4) | 4705 (56.7) | <.001 |
| ≥3,000 | 221 (32.6) | 3587 (43.3) | |
| Employment during pregnancy | |||
| No | 368 (54.4) | 3786 (45.7) | <.001 |
| Yes | 309 (45.6) | 4506 (54.3) | |
| Pre-pregnancy BMI | |||
| ≤18.5 | 133 (19.7) | 1708 (20.6) | .006 |
| 18.5–24.0 | 448 (66.2) | 5502 (69.1) | |
| ≥24.0 | 96 (14.2) | 851 (10.3) | |
| Smoking during pregnancy | |||
| No | 532 (78.6) | 6732 (81.2) | .10 |
| Yes | 145 (21.4) | 1560 (18.8) | |
| Season of conception | |||
| Fall | 176 (26.0) | 2280 (27.5) | .29 |
| Winter | 169 (25.0) | 1882 (22.7) | |
| Spring | 151 (22.3) | 1723 (20.8) | |
| Summer | 181 (26.7) | 2407 (29.0) | |
| Pre-history of preterm | |||
| No | 644 (95.1) | 8267 (99.7) | <.001 |
| Yes | 33 (4.9) | 25 (0.3) | |
| Parity | |||
| Primiparous | 452 (66.8) | 6282 (75.8) | <.001 |
| Multiparous | 225 (33.2) | 2010 (24.2) | |
| C-section | |||
| No | 366 (54.1) | 5307 (64.0) | <.001 |
| Yes | 311 (46.0) | 2985 (36.0) | |
| Preeclampsia | |||
| No | 612 (90.4) | 8152 (98.3) | <.001 |
| Yes | 65 (9.6) | 140 (1.7) | |
| Cooking fuel | |||
| Gas or electricity | 538 (79.5) | 7173 (86.5) | <.001 |
| Biomass or coal | 41 (6.1) | 196 (2.4) | |
| Others | 98 (14.5) | 923 (11.1) | |
Per 10µg/m3 increase in PM10 during the last four, six or eight weeks before delivery, the risk of PB increased 1%–2% (OR, 1.02; 95% CIs, 1.00–1.04, 0.99–1.04, and 1.01; 0.98–1.04, respectively; Table 3). A significantly increased risk was mainly seen for very PB (1.07; 1.02–1.13, 1.09; 1.02–1.15, and 1.10; 1.03–1.17 respectively). A larger association was observed for medically indicated PB during the entire pregnancy (1.14; 1.02–1.28), first trimester (1.07; 1.01–1.14), third trimester (1.05; 0.99–1.12), last 4 weeks (1.04; 1.00–1.09), and last 6 weeks (1.04; 1.00–1.09).
Table 3.
Associations between air pollutant PM10 and risk of preterm by exposure window
| PM10 per 10 µg/m3 increase | Preterm | Moderate preterm |
Very preterm | Medically indicated preterm |
Spontaneous preterm |
|---|---|---|---|---|---|
| ORa(95%CI) | ORa(95%CI) | ORa(95%CI) | ORa(95%CI) | ORa(95%CI) | |
| Entire pregnancy | 1.02(0.96–1.08) | 1.03(0.96–1.10) | 0.96(0.82–1.12) | 1.14(1.02–1.28) | 1.02(0.94–1.10) |
| 1st trimester | 1.00(0.97–1.04) | 1.01(0.97–1.05) | 0.97(0.87–1.07) | 1.07(1.01–1.14) | 0.97(0.93–1.02) |
| 2nd trimester | 1.01(0.97–1.05) | 1.01(0.97–1.06) | 1.02(0.92–1.13) | 1.01(0.94–1.08) | 1.02(0.97–1.07) |
| 3rd trimester | 0.99(0.96–1.03) | 0.98(0.95–1.02) | 1.06(0.97–1.16) | 1.05(0.99–1.12) | 0.97(0.93–1.01) |
| Last 4 weeks before delivery | 1.02(1.00–1.04) | 1.01(0.99–1.04) | 1.07(1.02–1.13) | 1.04(1.00–1.09) | 1.01(0.99–1.04) |
| Last 6 weeks before delivery | 1.02(0.99–1.04) | 1.01(0.98–1.03) | 1.09(1.02–1.15) | 1.04(1.00–1.09) | 1.01(0.98–1.04) |
| Last 8 weeks before delivery | 1.01(0.98–1.04) | 1.00(0.97–1.03) | 1.10(1.03–1.17) | 1.03(0.98–1.09) | 1.00(0.97–1.04) |
Abbreviations: PM, particulate matter; OR, odds ratio; CI: confident interval
Adjusted for maternal age (<25, 25–35, >35 years), parity (primiparous, multiparous), active smoking during pregnancy (yes,no), passive smoking during pregnancy (yes,no), season of conception (fall/winter, summer/spring), prehistory of preterm (yes,no), education level (<college, ≥college), family monthly income per capita (<3000, ≥3000RMB), cooking fuel (gas/electricity, coal/biomass, others), and daily average temperature
Women with PM10 averages over pregnancy higher than the U.S. 24-h NAAQS had increased PB risk (Table 4, OR, 1.48; 95% CI, 1.22–1.81) compared to pregnancies that averaged below the NAAQS. The risk was slightly higher for medically indicated PB (1.80; 1.24–2.62). When examining the association by various exposure time windows, we found that exposure to higher PM10 concentration during the last six weeks was associated with increased risk of very PB (2.03; 1.11–3.72).
Table 4.
Associations between air pollutant PM10 and risk of preterm by exposure window
| PM10 (µg/m3) (U.S. NAAQS level) |
Controls | Preterm | Moderate preterm | Very preterm | Medically indicated preterm |
Spontaneous preterm | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | ORa(95%CI) | Cases | ORa(95%CI) | Cases | ORa(95%CI) | Cases | ORa(95%CI) | Cases | ORa(95%CI) | ||
| Entire pregnancy | |||||||||||
| <150 | 5687 | 419 | 1 | 355 | 1 | 64 | 1 | 111 | 1 | 308 | 1 |
| ≥150 | 2605 | 258 | 1.48(1.22–1.81) | 216 | 1.48(1.20–1.84) | 42 | 1.45(0.91–2.33) | 74 | 1.80(1.24–2.62) | 184 | 1.37(1.09–1.72) |
| 1st trimester | |||||||||||
| <150 | 4591 | 353 | 1 | 295 | 1 | 58 | 1 | 97 | 1 | 256 | 1 |
| ≥150 | 3701 | 324 | 1.17(0.86–1.60) | 276 | 1.30(0.92–1.83) | 48 | 0.71(0.35–1.44) | 88 | 1.06(0.59–1.88) | 236 | 1.25(0.87–1.79) |
| 2nd trimester | |||||||||||
| <150 | 4102 | 343 | 1 | 289 | 1 | 54 | 1 | 93 | 1 | 250 | 1 |
| ≥150 | 4190 | 334 | 0.99(0.75–1.31) | 282 | 1.09(0.80–1.48) | 52 | 0.67(0.35–1.28) | 92 | 1.43(0.84–2.44) | 242 | 0.87(0.64–1.20) |
| 3rd trimester | |||||||||||
| <150 | 4520 | 392 | 1 | 322 | 1 | 70 | 1 | 103 | 1 | 289 | 1 |
| ≥150 | 3772 | 285 | 0.75(0.56–0.99) | 249 | 0.92(0.67–1.26) | 36 | 0.29(0.16–0.52) | 82 | 1.02(0.58–1.79) | 203 | 0.65(0.47–0.90) |
| Last 4 weeks before delivery | |||||||||||
| <150 | 5068 | 400 | 1 | 340 | 1 | 60 | 1 | 111 | 1 | 286 | 1 |
| ≥150 | 3224 | 277 | 1.19(0.94–1.49) | 231 | 1.11(0.86–1.42) | 46 | 1.70(0.96–3.04) | 74 | 1.07(0.69–1.64) | 206 | 1.23(0.94–1.61) |
| Last 6 weeks before delivery | |||||||||||
| <150 | 4916 | 397 | 1 | 340 | 1 | 57 | 1 | 103 | 1 | 294 | 1 |
| ≥150 | 3376 | 280 | 1.08(0.85–1.37) | 231 | 0.96(0.74–1.24) | 49 | 2.03(1.11–3.72) | 82 | 1.40(0.88–2.21) | 198 | 0.99(0.75–1.30) |
| Last 8 weeks before delivery | |||||||||||
| <150 | 4757 | 381 | 1 | 324 | 1 | 57 | 1 | 104 | 1 | 277 | 1 |
| ≥150 | 3535 | 296 | 1.15(0.88–1.48) | 247 | 1.06(0.80–1.40) | 49 | 1.73(0.89–3.33) | 81 | 1.14(0.70–1.87) | 215 | 1.15(0.85–1.55) |
Abbreviations: PM, particulate matter; OR, odds ratio; CI: confident interval
Adjusted for maternal age (<25, 25–35, >35 years), parity (primiparous, multiparous), active smoking during pregnancy (yes, no), passive smoking during pregnancy (yes, no), season of conception (fall/winter, summer/spring), prehistory of preterm (yes, no), education level (<college, ≥college), family monthly income per capita (<3000, ≥3000RMB), cooking fuel (gas/electricity, coal/biomass, others), and daily average temperature
We conducted sensitivity analysis by excluding the subjects with estimated conception dates 20 weeks before the data collection started or 43 weeks before it ended to avoid “fixed-cohort bias”(Barnett,2011) and reached the same conclusion (data not shown). Similar results were observed after exclusion of subjects with birth defects (data not shown). Similar results were also observed based on the nearest monitors and by using data for the women lived within 5.5 km (90% of the subjects) or 12.9 km (95% of the subjects) to the nearest monitors (data not shown).
4. Discussion and conclusions
Our study results support the hypothesis that maternal exposure to high levels of ambient PM10 is associated with an increased risk of PB. We also found that the risk was higher for very PB and medically indicated PB.
PB is a syndrome initiated by multiple mechanisms, including infection or inflammation, uteroplacental ischaemia or haemorrhage, uterine overditension, stress and other immunologically mediated processes (Romero et al.,2006). Higher particulate matter exposure during pregnancy has been linked to impaired immune competence and subsequently enhanced susceptibility to infection (Sagiv et al.,2005). It has been reported that exposure to PM10 during pregnancy is associated with higher natural killer cell fractions in cord blood and lower percentages of T-cells and CD3+ CD4+ cells, and CD4+: CD8+ cell ratio in maternal blood (Hertz-Picciotto et al.,2002). Thus, it is biologically plausible that exposure to high levels of PM10 is associated with an increased risk of PB.
Several studies have investigated the relationship between ambient PM10 and risk of PB and reached inconsistent results (Brauer et al.,2008; Darrow et al.,2009; Hansen et al.,2006; Jiang et al.,2007; Kim et al.,2007; Ritz et al.,2000; Sagiv et al.,2005; Schifano et al.,2013; Suh et al.,2009; van den Hooven et al.,2012; Wilhelm and Ritz,2005; Zhao et al.,2011). Two registry-based studies in California reported about a 15% increase in PB per 50 µg/m3 increase in PM10 averaged over 6 weeks before birth or over the first month of pregnancy (Ritz et al.,2000; Wilhelm and Ritz,2005). A study from Pennsylvania observed a non-significant 7 to 10% elevated risk for preterm per 50 µg/m3 increase in PM10 in the 6 weeks before birth (Sagiv et al.,2005). A study from Atlanta found no association (Darrow et al.,2009). Five non-US population registry-based studies also reported mixed results (Brauer et al.,2008; Hannam et al.,2014; Hansen et al.,2006; Schifano et al.,2013; Suh et al.,2009). A study from Canada reported a non-significant 13% increase in PB per 1 µg/m3 increase in PM10 (Brauer et al.,2008); a study from Australia reported a 15% increased risk of PB per (4.5µg/m3) increase in PM10 during the first trimester (Hansen et al.,2006); a Korean study found a 7% increase in PB per 16.53µg/m3 increase in PM10 during the first or third trimesters (Suh et al.,2009); while a study from a UK cohort observed no association (Hannam et al.,2014). Our study addresses some key limitations in these earlier studies using registry or administrative databases, by assessing exposure based on work and residential addresses throughout pregnancy and with detailed confounder information from a cohort.
In addition, three previous cohort studies were conducted: a hospital-based birth cohort study in Seoul (Kim et al.,2007) and two prospective cohort studies in Netherlands (van den Hooven et al.,2012) and Rome (Schifano et al.,2013). Kim et al. (Kim et al.,2007) observed a 5% increase in the risk of PB per 10 µg/m3 increase in PM10 during the third trimester, which is consistent with our results. Van den Hooven et al. (van den Hooven et al.,2012) reported that the third and fourth quartiles of PM10 exposure during entire pregnancy were associated with 40% and 32% increased risk of PB, respectively. Schifano et al. (Schifano et al.,2013) detected a non-significant 69% increased risk of PB per 1 µg/m3 increase of PM10 at a lag period of 12–22 days during the warm season in the month preceding delivery in Rome. However, such associations were not replicated in our study.
To date, only two studies on preterm and PM10 have been conducted in China. Zhao et al. (Zhao et al.,2011) reported a 7% increased risk of PB associated with per 100 µg/m3 increase in PM10 on day 4 of the week before delivery in Guangzhou. Jiang et al. (Jiang et al.,2007) observed about a 4% increased risk of PB associated with per 10 µg/m3 increase in PM10 averaged over 8 weeks before birth in Shanghai. However, both studies were based on registry databases.
A variety of exposure assessment approaches have been used in different studies, which complicates comparison of results across studies. Seven studies used city-level average of PM10 (Darrow et al.,2009; Hansen et al.,2006; Jiang et al.,2007; Sagiv et al.,2005; Schifano et al.,2013; Suh et al.,2009; Zhao et al.,2011), five studies assigned individual exposure level from air monitors based on home address or zip code of residence at delivery (Brauer et al.,2008; Kim et al.,2007; Ritz et al.,2000; van den Hooven et al.,2012; Wilhelm and Ritz,2005). One study estimated residential exposures using land use regression modeling, which provided improved local spatial resolution (Brauer et al.,2008). However, land use regression models are not developed in the current study area. To evaluate whether the four monitors provided reasonable spatial coverage in our study, we calculated coefficient of divergence, which provides the diversity between concentrations at sampling site-pairs (Pinto et al.,2004). We found that coefficients of divergence for all site-pairs were lower than 0.20, indicating low spatial heterogeneity of PM10 in our study (Figure 1). In our study, air pollution data for two of the four monitors was unavailable for the first 21 months of the study timeframe; results using all available data were similar to those using data from only two monitors with data for the full study. We conclude that potential exposure misclassification resulting from possible spatial heterogeneity and fewer monitors seems unlikely.
Most of the early studies were conducted in areas with low air pollutant levels. For example, mean PM10 exposure over gestation for previous studies on PB ranged from 13µg/m3 to 90µg/m3 (Brauer et al.,2008; Darrow et al.,2009; Hansen et al.,2006; Kim et al.,2007; Ritz et al.,2000; Sagiv et al.,2005; Schifano et al.,2013; Suh et al.,2009; van den Hooven et al.,2012; Wilhelm and Ritz,2005), versus 142.1 µg/m3 in the current study. In addition, chemical compositions of PM10 may vary in different areas, which could affect health effects (Furness et al.,2012). Health impacts associated with particulate matters can vary by area (Dominici et al.,2006), which may relate to differences in chemical composition or populations. Given that a large percent of the world’s population is exposed to high level of air pollution and that relatively few studies have been conducted in such areas, our study is a timely effort to address this understudied issue.
Previous studies mainly focused on overall PB (<37 gestational weeks) (Darrow et al.,2009; Hansen et al.,2006; Jiang et al.,2007; Ritz et al.,2000; Sagiv et al.,2005; van den Hooven et al.,2012; Wilhelm and Ritz,2005; Zhao et al.,2011). Only three previous studies explored the effect of PM10 exposure on very PB (Brauer et al.,2008; Schifano et al.,2013; Suh et al.,2009) and two of them suggested a higher association with very PB (Brauer et al.,2008; van den Hooven et al.,2012), which was consistent with our study. We also found a higher association for medically indicated PB, which has not been explored in previous studies. While it is unknown why associations could vary by different clinical subtypes of PB, different clinical subtypes of PB might have different etiology.
We observed that various exposure time windows might have different impact on the risk of PB. Consistently, several early studies suggested that exposure to high levels of PM10 during the first trimester and/or the third trimester had a stronger impact on PB than exposure during the second trimester (Hansen et al.,2006; Jiang et al.,2007; Kim et al.,2007; Suh et al.,2009; Wilhelm and Ritz,2005).
Our study included a relatively large sample size (N=8,969), which allowed exploration of associations by various clinical subtypes. A key strength of the study was assessment of exposure based on both home and work addresses, as well as residential mobility, which minimized potential exposure misclassification, whereas most previous studies used residence at birth to assign exposure for the whole pregnancy. Detailed information on potential confounding factors such as smoking and cooking fuels were collected and controlled for in our analysis. Birth outcomes and maternal complications during pregnancy were obtained from medical records, which minimized potential disease misclassification.
Limitations should be considered when interoperating the study results. The accuracy of exposure estimation might be limited by the lack of monitors in rural areas in Lanzhou city. However, more than 90% of the women lived within 5.5 km of a monitor. Sensitivity analyses using the data from different exposure assessment approaches (nearest monitors, inverse-distance weighting using two or four monitors, using data for the women lived within 5.5 km or 12.9 km of a monitor) showed consistent results. PM10 exposure estimated using data from government monitors, which although the most commonly used method in air pollution epidemiology, may not represent the actual individual exposure level, which would include differences in indoor/outdoor activity patterns. However, measurements through personal air monitors in this setting (over pregnancy in a large population) may be impractical as well as expensive and numerous studies have used the monitoring approach successfully. Future work may investigate PM sources, which have different chemical structures and possibly different health impacts. Finally, the study population was recruited from the largest maternity and child hospital in Lanzhou, the capital city of Gansu Province. Although the study was hospital-based, which might affect generalizability, the preterm rate (8.2%) in our study population was within the range of the reported preterm rate (4.1%–18.9%) in other Chinese populations (Blencowe et al.,2012).
In conclusion, our study supports the hypothesis that exposure to high levels of ambient PM10 during pregnancy increases the risk of PB. The risk might vary by different clinical subtypes and by different exposure time windows. The findings from our study have important public health implications and are relevant for policy makers who design air pollution policies for China and other high air pollution regions to protect public health from air pollution. Future multi-site studies with different types of air pollutants are needed to fully understand the impacts of air pollution mixture on PB in China.
Highlights.
Very few studies have been conducted in areas with high air pollution levels.
Exposure to high levels of ambient PM10 increases the risk of preterm birth.
The risks vary by clinical subtypes of preterm birth and exposure time windows.
Acknowledgements
Funding/support: The study was supported by internal funding from the Gansu Provincial Maternity and Child Care Hospital, Gansu Provincial Science and Technology Department Grant (1204WCGA021) and National Institutes of Health Grants (K02HD70324, R01ES016317, and R01ES019587).
Role of the sponsor:
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. Yawei Zhang and Qin Liu had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors had final responsibility for the decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions:
JQ, QL, and YZ designed the research; XH, MZ, ML, XX, HC, LL, XL, CZ, HZ, RX, XH, DZ, RL, TY, YD, YC conducted the birth cohort study; NZ, JS, HZ, HB, ZT, WW, YW, XL, MB, SL, WQ, HH, JL, QC, MJ performed statistical analysis; LJ constructed the figure; NZ, JQ, YZ, QL, YZ wrote the first draft and all authors contributed to the final draft and approved the manuscript.
Conflict of interest disclosures: None reported.
References
- Barnett AG. Time-dependent exposures and the fixed-cohort bias. Environmental health perspectives. 2011;119:A422–A423. doi: 10.1289/ehp.1103885. author reply A423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, et al. The worldwide incidence of preterm birth: A systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88:31–38. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- Brauer M, Lencar C, Tamburic L, Koehoorn M, Demers P, Karr C. A cohort study of traffic-related air pollution impacts on birth outcomes. Environmental health perspectives. 2008;116:680–686. doi: 10.1289/ehp.10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadvand P, Basagana X, Figueras F, Martinez D, Beelen R, Cirach M, et al. Air pollution and preterm premature rupture of membranes: A spatiotemporal analysis. American journal of epidemiology. 2013 doi: 10.1093/aje/kwt240. [DOI] [PubMed] [Google Scholar]
- Darrow LA, Klein M, Flanders WD, Waller LA, Correa A, Marcus M, et al. Ambient air pollution and preterm birth: A time-series analysis. Epidemiology. 2009;20:689–698. doi: 10.1097/EDE.0b013e3181a7128f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA : the journal of the American Medical Association. 2006;295:1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA US. National ambient air quality standards. [accessed 11 july 2012];2012 Available: Http://www.Epa.Gov/air/criteria.Html.
- Falah N, McElroy J, Snegovskikh V, Lockwood CJ, Norwitz E, Murray JC, et al. Investigation of genetic risk factors for chronic adult diseases for association with preterm birth. Hum Genet. 2013;132:57–67. doi: 10.1007/s00439-012-1223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Meeker JD. Environmental phthalate exposure and preterm birth. JAMA pediatrics. 2014;168:61–67. doi: 10.1001/jamapediatrics.2013.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness DL, Yasin N, Dekker GA, Thompson SD, Roberts CT. Maternal red blood cell folate concentration at 10–12 weeks gestation and pregnancy outcome. J Matern Fetal Neonatal Med. 2012;25:1423–1427. doi: 10.3109/14767058.2011.636463. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg JA, Bell SJ, Guan Y, Yu YH. Folic acid supplementation and pregnancy: More than just neural tube defect prevention. Rev Obstet Gynecol. 2011;4:52–59. [PMC free article] [PubMed] [Google Scholar]
- Ha S, Hu H, Roussos-Ross D, Haidong K, Roth J, Xu X. The effects of air pollution on adverse birth outcomes. Environmental research. 2014;134C:198–204. doi: 10.1016/j.envres.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannam K, McNamee R, Baker P, Sibley C, Agius R. Air pollution exposure and adverse pregnancy outcomes in a large uk birth cohort: Use of a novel spatio-temporal modelling technique. Scandinavian journal of work, environment & health. 2014;40:518–530. doi: 10.5271/sjweh.3423. [DOI] [PubMed] [Google Scholar]
- Hansen C, Neller A, Williams G, Simpson R. Maternal exposure to low levels of ambient air pollution and preterm birth in brisbane, australia. BJOG. 2006;113:935–941. doi: 10.1111/j.1471-0528.2006.01010.x. [DOI] [PubMed] [Google Scholar]
- Harris G, Thompson WD, Fitzgerald E, Wartenberg D. The association of pm with full term low birth weight at different spatial scales. Environmental research. 2014;134C:427–434. doi: 10.1016/j.envres.2014.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmfrid I, Berglund M, Lofman O, Wingren G. Health effects and exposure to polychlorinated biphenyls (pcbs) and metals in a contaminated community. Environment international. 2012;44:53–58. doi: 10.1016/j.envint.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Dostal M, Dejmek J, Selevan SG, Wegienka G, Gomez-Caminero A, et al. Air pollution and distributions of lymphocyte immunophenotypes in cord and maternal blood at delivery. Epidemiology. 2002;13:172–183. doi: 10.1097/00001648-200203000-00012. [DOI] [PubMed] [Google Scholar]
- Hwang BF, Lee YL, Jaakkola JJ. Air pollution and stillbirth: A population-based case-control study in taiwan. Environmental health perspectives. 2011;119:1345–1349. doi: 10.1289/ehp.1003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder A, Lee HJ, Ebisu K, Koutrakis P, Belanger K, Bell ML. Pm2.5 exposure and birth outcomes: Use of satellite- and monitor-based data. Epidemiology. 2014;25:58–67. doi: 10.1097/EDE.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang LL, Zhang YH, Song GX, Chen GH, Chen BH, Zhao NQ, et al. A time series analysis of outdoor air pollution and preterm birth in shanghai, china. [accessed 2007 oct];Biomed Environ Sci. 2007 20:426–431. Available: Http://www.Ncbi.Nlm.Nih.Gov/pubmed/18188997. [PubMed] [Google Scholar]
- Kadhel P, Monfort C, Costet N, Rouget F, Thome JP, Multigner L, et al. Chlordecone exposure, length of gestation, and risk of preterm birth. American journal of epidemiology. 2014;179:536–544. doi: 10.1093/aje/kwt313. [DOI] [PubMed] [Google Scholar]
- Kim OJ, Ha EH, Kim BM, Seo JH, Park HS, Jung WJ, et al. Pm10 and pregnancy outcomes: A hospital-based cohort study of pregnant women in seoul. J Occup Environ Med. 2007;49:1394–1402. doi: 10.1097/JOM.0b013e3181594859. [DOI] [PubMed] [Google Scholar]
- Lee PC, Roberts JM, Catov JM, Talbott EO, Ritz B. First trimester exposure to ambient air pollution, pregnancy complications and adverse birth outcomes in allegheny county, pa. Matern Child Health J. 2013;17:545–555. doi: 10.1007/s10995-012-1028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker MP, Klebanoff MA, Zhou H, Brock JW. Association between maternal serum concentration of the ddt metabolite dde and preterm and small-for-gestational-age babies at birth. Lancet. 2001;358:110–114. doi: 10.1016/S0140-6736(01)05329-6. [DOI] [PubMed] [Google Scholar]
- Mathews TJ, MacDorman MF. Infant mortality statistics from the 2003 period linked birth/infant death data set. Natl Vital Stat Rep. 2006;54:1–29. [PubMed] [Google Scholar]
- Pereira G, Belanger K, Ebisu K, Bell ML. Fine particulate matter and risk of preterm birth in connecticut in 2000–2006: A longitudinal study. American journal of epidemiology. 2014;179:67–74. doi: 10.1093/aje/kwt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto JP, Lefohn AS, Shadwick DS. Spatial variability of pm2.5 in urban areas in the united states. Journal of the Air & Waste Management Association. 2004;54:440–449. doi: 10.1080/10473289.2004.10470919. [DOI] [PubMed] [Google Scholar]
- Qiu J, He X, Cui H, Zhang C, Zhang H, Dang Y, et al. Passive smoking and preterm birth in urban china. American journal of epidemiology. 2014;180:94–102. doi: 10.1093/aje/kwu092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B, Yu F, Chapa G, Fruin S. Effect of air pollution on preterm birth among children born in southern california between 1989 and 1993. Epidemiology. 2000;11:502–511. doi: 10.1097/00001648-200009000-00004. [DOI] [PubMed] [Google Scholar]
- Rojas-Rueda D, de Nazelle A, Teixido O, Nieuwenhuijsen MJ. Health impact assessment of increasing public transport and cycling use in barcelona: A morbidity and burden of disease approach. Prev Med. 2013;57:573–579. doi: 10.1016/j.ypmed.2013.07.021. [DOI] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, et al. The preterm parturition syndrome. BJOG. 2006;113(Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudra CB, Williams MA, Sheppard L, Koenig JQ, Schiff MA. Ambient carbon monoxide and fine particulate matter in relation to preeclampsia and preterm delivery in western washington state. Environmental health perspectives. 2011;119:886–892. doi: 10.1289/ehp.1002947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, Mendola P, Loomis D, Herring AH, Neas LM, Savitz DA, et al. A time-series analysis of air pollution and preterm birth in Pennsylvania, 1997–2001. Environmental health perspectives. 2005;113:602–606. doi: 10.1289/ehp.7646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifano P, Lallo A, Asta F, De Sario M, Davoli M, Michelozzi P. Effect of ambient temperature and air pollutants on the risk of preterm birth, rome 2001–2010. Environment international. 2013;61:77–87. doi: 10.1016/j.envint.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Shiono PHKM, Nugent RP, Cotch MF, Wilkins DG, Rollins DE, Carey CJ, Behrman RE. Fetus-placenta-newborn: The impact of cocaine and marijuana use on low birth weight and preterm birth: A multicenter study. American Journal of Obstetrics & Gynecology. 1995;172:19–27. doi: 10.1016/0002-9378(95)90078-0. [DOI] [PubMed] [Google Scholar]
- Suh YJ, Kim H, Seo JH, Park H, Kim YJ, Hong YC, et al. Different effects of pm10 exposure on preterm birth by gestational period estimated from time-dependent survival analyses. Int Arch Occup Environ Health. 2009;82:613–621. doi: 10.1007/s00420-008-0380-7. [DOI] [PubMed] [Google Scholar]
- Taylor PR, Lawrence CE, Hwang HL, Paulson AS. Polychlorinated biphenyls: Influence on birthweight and gestation. American journal of public health. 1984;74:1153–1154. doi: 10.2105/ajph.74.10.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hooven EH, Pierik FH, de Kluizenaar Y, Willemsen SP, Hofman A, van Ratingen SW, et al. Air pollution exposure during pregnancy, ultrasound measures of fetal growth, and adverse birth outcomes: A prospective cohort study. Environmental health perspectives. 2012;120:150–156. doi: 10.1289/ehp.1003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinikoor-Imler LC, Davis JA, Meyer RE, Messer LC, Luben TJ. Associations between prenatal exposure to air pollution, small for gestational age, term low birthweight in a state-wide birth cohort. Environmental research. 2014;132:132–139. doi: 10.1016/j.envres.2014.03.040. [DOI] [PubMed] [Google Scholar]
- WHO. The worldwide incidence of preterm birth: A systematic review of maternal mortality and morbidity. [Accessed 25 September 2009];2009 doi: 10.2471/BLT.08.062554. Available: http://www.who.int/bulletin/volumes/88/1/08-062554/en/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Preterm birth. [Accessed November 2013];2012a Available: http://www.who.int/mediacentre/factsheets/fs363/en/ [Google Scholar]
- WHO. March of dimes, pmnch, save the children, who. Born too soon: The global action report on preterm birth. The Global Action Report on Preterm Birth. 2012b http://www.marchofdimes.org/materials/born-too-soon-the-global-action-report-on-preterm-birth.pdf. [Google Scholar]
- Wilhelm M, Ritz B. Local variations in co and particulate air pollution and adverse birth outcomes in los angeles county, california, USA. Environmental health perspectives. 2005;113:1212–1221. doi: 10.1289/ehp.7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm M, Ghosh JK, Su J, Cockburn M, Jerrett M, Ritz B. Traffic-related air toxics and preterm birth: A population-based case-control study in los angeles county, california. Environ Health. 2011;10:89. doi: 10.1186/1476-069X-10-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li M, Bravo MA, Jin L, Nori-Sarma A, Xu Y, et al. Air quality in lanzhou, a major industrial city in china: Characteristics of air pollution and review of existing evidence on air pollution and health. Water, air, soil pollution. (accepted) Water Air Soil Pollut. 2014;225:2187. doi: 10.1007/s11270-014-2187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorifuji T, Naruse H, Kashima S, Ohki S, Murakoshi T, Takao S, et al. Residential proximity to major roads and preterm births. Epidemiology. 2011;22:74–80. doi: 10.1097/EDE.0b013e3181fe759f. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Liang Z, Tao S, Zhu J, Du Y. Effects of air pollution on neonatal prematurity in guangzhou of china: A time-series study. Environ Health. 2011;10:2. doi: 10.1186/1476-069X-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]