Abstract
INTRODUCTION
Cortisol levels in adults show a sharp decrease from midmorning to midafternoon. Most toddlers take afternoon naps, which is associated with a less mature diurnal pattern characterized by a midday plateau in cortisol secretion. Napping in preschoolers produces a robust cortisol awakening response (CAR), which may account for such maturational differences. This experimental study extends prior work by examining whether the presence and timing of the nap-dependent CAR influences the diurnal cortisol pattern in toddlers.
METHODS
Toddlers (n=28; 13 females; 30–36 months) followed a strict biphasic sleep schedule (≥12.5h time in bed; ≥90 min nap) for ≥3 days before each of four randomly ordered, in-home cortisol assessments. For each assessment, saliva samples were obtained at morning awakening, ~09:30, pre-nap, 0, 15, 30, 45, 90, 135 min post-nap awakening (verified with actigraphy), and ~19:30. On one day, children napped at their scheduled time, and parents collected saliva samples. On another day, children missed their nap, and parents collected saliva samples at matched times. On two other days, children napped 4 h (morning) and 7 h (afternoon) after awakening in the morning, during which time researchers collected pre- and post-nap saliva samples. Saliva was assayed for cortisol (μg/dl).
RESULTS
Three-level multilevel models were used to estimate the CAR and diurnal cortisol patterns in all four conditions. Compared to the no-nap condition (no observed CAR; b= −0.78, p=0.65), we found a pronounced cortisol rise following the morning nap (b=11.00, p<0.001) and both afternoon naps whether samples were collected by parents (b=5.19, p<0.01) or experimenters (b= 4.97, p<0.01). Napping in the morning resulted in the most robust post-nap cortisol rise (b=10.21, p<0.001). Diurnal patterns were analyzed using piecewise growth modeling that estimated linear coefficients for five separate periods throughout the day (corresponding to morning decline, noon decline, post-nap rise, post-nap decline, and evening decline). We observed a significant post-nap rise in cortisol values on the parent-collected afternoon nap (b=3.41, p<0.01) and the experimenter-collected morning nap (b=7.50, p<0.01) days as compared to the no-nap day (b=−0.17, p=0.82). No other differences in diurnal profiles were observed between the parent-collected nap and no-nap conditions; however, toddlers had a steeper evening decline on the day of the morning nap compared to the parent-collected afternoon nap (b=0.30, p<0.05) and no-nap conditions (b=0.27, p<0.05).
DISCUSSION
These well-controlled findings suggest that the presence and timing of daytime naps influence the pattern of diurnal cortisol secretion in toddlers. They also provide support for the hypothesis that napping is the primary state driving the immature midday plateau in cortisol secretion, which becomes more adult-like across childhood. Prior studies of the diurnal cortisol pattern have employed a cubic model, and therefore, have not detected all possible variations due to napping. Our experimental data have important methodological implications for researchers examining associations between the slope of the diurnal cortisol pattern and developmental outcomes, as well as those utilizing afternoon cortisol reactivity protocols in napping children.
Keywords: cortisol awakening response, CAR, diurnal cortisol pattern, napping, hildren, toddlers
1. INTRODUCTION
An adaptive response to stressors is facilitated by cortisol, the effector hormone produced by the adrenocortical system in humans (Gunnar, 1989). Cortisol secretion is under the regulatory control of circadian oscillators and follows a 24-hour rhythm (Hellman et al., 1970). Daily cortisol rhythms are well established in adults, with the highest values approximately 30-min after awakening followed by a sharp decrease by mid-morning and a more gradual decline across the afternoon and evening hours (Kirschbaum and Hellhammer, 2000). Although this adult like pattern is evident in children 6 years of age (Davis et al., 2002), it is on average absent in 1- to 3-year-olds, who instead show a more immature pattern characterized by a midday plateau in cortisol secretion (Watamura et al., 2004). A common characteristic among toddlers and preschool-age children is that they are biphasic sleepers, taking an afternoon nap. Data from longitudinal studies indicate that daytime napping declines across early childhood (Iglowstein et al., 2003). In the U.S., nearly all 2-year-olds, 72% of 3-year-olds, and 14.3% of 5-year-olds meet part of their sleep need during the day; however, the time course of the decline in napping differs by ethnicity (Crosby et al., 2005; Weissbluth, 1995). Although a more mature pattern of cortisol secretion may emerge as children begin to drop their daytime naps (Watamura et al., 2004), current understanding of such a relationship is limited. Only a handful of studies observing the diurnal cortisol pattern in healthy young children have been published. In these, minimal samples were obtained across the day (e.g., wake-up, midmorning, midafternoon, bedtime), and napping was either not assessed at all or measured by subjective reports or behavioral observations (Sumner et al., 2010; Van Ryzin et al., 2009; Watamura et al., 2004; Watamura et al., 2002).
One feature of the diurnal pattern of cortisol is the cortisol awakening response (CAR), which is characterized by a period of increased hormonal secretion 30–45 min post-awakening (Pruessner et al., 1997). Research on the CAR has primarily focused on adults (Clow et al., 2010; Elder et al., 2013; Fries et al., 2009); however, recent work has examined the CAR in young children with conflicting findings (Baumler et al., 2013; Freitag et al., 2009; Gribbin et al., 2011; Michels et al., 2011; Scher et al., 2010). For example, in studies using subjective reports of awakening time, the CAR was observed in only ~50% of children (Freitag et al., 2009; Michels et al., 2011). In contrast, studies using objective measures to verify awakening and sampling times found that the great majority (86% – 97%) of children exhibited a CAR after morning wake time (Baumler et al., 2013; Stalder et al., 2013). Importantly, well-controlled, experimental research using sleep electroencephalography (EEG) to objectively verify awakening time in children ages 2- to 4-years-old (Gribbin et al., 2011) and adults (Wilhelm et al., 2007) showed robust CARs in 100% of study participants. Thus, sensitive measurement of the CAR necessitates objective assessment of wake time, whether researchers, participants, or caregivers collect samples in laboratory or naturalistic settings.
Despite recent work establishing that young children display a CAR, published reports on the association between the CAR and sleep, specifically napping, are limited. In our previously published study, we found that late morning and mid-afternoon naps both elicited a substantial CAR in preschoolers. In contrast, we did not find a CAR after children awakened from an early evening nap (Gribbin et al., 2011), which is consistent with findings of adults napping at a similar time of day (Federenko et al., 2004). Evening naps, which are close to the circadian cortisol nadir may result in less activation of the hypothalamic pituitary adrenal (HPA) axis. Thus, the circadian system likely modulates the CAR, allowing phasic activation of the HPA axis in response to the transition from sleep to wake. Interestingly, an additional study reported that adults who took midafternoon naps following one-night of sleep deprivation showed a robust increase in plasma cortisol levels characteristic of the CAR, which were not present in others who napped without nighttime sleep deprivation (Vgontzas et al., 2007). Clearly, more research using rigorous methods is needed to develop a comprehensive understanding of the nap-dependent CAR and interactions with the circadian and homeostatic sleep systems.
To our knowledge, no published reports on the napping CAR and its relationship to the diurnal pattern of cortisol secretion using objective sleep measures in toddlers exist. To address this gap in the literature, we first employed time-locked samples on nap and no-nap days to verify that the CAR was nap-dependent. Second, we assessed if the slope of the diurnal cortisol pattern was associated with the napping CAR. Finally, using information from our first two objectives, we determined whether a difference in the slope of the diurnal pattern of cortisol between napping and non-napping days exists and if this difference was accounted for by the presence of the post-nap CAR.
2. METHODS
2.1 Recruitment and screening of participants
Participants were 28 children aged 30–36 months old (33.2 ± 2.3 months; 13 females) recruited via flyers, a laboratory website, and personal contact at community events. Screening included parents completing a telephone interview and a set of questionnaires. Study inclusion required that children were regularly following a biphasic sleep schedule (nighttime sleep period of at least 10.5h and one daytime nap of at least 45 min time in bed), during which they fell asleep at least 3 days per week during their nap opportunity. Details regarding study exclusionary criteria have been previously published (Gribbin et al., 2011). In general, participants were healthy, with no chronic illnesses, regular use of medications affecting sleep, circadian rhythms, or HPA axis activity, or behavioral, emotional, or sleep problems.
All families signed a University of Colorado Institutional Review Board approved consent form. At study completion, parents received $220 in cash, and children received a $100 United States Savings Bond. Children were also rewarded with small non-monetary gifts throughout the duration of the study.
2.2 Protocol
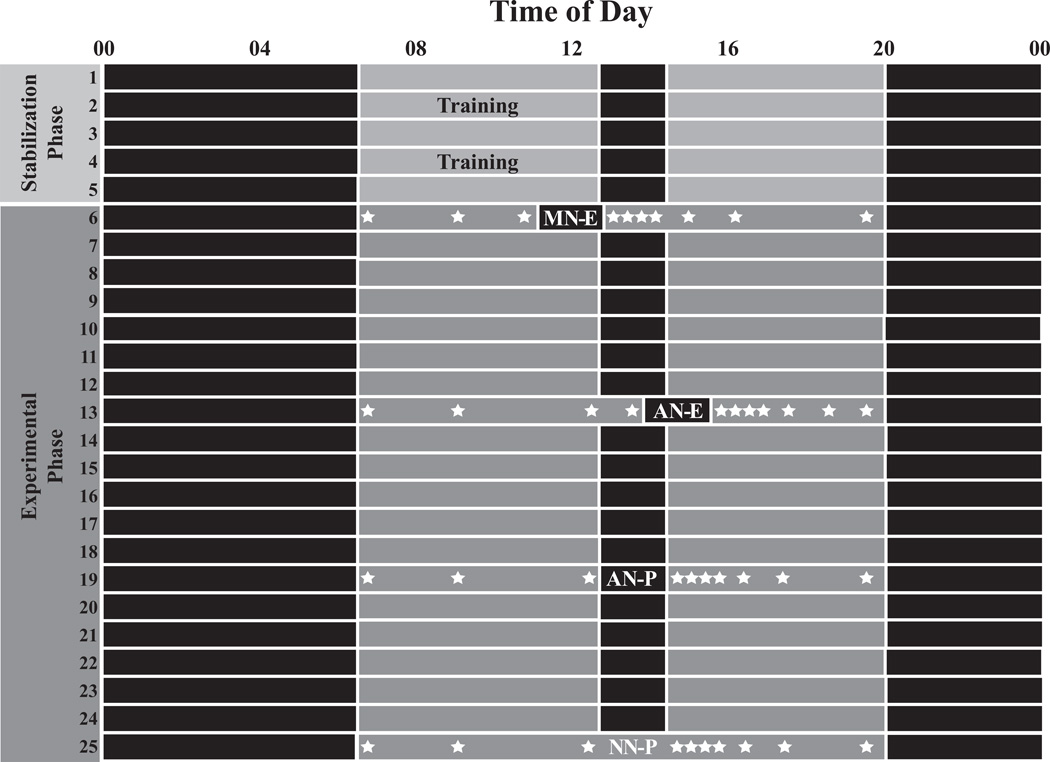
During the study, participants were required to sleep at home or childcare and to refrain from caffeine consumption, including chocolate. Children followed an individualized, prescribed sleep schedule, which allowed us to tailor schedules for each toddler, provide consistency prior to the experimental phase of the study, and control for potential sleep restriction and circadian effects on CAR parameters. Schedule compliance was verified with actigraphy, sleep diaries, and daily email/telephone contact with parents. As shown in Figure 1, the stabilization phase of the protocol included at least 2 training visits, which ensured parents were familiar with the study protocol and could obtain adequate saliva samples from their toddlers. During the experimental phase, children continued to follow their prescribed sleep schedules; however, the timing of the nap varied, as shown on study days 6, 13, and 19. Four randomly-ordered, in-home cortisol assessments occurred during the experimental phase, including a morning nap with experimenters collecting pre- and post-nap saliva samples (MN-E; lights-out 4 h post morning awakening), an afternoon nap with experimenters collecting pre- and post-nap saliva samples (AN-E; lights-out 7 h post morning awakening), an afternoon nap with parents collecting all salivary cortisol samples (AN-P; set to scheduled nap time), and a no-nap condition (NN-P) with the timing of all samples matched to the AN-P condition. Thus, parents or experimenters collected a total of 10 saliva samples across each cortisol assessment day: morning awakening, ~09:30, pre-nap, 0, 15, 30, 45, 90, and 135 min post-nap awakening, and at ~19:30 (before bedtime). On MN-E and AN-E assessment days, experimenters collected only the pre-nap and 6 post-nap samples; parents collected the morning and evening samples. Parents and researchers recorded children’s nap wake times, as well as the start and finish time of each saliva sample. The accuracy of the nap wake time was checked with actigraphy. Researchers subsequently verified that the first sample was taken within 15 minutes post nap awakening to avoid the appearance of a “flat” CAR (Dockray et al., 2008; Okun et al., 2010). The MN-E and AN-E conditions involved sleep EEG recordings as described previously (Gribbin et al., 2011). Scalp electrodes were not removed until the completion of the post-nap CAR samples to avoid potential stress to the participants during the salivary cortisol collection.
Figure 1.
Sample protocol for one study participant. Days 1–5 represent the stabilization phase, and days 6–25 represent the experimental phase during which time children participated in 4 randomly-ordered cortisol assessments: i) morning nap with experimenters collecting pre- and post-nap samples (MN-E); ii) afternoon nap with experimenters collecting pre- and post-nap samples (AN-E); iii) afternoon nap with parents collecting all samples (AN-P); and iv) no-nap with parents collecting all samples (NN-P). Solid black bars represent time in bed, and gray bars represent periods of wakefulness. Note. indicates salivary cortisol collection times.
Insert Figure 1
2.3 Field-based measures
2.3.1 Actigraphy
Standard laboratory procedures for scoring actigraphy data have been published in detail elsewhere (Berger et al., 2012; Gribbin et al., 2011). Briefly, parents completed a sleep diary and toddlers wore actigraphs (model AW2 or Actiwatch Spectrum) on their non-dominant wrist throughout the study (Philips/Respironics, Pittsburg, PA, USA). We used actigraphy to calculate nap lights-out time, nap sleep start time (sleep onset), nap sleep end time (sleep offset), nap period (lights-off to lights-on), nap duration (sleep onset to sleep offset), and latency to the first post-nap wake sample (minutes between sleep offset and first post-wake sample). Compliance with the timing of the cortisol study protocol was also verified with actigraphy. That is, latency to the first cortisol sample occurring at nap awakening was calculated to determine if parents accurately reported wake times on saliva collection days. Daily diary data were used to compute napping variables when actigraphy data was missing due to noncompliance (n=1 day), technical failure (n=5 days), or researcher error (n=1 day). Missing data were from seven different participants on distinct assessment days.
2.3.2 CAR and diurnal salivary cortisol assessments
Parents and researchers followed the same procedures, including withholding all milk products for ≥20 min and all food and liquids ≥15 min before sampling. Saliva was obtained with 6-inch cotton dental rolls (Henry Schein Inc., Melville, NY, USA), which children mouthed for 1–2 min. The cotton roll was then placed into a tube and the excess was cut off with safety scissors that were disinfected with alcohol between samples to avoid contamination. Samples were then capped, refrigerated on-site, and transported to the laboratory within 48 h, where they were centrifuged and frozen at −20° Celsius. Cortisol samples were shipped to Trier, Germany and analyzed using a time resolved fluorescence immunoassay (Dressendörfer et al., 1992); the sensitivity was 6.27× 10−3 µg/dl. The intra-assay coefficient of variation for duplicates of the same sample was 3.26% and the inter-assay coefficients of variation for known cortisol concentrations were 6.21% for low, 6.56% for medium and 7.56% for high concentrations.
2.3.3 Data analysis
Crossover-conditions were incomplete for 8 participants due to non-compliance or scheduling difficulties; thus, 13 of the possible 112 (11.6%) cortisol assessments were not attempted. Because HLM estimates cortisol profiles for each child on individual days, missing data for one experimental condition did not preclude us from utilizing a child’s data for other non-missing conditions. Thus, every participant was retained for analysis. Of the remaining 990 possible data points, 63 (6.4%) saliva samples were missing due to participant non-compliance (n=7), low sample volume (n=18), parent (n=23) or researcher error (n=4), child napping (sleeping) at planned sample time (n=10), or a change in protocol (n=1). All the missing values were replaced with the average of samples obtained by the same subject at the same time on a different sampling day (n=27), at a previous time on the same saliva sampling day (n=22), or the average between two samples occurring before and after the missing sample on the same day (n=14).
Preliminary analyses examined area under the curve with respect to ground (AUCg) (Pruessner et al., 2003) and Wake-Max Change. The Wake-Max Change dynamic of the CAR was computed as the difference between the waking (0 min) and maximum post-wake value wherever it occurred within subjects (15, 30, 45, 90, 135 min; wake-max change). Eight of the 112 possible assessments exhibited a negative post-nap wake-max change due to the highest cortisol concentration occurring upon awakening (0 min). Also, 12 toddlers exhibited a negative wake-max change when not given a nap. This known issue was addressed by setting the wake-max change values to 0, a method used in our previous published work (Gribbin et al., 2011).
The main analyses employed multilevel modeling using HLM Version 6 (Raudenbush et al., 2004). Three level multilevel modeling was used to explore the post-nap CAR and the diurnal cortisol pattern across the four different conditions (i.e., MN-E, AN-E, AN-P, and NN-P). First, a three-level model explored the effects of napping condition on the post-nap CAR based on cortisol values obtained upon awakening from nap (time = 0) and 15 (time = 0.25), 30 (time = 0.5), 45 (time = 0.75), 90 (time = 1.5), and 135 minutes post-nap awakening (time = 2.25). In order to evaluate whether the pattern of the post-nap CAR carried over to a day without a nap, samples were time-locked on NN-P and AN-P days. Cortisol change was modeled with a cubic model that controlled for the effects of sex and age on the cortisol profile. The nap condition variable was dummy-coded, with the NN-P condition serving as a reference condition. Subsequently, two more models estimated the effects of experimental conditions, with MN-E and AN-P as reference conditions. Thus, pairwise comparisons can be drawn about each pair of the four conditions.
Second, a piecewise hierarchical linear model estimated patterns of change in cortisol over the course of the day. The advantage of the piecewise model over a cubic model is that it estimates separate linear slope parameters for different parts of the cortisol rhythm, allowing us to explore the complex dynamics of the daily pattern. As such, our model estimated cortisol at awakening (i.e., intercept, π0), as well as separate slopes for the morning decline (slope1, π1), late-morning decline (slope2, π2), nap-awakening rise (slope 3, π3), post-nap recovery (slope 4, π4), and evening decline (slope 5, π5). Once again, one model used the NN-P condition as a reference, and two subsequently tested models used MN-E and AN-P as reference conditions. Detailed model descriptions can be found in the e-only supplementary file.
3. RESULTS
3.1 Summary statistics and objective protocol verification
Table 1 provides descriptive statistics of objective measures of sleep (actigraphy), latency to the 1st post-wake saliva sample, and the CAR. Average morning wake time on all cortisol collection days was 06:34 (00:42). Nap sleep duration between the three naps did not differ (p>0.05), with an average duration of 91.7 (20.0) minutes. On average, the first sample for all three napping conditions was collected before the 15 min threshold as measured by actigraphy (Dockray et al., 2008; Okun et al., 2010) and did not differ between napping conditions (p>0.05) (see Table 1), with the first post nap sample occurring on average 6.8 (6.0) minutes after nap awakening. In some cases, actigraphy overestimated sleep because toddlers were sitting still in bed while providing post-nap awakening saliva samples, thus, creating a negative latency to first sample value. This was corrected by setting these values to zero (see Data Analysis).
Table 1.
Descriptive statistics presented as Mean (SD) for actigraphic sleep measures, actigraphic estimates of the latency to first saliva sample, and cortisol awakening response (CAR) measures for parent collected (P) and experimenter collected (E) napping conditions.
| Napping Condition | ||||
|---|---|---|---|---|
| Morning Nap-E | Afternoon Nap-E | Afternoon Nap-E | No-Nap-P | |
| Actigraphy Measures | ||||
| Nap lights-out time | 11:07 (0:42) | 13:42 (0:35) | 13:11 (0:41) | ---- |
| Nap sleep start time | 11:26 (0:46) | 13:59 (0:31) | 13:33 (0:55) | ---- |
| Nap sleep end time | 12:57 (0:59) | 15:33 (0:36) | 15:05 (0:49) | ---- |
| Nap period (min) | 116.7 (31.4) | 119.0 (32.0) | 120.0 (24.9) | ---- |
| Nap duration (min) | 90.5 (33.4) | 94.8 (25.1) | 91.8 (23.6) | ---- |
| Latency to 1st sample (min) | 5.8 (5.8) | 6.2 (4.9) | 8.2 (7.5) | ---- |
| CAR Measures | ||||
| AUCg (µg/dl) | 21.72(9.92) | 13.16 (4.27) | 11.72 (4.79) | 8.41(4.18) |
| Wake-Max change (µg/dl) | 0.16 (.13) | 0.11 (.07) | 0.10 (.06) | 0.01 (.02) |
Key: AUCg = area under the curve with respect to ground; Wake-Max change = difference between awakening sample (0 min) and maximum sample at 15, 30, 45, 90, or 135 min.
3.2 CAR profiles
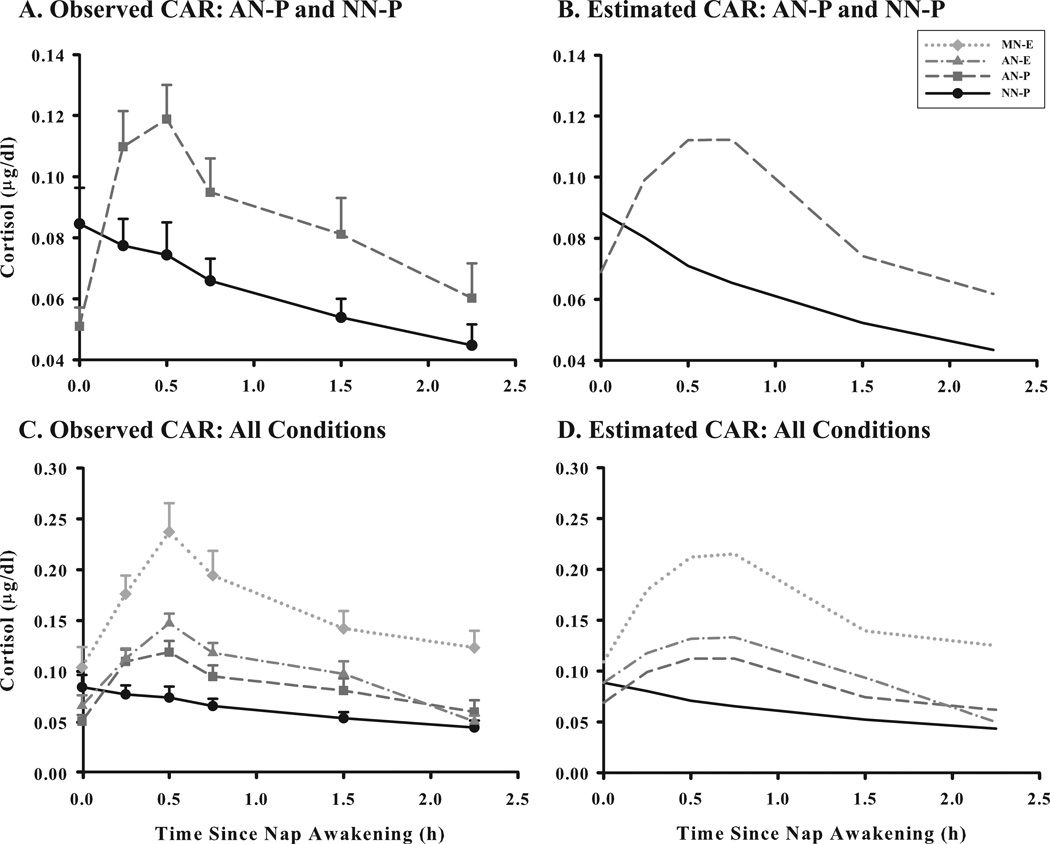
The four napping conditions produced distinct CAR profiles (see Figure 2). The MN-E condition led to the most robust post-nap rise, whereas the NN-P condition was not characterized by a cortisol rise around regular nap times. As shown in Table 2, napping at either time (morning or afternoon) was associated with a significant linear cortisol rise (as indicated by linear slope) and significant quadratic deceleration (as indicated by significant negative quadratic change parameters). Compared to all other conditions, the MN-E condition was associated with the most pronounced linear rise, the greatest quadratic deceleration around 30 minutes to 1 hour post-nap, and a slower cubic decline resulting in higher cortisol values around 1.5–2 hours post-awakening. The cortisol profile for the AN-P condition was not significantly different from the AN-E condition (based on values of the intercept and the three change parameters). The magnitudes of these parameters were significantly greater than those for the NN-P condition, but smaller than those for the MN-E condition.
Figure 2.
Average cortisol awakening response (CAR) profiles (n=28) for four napping conditions: i) morning nap – experimenter collected samples (MN-E); ii) afternoon nap – experimenter collected samples (AN-E); iii) afternoon nap – parent collected samples (AN-P); and iv) no-nap – parent collected samples (NN-P; sample times matched to AN-P). Salivary cortisol (µg/dl) samples were obtained at 0, 15, 30, 45, 90, and 135 minutes after nap wake time (verified objectively with actigraphy). Profiles for observed (A) and estimated (B) values of the CAR for AN-P and NN-P conditions and observed (C) and estimated (D) values for all CAR conditions using 3 models in HLM 6. Error bars represent SEM.
Table 2.
The effects of napping conditions on the cortisol awakening response (CAR) growth curve parameters.
| Reference Condition | ||||||
|---|---|---|---|---|---|---|
| No-Nap-P | Morning Nap-E | Afternoon Nap-P | ||||
| B | SE | B | SE | B | SE | |
| Intercept | 2.31 | 0.28 | 3.02 | 0.38 | 1.91 | 0.37 |
| Morning nap-E | 0.70 | 0.44 | -- | -- | 1.11 | 0.60 |
| Afternoon nap-P | −0.41 | 0.40 | −1.11* | 0.49 | -- | -- |
| Afternoon nap-E | 0.13 | 0.36 | −0.57 | 0.46 | 0.54 | 0.40 |
| No-Nap-P | -- | -- | −0.70 | 0.47 | 0.41 | 0.40 |
| Slope | −0.78 | 0.83 | 10.21*** | 1.64 | 4.41** | 1.30 |
| Morning nap-E | 11.00*** | 2.62 | -- | -- | 5.80* | 2.68 |
| Afternoon nap-P | 5.19** | 1.53 | −5.80* | 2.29 | ||
| Afternoon nap-E | 4.97** | 1.68 | −6.02** | 2.16 | −0.22 | 2.08 |
| No-Nap-P | -- | -- | −11.00*** | 2.23 | −5.19** | 1.53 |
| Quadratic change | 0.15 | 0.82 | −10.41*** | 1.73 | −4.63** | 1.34 |
| Morning nap-E | −10.56*** | 2.77 | -- | -- | 4.77** | 1.65 |
| Afternoon nap-P | −4.77** | 1.65 | 5.79* | 2.37 | ||
| Afternoon nap-E | −4.20* | 1.90 | 6.36** | 2.23 | 0.57 | 2.14 |
| No-Nap-P | -- | -- | 10.56*** | 2.30 | 4.77** | 1.65 |
| Cubic change | −0.01 | 0.21 | 2.65*** | 0.47 | 1.17** | 0.37 |
| Morning nap-E | 2.66** | 0.74 | -- | -- | 1.48* | 0.67 |
| Afternoon nap-P | 1.18* | 0.45 | −1.48* | 0.64 | -- | -- |
| Afternoon nap-E | 0.89 | 0.54 | −1.76** | 0.60 | −0.28 | 0.58 |
| No-Nap-P | -- | -- | −2.66*** | 0.62 | −1.18* | 0.45 |
Note. The four nap conditions were dummy coded. Three models were estimated, each with the parent collected no-nap (No-Nap-P), experimenter collected morning nap (Morning Nap-E), or parent collected afternoon nap (Afternoon Nap-P) as a reference condition in order to evaluate all pairwise comparisons among the four cortisol profiles.
p < 0.05;
p < 0.01;
p < 0.001
In order to understand the time course of the CAR, we computed for each participant the latency from nap awakening and the time at which cortisol levels returned to their pre-nap levels using linear interpolation between successive samples. Post-nap cortisol levels returned to pre-nap levels 94.5 (32.3) minutes after the AN-P wake time and 103.7 (36.7) minutes after the AN-E wake time. For the MN-E, the latency between nap wake time and pre-nap cortisol levels of 126.4 (76.7) min, with 4 children not reaching their pre-nap level until the evening sample.
3.4 Diurnal cortisol profiles
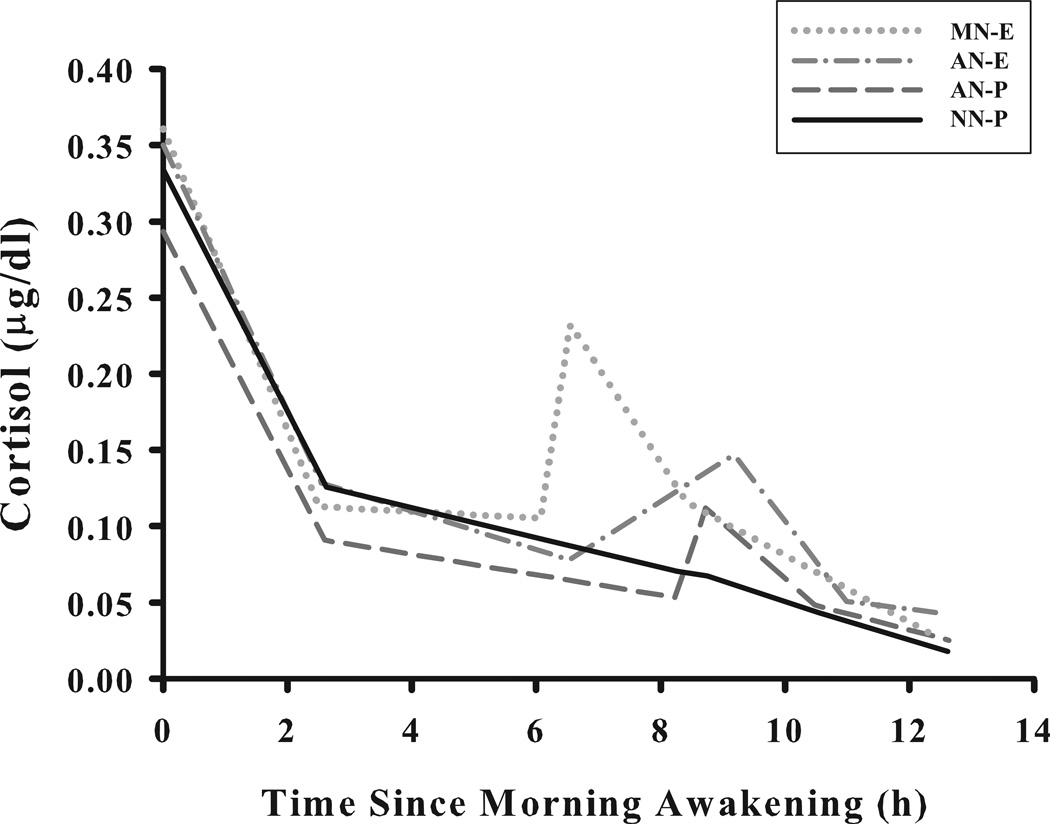
As illustrated in Figure 3, the four napping conditions also contributed to distinct diurnal cortisol patterns. The differences were mostly due to variability in the post-nap awakening cortisol response with MN-E producing the highest rise and subsequent recovery, and both AN-E and AN-P producing intermediary rise and recovery values (see Figure 2 for comparisons of rise and recovery slopes within the diurnal model). These findings replicate the differences observed in the model of the post-nap CAR (see Figure 2); however, we found additional differences in the rates of evening cortisol decline. MN-E was associated with a 0.27 (p < 0.01) µg/ml per hour steeper evening decline than the NN-P condition and a 0.30 (p < 0.01) µg/ml per hour steeper decline than the AN-P condition (see Figure 3). The direction of differences was similar for the AN-E vs. MN-E nap comparison (with the morning nap having a steeper decline), but it did not attain significance.
Figure 3.
The diurnal secretory pattern of cortisol (n=28) on four napping condition days: i) morning nap – experimenter collected samples (MN-E); ii) afternoon nap – experimenter collected samples (AN-E); iii) afternoon nap – parent collected samples (AN-P); and iv) no-nap – parent collected samples (NN-P; sample times matched to AN-P). Salivary cortisol samples (µg/dl) were obtained at morning awakening, 09:30, pre-nap, 0, 15, 30, 45, 90, and 135 min post-nap awaking and ~19:30 (nap wake time verified with actigraphy). Piecewise growth modeling estimated linear coefficients for five separate periods throughout the day (corresponding to morning decline, noon decline, post-nap rise, post-nap decline, and evening decline).
4. DISCUSSION
This research produced several significant findings about the napping CAR and its relationship to the diurnal pattern of cortisol secretion in healthy toddlers. First, when appropriate controls are used, a robust nap-dependent difference in the CAR exists: we observed a robust awakening response after napping that was absent on a day without a nap. Second, nap timing influenced the CAR and the diurnal pattern of cortisol secretion, with the morning nap resulting in the most robust CAR and a slower evening decline compared to the afternoon nap. Third, the slope of the diurnal cortisol pattern was influenced by the nap for at least 90 minutes, with the post-nap rise and post-nap decline contributed to differences in the diurnal secretory pattern between a day with an afternoon nap and a day without a nap. To our knowledge, this is the first published data employing extensive sampling and experimental control to describe the impact of not napping, as well as napping in the morning and afternoon on the CAR and the diurnal pattern of cortisol secretion in healthy toddlers.
The process of awakening is hypothesized to involve a “flip-flop” mechanism in which quick reciprocal switching between activation in cortical and sub-cortical brain regions is associated with the transition between sleep and consciousness (Saper et al., 2001). This switching of brain circuitry may drive the CAR phenomenon, as the process of awakening is a potent stimulus (Späth-Schwalbe et al., 1992; Wilhelm et al., 2007). Our well-controlled results revealed that a robust CAR is present after morning and afternoon naps but absent on a day without a nap when time-locked samples were obtained. This fundamental finding suggests that cortisol is not only regulated by the circadian system (Czeisler and Klerman, 1999) but also is nap-dependent. It is important to note that our results are not consistent with earlier research indicating the absence of a CAR following a nap in older infants (Bright et al., 2012); however, that study did not use objective assessment of sleep or nap wake time, which may have resulted in parents not accurately recognizing their child’s waking state and thus missing the opportunity to capture the CAR. Whether the CAR is already present in infancy or develops sometime before the toddler years is an important empirical question that will necessitate further study using objective measures of wake time.
Because our published work showed that preschoolers had relatively high cortisol secretion up to 60 min after nap awakening (Gribbin et al., 2011) and our sampling approach did not allow us to capture a return to pre-nap levels in all children, we utilized an expanded protocol to more comprehensively capture the CAR time course by sampling at 0, 15, 30, 45, 90, and 135 min post-nap awakening. On average, we found that the maximum post-nap waking cortisol value occurred at 30 min; however, some toddlers obtained their max value at 45 or 90 min after awakening. Additionally, average cortisol levels remained elevated and did not return to pre-nap levels until ~100 min after awakening from both the afternoon naps. For the morning nap, the return to pre-nap levels after awakening took just over 2 hours, with several participants not diminishing to their pre-nap levels until just before bedtime. These findings have significant implications for researchers utilizing stress paradigms to examine cortisol reactivity in young children during the afternoon following a nap (Blair and Razza, 2007; Luby et al., 2003) and perhaps even more so for studies examining diurnal patterning on days with different activities, for example child care and home days (Watamura et al., 2003). Whether the nap-dependent CAR would change HPA axis activity during an afternoon challenge context (e.g., because cortisol is already elevated on nap days, would children be more or less reactive during a stress protocol?) is an important empirical question; however, our results showing the time course of cortisol levels after nap awakening suggest that researchers should at the very least document nap timing and duration, and perhaps consider waiting 100–120 minutes before eliciting a stress response. If the baseline cortisol level restricts the magnitude and time course of the subsequent response, and if the study design evaluates differences between children of different ages or in different contexts as central questions, a failure to document and potentially avoid nap time influences on the subsequent response could confound the results. Indeed, this concern was a key motivation for the current study design.
In this study, we observed no differences in afternoon nap CAR parameters when experimenters were present and when parents collected samples, a finding suggesting that experimenter presence did not alter children’s levels of stress or account for the subsequent CAR. We did, however, find a less robust CAR after the afternoon than the morning nap. Because slow-wave sleep (SWS) is known to inhibit HPA secretion during the night (Gronfier et al., 1997; Späth-Schwalbe et al., 1992), it is possible that the same process unfolds during daytime naps, especially those rich with deep sleep. Based upon the two-process model of sleep regulation (Borbely, 1982), the morning nap (4 h prior wakefulness) of our toddlers should have included less SWS than afternoon naps (7 h prior wakefulness). Thus, we evaluated data from the sleep EEG recordings of 21 children collected during naps when experimenters were present using a two-tailed paired samples t-test. As expected, toddlers experienced significantly more slow-wave (deep) sleep during the afternoon nap (39.9%) compared to the morning nap (27.2%; t(20) = −2.95, d = 0.75, p = 0.008), which may be an important pathway to variability in post-nap cortisol secretion. We also acknowledge that light levels, which were not controlled in our study, could have in part accounted for differences between the morning and afternoon CAR parameters.
All toddlers in our sample demonstrated a clear pattern of daytime cortisol secretion; however, the dynamic of this pattern differed on napping and non-napping days. Across all napping conditions, the highest values on average were obtained at morning awakening (not different across conditions) and the lowest values occurred close to bedtime. For the morning and afternoon nap assessments, cortisol levels decreased significantly from morning to noon, increased post nap awakening, and then decreased following the post-nap increase, with an additional decline in the evening hours. This immature diurnal pattern of cortisol secretion was not observed on the no-nap day, which instead resembled the adult pattern, with a rapid decrease during the morning hours and a more gradual decline throughout the day (Edwards et al., 2001; Van Cauter, 2005). One study of the diurnal cortisol pattern at childcare showed that cortisol levels were higher on napping days compared to non-napping days in a small subsample of 2- to 4-year-olds who were observed to nap on one day but not another at child care (Watamura et al., 2002). Our experimental in-home findings suggest that this prior observation is due to the post-nap CAR, although the environmental context may also contribute to differences in the pattern of cortisol across the day (Vermeer and van IJzendoorn, 2006; Watamura et al., 2003) because variations between home and child care are more difficult to explain as nap driven. It is also the case that factors like child temperament, child care quality, and attachment to parents and teachers are related to differences in cortisol patterning between home and child care, and these effects would also be difficult to explain with nap differences alone. The current data do suggest, however, that context differences which do not control for post-nap CAR differences may need to be reevaluated, as an important component of the variance in cortisol may be missed when objectively verified sleep timing and duration is not recorded. Whether adults would also show an immature pattern of cortisol secretion on days napping is unknown: The one published study did not find a CAR after an early evening nap (Federenko et al., 2004), a time of day when we also did not observe a nap-dependent CAR in toddlers (Gribbin et al., 2011). Studies examining afternoon napping in young and older adults are needed to determine whether a CAR exists after naps occurring at earlier times in the day and whether this impacts subsequent behavior or physiology.
The findings of the current study provide strong evidence that the difference in cortisol profiles between napping and non-napping days at home is accounted for by the presence of the post nap CAR. Prior studies of the diurnal cortisol pattern used fewer saliva samples, employed a cubic model (Sumner et al., 2010; Van Ryzin et al., 2009; Watamura et al., 2004), and therefore, did not detect all possible variations in the diurnal patterns that are due to napping. To our knowledge, this study is the first to employ an experimental protocol obtaining 10 samples across the day, and thus, piecewise growth modeling was used to grasp subtle changes in the diurnal pattern that a cubic model would otherwise miss. Previous studies have shown developmental changes in cortisol activity throughout early childhood and adolescence (Kiess et al., 1995; Walker et al., 2001), with one showing more adult like decreases in cortisol in 30–36 month olds with shorter naps (Watamura et al., 2004). Thus, napping could play a critical role in the early development of the HPA axis; however, whether developmental changes in the cortisol profiles are due to decreasing frequency and duration of naps needs to be addressed through longitudinal studies using objective assessments of napping.
Although our experimental data contribute to the current understanding of the development of the HPA axis and its relation to napping during early childhood, our study had several limitations. Foremost, the generalizability of our findings is restricted because we studied only healthy, good-sleeping toddlers who had relatively stable sleep-wakefulness schedules and were in families willing and able to complete the extensive month-long experimental sleep manipulation. Additionally, although actigraphy allowed us to estimate the timing of the first sample post-nap awakening, many samples were obtained by parents, who were entrusted to accurately record their timing using a tab sheet rather than electronic monitoring containers (Kudielka et al., 2003). Also, as noted above, light levels were not controlled during sampling or during the napping periods, which may have produced more error variance in our CAR measures.
Collectively, our experimental data showing that the nap-dependent CAR influences the diurnal pattern of cortisol secretion are important for understanding the early maturation of the HPA axis. We believe they also provide a foundation for future studies examining the diurnal slope in clinical populations, such as depressed preschoolers, who may also have flattened cortisol profiles similar to adolescents and adults (Forbes et al., 2006; Van den Bergh et al., 2008). Additionally, the diurnal slope of cortisol and the CAR as potential disease biomarkers are of ongoing interest due to their association with poor health outcomes (Champaneri et al., 2013; Cullen et al., 2014; Imeraj et al., 2012). Finally, our findings make an important methodological contribution to researchers studying cortisol reactivity in early childhood, a time of dramatic change in napping patterns (Crosby et al., 2005; Weissbluth, 1995).
Supplementary Material
Table 3.
The effects of napping on the diurnal cortisol pattern.
| Reference Condition | ||||||
|---|---|---|---|---|---|---|
| No-Nap-P | Morning Nap-E | Afternoon Nap-P | ||||
| B | SE | B | SE | B | SE | |
| Intercept | 9.22 | 0.82 | 9.95 | 1.04 | 8.09 | 0.84 |
| Morning nap-E | 0.73 | 0.96 | -- | -- | 1.86 | 1.13 |
| Afternoon nap-P | −1.13 | 1.02 | −1.86 | 1.13 | -- | -- |
| Afternoon nap- E | 0.44 | 1.06 | −0.29 | 1.12 | 1.57 | 0.60 |
| No-Nap-P | -- | -- | −0.73 | 0.96 | 1.13 | 0.02 |
| Morning decline | −2.19*** | 0.37 | −2.72*** | 0.42 | −2.15*** | 0.36 |
| Morning nap-E | −0.53 | 0.45 | -- | -- | −0.57 | 0.52 |
| Afternoon nap-P | 0.04 | 0.36 | 0.57 | 0.52 | -- | -- |
| Afternoon nap-E | −0.21 | 0.40 | 0.32 | 0.50 | −0.26 | 0.29 |
| No-Nap-P | -- | -- | 0.53 | 0.45 | −0.04 | 0.36 |
| Noon decline | −0.27*** | 0.07 | −0.06 | 0.16 | −0.19* | 0.09 |
| Morning nap-E | 0.21 | 0.17 | -- | -- | 0.12 | 0.16 |
| Afternoon nap-P | 0.09 | 0.10 | −0.12 | 0.16 | -- | -- |
| Afternoon nap-E | −0.08 | 0.09 | −0.29 | 0.16 | −0.17 | 0.11 |
| No-Nap-P | -- | -- | −0.21 | 0.17 | −0.09 | 0.10 |
| Nap-awakening rise | −0.17 | 0.49 | 7.32*** | 1.54 | 3.24*** | 0.76 |
| Morning nap-E | 7.50*** | 1.64 | -- | -- | 4.08* | 1.70 |
| Afternoon nap-P | 3.41*** | 0.84 | −4.08* | 1.70 | -- | -- |
| Afternoon nap-E | 0.89 | 0.50 | −6.61*** | 1.52 | −2.53** | 0.77 |
| No-Nap-P | -- | -- | −7.50*** | 1.64 | −3.41*** | 0.84 |
| Post-nap decline | −.38* | 0.18 | −1.71*** | 0.41 | −1.00*** | 0.23 |
| Morning nap-E | −1.34** | 0.47 | -- | -- | −0.71 | 0.40 |
| Afternoon nap-P | −0.63* | 0.27 | 0.71 | 0.40 | -- | -- |
| Afternoon nap-E | −1.10*** | 0.25 | 0.24 | 0.41 | −0.47 | 0.26 |
| No-Nap-P | -- | -- | 1.34** | 0.47 | 0.63* | 0.27 |
| Evening decline | −0.33** | 0.09 | −0.60*** | 0.12 | −0.30** | 0.11 |
| Morning nap-E | −0.27** | 0.10 | -- | -- | −0.30* | 0.14 |
| Afternoon nap-P | 0.04 | 0.15 | 0.30* | 0.14 | -- | -- |
| Afternoon nap-E | 0.20 | 0.29 | 0.47 | 0.29 | 0.16 | 0.27 |
| No-Nap-P | -- | -- | 0.27* | 0.10 | −0.04 | 0.15 |
Note: The four nap conditions were dummy coded. Three models were estimated, each with either the parent collected no-nap (No-Nap-P), experimenter collected morning nap (Morning Nap-E), or parent collected afternoon nap (Afternoon Nap-P) as a reference condition in order to evaluate all pairwise comparisons among the four cortisol profiles. Piecewise growth modeling was used to estimate linear coefficients for five separate periods.
p < .05,
p < .01,
p < .001
HIGHLIGHTS.
The cortisol awakening response is nap-dependent in toddlers.
Napping leads to a less mature diurnal pattern of cortisol secretion.
Napping influences the slope of the diurnal cortisol pattern for 1.5–2.0 hours.
ACKNOWLEDGEMENTS
We are most grateful to the children and families for their generosity, time, and effort in making this study possible. We also appreciate all the University of Colorado Boulder undergraduate students and research assistants who collected these data. Hannah LeBourgeois assisted in the scoring and management of actigraphy data. This research was supported with funds from the National Institute of Mental Health (R01-MH086566).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
MKL and SEW developed the study design.
MKL and RCT carried out the data collection.
MKL, JD, SEW, and RCT performed the data analysis.
MKL, RCT, JD, and SEW prepared the manuscript.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- Baumler D, Kirschbaum C, Kliegel M, Alexander N, Stalder T. The cortisol awakening response in toddlers and young children. Psychoneuroendocrinology. 2013 doi: 10.1016/j.psyneuen.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Berger RH, Miller AL, Seifer R, Cares SR, LeBourgeois MK. Acute sleep restriction effects on emotion responses in 30- to 36-month-old children. Journal of sleep research. 2012;21:235–246. doi: 10.1111/j.1365-2869.2011.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Razza RP. Relating Effortful Control, Executive Function, and False Belief Understanding to Emerging Math and Literacy Ability in Kindergarten. Child Dev. 2007;78:647–663. doi: 10.1111/j.1467-8624.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- Borbely AA. A two process model of sleep regulation. Human neurobiology. 1982;1:195–204. [PubMed] [Google Scholar]
- Bright MA, Granger DA, Frick JE. Do infants show a cortisol awakening response? Developmental Psychobiology. 2012;54:736–743. doi: 10.1002/dev.20617. [DOI] [PubMed] [Google Scholar]
- Champaneri S, Xu XQ, Carnethon MR, Bertoni AG, Seeman T, DeSantis AS, Roux AD, Shrager S, Golden SH. Diurnal Salivary Cortisol Is Associated with Body Mass Index and Waist Circumference: The Multiethnic Study of Atherosclerosis. Obesity. 2013;21:E56–E63. doi: 10.1002/oby.20047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clow A, Hucklebridge F, Stadler T, Evans P, Thorn L. The cortisol awakening response: More than a measure of HPA axis function. Neurosci Biobehav Rev. 2010;35:97–103. doi: 10.1016/j.neubiorev.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Crosby B, LeBourgeois MK, Harsh J. Racial differences in reported napping and nocturnal sleep in 2- to 8-year-old children. Pediatrics. 2005;115:225–232. doi: 10.1542/peds.2004-0815D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen AE, Zunszain PA, Dickson H, Roberts RE, Fisher HL, Pariante CM, Laurens KR. Cortisol awakening response and diurnal cortisol among children at elevated risk for schizophrenia: Relationship to psychosocial stress and cognition. Psychoneuroendocrinology. 2014;46:1–13. doi: 10.1016/j.psyneuen.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeisler CA, Klerman EB. Circadian and sleep-dependent regulation of hormone release in humans. Recent Prog Horm Res. 1999;54:97–130. discussion 130–132. [PubMed] [Google Scholar]
- Davis EP, Bruce J, Gunnar MR. The anterior attention network: Associations with temperament and neuroendocrine activity in 6-year-old children. Developmental Psychobiology. 2002;40:43–56. doi: 10.1002/dev.10012. [DOI] [PubMed] [Google Scholar]
- Dockray S, Bhattacharyya MR, Molloy GJ, Steptoe A. The cortisol awakening response in relation to objective and subjective measures of waking in the morning. Psychoneuroendocrinology. 2008;33:77–82. doi: 10.1016/j.psyneuen.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Dressendörfer R, Kirschbaum C, Rohde W, Stahl F, Strasburger C. Synthesis of a cortisolbiotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. The Journal of steroid biochemistry and molecular biology. 1992;43:683–692. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- Edwards S, Clow A, Evans P, Hucklebridge F. Exploration of the awakening cortisol response in relation to diurnal cortisol secretory activity. Life Sci. 2001;68:2093–2103. doi: 10.1016/s0024-3205(01)00996-1. [DOI] [PubMed] [Google Scholar]
- Elder GJ, Wetherell MA, Barclay NL, Ellis JG. The cortisol awakening response– Applications and implications for sleep medicine. Sleep Medicine Reviews. 2013 doi: 10.1016/j.smrv.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Federenko I, Wust S, Hellhammer DH, Dechoux R, Kumsta R, Kirschbaum C. Free cortisol awakening responses are influenced by awakening time. Psychoneuroendocrinology. 2004;29:174–184. doi: 10.1016/s0306-4530(03)00021-0. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Williamson DE, Ryan ND, Birmaher B, Axelson DA, Dahl RE. Peri-sleep-onset cortisol levels in children and adolescents with affective disorders. Biol Psychiatry. 2006;59:24–30. doi: 10.1016/j.biopsych.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag CM, Hanig S, Palmason H, Meyer J, Wust S, Seitz C. Cortisol awakening response in healthy children and children with ADHD: impact of comorbid disorders and psychosocial risk factors. Psychoneuroendocrinology. 2009;34:1019–1028. doi: 10.1016/j.psyneuen.2009.01.018. [DOI] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Gribbin CE, Watamura SE, Cairns A, Harsh JR, LeBourgeois MK. The cortisol awakening response (CAR) in 2- to 4-year-old children: Effects of acute nighttime sleep restriction, wake time, and daytime napping. Developmental Psychobiology. 2011 doi: 10.1002/dev.20599. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronfier C, Luthringer R, Follenius M, Schaltenbrand N, Macher JP, Muzet A, Brandenberger G. Temporal relationships between pulsatile cortisol secretion and electroencephalographic activity during sleep in man. Electroen Clin Neuro. 1997;103:405–408. doi: 10.1016/s0013-4694(97)00013-1. [DOI] [PubMed] [Google Scholar]
- Gunnar MR. Studies of the human infant's adrenocortical response to potentially stressful events. New directions for child development. 1989:3–18. doi: 10.1002/cd.23219894503. [DOI] [PubMed] [Google Scholar]
- Hellman L, Nakada F, Curti J, Weitzman ED, Kream J, Roffwarg H, Ellman S, Fukushima DK, Gallagher T. Cortisol is secreted episodically by normal man. The Journal of Clinical Endocrinology & Metabolism. 1970;30:411–422. doi: 10.1210/jcem-30-4-411. [DOI] [PubMed] [Google Scholar]
- Iglowstein I, Jenni O, Molinari L, Largo R. Sleep duration from infancy to adolescence: reference values and generational trends. Pediatrics. 2003;111:302–307. doi: 10.1542/peds.111.2.302. [DOI] [PubMed] [Google Scholar]
- Imeraj L, Antrop I, Roeyers H, Swanson J, Deschepper E, Bal S, Deboutte D. Time-of-day effects in arousal: disrupted diurnal cortisol profiles in children with ADHD. J Child Psychol Psyc. 2012;53:782–789. doi: 10.1111/j.1469-7610.2012.02526.x. [DOI] [PubMed] [Google Scholar]
- Kiess W, Meidert A, Dressendörfer R, Schriever K, Kessler U, Köunig A, Schwarz H, Strasburger C. Salivary cortisol levels throughout childhood and adolescence: relation with age, pubertal stage, and weight. Pediatr Res. 1995;37:502–506. doi: 10.1203/00006450-199504000-00020. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol. Encyclopedia of stress. 2000;3 [Google Scholar]
- Kudielka BM, Broderick JE, Kirschbaum C. Compliance with saliva sampling protocols: electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosom Med. 2003;65:313–319. doi: 10.1097/01.psy.0000058374.50240.bf. [DOI] [PubMed] [Google Scholar]
- Luby JL, Heffelfinger A, Mrakotsky C, Brown K, Hessler M, Spitznagel E. Alterations in stress cortisol reactivity in depressed preschoolers relative to psychiatric and no-disorder comparison groups. Arch Gen Psychiatry. 2003;60:1248–1255. doi: 10.1001/archpsyc.60.12.1248. [DOI] [PubMed] [Google Scholar]
- Michels N, Sioen I, De Vriendt T, Huybrechts I, Vanaelst B, De Henauw S. Children's morning and evening salivary cortisol: pattern, instruction compliance and sampling confounders. Hormone research in paediatrics. 2011;77:27–35. doi: 10.1159/000334412. [DOI] [PubMed] [Google Scholar]
- Okun ML, Krafty RT, Buysse DJ, Monk TH, Reynolds CF, 3rd, Begley A, Hall M. What constitutes too long of a delay? Determining the cortisol awakening response (CAR) using self-report and PSG-assessed wake time. Psychoneuroendocrinology. 2010;35:460–468. doi: 10.1016/j.psyneuen.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner J, Wolf O, Hellhammer D, Buske-Kirschbaum A, Von Auer K, Jobst S, Kaspers F, Kirschbaum C. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61:2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS, Congdon R. HLM 6 for Windows [Computer software] Lincolnwood, IL: Scientific Software International; 2004. [Google Scholar]
- Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- Scher A, Hall WA, Zaidman-Zait A, Weinberg J. Sleep quality, cortisol levels, and behavioral regulation in toddlers. Developmental Psychobiology. 2010;52:44–53. doi: 10.1002/dev.20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Späth-Schwalbe E, Schöller T, Kern W, Fehm H, Born J. Nocturnal adrenocorticotropin and cortisol secretion depends on sleep duration and decreases in association with spontaneous awakening in the morning. The Journal of clinical endocrinology and metabolism. 1992;75:1431–1435. doi: 10.1210/jcem.75.6.1334495. [DOI] [PubMed] [Google Scholar]
- Stalder T, Bäumler D, Miller R, Alexander N, Kliegel M, Kirschbaum C. The cortisol awakening response in infants: Ontogeny and associations with development-related variables. Psychoneuroendocrinology. 2013;38:552–559. doi: 10.1016/j.psyneuen.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Sumner MM, Bernard K, Dozier M. Young children's full-day patterns of cortisol production on child care days. Archives of pediatrics & adolescent medicine. 2010;164:567–571. doi: 10.1001/archpediatrics.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauter E. Endocrine physiology. Principles and practice of sleep medicine. 2005;4:266–282. [Google Scholar]
- Van den Bergh BR, Van Calster B, Pinna Puissant S, Van Huffel S. Self-reported symptoms of depressed mood, trait anxiety and aggressive behavior in post-pubertal adolescents: Associations with diurnal cortisol profiles. Hormones and behavior. 2008;54:253–257. doi: 10.1016/j.yhbeh.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Van Ryzin MJ, Chatham M, Kryzer E, Kertes DA, Gunnar MR. Identifying atypical cortisol patterns in young children: the benefits of group-based trajectory modeling. Psychoneuroendocrinology. 2009;34:50–61. doi: 10.1016/j.psyneuen.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer HJ, van IJzendoorn MH. Children's elevated cortisol levels at daycare: A review and meta-analysis. Early Child Res Q. 2006;21:390–401. [Google Scholar]
- Vgontzas AN, Pejovic S, Zoumakis E, Lin H-M, Bixler EO, Basta M, Fang J, Sarrigiannidis A, Chrousos GP. Daytime napping after a night of sleep loss decreases sleepiness, improves performance, and causes beneficial changes in cortisol and interleukin-6 secretion. American Journal of Physiology-Endocrinology and Metabolism. 2007;292:E253–E261. doi: 10.1152/ajpendo.00651.2005. [DOI] [PubMed] [Google Scholar]
- Walker EF, Walder DJ, Reynolds F. Developmental changes in cortisol secretion in normal and at-risk youth. Development and Psychopathology. 2001;13:721–732. doi: 10.1017/s0954579401003169. [DOI] [PubMed] [Google Scholar]
- Watamura SE, Donzella B, Alwin J, Gunnar MR. Morning-to-afternoon increases in cortisol concentrations for infants and toddlers at child care: Age differences and behavioral correlates. Child Development. 2003;74:1006–1020. doi: 10.1111/1467-8624.00583. [DOI] [PubMed] [Google Scholar]
- Watamura SE, Donzella B, Kertes DA, Gunnar MR. Developmental changes in baseline cortisol activity in early childhood: relations with napping and effortful control. Dev Psychobiol. 2004;45:125–133. doi: 10.1002/dev.20026. [DOI] [PubMed] [Google Scholar]
- Watamura SE, Sebanc AM, Gunnar MR. Rising cortisol at childcare: relations with nap, rest, and temperament. Dev Psychobiol. 2002;40:33–42. doi: 10.1002/dev.10011. [DOI] [PubMed] [Google Scholar]
- Weissbluth M. Naps in children: 6 months-7 years. Sleep. 1995;18:82–87. doi: 10.1093/sleep/18.2.82. [DOI] [PubMed] [Google Scholar]
- Wilhelm I, Born J, Kudielka BM, Schlotz W, Wüst S. Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology. 2007;32:358–366. doi: 10.1016/j.psyneuen.2007.01.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.